Abstract
Nucleocytoplasmic shuttling of the Rous sarcoma virus (RSV) Gag polyprotein is an integral step in virus particle assembly. A nuclear export signal (NES) was previously identified within the p10 domain of RSV Gag. Gag mutants containing deletions of the p10 NES or mutations of critical hydrophobic residues at positions 219, 222, 225, or 229 become trapped within the nucleus and exhibit defects in the efficiency of virus particle release. To investigate other potential roles for Gag nuclear trafficking in RSV replication, we created viruses bearing NES mutant Gag proteins. Viruses carrying p10 mutations produced low levels of particles, as anticipated, and those particles that were released were noninfectious. The p10 mutant viruses contained approximately normal amounts of Gag, Gag-Pol, and Env proteins and genomic viral RNA (vRNA), but several major structural defects were found. Thin-section transmission electron microscopy revealed that the mature particles appeared misshapen, while the viral cores were cylindrical, horseshoe-shaped, or fragmented, with some particles containing multiple small, electron-dense aggregates. Immature virus-like particles produced by the expression of Gag proteins bearing p10 mutations were also aberrant, with both spherical and tubular filamentous particles produced. Interestingly, the secondary structure of the encapsidated vRNA was altered; although dimeric vRNA was predominant, there was an additional high-molecular-weight fraction. Together, these results indicate that the p10 NES domain of Gag is critical for virus replication and that it plays overlapping roles required for the nuclear shuttling of Gag and for the maintenance of proper virion core morphology.
Retroviral assembly is directed by the viral Gag polyprotein precursor. Despite containing very little sequence homology, all Gag proteins share three canonical domains, the MA (matrix), CA (capsid), and NC (nucleocapsid) domains, as well as additional regions that vary for each retrovirus. Immature virus particles contain unprocessed Gag and Gag-Pol fusion proteins arranged in a concentric array just inside the viral envelope, with the NC domain of Gag toward the center, bound to the dimeric, positive-sense viral RNA (vRNA) genome (4, 18, 51). The cleavage of Gag during the maturation process occurs during or immediately after budding, leading to a dramatic change in morphology, with the appearance of an electron-dense core near the particle center. This dense core is comprised of vRNA coated by the NC protein, together forming the ribonucleoprotein (RNP) complex. The RNP is enclosed by a shell composed of CA multimers forming the virion core, which is in turn surrounded by the MA protein in association with the lipid envelope (reviewed in references 45 and 46). The molecular events that govern RNP and core formation during virus assembly and maturation are still not well understood.
Functional mapping of the Gag polyprotein identified three discrete motifs, known as assembly domains, that coordinate virion formation and budding: the interaction (I) domains that are involved in Gag-Gag and Gag-RNA interactions, the membrane-binding (M) domain, which targets Gag to the plasma membrane, and the late (L) domain that interacts with host cell machinery to facilitate the final steps in budding. The M domain resides in the N-terminal portion of MA, while I domains are within CA and NC. The L domain has variable locations in different retroviral Gag proteins, but typically maps to regions outside the MA, CA, and NC domains (reviewed in references 13, 16, and 48). These domains are insufficient, however, to determine the normal organization of the mature virus core. Additional sequences, including CA and its flanking regions, are also necessary (21, 32, 35, 49, 52).
In addition to these defined assembly domains, independent subcellular trafficking signals that provide important functions in the virus life cycle have been identified in Gag proteins. In the Gag protein of Rous sarcoma virus (RSV), there are two distinct nuclear localization signals (NLSs) within the MA and NC domains and a nuclear export signal (NES) in the p10 cleavage product located between p2 and CA (Fig. 1) (7, 42). The NLS in MA overlaps the M domain, and the NC NLS resides within the I domain. The p10 NES, however, is not located within a previously identified assembly domain. Gag proteins from other retroviruses contain nuclear trafficking signals as well; putative NLS and NES sequences were reported for the human immunodeficiency virus type 1 (HIV-1) Gag protein (5, 14). The murine leukemia virus (MLV) Gag polyprotein is present in the nucleus, the MLV NC protein localizes to the nucleoli (39), and the p12 domain was implicated to have a role in the nuclear import of the viral genome, presumably owing to intrinsic karyophilic properties (1, 53). Thus, the subcellular targeting signals within Gag proteins, although poorly characterized, are likely to play important roles during infection.
FIG. 1.
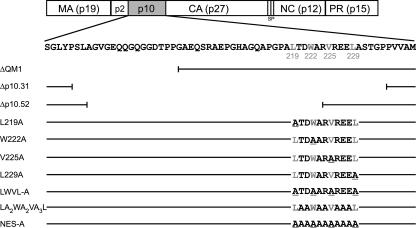
Schematic of NES mutant viruses. The wild-type RSV Gag polyprotein is depicted at the top with the MA, p2, p10, CA, SP, NC, and PR domains indicated. Below is the amino acid sequence of the p10 domain, with the four hydrophobic amino acids comprising the NES shown in gray. The p10 mutants are listed below, with horizontal lines depicting unaltered sequence and vertical lines delineating the last amino acid present in each deletion construct. Amino acid sequences for the NES substitutions are indicated. Deletion ΔQM1 removes codons 176 to 200, but preserves the Gag NES. The Δp10.52 and Δp10.31 mutations delete codons 183 to 234 and 193 to 223, respectively, including all or most of the export sequence. The alanines that replace each critical hydrophobic residue are underlined.
The p10 region of RSV is critical for virus infectivity, and accumulated evidence suggests several possible roles it may play in replication. The organization of Gag proteins in the particles may be partially determined by the p10 domain, as the second half of p10 strongly influences the morphology of particles formed in vitro by Gag fragments. Specifically, the addition of 25 amino acids of p10 to the N terminus of a CA-NC fusion protein converts the morphology of particles from tubes to spheres in Escherichia coli (9, 26). This 25-amino-acid sequence, which spans the NES, is very sensitive to mutation or deletion in bacterial and baculovirus expression systems, presumably because these residues participate in an extensive interface with the CA domain of Gag and, thus, promote Gag multimerization (25, 26, 32). In addition, in the context of proviral expression, insertions and deletions in this region of p10 result in a 20- to 50-fold reduction in particle assembly and a loss of infectivity, although particle morphology was not examined in that report (15).
The identification of an NES within p10 led us to ask whether disruption of the nuclear export activity of Gag by mutation would impair virion formation or virus replication. We previously identified four hydrophobic amino acids (L219, W222, V225, and L229) that are critical for both NES activity and virus-like particle (VLP) release (43), although these mutants had not yet been studied in the context of the viral genome. In this study, we have examined the effects of a set of mutations in p10 on both virus infectivity and morphology. We sought to determine whether the p10 nuclear export function could be genetically separated from its role in governing particle morphogenesis, thereby yielding insights into whether nuclear transport and virion assembly are linked in vivo.
MATERIALS AND METHODS
Proviral expression vectors, plasmids, and cells.
The proviral vector pRC.V8, containing the Prague C gag sequence from pATV8, and its derivative pRC.Myr1E were described previously (12, 36). Deletions within the p10 region (Δp10.31, Δp10.52, and ΔQM1) were transferred into pRC.V8 by SstI-HpaI fragment exchange from simian virus 40-based expression vectors (28), while the L219A, W222A, V225A, and L229A mutations were introduced by SstI-SdaI fragment exchange from pGag-green fluorescent protein (GFP) constructs (42). Mutants pGag-GFP.NES-A (NES-A), pGag-GFP.LWVL-A (LWVL-A), and pGag-GFP.LA2WA2VA3L (LA2WA2VA3L) were made using QuikChange mutagenesis (Stratagene) and transferred into the proviral vector pRS.V8-EGFP (8) using SstI-SdaI, and all mutations were confirmed by DNA sequencing. Plasmid pGagΔPR, containing the complete Gag coding sequence except for PR, and its derivatives were constructed by inserting an SstI-NotI PCR-amplified product into the pGag-GFP vector, with expression under the control of the cytomegalovirus promoter (43). All experiments were performed by using the chemically transformed quail fibroblast QT6 cell line, primary turkey embryo fibroblasts (TEFs), or DF-1 cells, each maintained as previously described (23, 36). Transfections were performed by the calcium phosphate method for QT6 cells, using DEAE-dextran for TEFs, and using Fugene (Roche Diagnostics) for DF-1 cells.
Radioimmunoprecipitation assays.
Budding assays were performed as previously described (37, 47). Briefly, transfected cells expressing proviral genomes were labeled for 2.5 h with l-[35S]methionine and cysteine (0.1 μCi/μl; >1,000 Ci/mmol) in Dulbecco's modified Eagle's medium lacking these amino acids. The cell culture medium was removed, cells were lysed in radioimmunoprecipitation buffer, viral proteins from the postnuclear cell lysates and medium samples were immunoprecipitated with polyclonal RSV antisera, and proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by using a PhosphorImager (Molecular Dynamics). The budding efficiency was calculated as the ratio of the amount of CA protein in the medium to the sum of the amount of CA protein in the medium plus the amount of Gag polyprotein precursor in the lysate.
Virus infectivity analysis.
For RC.V8 constructs, virus particles were obtained from the supernatants of QT6 or DF-1 cells approximately 40 h after transfection. A portion of the supernatant was analyzed for the level of exogenous reverse transcriptase (RT) activity and normalized to the wild-type level. Equivalent amounts of particles were added to QT6 or TEF cells for 2 h at 37°C, the medium was changed, and cell supernatants were collected every 3 days, pelleted through 25% sucrose at either 55,000 rpm for 40 min in a Beckman TLA100.4 rotor or 18,500 rpm for 90 min in a Sorvall SL-50T rotor, resuspended in phosphate-buffered saline, and stored at −70°C. After all samples were collected, the stored aliquots were subjected to RT analysis as described previously (12). For RS.V8 constructs, which contain the enhanced GFP coding sequence in place of src, infectivity was determined by using fluorescence-activated cell sorting (FACScan; BD Biosciences) as described previously (8).
Analysis of vRNA content.
RNA packaging assays were performed as described previously (20). Briefly, virions obtained from cell culture supernatants following transient transfection of QT6 or DF-1 cells were concentrated by ultracentrifugation (1 h 20 min at 27,000 rpm in a Beckman SW28 rotor; 4°C) and resuspended in TNE buffer (10 mM Tris-Cl [pH 7.5], 100 mM NaCl, 1 mM EDTA), and the relative numbers of viral particles were assayed by RT assay. RNA from equivalent amounts of virus particles was extracted and hybridized to an in vitro transcribed riboprobe spanning the 3′ splice site junction of the RSV genome (nucleotides 4998 to 5257) using the RPA III protocol according to the manufacturer's specifications (Ambion). Following treatment with RNases A and T1, undigested and digested RNAs were separated on 5% acrylamide-8 M urea gels and analyzed by phosphorimager analysis. The relative RNA content was determined by the intensity of signal generated by the protected fragment for each mutant relative to the signal for the wild-type sample. Nondenaturing vRNA Northern blotting was performed as previously described (20), and quantification was performed by using phosphorimager analysis to compare the relative amounts of monomers, dimers, or high-molecular-weight (HMW) fractions within each lane.
Electron microscopy analysis.
QT6 cells were transfected with plasmid DNA in Permanox dishes (EM Sciences), washed in 0.1 M sodium cacodylate (pH 7.4) and fixed in 4% paraformaldehyde-0.5% glutaraldehyde for 1 h at 4°C. Cells were postfixed in 1% osmium tetroxide-1.5% potassium ferrocyanide overnight at 4°C, washed in 0.1 M sodium cacodylate, and serially dehydrated in ethanol. Monolayers were embedded in Epon 812, thin sectioned, stained with uranyl acetate and lead citrate, and viewed with a Phillips 400 electron microscope. Images were captured using conventional photography, the negatives were scanned at high resolution, and some images were adjusted for optimal exposure using Corel Draw (Corel Corp, Ottawa, Canada).
RESULTS
The p10 domain of RSV contains a CRM1-dependent NES containing four essential hydrophobic residues: L219, W222, V225, and L229 (7, 43). The alteration of any individual hydrophobic residue to alanine results in the accumulation of Gag within the nucleus and the reduction of Gag-directed VLP release. Furthermore, mutant VLPs are released with delayed kinetics, suggesting that nuclear export might be a rate-limiting step for particle assembly (43). Intriguingly, the same four amino acids play key roles in an intersubunit interaction with the CA domain of Gag that was identified in a crystallized Gag fragment and implicated in Gag protein dimerization during assembly (32).
Although mutation of any one of the four essential hydrophobic residues reduces particle release in the VLP assays, the effects are not absolute. It is, therefore, not straightforward to predict the behavior of the same mutant Gag proteins when expressed in the context of a complete proviral genome. The presence of the full complement of viral proteins and vRNA and the possibility of additional interactions with cellular trafficking pathways add complexities that cannot be easily foreseen. Therefore, we initiated studies of the assembly, morphology, and replication of mutant viruses bearing alterations in the NES sequence. In addition to the single substitutions at the hydrophobic positions, several new NES mutants were examined, including three deletion mutants, one substitution mutant in which each of the 11 residues in the p10 NES was replaced by alanine (NES-A), and another with all four essential hydrophobic residues changed to alanines (LWVL-A) (Fig. 1). An additional mutant in which alanines were substituted for the amino acids other than the four hydrophobic residues (LA2WA2VA3L) was created to address the role of intervening residues in nuclear export, virus morphogenesis, and replication. Because at least two functions, those of nuclear export and particle structure, have been attributed to the same amino acids in p10, we asked whether it would be possible to genetically separate these activities with any of the NES mutations.
Subcellular localization of novel NES mutant Gag-GFP fusion proteins.
Previously, Gag p10 NES mutants with single hydrophobic-residue-to-alanine substitutions were analyzed for their subcellular localization and levels of VLP release by using Gag-GFP fusion proteins. Although the majority of the mutant Gag-GFP protein was localized within the nucleus, some fluorescent protein could be seen at the plasma membrane, and budding was reduced only to 25 to 30% of the wild-type levels (43). Because of this residual level of particle release, we wondered whether the four hydrophobic residues act in concert to promote Gag nuclear export. If so, then mutating all four hydrophobic amino acids simultaneously would be expected to prevent more Gag protein from reaching the plasma membrane, further reducing VLP release. Moreover, if residues located between the hydrophobic residues within the NES were involved in the export activity, then they might also be sensitive to mutation.
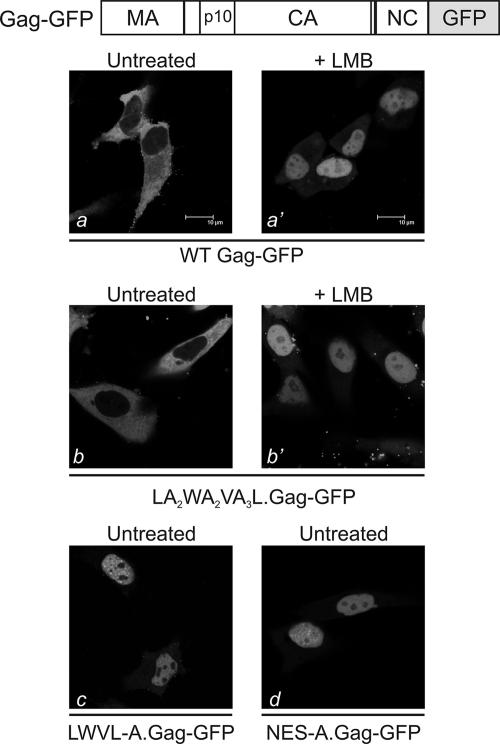
To address these questions, mutant proteins LWVL-A.Gag-GFP and NES-A.Gag-GFP were examined for their subcellular localization by confocal microscopy (Fig. 1 and 2). As previously reported, wild-type Gag-GFP is predominantly located in the cytoplasm, with punctuate foci along the plasma membrane (42, 43) (Fig. 2a). Treatment with leptomycin B (LMB), which prevents the interaction of Gag with its cellular export receptor CRM1, blocked the nuclear export of Gag-GFP (panel a'). Similarly, changing all four hydrophobic residues in the NES to alanine residues (LWVL-A) or substituting a span of alanines for the entire NES sequence (NES-A) resulted in the accumulation of the mutant Gag-GFP proteins within the nucleus (panels c and d). The amounts of residual LWVL-A and NES-A Gag proteins in the cytoplasm or at the plasma membrane were not markedly different from the amounts seen with mutants in which single hydrophobic residues were replaced by alanines (43). Taken together, these results suggest that altering a single hydrophobic residue (L219, W222, V225, or L229) is sufficient to maximally block the nuclear export activity of Gag.
FIG. 2.
Subcellular localization of Gag-GFP fusion proteins. The Gag-GFP protein is depicted above, illustrating the in-frame fusion of GFP in place of PR, with the additional loss of the C-terminal seven residues of NC during cloning. Below are confocal microscopy images of QT6 cells expressing the indicated Gag-GFP fusion proteins, either with or without the addition of 18 nM LMB 2 h prior to imaging. WT, wild type.
To assess whether nonhydrophobic residues within the NES influenced the export function, a mutant with intervening amino acids changed to alanines was analyzed. Nuclear export activity was retained in this case, as the LA2WA2VA3L Gag-GFP fusion protein localized to the cytoplasm and plasma membrane under steady-state conditions but became concentrated within the nucleus after the incubation of cells in LMB (Fig. 2, panels b and b'). Thus, for nuclear export activity per se, amino acids in Gag other than the hydrophobic residues did not appear to contribute to NES activity. However, these amino acids could be required for a second function that is essential for virus particle release or infectivity.
Release of virus particles.
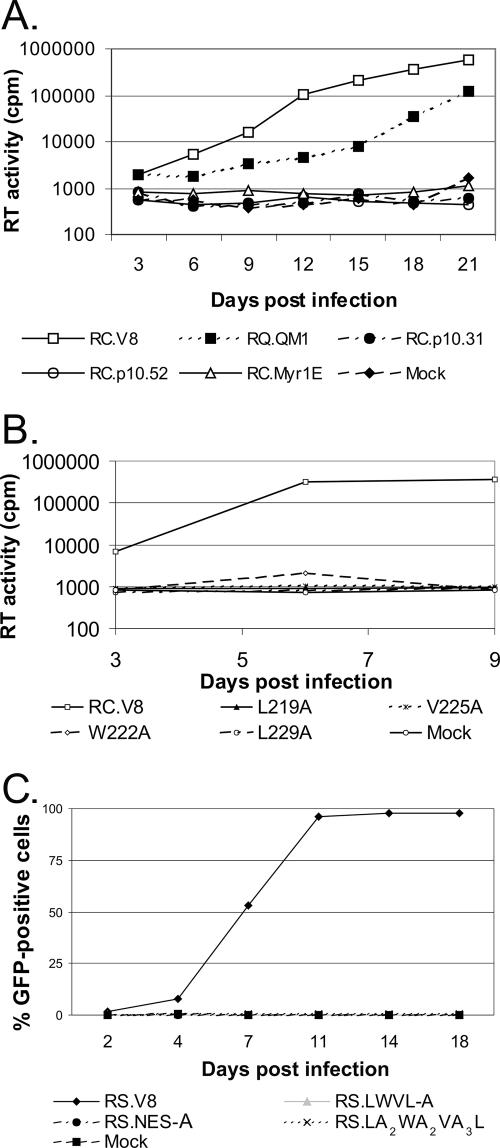
To determine whether the Gag NES mutants were capable of forming virus particles when expressed in the context of an otherwise infectious genome, each of the NES mutants shown in Fig. 1 was transferred into the proviral clone pRC.V8 (36) or pRS.V8 (8). Both proviral vectors express the Prague C gag gene, although RS.V8 introduces a silent SpeI site at nucleotides 434 to 439 and the enhanced GFP gene in place of src. The resultant plasmids were used to assess virus particle production in QT6 and DF-1 cells. The wild-type Gag protein of RC.V8 (Pr76gag) was efficiently expressed in cells, and its budding efficiency was set as 100% to allow comparison of the mutants (Fig. 3A). Mutant RC.ΔQM1, which contains a deletion in p10 upstream of the NES, was released from the cells with higher efficiency than RC.V8 (166%; P = 0.07) (Fig. 3A); however, an additional processing intermediate of ∼35 kDa was detected in the virus particles, reflecting the loss of the p2-p10 cleavage site (data not shown).
FIG. 3.
Virus particle assembly. (A) Quail fibroblasts were transfected with proviral constructs expressing variants of Gag in the context of the RC.V8 plasmid, and budding assays were performed. Budding efficiency was calculated as described in Materials and Methods, and the wild-type level of budding was arbitrarily set at 100%. Error bars represent standard deviations, and each experiment was performed in at least three replicates. (B) The budding assay was performed and analyzed in a fashion identical to that described for panel A except that the gag mutations were introduced into the pRS.V8 background. (C) Gag proteins were immunoprecipitated from lysates or media of transfected cells and analyzed by SDS-PAGE. Molecular mass standards (kDa) are indicated to the left, and the positions of CA-S, CA, and CA-SP are shown to the right.
Gag proteins containing deletions of the p10 NES were stably expressed; however, virus particles bearing these mutants were released with much-lower efficiencies than virus particles with the wild-type protein (Δp10.31, 23%, and Δp10.52, 47%; P values, <0.0001) (Fig. 3A). Similarly, the mutation of each individual codon of an essential hydrophobic residue in the NES resulted in significantly lower levels of particle release (RC.L219A, 35%; RC.W222A, 21%; RC.V225A, 19%; and RC.L229A, 31%; P values, <0.0001). Mutating all of the NES residues to alanines or changing each of the four hydrophobic residues resulted in a budding defect similar to those from the single hydrophobic substitutions (RS.NES-A, 26%, and RS.LWVL-A, 17%) (Fig. 3B). In addition to potential structural issues, the defect in particle production clearly is explained in part by a lower concentration of Gag in the cytoplasm because the majority of the protein is trapped in the nucleus (42, 43).
When the processing of Gag into its mature cleavage products was assessed by metabolic labeling and immunoprecipitation, mutants RC.L219A and RC.W222A were noted to be impaired in CA maturation (Fig. 3C), a phenotype that is associated with lack of infectivity (2, 50). For wild-type RSV, the CA protein has three forms that can be distinguished by SDS-PAGE analysis: CA-SP, the longest CA protein, is a transient processing intermediate consisting of CA linked to 12 residues of the spacer between CA and NC; CA-S contains CA plus three amino acids from the spacer; and CA, the shortest species, lacks the spacer entirely. CA-SP appears earliest and migrates fastest through the SDS-polyacrylamide gel, even though it is the largest protein by mass (38). As maturation proceeds, the CA-S and CA bands appear (11, 50).
The CA-S band was the principle isoform for RC.V8 (wild type) under the labeling conditions used here, while both CA-S and CA were visible for the infectious ΔQM1 mutant (Fig. 3C). Deletion mutant Δp10.52 had an increased proportion of CA-SP, a finding that was even more striking in the Δp10.31 virus particles. Point mutants L219A, V225A, and L229A demonstrated an increase in CA-SP, and W222A contained the highest ratio of the immature CA-SP species. Thus, the processing of SP from CA-SP was impaired in all of the mutants with deletions or mutations in the C-terminal sequence of p10, arguing that the alterations in p10 have global effects on Gag packing and orderly maturation.
To examine whether the nuclear export of Gag was sufficient to direct efficient particle release, viral mutant LA2WA2VA3L, which was competent for nuclear shuttling when assessed by confocal microscopy (Fig. 2b), was tested for its ability to mediate budding (Fig. 3B). Even though this Gag mutant appeared to be exported normally from the nucleus, budding was still compromised when the amino acids situated between the critical hydrophobic residues were altered (58% of the wild-type level; P = 0.0023); however, the level of particle release was substantially greater than that for any of the NES mutations that targeted hydrophobic residues. Thus, we identified a mutant of the Gag NES that appears to genetically separate nuclear export and virion release, consistent with these overlapping functions mapping to this region.
Effect of the p10 NES sequence on virus infectivity.
To determine whether disruption of the p10 NES alters virus replication, particles produced by transient transfection were normalized by RT assay and used to infect new cells. While the wild-type virus (RC.V8) spread quickly through the culture, as evidenced by the rise in RT activity during serial passages of the cells, the mutant RC.ΔQM1 replicated more slowly but remained within one log of the wild-type level (Fig. 4A). Mutant RC.Myr1E, previously reported as noninfectious, was included as a negative control (36). All other deletions of the p10 NES, as well as the single hydrophobic amino acid substitutions, abolished viral replication; none of the mutant viruses produced detectable levels of RT activity in cells challenged with equivalent numbers of virus particles (Fig. 4A and B). In addition, the RS.NES-A and RS.LWVL-A NES substitution mutants also lacked any detectable infectivity, as did the LA2WA2VA3L mutant virus, containing substitutions for the nonhydrophobic residues within the NES sequence (Fig. 4C). We also found that the NES mutant viruses were unable to replicate in primary TEFs (data not shown).
FIG. 4.
Infectivity of viruses with mutations in the Gag p10 NES sequence. Equal amounts of viral particles produced by transfection with the indicated proviral plasmids were added to either QT6 (panels A and B) or TEF cells (panel C). The ability of the viruses to spread throughout the culture was assayed by the RT activity present in cell culture supernatants collected every 3 days (A and B) or by the percentage of cells expressing GFP (C) over time.
Characterization of defective viruses.
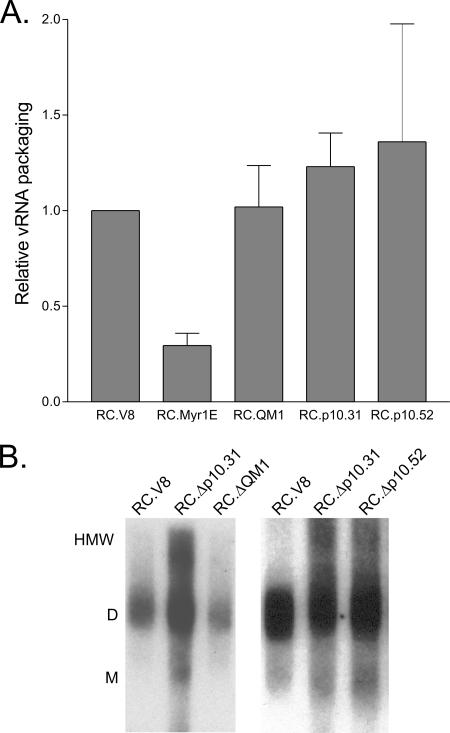
To determine whether the mutant virus particles were noninfectious due to a failure to incorporate Gag-Pol, Env (viral glycoproteins), or vRNA, virus particles were analyzed biochemically. We found that the Env, Gag-Pol, and exogenous RT levels in the mutant particles did not differ substantially from those in the wild-type virus (data not shown). Quantitative analysis of vRNA content performed using an RNase protection assay revealed that mutants RC.ΔQM1, RC.Δp10.31, and RC.Δp10.52 contained levels of vRNA that were the same as or slightly higher than those in the wild type (Fig. 5A). Mutant Myr1E was included as a control, as this virus is known to incorporate ∼30% of the wild-type levels of vRNA (20, 36).
FIG. 5.
Virion RNA content and analysis of RNA dimer structure. (A) Viral particles were normalized based on RT activity, equivalent numbers of particles were lysed, and vRNA incorporation was quantitatively assessed by RNase protection assay using a labeled probe spanning the 3′ splice site of the vRNA (nucleotides 4998 to 5257). The amount of vRNA detected for each mutant construct was compared to that in the wild type, which was set at 1.0. Each bar represents the average for at least three independent experiments with standard deviations shown. (B) Nondenaturing Northern blotting analysis of vRNA isolated from virus particles. The positions of the HMW species, dimers (D), and monomers (M) are indicated to the left.
To examine the secondary structures of the viral genomes, vRNA was isolated from mutant or wild-type particles and analyzed by nondenaturing Northern blotting. The migration of the vRNA isolated from RC.Δp10.31 and RC.Δp10.52 virus particles was aberrant (Fig. 5B); while dimers were present in all lanes, there was an increase in HMW forms of vRNA extending upward from the dimer band. For mutants Δp10.52 and Δp10.31, the HMW fractions were increased to 41% and 52% of the total, respectively, compared to only 6% for the wild type (Table 1). The fraction of RNA present in monomer form was similar for each virus tested, in contrast to other RSV mutants that have defects in subcellular localization of Gag and package primarily monomers (20, 36). Instead, the alteration of the higher-order structure of the vRNA suggests that Gag-vRNA interactions were perturbed. Because disruption of the normal Gag-vRNA conformation could lead to ultrastructural changes in the RNP core that might be evident by electron microscopy, the morphology of the particles was evaluated.
TABLE 1.
Distribution of viral RNA species isolated from viral particles under nondenaturing conditions
| Virus particle | RNA fraction (% of total)
|
||
|---|---|---|---|
| HMW | Dimers | Monomers | |
| RC.V8 (wild type) | 6 | 85.5 | 8.5 |
| RC.Δp10.31 | 50 | 40.5 | 9.5 |
| RC.Δp10.52 | 38.3 | 52.3 | 9.5 |
Virion morphology analysis.
Thin-section electron microscopy images revealed that mature wild-type RSV particles generally contained a spherical, electron-dense core and a thin lucent region between the core and the viral envelope (Fig. 6a). However, elongated cores were observed occasionally. Particles produced by the infectious ΔQM1 virus retained a morphology that was similar to that of the wild type (panel b). These virus particles were relatively uniform, and their single electron-dense RNP cores were centrally located and approximately spherical. In contrast, the various mutant NES virus particles displayed striking variability in particle and core structure. Although some of the RC.Δp10.31 and RC.Δp10.52 particles were relatively normal in appearance, others were large and irregular, and the RNP cores appeared elongated or cylindrical (panels c to f). In addition, large particles containing multiple or fragmented cores were observed.
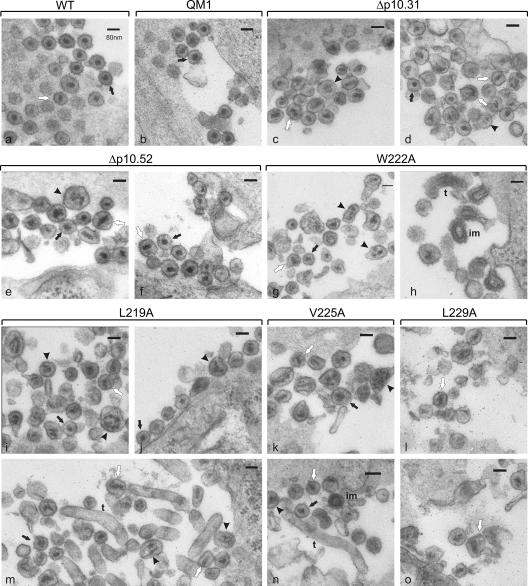
FIG. 6.
Thin-section electron microscopy analysis of mature viral particles. QT6 cells expressing the wild-type virus (a) or mutants ΔQM1 (b), Δp10.31 (c and d), Δp10.52 (e and f), W222A (g and h), L219A (i, j, and m), V225A (k and n) or L229A (l and o) were fixed, stained, thin sectioned and visualized by transmission electron microscopy. The scale bar represents 80 nm for each image. Black arrows show particles with wild-type morphology (spherical, electron-dense core and a thin lucent region between the core and the viral envelope); arrowheads indicate diffuse, multiple cores; white arrows point to particles with tubular or cylindrical cores; “t” specifies tubular or filamentous particles; and “im” indicates cores with immature morphology.
Virus particles bearing single-amino-acid substitutions in the hydrophobic sequence of the p10 NES displayed even more dramatic defects in particle morphology. Although there were a few particles that appeared similar to wild-type virions, the mutant particles were generally more heterogeneous, and the RNP cores were misshapen (Fig. 6g to o). Some mutant particles contained elongated RNP cores, while other cores appeared granular and less condensed, with multiple or segmented densities visible. For the W222A mutant (panels g and h), tube-like budding structures with dense aggregates of RNP complexes visible under the membrane were observed, and there were membrane-enclosed particles with immature core morphology. Tubular structures were also observed in cells expressing L219A (panel m), in addition to particles with normal morphology, multiple cores, and elongated cores (panels i and j). Rare immature cores were observed for V225A (panel n), and tubes lacking electron-dense aggregates were seen in longitudinal sections. For mutant L229A (panels l and o), few particles could be found, and those that were seen were misshapen, with disorganized, elongated, cylindrical cores.
The heterogeneity in virus particle diameter and the aberrant core morphology of the p10 mutants supports the idea that mutations spanning the p10 NES sequence strongly influence the configuration of the mature virion core. Importantly, however, mutations in this region do not absolutely prevent spherical particles and cores from forming.
Morphology of immature VLPs.
Having observed striking defects in the mature virion core ultrastructure, we asked whether the p10 NES region might also regulate the assembly of immature particles in QT6 cells. Although the structures of VLPs derived from mutant Gag proteins with changes in p10 have not been previously examined in avian cells, Vogt and coworkers studied the morphology of p10 mutant particles in E. coli and baculovirus expression systems (9, 25, 26). In those reports, only immature particles were studied; the deletion of p10 was reproducibly associated with tubular particles, while mutants generated by random mutagenesis of the C-terminal region of p10 resulted in the formation of either tubes or spheres, but not both.
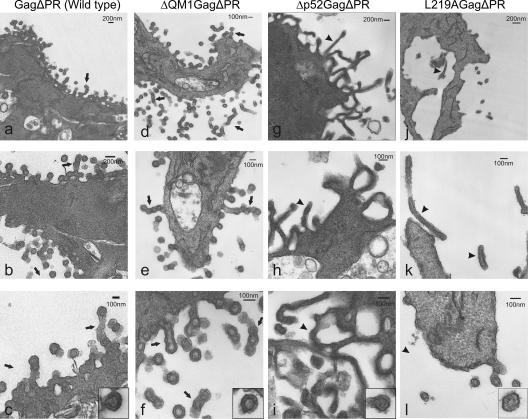
QT6 cells were transfected with plasmids expressing GagΔPR derivatives and examined by thin-section electron microscopy (Fig. 7). For wild-type GagΔPR (Fig. 7a to c), spherical VLPs were observed in close proximity to the cell membrane. Several regions were noted to have a series of multiple particles connected in chains. These aggregates had the appearance of filamentous structures containing individual particles that had not yet separated from one another, possibly due to successive budding of VLPs from one particular site on the plasma membrane, as suggested for MLV (52). Higher magnification of individual particles (see representative image, panel c inset) demonstrated a complete ring of electron-dense staining representing immature Gag proteins under the virion envelope. VLPs released from cells expressing the N-terminal p10 deletion ΔQM1.GagΔPR (panels d to f) were also spherical and appeared similar to the wild type, although the membranous filaments containing chains of individual particles were more numerous, and there was less distinction between individual particles. However, discrete particles appeared to contain intact rings of Gag proteins, as shown in the inset of panel f.
FIG. 7.
Electron microscopy images of cell-associated immature virus-like particles. Quail fibroblasts were processed exactly as described in the Fig. 6 legend. Cells were transfected with plasmids expressing wild-type or mutant Gag proteins (as labeled) with C-terminal truncations that remove the PR sequence. Scale bars are labeled with dimensions. Arrows point to chains of spherical particles and arrowheads indicate elongated tubular particles. Higher magnification insets of individual particles in panels c, f, i, and l are included to show the detailed structure of the immature cores.
Cells expressing Gag proteins bearing the Δp10.52.GagΔPR deletion appeared dramatically different from wild-type Gag-expressing cells (Fig. 7g to i). Cells with high-level Gag expression contained an increased amount of electron-dense material throughout the cell. Long filamentous projections with densities just inside the membrane extended from the plasma membrane. These tubular structures were slightly smaller in diameter (∼80 to 100 nm) than the VLPs (∼100 nm), although rare discrete particles were observed. Higher magnification of an individual particle (inset, panel i) revealed an irregular, less-dense, immature core with poorly defined interior organization, although very few single particles were available for examination.
VLP formation was greatly reduced for the point mutant L219A.GagΔPR (Fig. 7j to l). However, both tubular and spherical particles were released from cells. It is possible that the filamentous structures are artifacts due to overexpression of the Gag mutants, although the tubular structures shown in Fig. 7 are remarkably similar in appearance to a p10 deletion mutant expressed using the baculovirus system (25). In panel l, a few single particles were seen emerging from the plasma membrane, although on close examination, the immature cores appeared to be less electron dense than wild-type cores, and the dense rings were discontinuous (inset). Thus, it appears that mutations within the p10 NES that disrupt the nuclear egress of Gag also result in disorganized immature cores and aberrant mature RNP complexes, suggesting that this region of p10 plays overlapping critical roles in virus assembly.
DISCUSSION
The nuclear trafficking of the RSV Gag protein is an integral step in the virus assembly pathway. However, whether the transit of the Gag polyprotein through the nucleus is essential for the production of infectious virions is less clear. Nuclear transport is not required for the assembly of particles, because mutants that bypass the nuclear compartment are budding competent; however, these mutant viruses are noninfectious, and their defect is in vRNA packaging and genome dimerization (8, 42). Thus, Gag nuclear trafficking and genome incorporation are functionally linked, leading to the hypothesis that Gag proteins in the nucleus might interact with vRNA to select the genome for packaging into virions. In an attempt to examine the role of Gag nuclear transport in virus replication, we studied viruses with mutations that impaired nuclear egress. Our results revealed important new insights into the role of p10 in virus particle assembly, core morphology, and Gag-vRNA interactions.
Due to the overlap of the NES with a crucial structural domain of Gag, it was not possible to examine nuclear export activity in isolation. Single-point mutations, as well as the multiple-substitution and deletion mutations that altered the NES, all caused severe and pleiotropic effects in both the virus and immature particles. In particular, we found alterations in Gag nuclear-trafficking behavior, aberrant particle morphology, and complete loss of infectivity. Neither multiple substitutions of the essential hydrophobic residues nor deletion of the entire NES caused further impairment of nuclear export or budding beyond the levels seen for single substitutions within the NES sequence. In contrast, the ΔQM1 mutant, which lacks much of p10 but preserves the NES, appeared much like the wild type in each of the assays. Thus, there are multiple critical determinants of virus replication that reside within the same amino acids in the C-terminal region of the p10 domain.
Separation of the two major functions, nuclear trafficking and particle morphogenesis, was not possible with most of these mutations. In fact, no mutants were identified that were defective in nuclear export but assembled virus particles normally. However, a mutant with substitutions in the residues between the critical hydrophobic amino acids (LA2WA2VA3L) showed apparently normal nuclear trafficking but was still reduced in particle release, although its budding efficiency was considerably greater than that of any of the single-substitution, NES-A, or LWVL-A mutants. Importantly, those virus particles that were released had undetectable infectivity, revealing that although the residues between the hydrophobic amino acids are not required for nuclear export, they play a crucial role in infectivity, likely for structural reasons.
Intriguingly, not only was the overall particle shape sensitive to p10 mutations, but the internal organization of mature particles was also disturbed by each of the mutations, even though the CA sequence itself was unaltered. The capsid shell in RSV is typically not well preserved by standard thin-section electron microscopy preparation techniques. In some wild-type particles, however, a clear region can be seen between the core and the membrane. Electron density in the interior of wild-type particles is believed to reflect primarily the RNP, with perhaps some contribution by CA. Thus, the effects of the p10 mutations on the integrity of the capsid shell cannot be directly assessed in this manner, but the serious malformation of internal structures suggests that there is not a normal capsid structure in many of the particles. This is likely due to perturbation of the normal Gag-Gag and/or Gag-vRNA associations in the immature particle, the consequences of which become more drastic during maturation. This conclusion is supported not only by the internal structure as seen by electron microscopy but also by the slow or incomplete processing of cleavage sites at the CA-SP junctions and the disordered vRNA structure.
The finding that the NES activity of p10 overlaps precisely with a structural domain that is required for the assembly of infectious virus particles suggests that both functions are under significant selective pressure to be maintained in the genome. The requirement for the same exact residues of p10 to participate in two competing activities raises a compelling question: how can the hydrophobic residues that are involved in binding CRM1 during nuclear export also be available to promote CA-CA interactions at the proposed Gag dimer interface? Possible explanations are that (i) CRM1 binding and Gag-Gag dimerization are temporally separated (e.g., the Gag-CRM1 interaction may precede the formation of Gag-Gag interactions); (ii) Gag proteins that undergo nuclear trafficking are in a separate pool or different subcellular compartment from those engaged in Gag oligomerization; and (iii) Gag may exist in at least two conformational states, one in which the CRM1-binding sites are exposed and a second in which p10-CA interactions are favored. These scenarios are not mutually exclusive, and future experiments will be pursued to define these early events in Gag trafficking and dimerization.
The C-terminal portion of the Gag p10 domain was previously shown in several nonviral systems to be a “shape-determining” domain (9, 25, 26). Consistent with this, we found that immature VLPs produced from a Gag p10 deletion mutant (Δp10.52ΔPR) appeared almost exclusively as long filaments, and very few individual spherical particles were found. These data also indicate that the same immature Gag protein (e.g., in the L219A mutant) was capable of forming both spheres and filamentous enveloped particles. Similarly, a mutant of Moloney MLV that lacks the p12 domain, analogous in position to p10 in RSV, forms both tubes and spherical enveloped particles in vivo (52). As well, there are several examples of HIV Gag mutants that form tubes, cones, and spherical cores, including deletions in the p2/SP1 spacer (19, 21, 22, 30). Together with these findings, our data favor the interpretation that Gag molecules are capable of forming either cylindrical or spherical particles, and the regions flanking CA (p10 in RSV, p12 in Moloney MLV, and MA and SP1 in HIV) serve as “conformational switches” that provide the flexibility to allow the intermolecular rearrangements that are needed for capsid assembly (21, 32, 35, 49, 52).
The ability to form filaments and spheres is similar to the observation that influenza virus forms both types of particles, depending on culture conditions and cellular or viral factors (3, 6, 10, 24, 27, 31, 40, 41, 44). Thus, there is support for the idea that mixed morphogenetic populations of particles can be formed from identical viral structural components. Interestingly, one factor controlling spherical versus filamentous particle formation in influenza virus is the strength of the M1 binding to the RNP, which also promotes M1-mediated nuclear export of the RNP (29). Thus, there are intriguing parallels between the involvement of the influenza virus M1-RNP complex and the RSV Gag-RNP core interaction in determining the morphology of virus particles and cores, highlighting the notion that the assembly and budding of spherical enveloped viruses share many fundamental features.
In mature RSV particles, it is intriguing that mutations in the p10 NES sequence induced elongated and grossly misshapen cores. The simplest explanation is that the mutants have a partial processing defect, and those particles that undergo complete cleavage of CA are spherical, while those that remain unprocessed form elongated, cylindrical cores (Fig. 8). The presence of immature particles as observed in electron micrographs of the W222A and V225A mutants (Fig. 6, panels h and n) is consistent with the predominance of unprocessed CA-SP in these virus particles. If p10 performs a critical function that affects the ordered arrangement of immature Gag proteins, then the formation of PR dimers might be hindered or Gag cleavage sites might be less accessible, impairing processing (49).
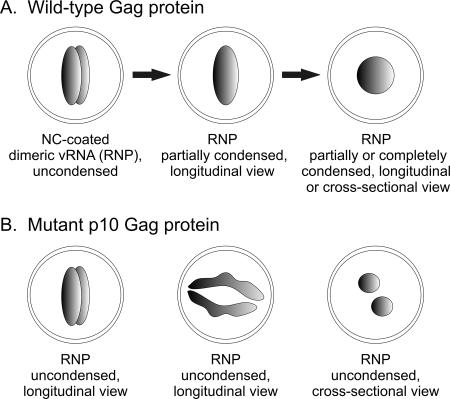
FIG. 8.
Model of mature virion core morphology. (A) In wild-type virions, the vRNP complex begins as an elongated, uncondensed structure, similar in appearance to influenza virus RNPs (38). The vRNA dimer and NC undergo a conformational change during maturation, and the core condenses into a sphere. (B) With alteration of the p10 domain, maturation of the vRNA and the Gag protein are incomplete. Condensation of the RNP complex is impaired, and viral cores adopt a variety of different appearances, depending on the angle from which they are viewed. This illustration suggests a possible mechanism for how multiple cores, elongated cores, and fragmented or horseshoe-shaped cores might be formed in p10 mutant virus particles.
Unexpectedly, the p10 domain also influences vRNA secondary structure; an unusual amount of HMW RNA was seen on the nondenaturing-gel blots. Because p10 is not known to bind vRNA, it is likely that alterations in p10 indirectly affect vRNA conformation in several possible ways. The observation of some individual particles that are larger than normal suggests that more than two genome segments could be packaged. Also, it is possible that the mutations in p10 alter Gag-vRNA interactions that then distort the RNA secondary structure or impede the normal PR-mediated maturation of the vRNA, resulting in the disorganized structure of the RNP core (Fig. 8). As a result, RNP condensation would be impaired and incomplete, leading to cores that remain elongated and diffuse. Support for this idea is provided by studies of MLV that link virion core maturation and proper maturation of the vRNA dimer (17). Also, high-resolution images of influenza virions show elongated, rod-shaped RNP complexes in longitudinal sections that appear circular when viewed in cross-section (34). If retroviruses assemble elongated RNPs that condense into spheres (in the case of RSV), then it is feasible that the elongated cores seen in the p10 mutants may represent uncondensed RNPs. The small, discrete, multiple cores seen in some of the p10 mutants could be rods cut transversely (Fig. 8). For the wild-type virus, maturation might lead to RNP condensation, resulting in a spherical core. Although this model is speculative and extrapolated from the influenza virus data, it could explain the heterogeneous mature core morphologies observed for the p10 mutant viruses.
In summary, we found that p10, which is not known to be a component of the mature virion core, has a critical role in core assembly. Importantly, our results indicate that immature and mature core assembly are not independent events but, rather, are linked sequentially. We conclude that contact points between immature Gag proteins, identified in the crystal structure of p10-CA (32), might help to direct normal core maturation and affect subsequent CA-CA, NC-NC, and NC-vRNA interactions, which in turn determine the condensation and shape of the mature vRNP core. It is also conceivable that the p10 peptide itself or cellular factors that associate with the p10 NES play a fundamental role in viral core morphogenesis.
Although these studies could not directly assess the role of Gag nuclear trafficking in replication, the data do reveal a critical role for the p10 domain in Gag-Gag and Gag-vRNA interactions. The simplicity of the RSV genome necessitates that its gene products have multiple functions. Thus, two critical functions, one required for the nuclear export of Gag and the other for proper assembly of the mature vRNP core, both reside in the same region. For RSV, this is an effective strategy to guarantee that these essential overlapping features within the p10 NES sequence remain conserved, ensuring successful virus propagation.
Acknowledgments
This research was supported by NIH grants R01 CA76534 to L.J.P. and R01 CA100322 to R.C.C. and a National Science Foundation graduate research fellowship to L.Z.S.
We thank scientists at the Penn State College of Medicine Core Facilities for their technical expertise, specifically, Roland Myers for electron microscopy, Rhona Ellis and Alistair Barber for confocal microscopy, and Nate Sheaffer for fluorescence-activated cell sorting, and we thank Andrea Beyer for critical review of the manuscript.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Auerbach, M. R., C. Shu, A. Kaplan, and I. R. Singh. 2003. Functional characterization of a portion of the Moloney murine leukemia virus gag gene by genetic footprinting. Proc. Natl. Acad. Sci. USA 100:11678-11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, R. P., S. Rhee, R. C. Craven, E. Hunter, and J. W. Wills. 1991. Amino acids encoded downstream of gag are not required by Rous sarcoma virus protease during Gag-mediated assembly. J. Virol. 65:272-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourmakina, S. V., and A. Garcia-Sastre. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517-527. [DOI] [PubMed] [Google Scholar]
- 4.Briggs, J. A., M. C. Johnson, M. N. Simon, S. D. Fuller, and V. M. Vogt. 2006. Cryo-electron microscopy reveals conserved and divergent features of gag packing in immature particles of Rous sarcoma virus and human immunodeficiency virus. J. Mol. Biol. 355:157-168. [DOI] [PubMed] [Google Scholar]
- 5.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burleigh, L. M., L. J. Calder, J. J. Skehel, and D. A. Steinhauer. 2005. Influenza A viruses with mutations in the M1 helix six domain display a wide variety of morphological phenotypes. J. Virol. 79:1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield-Gerson, K. L., L. Z. Scheifele, E. P. Ryan, A. K. Hopper, and L. J. Parent. 2006. Importin-β family members mediate alpharetrovirus Gag nuclear entry via interactions with matrix and nucleocapsid. J. Virol. 80:1798-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan, E. M., and J. W. Wills. 2003. Link between genome packaging and rate of budding for Rous sarcoma virus. J. Virol. 77:9388-9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, S., and V. M. Vogt. 1997. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J. Virol. 71:4425-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choppin, P. W., J. S. Murphy, and I. Tamm. 1960. Studies of two kinds of virus particles which comprise influenza A2 virus strains. III. Morphological characteristics: independence to morphological and functional traits. J. Exp. Med. 112:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craven, R. C., A. E. Leure-duPree, C. R. Erdie, C. B. Wilson, and J. W. Wills. 1993. Necessity of the spacer peptide between CA and NC in the Rous sarcoma virus Gag protein. J. Virol. 67:6246-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craven, R. C., A. E. Leure-duPree, R. A. Weldon, Jr., and J. W. Wills. 1995. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J. Virol. 69:4213-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craven, R. C., and L. J. Parent. 1996. Dynamic interactions of the Gag polyprotein. Curr. Top. Microbiol. Immunol. 214:65-94. [DOI] [PubMed] [Google Scholar]
- 14.Dupont, S., N. Sharova, C. DeHoratius, C. M. Virbasius, X. Zhu, A. G. Bukrinskaya, M. Stevenson, and M. R. Green. 1999. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature 402:681-685. [DOI] [PubMed] [Google Scholar]
- 15.Dupraz, P., and P. F. Spahr. 1993. Analysis of deletions and thermosensitive mutations in Rous sarcoma virus Gag protein p10. J. Virol. 67:3826-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu, W., Q. Dang, K. Nagashima, E. O. Freed, V. K. Pathak, and W. S. Hu. 2006. Effects of Gag mutation and processing on retroviral dimeric RNA maturation. J. Virol. 80:1242-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller, S. D., T. Wilk, B. E. Gowen, H. G. Krausslich, and V. M. Vogt. 1997. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr. Biol. 7:729-738. [DOI] [PubMed] [Google Scholar]
- 19.Ganser, B. K., S. Li, V. Y. Klishko, J. T. Finch, and W. I. Sundquist. 1999. Assembly and analysis of conical models for the HIV-1 core. Science 283:80-83. [DOI] [PubMed] [Google Scholar]
- 20.Garbitt, R. A., J. A. Albert, M. D. Kessler, and L. J. Parent. 2001. trans-Acting inhibition of genomic RNA dimerization by Rous sarcoma virus matrix mutants. J. Virol. 75:260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross, I., H. Hohenberg, T. Wilk, K. Wiegers, M. Grattinger, B. Muller, S. Fuller, and H. G. Krausslich. 2000. A conformational switch controlling HIV-1 morphogenesis. EMBO J. 19:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross, I., H. Hohenberg, C. Huckhagel, and H. G. Krausslich. 1998. N-terminal extension of human immunodeficiency virus capsid protein converts the in vitro assembly phenotype from tubular to spherical particles. J. Virol. 72:4798-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248:295-304. [DOI] [PubMed] [Google Scholar]
- 24.Iwatsuki-Horimoto, K., T. Horimoto, T. Noda, M. Kiso, J. Maeda, S. Watanabe, Y. Muramoto, K. Fujii, and Y. Kawaoka. 2006. The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J. Virol. 80:5233-5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, M. C., H. M. Scobie, Y. M. Ma, and V. M. Vogt. 2002. Nucleic acid-independent retrovirus assembly can be driven by dimerization. J. Virol. 76:11177-11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi, S. M., and V. M. Vogt. 2000. Role of the Rous sarcoma virus p10 domain in shape determination of Gag virus-like particles assembled in vitro and within Escherichia coli. J. Virol. 74:10260-10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilbourne, E. D., and J. S. Murphy. 1960. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J. Exp. Med. 111:387-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishna, N. K., S. Campbell, V. M. Vogt, and J. W. Wills. 1998. Genetic determinants of Rous sarcoma virus particle size. J. Virol. 72:564-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, T., J. Muller, and Z. Ye. 2002. Association of influenza virus matrix protein with ribonucleoproteins may control viral growth and morphology. Virology 304:89-96. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa, Y., D. J. Hockley, M. V. Nermut, and I. M. Jones. 2000. Roles of matrix, p2, and N-terminal myristoylation in human immunodeficiency virus type 1 Gag assembly. J. Virol. 74:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muraki, Y., H. Washioka, K. Sugawara, Y. Matsuzaki, E. Takashita, and S. Hongo. 2004. Identification of an amino acid residue on influenza C virus M1 protein responsible for formation of the cord-like structures of the virus. J. Gen. Virol. 85:1885-1893. [DOI] [PubMed] [Google Scholar]
- 32.Nandhagopal, N., A. A. Simpson, M. C. Johnson, A. B. Francisco, G. W. Schatz, M. G. Rossmann, and V. M. Vogt. 2004. Dimeric Rous sarcoma virus capsid protein structure relevant to immature Gag assembly. J. Mol. Biol. 335:275-282. [DOI] [PubMed] [Google Scholar]
- 33.Nash, M. A., M. K. Meyer, G. L. Decker, and R. B. Arlinghaus. 1993. A subset of Pr65gag is nucleus associated in murine leukemia virus-infected cells. J. Virol. 67:1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda, T., H. Sagara, A. Yen, A. Takada, H. Kida, R. H. Cheng, and Y. Kawaoka. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490-492. [DOI] [PubMed] [Google Scholar]
- 35.Oshima, M., D. Muriaux, J. Mirro, K. Nagashima, K. Dryden, M. Yeager, and A. Rein. 2004. Effects of blocking individual maturation cleavages in murine leukemia virus Gag. J. Virol. 78:1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parent, L. J., T. M. Cairns, J. A. Albert, C. B. Wilson, J. W. Wills, and R. C. Craven. 2000. RNA dimerization defect in a Rous sarcoma virus matrix mutant. J. Virol. 74:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent, L. J., C. B. Wilson, M. D. Resh, and J. W. Wills. 1996. Evidence for a second function of the MA sequence in the Rous sarcoma virus Gag protein. J. Virol. 70:1016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepinsky, R. B., I. A. Papayannopoulos, E. P. Chow, N. K. Krishna, R. C. Craven, and V. M. Vogt. 1995. Differential proteolytic processing leads to multiple forms of the CA protein in avian sarcoma and leukemia viruses. J. Virol. 69:6430-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risco, C., L. Menendez-Arias, T. D. Copeland, P. Pinto da Silva, and S. Oroszlan. 1995. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J. Cell Sci. 108:3039-3050. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, P. C., and R. W. Compans. 1998. Host cell dependence of viral morphology. Proc. Natl. Acad. Sci. USA 95:5746-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts, P. C., R. A. Lamb, and R. W. Compans. 1998. The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology 240:127-137. [DOI] [PubMed] [Google Scholar]
- 42.Scheifele, L. Z., R. A. Garbitt, J. D. Rhoads, and L. J. Parent. 2002. Nuclear entry and CRM1-dependent nuclear export of the Rous sarcoma virus Gag polyprotein. Proc. Natl. Acad. Sci. USA 99:3944-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheifele, L. Z., E. P. Ryan, and L. J. Parent. 2005. Detailed mapping of the nuclear export signal in the Rous sarcoma virus Gag protein. J. Virol. 79:8732-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smirnov, Y., M. A. Kuznetsova, and N. V. Kaverin. 1991. The genetic aspects of influenza virus filamentous particle formation. Arch. Virol. 118:279-284. [DOI] [PubMed] [Google Scholar]
- 45.Swanstrom, R., and J. W. Wills. 1997. Synthesis, assembly and processing of viral proteins., p. 263-334. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 46.Vogt, V. M. 1997. Retroviral virions and genomes, p. 27-70. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 47.Weldon, R. A., Jr., C. R. Erdie, M. G. Oliver, and J. W. Wills. 1990. Incorporation of chimeric Gag protein into retroviral particles. J. Virol. 64:4169-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wills, J. W., and R. C. Craven. 1991. Form, function, and use of retroviral gag proteins. AIDS 5:639-654. [DOI] [PubMed] [Google Scholar]
- 49.Wright, E. R., J. B. Schooler, H. J. Ding, C. Kieffer, C. Fillmore, W. I. Sundquist, and G. J. Jensen. 2007. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 26:2218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang, Y., R. Thorick, M. L. Vana, R. Craven, and J. Leis. 2001. Proper processing of avian sarcoma/leukosis virus capsid proteins is required for infectivity. J. Virol. 75:6016-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeager, M., E. M. Wilson-Kubalek, S. G. Weiner, P. O. Brown, and A. Rein. 1998. Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: implications for retroviral assembly mechanisms. Proc. Natl. Acad. Sci. USA 95:7299-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan, B., S. Campbell, E. Bacharach, A. Rein, and S. P. Goff. 2000. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J. Virol. 74:7250-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yueh, A., and S. P. Goff. 2003. Phosphorylated serine residues and an arginine-rich domain of the Moloney murine leukemia virus p12 protein are required for early events of viral infection. J. Virol. 77:1820-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]