Abstract
The induction of lytic infection has been proposed as a therapeutic strategy for treating Epstein-Barr virus (EBV)-positive malignancies. To succeed, efficient methods are needed for activating the EBV immediate-early (IE) promoters, Zp and Rp. Here we compared factors which regulate Zp and Rp in AGS gastric carcinoma cells that support a remarkably high level of persistently lytic EBV infection with HeLa cervical cells that permit only tightly latent infection. We found that the level of Zp activity assayed by transient transfection assays with reporter plasmids was high in AGS cells but low in HeLa cells. The level of Rp activity was low in both cell types. Mutational analysis indicated that sequences within Zp located between −70 and +27 relative to the transcription initiation site were sufficient to confer a high level of Zp activity in AGS cells. The Zp CRE motif was necessary for this constitutive activity, while the ZIA and ZIB MEF2D motifs were not. Consistent with these findings, immunoblot analysis indicated that phosphorylated c-Jun, which activates Zp through the CRE motif, was expressed at a much higher level in EBV-infected AGS cells than in EBV-infected HeLa cells. In contrast, ZEB1, which represses Zp via the ZV motif located near the transcription initiation site, was abundant in HeLa cells, while it was absent from AGS cells. Exogenous addition of ZEB1 led to the repression of Zp in AGS cells. We conclude that the unusually high Zp activity level in AGS cells is due to the high abundance of positively acting transcription factors such as c-Jun combined with the low abundance of negatively acting factors such as ZEB1.
Epstein-Barr virus (EBV) is a human herpesvirus that causes infectious mononucleosis, and EBV is associated with both epithelial and B-cell malignancies (reviewed in references 34 and 53). Like all herpesviruses, EBV can infect cells either latently or lytically. Lytic infection is required for the production of infectious viral particles, enabling the virus to spread from cell to cell and host to host. In the human host, lytic EBV infection is generally restricted to differentiated oropharyngeal epithelial cells and plasma cells. The switch from latent to lytic infection is mediated by the two viral immediate-early (IE) proteins, BZLF1 (also called Z, Zta, and ZEBRA) and BRLF1 (also called R and Rta). BZLF1 and BRLF1 are transcription factors that activate both each other's expression and, together, the entire lytic cascade of EBV gene expression (1, 9, 11, 12, 14, 24, 26, 33, 40, 52, 54, 67). High-level expression of the BZLF1 gene or, in some cell lines, the BRLF1 gene is sufficient to convert cells from a latent to a lytic form of viral infection. In latently infected cells, the EBV IE genes are not transcribed. Therefore, the activation of one or both of these IE promoters by cellular transcription factors is the first crucial step in reactivation of EBV out of latency into its lytic cycle of replication.
EBV infection of normal oropharyngeal epithelial cells in humans results in completely lytic infection (38; reviewed in references 34, 53, and 56). However, in EBV-associated epithelial malignancies, including nasopharyngeal and gastric carcinomas, most of the tumor cells contain one of the latent forms of viral infection (reviewed in references 29, 34, and 53). Presumably, the establishment of a predominantly latent form of EBV infection in these epithelial tumors helps both to ensure that the virus does not kill the tumor cells and to provide a selective growth advantage to the cells. Yet to be identified are the cellular and viral factors responsible for EBV infection of normal epithelial cells being completely lytic, yet converting to a latent form in epithelial tumor cells.
EBV infection of most transformed human epithelial cell lines in vitro also usually leads to the establishment of cells containing EBV in a highly latent form. A notable exception to this general rule is the gastric carcinoma cell line AGS. AGS cells stably infected with the B95-8 strain of EBV support persistently lytic infection (32). Not yet understood is why, among transformed epithelial cell lines, AGS cells are uniquely susceptible to maintaining EBV infection in a highly lytic form.
In this paper, we investigated viral and cellular factors that contribute to lytic EBV infection in AGS cells. We show that AGS cells stably infected with the B95-8 strain of EBV expressed a much higher level of viral lytic proteins than did stably infected HeLa cells, while expressing similar levels of EBNA-1, a latent protein. We examined the activities of the BZLF1 and BRLF1 promoters, Zp and Rp, respectively, in these two cell lines to determine whether their differential responses to infection were due, in part, to differential expression of these genes. We found that Zp activity was dramatically higher in AGS cells than in HeLa cells. The expression of Zp correlated with the presence of active (phosphorylated) c-Jun, a well-known positive regulator of Zp (39). It also inversely correlated with the presence of ZEB1 (also called δEF1, TCF8, AREB6, ZFHEP, ZFHX1A, and BZP) (20, 22, 48-51, 65), a zinc-finger E box-binding cellular transcription factor previously shown to function as a potent negative regulator of Zp in B-cell and epithelial cell lines (36). Thus, we conclude that the combination of high c-Jun activity, together with the loss of negative regulation by ZEB1, likely leads to high-level expression of the BZLF1 gene and, consequently, persistently lytic infection by EBV in AGS cells.
MATERIALS AND METHODS
Cell lines.
AGS (a human gastric carcinoma cell line), HeLa (a human cervical carcinoma cell line), and 293 (a human embryonic kidney cell line) cells were obtained from the American Type Culture Collection (Rockville, MD). Hone-EBV (a gift from Lawrence Young) is a human nasopharyngeal carcinoma cell line that stably maintains the EBV (Akata strain) genome under G418 selection (59). The cell line cell 666 (C666; a gift from Dolly Huang, Department of Anatomical and Cellular Pathology, the Chinese University of Hong Kong, Hong Kong, China) is an EBV-positive nasopharyngeal carcinoma (NPC) line that was derived from an undifferentiated NPC biopsy. 293-EBV, a generous gift from H.-J. Delecluse, is a derivative of 293 cells that is latently infected with p2089, a bacmid which contains the entire B95-8 strain of EBV along with the coding regions for hygromycin resistance and green fluorescence protein expressed from the cytomegalovirus-IE promoter (13). The AGS-EBV cell line was created by the infection of AGS cells with EBV virus made from these 293-EBV cells as previously described (13, 14, 32). The HeLa-EBV cell line was generated likewise except that the HeLa cells were pretransfected with a CD21 expression vector (64) prior to infection with EBV. AGS and AGS-EBV cells were maintained in F-12 medium supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. Hone-EBV and C666 cells were grown in RPMI 1640 medium (Sigma). HeLa, 293, and their corresponding EBV-positive derivative cells were grown in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with 10% fetal bovine serum, penicillin, and streptomycin. When EBV was present, the medium contained hygromycin as well. MCF-7/WS8 cells from a human mammary carcinoma cell line (a gift from V. Craig Jordan) (31) were cultured in Dulbecco's modified Eagle's medium/F-12 medium supplemented with 10% FBS, 6 ng insulin/ml, 3 μg glutamine/ml, penicillin, and streptomycin. DG75 cells (an EBV-negative Burkitt's lymphoma cell line; generously provided by Bill Sugden) were maintained in RPMI-1640 supplemented with 10% FBS, penicillin, and streptomycin.
Plasmids.
Plasmid DNAs were purified through QIAGEN columns as described by the manufacturer. The BZLF1 promoter sequence from nucleotides (nt) −495 to +27 (relative to the transcription initiation site) in the B95-8 strain of EBV was PCR amplified and inserted into the luciferase reporter vector pGL3-basic (Promega, Madison, WI) between the NheI and BglII sites to create pZp-Luc-WT. A series of 5′ deletion mutant variants of pZp-Luc-WT were constructed containing the BZLF1 promoter sequences from nt −50 to +27, −70 to +27, −90 to +27, and −221 to +27. Site-directed mutagenesis of pZp-Luc-WT was used to construct pZp-Luc-ZVmt, a mutant variant in which the previously described ZV element ZEB1-binding motif (35, 36) was altered from A to C at nt −12 relative to the Zp transcriptional initiation site. The plasmid pWTZpLUC and its mutant variant p-12CZpLUC contain the region consisting of nt −221 through +40 of the BZLF1 promoter driving expression of the luciferase reporter in pGL3-basic. Plasmid pCiZEB1, a generous gift from Douglas Dean, encodes the full-length chicken homologue of the human ZEB1, δEF1, with an amino-terminal FLAG tag expressed under the control of the cytomegalovirus-IE promoter (48). Plasmid pRp-Luc, containing the region consisting of nt −981 through +37 of the BRLF1 promoter relative to its transcription initiation site, was constructed by PCR amplification of this region of the B95.8 strain of EBV and insertion between the NheI and BglII sites of pGL3-basic.
Plasmid pZp-CAT, containing the wild-type (WT) BZLF1 promoter sequences of EBV strain B95.8 from nt −221 to +12 driving expression of CAT, variants of it containing cluster base pair substitution mutations in the Zp ZII CRE- and ZIA/ZIB MEF2D-binding motifs (6, 19, 40-42), and pBS-CAT, their empty parental reporter construct, were generous gifts from Erik Flemington. Plasmid pSG424, encoding only the Gal4 DNA-binding domain (DBD), and plasmids pGal4-CREB, pGal4-ATF1, and pGal4-ATF2 were generously provided by Michael Green (37). Plasmid pGal4-c-Jun was a gift from A. Baldwin (58). They express the indicated transcription factors fused in frame to the Gal4 DBD. Plasmid Gal4-E1B-CAT, a chloramphenicol acetyltransferase (CAT) reporter construct containing two copies of the Gal4 DNA-binding site linked to the adenovirus E1B promoter (37), was also provided by Michael Green. Plasmid pGal4-MEF2D, expressing the transcription factor MEF2D fused to the Gal4 DBD, and plasmid Gal4-TK-Luc, a luciferase reporter construct containing Gal4 DNA-binding sites linked to the herpes simplex virus-thymidine kinase promoter, were generous gifts from Xiang-Jiao Yang (23). Plasmid pCI-δEF1, encoding a FLAG-tagged version of the chicken homolog of ZEB1 expressed from a CMV promoter (49), was a generous gift of H. Kondoh and Douglas Dean.
Immunoblot analysis.
Immunoblots were performed as previously described (1). Briefly, whole-cell extracts were prepared by lysis in NP-40 lysis buffer supplemented with protease and phosphatase inhibitors. Similar amounts of protein from each extract were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After blocking, the membranes were incubated overnight at 4°C with the following primary antibodies diluted as indicated: anti-EBNA1 (1:1,000; a gift from Sarah Duellman and Richard Burgess, University of Wisconsin School of Medicine and Public Health), anti-BRLF1 (1:100; Argene, Varilhes, France), anti-BZLF1 (1:100; Argene), anti-BMRF1 (1:100; Capricorn, Baltimore, MD), and anti-β-actin (1:5,000; clone AC-15; Sigma, St. Louis, MO) antibodies diluted in 5% milk in 1× phosphate-buffered saline (PBS)-0.1% Tween 20 (PBS-T); anti-c-Jun (1:5,000; Upstate, Lake Placid, NY), anti-phospho-serine 73-c-Jun (1:1,000, Cell Signaling, Danvers, MA), and ATF-2 (1:250; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies diluted in 5% bovine serum albumin in 1× PBS-T; and anti-ZEB1 (1:500; H102, Santa Cruz Biotechnology) antibody diluted in 5% casein in PBS-T. For ZEB1, whole-cell extracts were prepared by lysis in 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 2 mM EDTA, and 50 mM phenylmethylsulfonyl fluoride, and the cell proteins were separated by an SDS 3% to 8% Novex NuPAGE gel (Invitrogen, Carlsbad, CA) in Tris-acetate buffer, electroblotted onto a nitrocellulose membrane, and blocked in 5% casein. The antibodies were visualized by use of ECL reagent (Amersham, London, United Kingdom) according to the manufacturer's instructions.
Luciferase assays.
Cells were transfected using FuGENE 6 (Roche, Indianapolis, IN) or TransIT LT1 (Mirus Corp., Madison, WI). Luciferase assays were performed with extracts prepared 48 h after transfection by freeze-thawing the cell pellet in reporter lysis buffer according to the manufacturer's instructions (Promega, Madison, WI). Luciferase activity was determined with an Auto Lumat LB953 luminometer (Berthold Technologies, Oak Ridge, TN) in buffer containing 12.5 mM glycylglycine, 2 mM EGTA, 7.5 mM MgSO4, 7.5 mM K2HPO4, 0.5 mM dithiothreitol, 1 mM ATP, 100 μM luciferin, and 50 mM Tris-HCl (pH 7.5).
CAT assays.
Cells were transfected using FuGENE 6. Two days posttransfection, cell extracts were prepared and incubated at 37°C with 14C-labeled chloramphenicol (Amersham, London, United Kingdom) in the presence of acetyl coenzyme A (Roche, Indianapolis, IN) as previously described (21). The percent acetylation of chloramphenicol was quantified by thin-layer chromatography using a PhosphorImager.
EBV real-time PCR.
The cell supernatant was removed and centrifuged at 2,000 × g for 5 min. One hundred microliters of clarified supernatant was digested with 2 U RQ1 RNase-free DNase (Promega) for 1 h at 37°C. Following digestion, DNase-resistant EBV genomic DNA was isolated from the supernatant and cell pellets by using a QIAGEN DNeasy tissue kit according to the manufacturer's instructions. Cell pellets were washed twice with 1× PBS, followed by extraction of total genomic DNA as described above. Real-time PCR was then performed as previously described (55).
BZLF1 immunofluorescence.
AGS-EBV cells were grown on glass coverslips and fixed in 100% ice-cold methanol. Cells were incubated with BZLF1-specific mouse monoclonal antibody (Argene) at a 1:50 dilution or an isotype control antibody and then incubated with a 1:1,000 dilution of Texas Red-conjugated goat anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA). Cell nuclei were then counterstained with DAPI (4′,6′-diamidino-2-phenylindole) (Sigma) and visualized using a fluorescence microscope.
RESULTS
AGS cells, but not HeLa cells, support persistently lytic EBV infection.
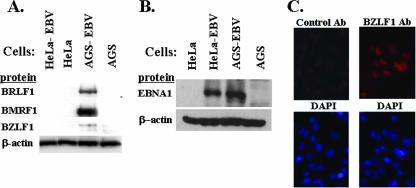
For most cell lines that can be infected by EBV, the virus either persists in a highly latent state or replicates and kills the cells. Thus, it has been difficult to establish cell lines in which EBV persists in a highly lytic state. EBV-infected AGS cells have been recently reported to express lytic viral proteins (32). However, the level of lytic viral gene expression in other epithelial cell lines has not been carefully examined. To quantify the relative levels of expression of EBV lytic genes in EBV-infected AGS and HeLa cells, we infected both cell types with virus derived from the B95-8 strain of EBV that includes a hygromycin resistance marker and the green fluorescent protein gene, and we selected for stable infection with hygromycin. As shown in Fig. 1A, the infection of AGS cells resulted in much higher levels of expression of lytic viral proteins than did the infection of HeLa cells. In contrast, the levels of expression of a latent protein, EBNA1, were similar in the two cell types (Fig. 1B). Imunofluorescence assays indicated that close to 50% of EBV-infected AGS cells expressed BZLF1 (Fig. 1C). Using quantitative EBV PCR assays, we found that the supernatant of EBV-AGS cells contained 1,000 to 3,000 copies/μl of DNase-resistant (virion-associated) EBV (data not shown). Similar results were obtained with two other independently derived cell lines (data not shown). Importantly, the high levels of expression of lytic EBV genes observed in the AGS-EBV cells persisted even after 6 months in culture (data not shown). These findings indicate that AGS cells are much more permissive for the lytic form of EBV gene expression than are HeLa cells.
FIG. 1.
EBV-infected AGS cells, but not HeLa cells, have persistently lytic viral gene expression. (A) Protein analysis for the expression of lytic viral genes in AGS-EBV and HeLa-EBV cell lines. Whole-cell extracts were prepared and assayed by immunoblot analysis for the presence of the indicated EBV IE (BZLF1 and BRLF1) and early (BMRF1) lytic proteins. The blot was also probed for β-actin as a loading control. (B) Immunoblot analysis was performed to examine EBNA1 expression in AGS-EBV and HeLa-EBV cells. (C) BZLF1 expression was examined in AGS-EBV cells by immunofluorescence using an anti-BZLF1 antibody (ab) or an isotype control antibody (upper panels). DAPI staining of nuclei is shown below each antibody stained panel.
Zp, but not Rp, is much more active in AGS cells than in HeLa cells.
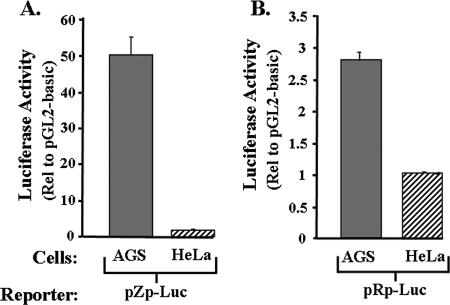
One hypothesis to explain the above finding is that one or both of the EBV IE promoters is much more efficiently expressed in AGS cells than in HeLa cells. To test the validity of this hypothesis, we transfected into these cell lines reporter plasmids in which luciferase expression was driven from either the BZLF1 (Zp) or the BRLF1 (Rp) promoter of the B95-8 strain of EBV. Cells were transfected in parallel with the promoterless parental plasmid, pGL3-basic, as a control. The cells were harvested 2 days later, and luciferase activity was measured. Strikingly, the BZLF1 promoter enhanced expression of the reporter 50-fold in AGS cells, yet two- to threefold at most in HeLa cells (Fig. 2A). In contrast, the BRLF1 promoter enhanced expression of the reporter threefold at most in either cell line (Fig. 2B). Thus, we conclude that the highly lytic EBV infection observed in B95-8-infected AGS cells is likely due, at least in part, to very high constitutive activity of the BZLF1 promoter in this particular cell line.
FIG. 2.
The EBV IE promoter Zp is highly active in AGS cells. EBV-negative AGS and HeLa cells were transiently transfected with 1 μg DNA per 3.5-cm-diameter well with a reporter plasmid DNA in which the luciferase-coding region was driven from (A) the region consisting of nt −495 through +27 of the BZLF1 promoter, Zp, or (B) the region consisting of nt −981 through +37 of the BRLF1 promoter, Rp. Forty-eight hours later, the cells were harvested, and luciferase activities was determined with normalization to the activity present in the same cell type transfected in parallel with pGL3-basic, the promoterless parental reporter plasmid. Transfections were performed in duplicate; shown here are representative data from one such experiment. Error bars indicate ranges.
Sequences between nt −70 and +27 are sufficient for high-level expression of Zp in AGS cells.
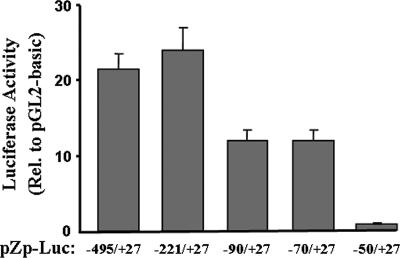
To localize the sequences within the region consisting of nt −495 to +27 of the BZLF1 promoter that are sufficient for its high-level expression in AGS cells, we constructed a series of 5′ deletion mutant variants of pZp-Luc and assayed them likewise by transient transfection in AGS cells. The deletion of the region consisting of nt −495 through −222 had little or no effect on the transcriptional activity of Zp (Fig. 3). The deletion of the region consisting of nt −221 through −71 as well led to only a modest, twofold decrease in the activity of the promoter (Fig. 3). However, the deletion of an additional 20 bp down to nt −50 led to the elimination of most, if not all, of the activity of Zp relative to the promoterless parental reporter in these cells (Fig. 3). Thus, we conclude that sequences lying within the region of Zp consisting of nt −70 to +27 are sufficient for high-level expression of Zp in AGS cells, with a crucial cis-acting element(s) mapping within the region consisting of nt −70 and −50.
FIG. 3.
Sequences between nt −70 and +27 are sufficient for high-level expression of the BZLF1 promoter in AGS cells. AGS cells were transfected in parallel with 1 μg DNA per 3.5-cm-diameter well of the pZp-luciferase reporter plasmids containing the indicated regions of Zp relative to the Zp transcription initiation site. Forty-eight hours later, cells were harvested and luciferase activities were determined with normalization to the activity obtained with the promoterless pGL3-basic plasmid transfected in parallel. Transfections were performed in duplicate; shown here are representative data from one such experiment. Error bars indicate ranges. Rel., relative.
The Zp CRE motif is required for high-level expression of Zp in AGS cells.
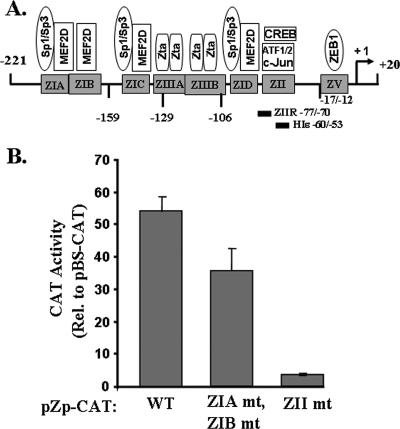
Sequences within the region consisting of nt −70 to −50 of the BZLF1 promoter contain a CRE motif which binds c-Jun, ATF-1, ATF-2, and CREB, known positive regulators of Zp (6, 19, 39, 41, 60, 61). Thus, we next examined whether a mutation of this CRE motif affects the expression of Zp in AGS cells within the context of BZLF1 promoter sequences from nt −221 to +12 driving a CAT reporter. As shown in Fig. 4B, a 4-bp substitution mutation in this motif, altering nt −65, −66, −67, and −69 of Zp (19), led to a dramatic reduction in Zp activity in AGS cells to within a fewfold of the empty reporter. In contrast, 4-bp substitution mutations in each of the two Zp MEF2D-binding motifs, ZIA and ZIB (19), had at most a minor effect on Zp activity in AGS cells (Fig. 4B). Thus, we conclude that the ZII region contains a cis-acting element(s) that functions as a strong positive regulator of Zp activity in AGS cells.
FIG. 4.
The Zp ZII element CRE motif is essential for high-level expression of the BZLF1 promoter in AGS cells. (A) Schematic diagram of the BZLF1 promoter showing known cis-acting elements and their trans-acting factors. (B) AGS cells were transfected in parallel with 1 μg DNA per 3.5-cm-diameter well of CAT reporter plasmids in which expression was driven by the region consisting of −221 through +12 of Zp (WT) or variants of it containing a cluster of 4-bp substitution mutations in (i) both the ZIA and ZIB MEF2D DNA-binding motifs (ZIA mt and ZIB mt), or (ii) the ZII region CRE motif (ZII mt). Cells were harvested 48 h after transfection, and CAT activities were determined with normalization to the activity observed with the promoterless parental plasmid, pBS-CAT. Transfections were performed in duplicate; shown here are representative data from one such experiment. Error bars indicate ranges. Rel. relative.
c-Jun and ATF-2 contribute to high-level expression of Zp in AGS cells.
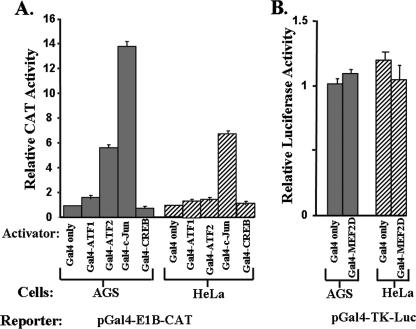
Cellular transcription factors known to activate Zp through its CRE motif include ATF-1, ATF-2, c-Jun, and CREB (6, 19, 39, 41, 61). To examine whether one or more of these factors can enhance the expression of Zp in AGS cells, we cotransfected AGS and HeLa cells with (i) expression plasmids encoding the Gal4 DBD fused to the transactivation domain of ATF-1, ATF-2, c-Jun, or CREB, and (ii) pGal4-E1B-CAT, a reporter plasmid containing a minimal adenovirus E1B gene promoter driving expression of the CAT gene activated from two upstream Gal4 DNA-binding sites. Because MEF2D is also a known regulator of Zp, in this case via its four ZI elements (Fig. 4A) (7, 25, 42), we also examined the effect of expression of a Gal4-MEF2D fusion protein on the activation in these cells of pGal4-TK-Luc, a reporter plasmid containing a minimal herpes simplex virus-thymidine kinase gene promoter driving expression of the luciferase gene activated from upstream Gal4 DNA-binding sites. Since binding to the promoter occurs in these experiments via the Gal4 DBD, any differences observed in the activation of the reporter genes would be a reflection solely of differences in the functionalities of the activation domains of these Gal4 fusion proteins in these cells.
The activities of the Gal4-CREB, Gal4-ATF1, and Gal4-MEF2D fusion proteins were found to be similar to that of the Gal4 DBD by itself in both of these cell lines (Fig. 5A and B). In contrast, the Gal4-ATF2 and Gal4-c-Jun fusion proteins enhanced transcription significantly more in AGS cells than they did in HeLa cells. Thus, the transcriptional activation domains of ATF-2 and c-Jun exhibit higher levels of functional activity in AGS cells than they do in HeLa cells.
FIG. 5.
Functional activities in AGS versus HeLa cells of the transcriptional activation domains of cellular transcription factors known to bind the Zp ZII motif. (A) AGS and HeLa cells were cotransfected in parallel with (i) pGal4-EIB-CAT, and (ii) an expression plasmid encoding the Gal4 DBD by itself or linked to the activation domain of the indicated cellular transcription factor. Cells were harvested 48 h after transfection and assayed for CAT activity with normalization to the activity obtained with the expression plasmid encoding only the Gal4 DBD. (B) AGS and HeLa cells were cotransfected in parallel with (i) pGal4-TK-Luc and (ii) an expression plasmid encoding the Gal4 DBD by itself or fused in frame to the activation domain of MEF2D. Cells were harvested 48 h later and assayed for luciferase activity. These transfections were performed in duplicate; shown here are representative data from one such experiment. Error bars indicate ranges.
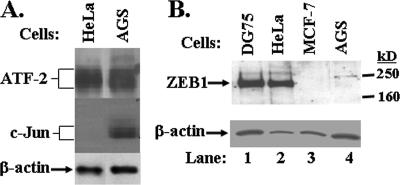
We also compared the endogenous levels of ATF-2 and c-Jun protein present in uninfected AGS and HeLa cells by immunoblot analysis. The two cell lines had similar high levels of ATF-2 protein (Fig. 6A). In contrast, AGS cells had significant amounts of c-Jun protein, while HeLa cells contain much less c-Jun (Fig. 6A). Thus, uninfected AGS cells have greatly enhanced expression of the transcription factor c-Jun in comparison to uninfected HeLa cells.
FIG. 6.
AGS cells contain little or no ZEB1 but express high level c-Jun. Whole-cell extracts were prepared from AGS, HeLa, DG75, and MCF-7 cells. Equivalent amounts of total protein from each extract were resolved by PAGE, blotted to membranes, and probed with antibodies specific to (A) ATF-2, c-Jun, and (B) ZEB1. The blots were reprobed with antibody specific to β-actin as a control.
AGS cells contain little or no ZEB1, a negative regulator of Zp.
ZEB1 is a cellular transcription factor that binds to the ZV element of Zp (Fig. 4A), functioning as a negative regulator in several different cell types, including ZEB1-positive B-lymphocytic DG75 cells and ZEB1-negative mammary carcinoma MCF-7 cells (36). One hypothesis to explain, in part, the persistently lytic nature of EBV infection observed in AGS cells is that Zp is expressed at high levels in AGS cells because ZEB1 is either (i) absent from AGS cells or (ii) functioning as a transcriptional activator (48, 51) rather than as a repressor in them. To test the validity of the first of these hypotheses, we prepared whole-cell extracts of uninfected AGS, HeLa, DG75, and MCF-7 cells, with the latter two serving as a positive and negative control for ZEB1 protein, respectively. The proteins in these extracts were separated by gradient SDS-PAGE, transferred to a membrane, and probed with a ZEB1-specific polyclonal antibody. Strikingly, ZEB1 was found to be quite abundant in HeLa cells, even more abundant than in DG75 cells when normalized to levels of β-actin protein present in the same extracts (Fig. 6B, lane 2 versus lane 1). In contrast, little or no cross-reacting material was detected migrating in the position of ZEB1 in the extract made from AGS cells (Fig. 6B, lane 4). Thus, AGS cells, like MCF-7 cells, accumulate little or no ZEB1.
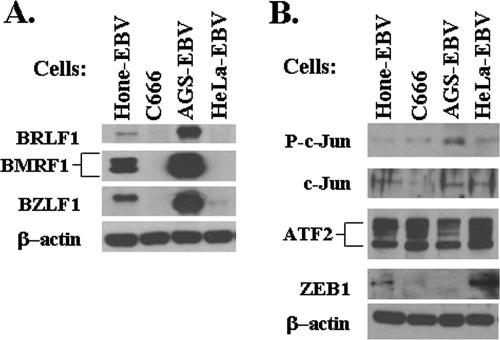
Activated c-Jun and ZEB1 may both contribute to stringency of viral latency in EBV-infected epithelial lines.
Selection for stable EBV-positive cell lines might result in lines which have altered levels of cellular transcription factors in comparison to the original EBV-negative cells. Therefore, we also examined the levels of total c-Jun, activated (phosphorylated) c-Jun, total ATF-2, and ZEB1 in four different stably infected EBV-positive epithelial cell lines which have dramatically different levels of lytic viral protein expression. As shown in Fig. 7, AGS-EBV cells, which support the highest level of persistent lytic EBV protein expression, also expressed the highest level of activated c-Jun, and the lowest level of ZEB1. Three stable EBV-positive epithelial cell lines (C666, HeLa-EBV, and Hone-EBV), which express much lower levels of lytic EBV proteins than do the AGS-EBV cells, each had a very low level of activated-c-Jun as well as detectable ZEB-1 expression. Relative to the uninfected HeLa cells (Fig. 6), HeLa cells selected for stable EBV infection unexpectedly expressed more total c-Jun, although the level of activated c-Jun remained low (Fig. 7B). Interestingly, C666 NPC cells, while tightly latent, contained a relatively low, albeit detectable, level of ZEB1. On the other hand, Hone-EBV cells, which had detectable lytic viral protein expression, contained a higher level of ZEB1. It is possible that other positive regulators of Zp activity, such as C/EBP proteins (30), are expressed at high levels in Hone-EBV cells, overcoming to some degree the repressive effect of ZEB1. The stringent viral latency observed in C666 cells (which, unlike most NPC lines, have retained the original tumor EBV genome even after many passages in culture) may be due in part to epigenetic modifications of the viral genome, such as the methylation of Zp, which promotes viral latency, and/or the low level of total and activated c-Jun in this line.
FIG. 7.
Comparison of lytic viral gene expression versus c-Jun, ATF2, and ZEB1 expression in different stably EBV-infected epithelial lines. Immunoblot analysis showing the relative levels in the indicated cell lines of (A) lytic viral protein expression, and (B) various cellular transcription factors known to regulate Zp activity. P-c-Jun was probed with an antibody which recognizes the phosphorylated (activated) form of c-Jun; c-Jun was probed with an antibody recognizing total c-Jun.
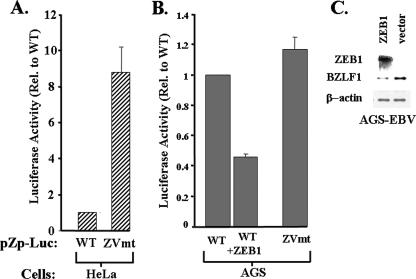
ZEB1 inhibits Zp activity when expressed in AGS cells.
Lastly, we examined the effect of the presence of ZEB1 on expression of Zp in AGS cells versus that in HeLa cells to determine whether it functions in these cells as a repressor or activator of transcription. A variant of pZp-Luc-WT was constructed containing a 1-bp substitution mutation of A to C with the ZV element at nt −12 relative to the Zp transcription initiation site, a mutation known to significantly reduce binding of ZEB1 to Zp (36) (Fig. 4A). This ZV element mutant and its parental WT reporter plasmid were assayed for transcriptional activity by transient transfection into AGS cells. As expected, the presence of the ZV element mutation had little, if any effect on expression of Zp in these ZEB1-negative cells (Fig. 8B), similar to what we previously observed with ZEB1-negative MCF-7 cells (35). In stark contrast, the presence of the −12C ZV element mutation led to a dramatic ninefold derepression of Zp in HeLa cells (Fig. 8A), consistent with the highly abundant endogenous ZEB1 in HeLa cells functioning as an efficient repressor of transcription of Zp in these cells. The addition of ZEB1 to AGS cells by cotransfection with a ZEB1 expression plasmid, pCI-δEF1, led to a twofold reduction in the expression of Zp (Fig. 8B). Similar results were obtained when AGS cells were transfected with pWTZpLUC versus p-12CZpLUC, i.e., WT and −12C ZV element mutant reporter plasmids, respectively, containing the region consisting of nt −221 through +40 of the BZLF1 promoter (data not shown). Finally, we also found that transfecting EBV-AGS cells with the ZEB1 expression vector significantly reduced the level of BZLF1 expression derived from the intact viral genome in these cells (Fig. 8C). This result was particularly impressive given that only about half of the cells were transfected (data not shown). Thus, we conclude that ZEB1 functions as a repressor of the BZLF1 promoter in AGS cells, albeit less potently than in HeLa cells, likely due to AGS cells containing a high level of activated c-Jun (Fig. 7B). The high level of Zp activity observed in AGS cells is due, in part, to little if any ZEB1 being present in these cells.
FIG. 8.
ZEB1 functions as a repressor of Zp in both HeLa and AGS cells but is normally absent from the latter cells. (A) HeLa cells were transfected by utilizing LT1 transfect reagent (Mirus Corp., Madison, WI) in parallel with 0.2 μg per 15-mm-diameter well of either the reporter plasmid pWTZpLUC (WT) or a ZV element mutant variant of it, p-12CZpLUC (ZVmt), containing a 1-bp A-to-C substitution mutation at nt −12. (B) AGS cells were cotransfected in parallel with (i) the reporter plasmid pZp-Luc WT or ZVmt (0.2 μg DNA per 15-mm-diameter well), and (ii) the ZEB1 expression plasmid pCiZEB1 or its empty parental plasmid (0.2 μg DNA per 15-mm-diameter well). Cells were harvested 48 h later, and luciferase activities were determined. Transfections were performed in triplicate; shown here are representative data from one such experiment. Error bars indicate standard deviations. (C) AGS-EBV cells were transfected utilizing Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with the ZEB1 expression plasmid pCiZEB1 or its empty parental plasmid (1.0 μg DNA per 35-mm-diameter well). Immunoblots were performed 2 days later on transfected cell extracts to examine the expression of ZEB1, BZLF1, and β-actin.
DISCUSSION
Although EBV infection is completely lytic in normal oropharyngeal epithelial cells (53, 57), the virus persists in a predominantly latent form in the epithelial cell tumors that contain EBV (34, 53, 66). The ability of EBV-infected epithelial tumor cells to repress lytic EBV gene expression is probably an early and essential step in the progression of these malignancies, since completely lytic EBV infection would likely be incompatible with tumor cell viability. As there is growing interest in the use of lytic induction as a novel therapeutic strategy for treating EBV-positive tumors (4, 15-18, 44, 45, 47, 62, 63), it is important to understand the various pathways that might allow the virus to reactivate in epithelial cell malignancies. Thus, one needs to identify the cellular factors that play roles in the activation and repression of the EBV IE promoters, Zp and Rp, which control the switch between latency and lytic replication of the virus.
As an initial step toward achieving this goal, we first established epithelial cell lines that support either persistently latent or lytic EBV infection (Fig. 1). We found that Zp and Rp were both quiescent in the latently infected HeLa cells; on the other hand, Zp was constitutively active in the persistently lytically infected AGS cells, consistent with its lytic state (Fig. 2). Deletion and base pair substitution analyses revealed that at least one of the cis-acting regulatory elements required for this high constitutive expression in AGS cells mapped to the region consisting of nt −70 to −50 of Zp within the ZII CRE motif-containing element (Fig. 3 and 4). Noteworthy was the finding that EBV-infected AGS cells contain much higher levels of activated c-Jun, a protein known to activate Zp via binding of this CRE motif (6, 39, 41, 61), than do EBV-infected HeLa cells (Fig. 7). We further showed that the activation domains of both c-Jun and ATF-2, another CRE motif-binding protein, can function in AGS cells as transcriptional activators (Fig. 5). Lastly, we found that the cellular transcription factor, ZEB1, previously shown to function as a major negative regulator of Zp activity in other cell types (36), is largely absent from AGS cells, but highly abundant in HeLa cells (Fig. 6 and 7), while still able to function as a repressor of Zp in either cell type (Fig. 8).
The fact that EBV can exist in a highly latent state in HeLa cells as well as in 293 cells (13, 14), another ZEB1-positive epithelial cell line (X. Yu., Z. Wang, and J. E. Mertz, submitted for publication), is consistent with ZEB1 functioning as a central gatekeeper of Zp expression. Nevertheless, our finding that C666 cells (an EBV-positive NPC line) express only a low level of ZEB1 yet are stringently latent suggests that reduced ZEB1 expression by itself is likely not sufficient to induce lytic EBV gene expression. Thus, we conclude that the unusually high level of expression of BZLF1 in EBV-infected AGS cells is probably due to its specific combination of the presence of positive regulatory transcription factors (i.e., activated c-Jun and ATF-2) and the absence of negative regulatory ones (i.e., ZEB1), with the precise molar ratio of activators to repressors determining the level of Zp activity (36).
The factors that regulate whether ZEB1 levels are high or low in cells are only beginning to be understood. Chua et al. (10) recently reported that minimally transformed MCF10A mammary epithelial cells also contain little ZEB1; however, the addition of the p65 subunit of NF-κB results in the expression of ZEB1, leading to repression of E-cadherin synthesis, followed by epithelial-to-mesenchymal transition of the cells. Likewise, ZEB1 levels are quite high in many Burkitt's lymphoma B-cell lines, including DG75 (Fig. 6B) (35, 36) and Akata (X. Yu and J. E. Mertz, unpublished data), and primary blood and memory B cells (Table 1), other physiologically relevant cell types in which EBV can exist in a highly latent state (5, 34, 53). ZEB1 levels are also low in some early-passage lymphocytic cell lines in which infection by EBV is quite lytic (28), yet higher in later passages of the same cell line in which infection has gone latent (A. Ellis, S. C. Kenney and J. E. Mertz, unpublished data). Interestingly, a 2-bp substitution mutation in the ZV element of Zp is sufficient to lead to spontaneous reactivation of the B95.8 strain of EBV in both epithelial and B cells under growth conditions in which its parental WT virus rarely, if ever, reactivates (Yu et al., submitted for publication). Also striking is the finding that ZEB1 expression rapidly drops to low levels following differentiation induced by activation with anti-immunoglobulin (Table 1), a condition that frequently leads to the reactivation of EBV out of latency into lytic replication (60). Thus, we conclude that a positive correlation exists between ZEB1 being absent or unable to bind the ZV element of Zp and EBV infection of the cells being lytic, a correlation likely due to ZEB1 playing a central role in regulating the expression of the BZLF1 promoter (35, 36). Given these findings, we speculate that variable levels of ZEB1 expression in different cell types likely contributes to the stringency of EBV latency versus lytic replication that occurs during natural EBV infection in humans.
TABLE 1.
Relative amounts of ZEB1 mRNA in primary human B cellsc
| B-cell type | Amt of ZEB1 mRNA relative to adult blood B cells in study:
|
|
|---|---|---|
| 1a | 2b | |
| Adult blood | 100 | 100 |
| Naïve blood | 134 | ND |
| Memory blood | 44 | ND |
| Germinal center tonsil | 10 | ND |
| Anti-IgM-activated blood at: | ||
| 3 h | ND | 36 |
| 6 h | 26 | 9 |
| 12 h | 9 | 7 |
Data for study 1 were extracted from Alizadeh et al. (3) using the supplementary information available at http://llmpp.nih.gov/lymphoma.
Data for study 2 were extracted from Cahir-McFarland et al. (8) using the supplementary information available at http://kiefflab.bwh.harvard.edu.
IgM, immunoglobulin M; ND, not determined.
Interestingly, the EBV-encoded protein latent membrane protein 1 (LMP1) is known to induce synthesis of NF-κB (8, 46). Thus, LMP1 may affect the expression of ZEB1 and, consequently, Zp via NF-κB effecting the expression of ZEB1. Consistent with this hypothesis is our preliminary finding that LMP1, known to repress Zp and promote viral latency (2), regulates Zp activity, in part, via ZEB1 binding to the ZV element (R. J. Kraus and J. E. Mertz, unpublished data). Furthermore, ZEB1 can function as either a positive or a negative regulator of transcription depending upon promoter context, cell type, and posttranslational modifications (e.g., phosphorylation and acetylation) induced by factors in the environment (e.g., tumor necrosis factor alpha and transforming growth factor beta) (48, 51) in which the cells are grown. Thus, there likely exist multiple determinants, in addition to cell type, that contribute to whether and how ZEB1 effects expression of the BZLF1 promoter.
Here we used cell lines stably infected with EBV due to the difficulty of efficiently infecting epithelial cells with virions of EBV. Thus, we cannot definitively exclude the possibility that HeLa cells are initially susceptible to lytic EBV infection, but most are killed by the primary infection and only the small percentage of cells which remained viable by managing to inhibit lytic infection went on to become the stably infected cells we studied here. Contrary to this hypothesis is our finding that both the Zp and Rp IE promoters have essentially no activity in HeLa cells in the absence of any selection.
Another question raised by our studies is why AGS cells infected with the B95-8 strain of EBV support long-term, persistently lytic infection. We speculate that the same cellular factors that contribute to high Zp activity (i.e., the presence of activated c-Jun and the lack of ZEB1) and, consequently, lytic infection may also contribute to the continued viability of the AGS cells. This is the first report of an epithelial cell line that can tolerate a high level of lytic EBV infection while remaining viable. Although the B95-8-infected AGS cells grow significantly more slowly than uninfected AGS cells do (data not shown), we have, nevertheless, observed that the infected cells can be maintained for many months in culture despite persistently lytic EBV gene expression (data not shown). Furthermore, we have found that up to half of the B95-8-infected AGS cells express the IE protein BZLF1 as well as the late viral protein VCA (32). The ability of AGS cells to tolerate highly lytic EBV infection suggests that lytic EBV induction by itself may not be sufficient to kill all tumor cells; rather, lytic replication may need to be combined with drugs such as ganciclovir, which is converted to a cytotoxic nucleotide analogue in lytically infected cells (16), to achieve the killing of tumor cells.
Whether either of the two cellular factors identified here as important for lytic EBV infection in AGS cells also plays a role during natural EBV infection of oropharyngeal epithelial cells in humans remains unknown. Lytic EBV infection in normal oropharyngeal cells likely requires epithelial cell differentiation (27, 29, 34, 38, 53, 56, 57, 66), something most epithelial tumor cells fail to do. MacCallum et al. (43) suggested that epithelial cell differentiation may increase Zp activity by enhancing the activities of CREB and ATF-1. The effects of epithelial cell differentiation on ZEB1 abundance and transcriptional activity have yet to be explored. The identification of pathways that can induce lytic EBV infection in undifferentiated epithelial tumor cells is important for designing lytic induction strategies for treating EBV-positive NPC and gastric cancers. Our results reported here suggest that finding ways to decrease ZEB1 expression or repressor activity, while simultaneously increasing the activity of transcription factors that bind to the Zp CRE motif, will be essential for identifying maximally effective lytic induction strategies.
Acknowledgments
We thank Henri-Jacque Delecluse, Dolly Huang, V. Craig Jordan, Lawrence Young, and Bill Sugden for cell lines; A. Baldwin, Douglas Dean, Erik Flemington, Michael Green, Lindsay Hutt-Fletcher, H. Kondoh, Douglas Dean, and Xiang-Jiao Yang for plasmids; Sarah Duellman and Richard Burgess for the antisera; and Todd Seaman for advice regarding the construction of plasmids.
This work was supported by NIH grants PO1CA19014, R01CA66519, and R01CA58853 to S.C.K.; RO1AI107034 to J.E.M.; and P30CA14520 to the UWCCC and by a grant from the Pardee Foundation to J.E.M.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., E. Schaadt, B. Kempkes, U. Zimber-Strobl, B. Baier, and G. W. Bornkamm. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc. Natl. Acad. Sci. USA 99:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alizadeh, A. A., M. B. Eisen, R. E. Davis, et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. [DOI] [PubMed] [Google Scholar]
- 4.Ambinder, R. F., K. D. Robertson, S. M. Moore, and J. Yang. 1996. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Semin. Cancer Biol. 7:217-226. [DOI] [PubMed] [Google Scholar]
- 5.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 6.Borras, A. M., J. L. Strominger, and S. H. Speck. 1996. Characterization of the ZI domains in the Epstein-Barr virus BZLF1 gene promoter: role in phorbol ester induction. J. Virol. 70:3894-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant, H., and P. J. Farrell. 2002. Signal transduction and transcription factor modification during reactivation of Epstein-Barr virus from latency. J. Virol. 76:10290-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahir-McFarland, E. D., K. Carter, A. Rosenwald, J. M. Giltnane, S. E. Henrickson, L. M. Staudt, and E. Kieff. 2004. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 78:4108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua, H. L., P. Bhat-Nakshatri, S. E. Clare, A. Morimiya, S. Badve, and H. Nakshatri. 2007. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26:711-724. [DOI] [PubMed] [Google Scholar]
- 11.Countryman, J., H. Jenson, R. Seibel, H. Wolf, and G. M. Miller. 1987. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J. Virol. 61:3672-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delecluse, H.-J., T. Hilsendegen, D. Pich, R. Zeidler, and W. Hammerschmidt. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 95:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, W.-H., G. Hong, H.-J. Delecluse, and S. C. Kenney. 2004. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J. Virol. 78:1893-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, W.-H., B. Israel, N. Raab-Traub, P. Busson, and S. C. Kenney. 2002. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 62:1920-1926. [PubMed] [Google Scholar]
- 17.Feng, W.-H., and S. C. Kenney. 2006. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 66:8762-8769. [DOI] [PubMed] [Google Scholar]
- 18.Feng, W.-H., E. Westphal, A. Mauser, N. Raab-Traub, M. L. Gulley, P. Busson, and S. C. Kenney. 2002. Use of adenovirus vectors expressing Epstein-Barr virus (EBV) immediate-early protein BZLF1 or BRLF1 to treat EBV-positive tumors. J. Virol. 76:10951-10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemington, E., and S. H. Speck. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genetta, T., D. Ruezinsky, and T. Kadesch. 1994. Displacement of an E-box-binding repressor by basic helix-loop-helix proteins: implications for B-cell specificity of the immunoglobulin heavy-chain enhancer. Mol. Cell. Biol. 14:6153-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray, S., and M. Levine. 1996. Transcriptional repression in development. Curr. Opin. Cell Biol. 8:358-364. [DOI] [PubMed] [Google Scholar]
- 23.Grégoire, S., and X.-J. Yang. 2005. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol. Cell. Biol. 25:2273-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruffat, H., E. Manet, and A. Sergeant. 2002. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 3:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildesheim, A., and P. H. Levine. 1993. Etiology of nasopharyngeal carcinoma: a review. Epidemiol. Rev. 15:466-485. [DOI] [PubMed] [Google Scholar]
- 28.Hong, G. K., M. L. Gulley, W. H., Feng, H.-J. Delecluse, E. Holley-Guthrie, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 79:13993-14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hording, U., H. W. Nielsen, H. Albeck, and S. Daugaard. 1993. Nasopharyngeal carcinoma: Histopathological types and association with Epstein-Barr virus. Eur. J. Cancer B Oral Oncol. 29:137-139. [DOI] [PubMed] [Google Scholar]
- 30.Huang, J., G. Liao, H. Chen, F. Y. Wu, L. Hutt-Fletcher, G. S. Hayward, and S. D. Hayward. 2006. Contribution of C/EBP proteins to Epstein-Barr virus lytic gene expression and replication in epithelial cells. J. Virol. 80:1098-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, S.-Y., D. M. Wolf, J. M. Yingling, C. Chang, and V. C. Jordan. 1992. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol. Cell. Endocrinol. 90:77-86. [DOI] [PubMed] [Google Scholar]
- 32.Jones, R. J., S. Dickerson, P. M. Bhende, H.-J. Delecluse, and S. C. Kenney. 2007. Epstein-Barr virus lytic infection induces retinoic acid-responsive genes through induction of a retinol metabolizing enzyme, DHRS9. J. Biol. Chem. 282:8317-8324. [DOI] [PubMed] [Google Scholar]
- 33.Kenney, S. C., J. Kamine, E. Holley-Guthrie, J.-C. Lin, E.-C. Mar, and J. Pagano. 1989. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J. Virol. 63:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2552. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields Virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 35.Kraus, R. J., S. J. Mirocha, H. M. Stephany, J. R. Puchalski, and J. E. Mertz. 2001. Identification of a novel element involved in regulation of the lytic switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 75:867-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus, R. J., J. G. Perrigoue, and J. E. Mertz. 2003. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 77:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemaigre, F. P., C. I. Ace, and M. R. Green. 1993. The cAMP response element binding protein, CREB, is a potent inhibitor of diverse transcriptional activators. Nucleic Acids Res. 21:2907-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Q. X., L. S. Young, and G. Niedobitek. 1992. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 39.Liang, L.-C., J.-L. Chen, Y.-P. P. Hsu, J. T. Ou, and Y.-S. Chang. 2002. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-β through cooperativity of Smads and c-Jun/c-Fos proteins. J. Biol. Chem. 277:23345-23357. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman, P. M., J. M. Hardwick, J. Sample, G. S. Hayward, and S. D. Hayward. 1990. The Zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J. Virol. 64:1143-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, P., S. Liu, and S. H. Speck. 1998. Identification of a negative cis element within the ZII domain of the Epstein-Barr virus lytic switch BZLF1 gene promoter. J. Virol. 72:8230-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, S., P. Liu, A. Borras, T. Chatila, and S. H. Speck. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 16:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacCallum, P., L. Karimi, and L. J. Nicholson. 1999. Definition of the transcription factors which bind the differentiation responsive element of the Epstein-Barr virus BZLF1 Z promoter in human epithelial cells. J. Gen. Virol. 80:1501-1512. [DOI] [PubMed] [Google Scholar]
- 44.Mentzer, S. J., J. Fingeroth, J. J. Reilly, S. P. Perrine, and D. V. Faller. 1998. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr virus (EBV)-associated lymphoma. Blood Cells Mol. Dis. 24:114-123. [DOI] [PubMed] [Google Scholar]
- 45.Mentzer, S. J., S. P. Perrine, and D. V. Faller. 2001. Epstein-Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological re-activation of viral target genes with arginine butyrate. Transplant Infect. Dis. 3:177-185. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell, T., and B. Sugden. 1995. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore, S. M., J. S. Cannon, Y. C. Tanhehco, F. M. Hamzeh, and R. F. Ambinder. 2001. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob. Agents Chemother. 45:2082-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postigo, A. A. 2003. Opposing functions of ZEB proteins in the regulation of the TGFβ/BMP signaling pathway. EMBO J. 22:2443-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postigo, A. A., and D. C. Dean. 1997. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J. 16:3935-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postigo, A. A., and D. C. Dean. 1999. Independent repressor domains in ZEB regulate muscle and T-cell differentiation. Mol. Cell. Biol. 19:7961-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postigo, A. A., J. L. Depp, J. J. Taylor, and K. L. Kroll. 2003. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 22:2453-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 54.Rooney, C. M., D. T. Rowe, T. Ragot, and P. J. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryan, J. L., H. Fan, S. L. Glaser, S. A. Schichman, N. Raab-Traub, and M. L. Gulley. 2004. Epstein-Barr virus quantitation by real-time PCR targeting multiple gene segments: a novel approach to screen for the virus in paraffin-embedded tissue and plasma. J. Mol. Diagn. 4:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sixbey, J. W. 1989. Epstein-Barr virus and epithelial cells. In G. Klein (ed.), Advances in viral oncology, p. 187-202. Raven Press, New York, NY.
- 57.Sixbey, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 58.Stein, B., A. S. Baldwin, D. W. Ballard, W. C. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-κB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart, S., C. W. Dawson, K. Takada, J. Curnow, C. A. Moody, J. W. Sixbey, and L. S. Young. 2004. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-κB transcription factor pathway. Proc. Natl. Acad. Sci. USA 101:15730-15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Y.-C. J., J.-M. Huang, and E. A. Montalvo. 1997. Characterization of proteins binding to the ZII element in the Epstein-Barr virus BZLF1 promoter: transactivation by ATF1. Virology 227:323-330. [DOI] [PubMed] [Google Scholar]
- 62.Westphal, E.-M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 63.Westphal, E. M., W. Blackstock, W. Feng, B. Israel, and S. C. Kenney. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781-5788. [PubMed] [Google Scholar]
- 64.Yang, L., S. Maruo, and K. Takada. 2000. CD21-mediated entry and stable infection by Epstein-Barr virus in canine and rat cells. J. Virol. 74:10745-10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasui, D. H., T. Genetta, T. Kadesch, T. M. Williams, S. L. Swain, L. V. Tsui, and B. T. Huber. 1998. Transcriptional repression of the IL-2 gene in Th cells by ZEB. J. Immunol. 160:4433-4440. [PubMed] [Google Scholar]
- 66.Yeung, W. M., Y. S. Zong, C. T. Chiu, K. H. Chan, J. S. Sham, D. T. Choy, and M. H. Ng. 1993. Epstein-Barr virus carriage by nasopharyngeal carcinoma in situ. Int. J. Cancer 53:746-750. [DOI] [PubMed] [Google Scholar]
- 67.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the IE BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]