Abstract
A candidate pediatric dengue virus (DENV) vaccine based on nonpropagating Venezuelan equine encephalitis virus replicon particles (VRP) was tested for immunogenicity and protective efficacy in weanling mice in the presence and absence of potentially interfering maternal antibodies. A gene cassette encoding envelope proteins prM and E from mouse-adapted DENV type 2 (DENV2) strain NGC was cloned into a VEE replicon vector and packaged into VRP, which programmed proper in vitro expression and processing of DENV2 envelope proteins upon infection of Vero cells. Primary immunization of 3-week-old weanling BALB/c mice in the footpad with DENV2 VRP resulted in high levels of DENV-specific serum immunoglobulin G antibodies and significant titers of neutralizing antibodies in all vaccinates. A booster immunization 12 weeks after the prime immunization resulted in increased neutralizing antibodies that were sustained for at least 30 weeks. Immunization at a range of doses of DENV2 VRP protected mice from an otherwise-lethal intracranial DENV2 challenge. To model vaccination in the presence of maternal antibodies, weanling pups born to DENV2-immune or DENV2-naïve dams were immunized with either DENV2 VRP or live DENV2 given peripherally. The DENV2 VRP vaccine induced neutralizing-antibody responses in young mice regardless of the maternal immune status. In contrast, live-DENV2 vaccination performed poorly in the presence of preexisting anti-DENV2 antibodies. This study demonstrates the feasibility of a VRP vaccine approach as an early-life DENV vaccine in populations with high levels of circulating DENV antibodies and suggests the utility of VRP-based vaccines in other instances where maternal antibodies make early vaccination problematic.
Dengue viruses (DENV) are members of the family Flaviviridae and one of the most important groups of emerging viruses of global significance today (36, 66). There are four distinct antigenic serotypes (DENV1, DENV2, DENV3, and DENV4), all of which are capable of causing a spectrum of diseases in humans ranging from asymptomatic infections to debilitating classical dengue fever and severe and often fatal dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) (36, 68). DENV is transmitted to humans primarily by the mosquito Aedes aegypti. The lack of effective mosquito control, as well as demographic and economic changes, has contributed to the dramatic expansion and worldwide distribution of DENV epidemic activity in tropical and subtropical areas (36). It is estimated that up to 100 million infections and several hundred thousand cases of DHF/DSS occur each year, with more than 2.5 billion people living in areas at risk of infection in 2004 (21, 68). DHF is a leading cause of hospitalization and death among children in many countries in Southeast and South Asia, and the WHO has reported a rising trend in disease over the past decade (68). At the peak of epidemic times, as many as 70 children with severe DHF may present to a single hospital in a day, 20 of them with potentially fatal DSS (58). Although DHF/DSS in infants has not been comprehensively studied, it is estimated that more than 5% of all DHF/DSS cases occur in infants (26, 33, 41, 43, 56, 67, 70).
In the absence of vector control effective on a global scale, there is a clear need for a DENV vaccine. However, the development of a DENV vaccine has faced significant challenges that have resulted in the lack of a licensed vaccine after 70 years of research (17). In many areas where there is cocirculation of two or more serotypes, there is a high probability that individuals will be infected more than once in their lifetimes. Preexisting homotypic immunity protects from a secondary infection with the same serotype, and this protection seems to last for life (24, 25). However, preexisting heterotypic nonneutralizing immunity to a secondary infection with a different DENV serotype is a risk factor for the development of severe DHF/DSS (23, 27, 61). These considerations suggest that a safe and efficacious DENV vaccine must be tetravalent and induce a long-term and balanced immune response to all four serotypes simultaneously in order to avoid sensitizing the vaccine recipient to a more severe outcome during a subsequent DENV infection. Additionally, primary infections during the first year of life that result in DHF/DSS have been associated with the presence of subneutralizing levels of maternal anti-DENV antibodies, which may increase the risk of enhanced infection and disease by antibody-mediated enhancement (26, 33, 41, 56). To protect infants and children in dengue-endemic countries from severe dengue, the ideal DENV vaccine should be given during the first 6 months of life. In addition, an infant DENV vaccine has to be effective in the face of circulating anti-DENV maternal antibodies, which in dengue-endemic countries are present in more than 95% of newborns and have disappeared by 12 months of age (63).
There are a number of DENV vaccine candidates in preclinical and clinical trials (reviewed in references 10 and 66), including live attenuated virus, DNA plasmids (49), subunit vaccines (11, 16), and adenovirus vectors (29, 31). Live attenuated virus vaccines are the more advanced candidates in phase I and II clinical trials. They have been attenuated either empirically (4), by engineering attenuating mutations into a DENV cDNA infectious clone (5, 15), or by chimerization with other flaviviruses (22, 39, 46). Further clinical development of these candidates has been delayed due to several problems. (i) Balanced immune responses to the four serotypes have proven difficult to achieve with tetravalent cocktails of live vaccine candidates, in which each component differs in its level of attenuation or in which interference among the live components of the vaccine may occur. (ii) Determination of virulence in primate models may not accurately predict attenuation for humans. In fact, an attenuated DENV3 candidate vaccine that was deemed safe in mice and primates produced dengue fever in human volunteers (51). (iii) In many DENV-endemic regions of Asia, the dengue seroprevalence is very high, and over 95% of children born have maternal dengue antibody. Human safety as assessed in a phase I trial in seronegative populations may not accurately reflect safety in persons seropositive for one of the DENV serotypes or infants with maternal antibodies. (iv) The presence of such antibodies also might interfere with live attenuated dengue vaccines. If vaccine is administered during the first year of life, passively transferred anti-DENV maternal antibodies would likely interfere with the replication and immunogenicity of one or more components of the tetravalent cocktail. If the vaccine is administered later in childhood or in adulthood, antibodies to an earlier natural infection may be boosted and yet interfere with the immunogenicity of a heterologous component of the multivalent live vaccine.
We propose that nonpropagating Venezuelan equine encephalitis virus (VEE) replicon particles (VRP) are well suited to address the difficulties faced in DENV vaccine development. Three properties of the VEE vectors may contribute to their ability to overcome maternal-antibody interference to a significant degree. (i) The DENV antigens are not exposed on the VRP surface; therefore, preexisting DENV-neutralizing antibodies should not affect delivery of the DENV genes to the target cells. (ii) Unlike live attenuated vaccines that depend on multiple rounds of replication and are thus more susceptible to interference by preexisting anti-DENV antibodies, nonpropagating VRP vectors express high levels of the heterologous gene in a single round of infection. (iii) Due to the tropism mediated by the VEE glycoproteins that targets the VRP to the lymph node (35), and due to the adjuvant activity of the VRP (57), antigen presentation is facilitated and enhanced.
The safety of nonpropagating VEE replicon vectors has been tested in many different animals, including over 2,000 rodents, 100 macaques, and more than 20 horses. No clinical signs of disease have been observed with any of these animals, including neonatal mice inoculated intracranially (i.c.) with 5 × 107 infectious units (IU) and RAG−/− mice inoculated with 107 IU of a VRP vaccine (48; A. West and N. Davis, personal communication). Safety has also been demonstrated in young adult volunteers in the United States, South Africa, and Botswana undergoing phase I clinical trials with a VRP expressing the Gag protein of clade C human immunodeficiency virus type 1. No serious adverse events were reported with doses as high as 108 IU (12). VRP vectors confer long-lived humoral and cellular immune responses to a wide variety of viral and bacterial antigens tested in animal models, resulting in strong and complete protective immune responses to influenza virus in rodents and chickens (48, 52), Lassa fever and ebola viruses in rodents (69), equine arteritis virus in rodents and horses (2), and Marburg virus in primates (28).
Here, we demonstrate the ability of VRP vaccine vectors to deliver the immunogenic membrane prM and E protein genes of DENV2 into young mice and to induce a protective humoral immune response, even in the presence of maternal antibodies that otherwise interfere with immunization with a model live DENV2 vaccine. This study shows the feasibility of a VRP vaccine approach as an early-life DENV vaccine to protect infants during that window of time when maternal antibodies are no longer protective but still may interfere with active immunization induced by a live attenuated vaccine.
MATERIALS AND METHODS
Cells.
BHK-21 and Vero-81 cells were obtained from the American Type Culture Collection (ATCC). BHK-21 cells were maintained in alpha minimal essential medium containing 10% donor calf serum, 10% tryptose phosphate broth, and 0.29 mg of glutamine per ml. Vero-81 cells were maintained in Dulbecco's modified Eagle's medium/F12 medium supplemented with 10% fetal calf serum and 0.29 mg of glutamine per ml. Insect C6/36 cells were obtained from the ATCC and maintained in alpha minimal essential medium containing 10% fetal calf serum.
Antibodies.
Hyperimmune mouse ascetic fluid (HMAF) specific for DENV2 strain New Guinea C (NGC) was obtained from the ATCC. Two monoclonal antibodies (MAbs) that recognize conformational epitopes on DENV2 E protein were used: 4G2 (ATCC) and 3H5-1-21/TCF (CDC). Human immune sera were collected by us for other studies of dengue epidemiology and immunology or from the National Institute for Biological Standards and Controls (Hertfordshire, United Kingdom).
Viruses.
The mouse-adapted, neurovirulent NGC strain of DENV2 used in these studies was provided by the late Robert Shope, University of Texas Medical Branch, Galveston, TX. The stock virus for immunization and challenge studies was amplified no more than two times in C6/36 cells and stored at −80°C at a concentration of 1 × 107 PFU/ml. The stock virus used for neutralization assays was propagated two or three times in C6/36 cells, titrated on Vero cells, and stored at −80°C at a concentration of 1 × 107 PFU/ml. The virus preparation used as an antigen to coat enzyme-linked immunosorbent assay (ELISA) plates was obtained by further purification and concentration of the C6/36-grown virus stock. Briefly, DENV2-infected C6/36 culture supernatants were subjected to centrifugation at 72,000 × g for 5 h through a 5-ml cushion of 20% (wt/vol) sucrose. The sedimented virus was further purified by density gradient centrifugation in a 10 to 40% iodixanol gradient at 163,700 × g for 120 min. Virus-containing fractions were pooled, and purified virus was concentrated by centrifugation at 72,000 × g for 5 h. The virus was resuspended in phosphate-buffered saline (PBS)-1% fetal bovine serum and stored at −80°C.
Cloning the DENV2 prM/E cassette into the VEE replicon plasmid.
cDNA of DENV2 prM/E genes was obtained from the mouse-neuroadapted DENV2 strain NGC RNA genome by reverse transcription-PCR. We engineered a start codon and a stop codon flanking the sequence encoding (5′ to 3′) the C-terminal domain of the capsid gene containing the prM signal sequence (20 amino acids), the prM gene, and the E gene. The sequences of the primers used to amplify this gene cassette are as follows: forward primer, 5′ AGTCTAGTCCGCCAAGATGTTGAACAGGAGACGCAGAACTGCAGG; reverse primer, 5′ GGCGCGCCTTAGGTCTGCACCATAACTCCCAAATACAGCGT. The amplified regions were initially cloned into PCR cloning plasmids, and their sequences were confirmed. The prM/E gene cassette was cloned into the multicloning site of the VEE replicon vector pVR21 (2) using ApaI and AscI sites upstream and downstream of the 26S subgenomic-RNA transcription start site, respectively, by overlapping extension PCR to generate pVRDENV2prM/E. The clone was linearized at a unique NotI site downstream of the VEE 3′ untranslated region and poly(A) tract, and full-length T7 transcripts were generated in vitro using an mMessage mMachine kit (Ambion) as previously described (14). To package the recombinant replicon genome into VRP for delivery in vitro and in vivo, the replicon RNA was mixed with two helper RNAs also transcribed in vitro using T7 polymerase. One helper encoded only the capsid gene, and the other encoded only the glycoproteins from a cDNA clone of VEE, V3000. The helper RNAs had the replicase genes and the cis-acting packaging signal deleted. Transcripts were cotransfected into BHK or Vero cells by electroporation. The culture medium was harvested at 22 to 24 h postelectroporation.
Purification and titration of VRP.
VRP-containing culture medium was clarified by centrifugation at 12,000 × g for 30 min, and the VRP were partially purified and concentrated by sedimentation at 72,000 × g for 3 h through a 5-ml cushion of 20% (wt/vol) sucrose dissolved in PBS. The pelleted VRP were resuspended overnight in endotoxin-free PBS with 1% donor calf serum at 4°C, followed by storage at −80°C. Each VRP preparation was safety tested to ensure the absence of replication-competent virus that could have arisen by nonhomologous recombination. Ten percent of the preparation was used to inoculate BHK cells, and the presence of cytopathic effect was monitored during two sequential passages as an indication of the presence of replication-competent virus. Preparations that resulted in cytopathic effect failed the safety test and were discarded. VRP were titrated in BHK cells by indirect immunofluorescence assays (IFA). Cells seeded in eight-well chamber slides were infected with serial dilutions of the concentrated VRP preparation for 18 h at 37°C, fixed in methanol for 10 min at 4°C, and incubated sequentially with mouse polyclonal anti-DENV antibody, biotinylated anti-mouse immunoglobulin G (IgG), and avidin conjugated to fluorescein isothiocyanate. Replicon-infected fluorescent cells were enumerated using a fluorescence microscope under UV illumination to determine the number of BHK IU per ml.
Radioimmunoprecipitation and polyacrylamide gel electrophoresis.
Vero or BHK cell monolayers grown in six-well plates were mock infected or infected with DENV2 VRP (multiplicity of infection [MOI] = 10 IU/cell) or DENV2 NGC (MOI = 10 PFU/cell). At 6 h postinfection (p.i.), VRP- and mock-infected cells were starved for 1 h in methionine- and cysteine-free medium (Sigma), followed by metabolic radiolabeling with 100 μCi of [35S]methionine and cysteine/ml (Pro-Mix; Amersham) for 4 h. Vero cells infected with DENV2 NGC were starved at 49 h p.i. and radiolabeled from 50 to 55 h p.i. At the end of the labeling period, the medium was clarified by centrifugation at 10,000 × g for 15 min in a microcentrifuge, followed by sedimentation through a 20% sucrose cushion in a TLA 100.3 rotor for 3 h at 60,000 rpm. The pellets were incubated in PBS for resuspension overnight at 4°C. The cells were lysed in NP-40 buffer in the presence of protease inhibitors. DENV proteins in the cell lysates and the pelleted media were immunoprecipitated with DENV2-specific HMAF (a 1:50 dilution of neat HMAF) or MAb 4G2 or 3H5 (a 1:50 dilution of 0.1 mg/ml), or normal mouse sera using protein A-Sepharose CL-4B beads (Sigma) according to standard protocols. Proteins were separated by electrophoresis in 10% polyacrylamide gels (sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) after being denatured in 1% SDS and 50 mM 2-β-mercaptoethanol. Molecular mass standards (14.3 to 220 kDa; Amersham) were included, and radiolabeled protein bands were visualized using a phosphorimager.
IFA.
BHK or Vero cells grown in eight-well chamber slides were mock infected or infected with DENV2 VRP at an MOI of 10. At 18 to 24 h p.i., the cells were rinsed with PBS and fixed with cold methanol for 10 min. The fixed cells were stained with one of the following primary antibodies: mouse polyclonal HMAF (1:200), MAb 3H5, or MAb 4G2 (1:400). Both MAbs recognize conformational epitopes on DENV2 E protein. Texas Red-conjugated goat anti-mouse IgG was used as the secondary antibody (1:200; Sigma). Stained cells were observed using a fluorescent microscope.
Mice immunizations and lethal challenge.
Specific-pathogen-free adult (6-week-old) and weanling (3-week-old) BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). Weanling mice were also generated in our breeding colony from DENV-naïve or DENV-immune female BALB/c mice crossed with DENV-naïve male BALB/c mice. Breeding cages were checked daily for new births. Pups were kept with their mothers until they were weaned at 3 weeks of age. Animal housing and care at the University of North Carolina were in accordance with all University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee guidelines.
For infant mouse immunizations, groups of six mice were immunized at 3 and 9 or at 3 and 15 weeks of age with one of three vaccine formulations: DENV2 VRP (105 or 106 IU subcutaneously [s.c.]), nonspecific control influenza virus hemagglutinin (HA) VRP (105 or 106 IU s.c.), or live-virus control DENV2 NGC (105 or 106 PFU intraperitoneally [i.p.]). VRP vaccines were administered by s.c. inoculation in the left rear footpad in a 10-μl volume after physically restraining the mouse. Virus immunization was delivered in 100 μl by i.p. injection. By this route, infection with this DENV2 strain results in subclinical infection and induction of humoral and cellular immune responses. The mice were bled by tail vein puncture 1 day prior to and periodically after immunizations at 2- to 3-week intervals. The sera were stored at −20°C for later analysis of the humoral response by virus neutralization assays and ELISA.
For protection studies, BALB/c mice (eight per group) were immunized once at 3 weeks of age or twice at 3 and 5 weeks of age with either 104, 105, or 106 IU of DENV2 VRP, 106 IU of HA VRP, or 106 PFU of DENV2 NGC. VRP and virus immunizations were delivered as described above. At 6 weeks of age, the mice were challenged i.c. with a lethal dose (104 PFU in a 10-μl inoculum) of DENV2 NGC, after being anesthetized by i.p. injection with a 4/1 mixture (vol/vol) of ketamine (50 μg/g body weight) and xylazine (15 μg/g body weight). Following challenge, the mice were monitored daily for 21 days for weight loss, morbidity, and mortality. Mice were euthanized if their weight dropped below 80% of their initial weight. In this challenge model, i.c. delivery of the mouse-adapted neurovirulent DENV2 strain NGC results in 100% fatal encephalitis in BALB/c mice 6 weeks old or younger.
Passive transfer of maternal antibodies and immune sera.
DENV-immune adult female mice were generated by two i.p. injections with 1 × 106 PFU each of DENV2 NGC 3 weeks apart to reach a DENV-specific neutralization titer (F-NEUT50 [see below]) of 160 or higher before pregnancy. DENV-immune and DENV-naïve BALB/c dams were mated with DENV-naïve BALB/c males. The pups were allowed to nurse for 21 days before being weaned. Each litter was divided into two groups (four to six pups per group), and each group received one of two vaccines: DENV2 VRP or live DENV2 NGC at doses of 105 IU s.c. or 105 PFU i.p., respectively. The immunized pups were bled at 3, 6, 9, 11, 13, and 17 weeks of age. A third group of pups born to DENV-immune dams were not immunized and were bled at the same intervals to measure passively transferred maternal antibodies and their rate of decay over time.
DENV neutralization assays.
DENV2-specific neutralizing antibodies in immunized mouse serum were quantified using a flow cytometry-based neutralization assay (F-NEUT) on Vero cells. This neutralization assay was adapted from a flow cytometry-based DENV titration assay as described previously (34; A. Kraus, W. Messer, L. Haymore, T. Morrison, and A. de Silva, submitted for publication). In the F-NEUT assay, the ability of immune serum to neutralize the infectivity of DENV on Vero cells is measured at 20 to 24 h p.i. by enumerating cells that are positive for intracellular staining of DENV E protein by flow cytometry. This assay can be used to measure the neutralization of clinical isolates and viral strains that have not been adapted to grow on Vero or BHK cells and that do not plaque well in the classical plaque reduction neutralization test (PRNT). In addition, the flow cytometry-based assay reduces the performance time from 1 week to 3 days. Briefly, heat-inactivated control or immune sera were diluted twofold with Dulbecco's modified Eagle's medium/F12 containing 1% bovine serum albumin, and each dilution was mixed with an equivalent volume of DENV2 NGC in a total volume of 110 μl. The dilution of DENV2 chosen ensured an infection rate within the linear range of the dose-response curve, i.e., 7 to 15% at 24 h, and allowed antibody to be in excess. The mixture was incubated for 1 h at 37°C in 5% CO2 before transferring it to Vero cell monolayers seeded the day before in 24-well plates at 105 cells/well. Each serum dilution was tested in duplicate wells. After 1 h of adsorption, complete medium was added, and the plates were incubated for 20 to 24 h at 37°C. To enumerate infected cells, monolayers were washed with PBS, trypsin treated, washed again with PBS, and transferred to 96-well plates with a V bottom (Corning, Inc.), where the cells were fixed and permeabilized with Cytofix/Cytoperm (BD-PharMingen, San Diego, CA) at 4°C for 20 min, washed twice with Perm-Wash solution, and stained for 1 h at 4°C with 50 μl of ALEXA 488-conjugated MAb 4G2 (4.2 nM final concentration). After two additional washes to remove unbound antibody, the cells were analyzed by flow cytometry in a FACS-SCAN II (Becton Dickinson). Controls included in each assay were (i) mock-infected cells, (ii) cells infected with DENV2 in the absence of immune sera or with normal mouse sera, and (iii) cells infected with DENV2 preincubated with a reference DENV-immune serum. The percent neutralization for each dilution was defined as the reduction in the number of E-antigen-positive cells in the test sera compared with the number of cells in the virus control. The neutralization titer for each serum sample was expressed as the reciprocal of the highest dilution of serum that neutralized the challenge virus by 50% (F-NEUT50). For a subset of mouse serum samples and a number of WHO reference sera, neutralizing-antibody titers were determined by PRNT, in addition to F-NEUT, and the titers obtained from both methods were comparable, as shown earlier (34; Kraus et al., submitted).
DENV-specific ELISA for IgG and IgG subclasses.
Serum IgG, IgG2a, and IgG1 against DENV2 were measured by ELISA. The DENV antigen used to coat the plates consisted of DENV2 NGC purified by iodixanol density gradient centrifugation as described above. Immulon 4HBX 96-well plates (Thermo Electron Corp.) were coated with DENV2 antigen at 0.5 μg/ml in carbonate buffer (pH 9) and incubated overnight at 4°C. After excess antigen was removed, the plates were blocked using 0.05% Tween 20 in PBS with 10% Sigmablock (Sigma) for 3 h at 4°C. After each incubation, the plates were washed five times with 0.05% Tween 20 in PBS. Serum samples were diluted in 10% Sigmablock and incubated for 3 h at 4°C. After being washed, the plates were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG, IgG2a, or IgG1 (Southern Biotechnology Associates) diluted 1/1,000 in 10% Sigmablock for 2 h at 4°C. The plates were washed, and O-phenylenediamine dihydrochloride substrate was added for 30 min, after which the reaction was stopped by the addition of 0.1 N NaF. Optical densities (OD) versus serum dilutions were analyzed by linear regression, and titers were calculated from the regression parameters as the inverse of the dilution that produced an OD at 450 nm (OD450) ≥0.2 above the blank.
VEE neutralization assay.
VEE-specific neutralization titers in sera from VRP-immunized mice were determined using a flow cytometry-based neutralization assay. Briefly, BHK cell monolayers were seeded in 24-well plates at a density of 105 cells per well. Control or immune sera were diluted twofold with minimal essential medium containing 1% bovine serum albumin, and each dilution was mixed with an equivalent volume of a VRP expressing green fluorescent protein (35) to give an MOI of 0.1 in a total volume of 110 μl. The mixture was incubated for 1 h at 37°C in 5% CO2 before being transferred to BHK cell monolayers in 24-well plates. Each serum dilution was tested in duplicate wells. After 1 h of adsorption, complete medium was added, and the plates were incubated for 24 h at 37°C. To enumerate infected cells, monolayers were washed with PBS, trypsin treated, washed again with PBS, and fixed in 2% paraformaldehyde. The cells were analyzed by flow cytometry in a FACS-SCAN II (Becton Dickinson). The percent neutralization for each dilution was defined as the reduction in the number of green fluorescent protein-expressing cells in the test sera compared with the number of cells in the VRP control. The neutralization titer for each serum sample was expressed as the reciprocal of the F-NEUT50.
Statistical analysis.
Antibody titers were evaluated for statistically significant differences by the Mann-Whitney test (INSTAT; GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Expression of DENV2 prM and E proteins in cells infected with DENV2 VRP.
We constructed a prototype DENV2 VRP vaccine expressing the immunogenic membrane proteins prM and E from the DENV2 strain NGC under the control of the VEE replicon 26S subgenomic promoter. The prM/E gene cassette included the signal sequence from the C terminus of the capsid protein to drive the insertion of prM/E into the endoplasmic reticulum membrane, where signal peptidases would cleave the precursor into the individual proteins prM and E as they progressed through the secretory pathway.
A single transfection reaction of 1.2 × 107 BHK-21 cells typically yielded between 0.5 × 108 and 5 × 108 BHK IU. The titers of unconcentrated culture supernatants ranged from 2.5 × 105 to 2.5 × 106 IU/ml. After concentration, the titers were typically between 0.5 × 108 and 5 × 108 IU/ml.
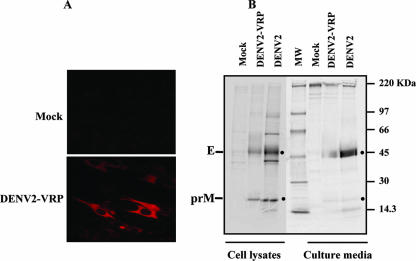
The in vitro expression of DENV2 prM and E proteins in VRP-infected cells was examined by IFA and by immunoprecipitation of metabolically radiolabeled infected cultures (Fig. 1). Specific staining of DENV2 proteins was observed in cells stained with MAb 3H5, which recognizes a conformational epitope on the E protein (Fig. 1A); with anti-DENV2 polyclonal HMAF; and with human convalescent antisera (data not shown). Cytoplasmic staining with these antibodies was detected in cells infected with DENV2-VRP but not in mock-infected cells (Fig. 1A) or in influenza virus HA VRP-infected cells (data not shown). The expression and processing of the prM/E gene cassette into final protein products, and possible secretion of subviral particles (10, 19), were further characterized by radiolabeling with [35S]methionine and cysteine, followed by immunoprecipitation of infected cell lysates and pelleted culture supernatants with anti-DENV2 HMAF. Analysis of radiolabeled DENV-specific proteins by 10% SDS-PAGE (Fig. 1B) revealed the presence of protein bands migrating at the apparent molecular sizes of DENV E and prM proteins in the cell lysates and pelleted supernatants of Vero cells infected with DENV2 VRP, but not in mock-infected cells. These bands comigrated with predicted E and prM proteins present in the cell lysates and supernatants of DENV2 NGC-infected cells. The E and prM proteins were also immunoprecipitated using MAb 4G2 and human convalescent-phase sera (data not shown). The presence of mature M protein was not consistently detected in our gels.
FIG. 1.
Expression of DENV2 prM and E proteins in Vero cells infected with DENV2 VRP. (A) Immunofluorescence. Vero cells were mock infected or infected with DENV2 VRP (MOI = 10); 24 h p.i., the cells were rinsed with PBS, fixed in cold methanol for 10 min, and stained with MAb 3H5 (1:400) specific for DENV2 E protein. Texas Red-conjugated anti-mouse IgG was used as a secondary antibody. (B) Immunoprecipitation. Vero cells were mock infected, infected with DENV2 prM/E-VRP, or infected with DENV2 (MOI = 10). VRP-infected cells were starved in methionine/cysteine-free medium for 1 h, followed by metabolic radiolabeling with 35S Pro-Mix (100 μCi/ml) for 4 h. DENV2-infected cells were starved at 49 h p.i. and labeled for 5 h. DENV-specific proteins were immunoprecipitated from the cell lysates and pelleted media using HMAF. Proteins were separated in a 10% SDS-PAGE gel.
These results indicate that the DENV2 membrane proteins are properly expressed and processed when a gene cassette containing the signal sequence from the C terminus of the capsid protein and the prM and E genes is cloned into a VEE replicon plasmid, packaged into VRP, and delivered into cultured cells. The presence of E and prM in the pelleted media of infected cells, although at low levels, suggests that the proteins are present in the form of extracellular subviral particles or some other aggregated form.
DENV2 VRP induces antibody responses in young mice.
To determine the immunogenicity of DENV2 prM/E proteins delivered by a nonpropagating VRP vector in 3-week-old infant mice, groups of six BALB/c female infant mice were immunized by s.c. injection into the footpad with 106 IU of DENV2-VRP in a 10-μl volume. A control group was immunized with 106 IU of HA VRP by s.c. injection. In order to compare the responses induced by the VRP vaccine to those induced by a replicating DENV2, a group of control mice were immunized i.p. with 106 PFU of DENV2 NGC, which undergoes limited replication and subclinical infection upon peripheral inoculation, enough to induce a protective immune response in the absence of disease signs, mimicking immunization with a live attenuated vaccine. Delivery of DENV2 NGC by the i.p. route allows a larger inoculum to be utilized and therefore is more immunogenic than the s.c. route. The mice were primed at 3 weeks of age and boosted with the same formulation 12 weeks later. Neutralizing-antibody titers and ELISA IgG titers were determined in sera obtained 1 day before each immunization and at 2- to 3-week intervals after each immunization.
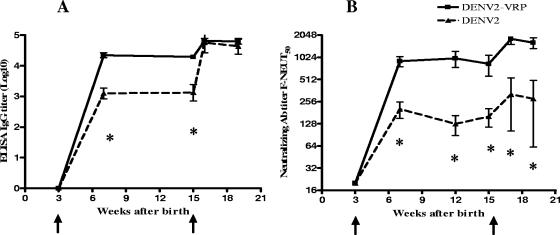
The time course of induction of serum antibodies to DENV2 was measured by IgG ELISA and F-NEUT, as shown in Fig. 2A and B, respectively. A single dose of 106 IU of DENV2-VRP induced DENV-specific binding antibody in all immunized mice. At 3 weeks postprime, average ELISA titers were 4.3 on a log10 scale. These titers remained high for another 12 weeks after a single dose and showed further increase after a second, booster immunization. Neutralizing-antibody titers induced by immunization with DENV2 VRP showed kinetics of induction similar to those of ELISA IgG titers. At 3 weeks postprime, the mean F-NEUT50 titer was 904, and it remained high for 12 weeks after the prime, showing further increase after the second immunization. Control mice vaccinated with 106 PFU of live DENV2 NGC responded with ELISA IgG and neutralizing titers significantly lower than those found in DENV2 VRP-vaccinated mice after the prime (P < 0.05). A second immunization resulted in a significant increase in serum IgG antibodies reactive with DENV2 by ELISA (P < 0.05); however, a parallel increase in neutralizing antibodies after the boost was not observed. This suggests that a larger proportion of the ELISA antibodies induced by the live-virus booster are nonneutralizing antibodies. This seems to be in contrast with the response induced by the VRP vaccine, in which the kinetics of DENV2 antibodies reactive in ELISA correlates with DENV2-specific neutralizing antibodies. The specificity of the antibody assays for DENV was shown by the responses of the HA VRP-immunized mice, which showed neither DENV2-specific ELISA antibody titers (OD450 < 0.2) nor neutralizing antibodies (F-NEUT50 < 10) at any time.
FIG. 2.
Kinetics of DENV2-specific serum antibodies in young mice after prime and boost immunizations. Groups of six weanling mice were immunized at 3 and 15 wks after birth with 106 IU of DENV2 VRP (squares) or 106 PFU of DENV2 NGC (triangles). The mice were bled at the indicated times for detection of serum-specific DENV2 antibodies. (A) Total serum IgG titers were determined by ELISA. The data represent IgG ELISA endpoint dilution GMTs (OD450 > 0.2). The error bars represent standard deviations. (B) Neutralizing-antibody (Ab) titers were measured by flow cytometry-based neutralization tests on Vero cells (F-NEUT). The data represent the F-NEUT50 GMTs. The error bars represent standard errors. *, significantly different at a P value of <0.05 by Mann-Whitney U test. The arrows indicate prime and boost immunization times.
A separate experiment was conducted to examine the speed at which immunity was induced after a priming VRP immunization. In a group of eight mice immunized with 106 IU of DENV2 VRP, as early as 1 week after prime, all of the mice had neutralizing-antibody titers above an F-NEUT50 of 20 (geometric mean titer [GMT], 26).
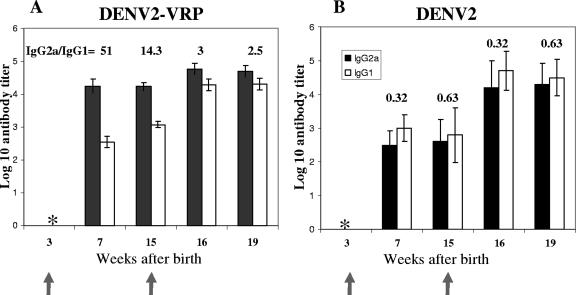
To better characterize the quality of the immune response induced by DENV2 VRP, the IgG subclass of antibodies induced by a VRP vaccination in infant mice were determined, as markers for Th1/Th2 responses, and then compared to those elicited by a live-virus immunization (Fig. 3). The VRP-based immunization induced predominantly IgG2a after the prime and the boost. However, the difference between IgG2a and IgG1 was more pronounced after the prime, when the IgG2a/IgG1 ratios were 51:1 at 7 weeks. This difference became less pronounced later after the prime (14.3:1 at week 15). Furthermore, after the boost, there was an increase in the IgG1 titers, resulting in lower IgG2a/IgG1 ratios of 3:1 and 2.5:1 at weeks 16 and 19, respectively. These results suggest a Th1-biased response after the first dose that becomes more balanced after the boost. Mice immunized with DENV2 NGC showed similar titers of both IgG subclasses at all time points, with a slight predominance of IgG1, suggesting a Th2 bias throughout the time course.
FIG. 3.
Kinetics of DENV-specific IgG subclass distribution as a marker for Th1/Th2 responses after DENV2 VRP immunization (A) and DENV2 immunization (B). Weanling mice were immunized at 3 weeks and 15 weeks after birth with 106 IU of DENV2 VRP or 106 PFU DENV2 NCG. The mice were bled at the times indicated on the x axis. The data represent mean DENV-specific IgG2a (solid bars) and IgG1 (empty bars) ELISA endpoint dilution titers. The error bars represent standard deviations. IgG2a/IgG1 ratios are shown above the histograms. *, below the level of detection.
The duration of DENV2-specific serum neutralizing antibodies after VRP immunization was addressed in a separate experiment in which a group of six mice immunized at ages of 3 and 12 weeks with DENV2 VRP (105 IU s.c.) were monitored up to 30 weeks post-booster vaccination. The F-NEUT50 titers were sustained through the later time points (GMTs ± standard errors of the mean were as follows: 6 weeks postprime, 160 ± 0; 10 weeks postprime, 190 ± 137; 6 weeks postboost, 1,076 ± 747; 30 weeks postboost, 906 ± 329).
Anti-VEE immunity induced by the VRP vector was measured after one or two immunizations with 106 IU of DENV2 VRP. Weanling mice (n = 6) were immunized at 3 and 15 weeks of age, and serum samples collected at 6 weeks postprime and 4 weeks postboost were analyzed for the presence of VEE-specific neutralizing antibodies. Moderate anti-VEE neutralization titers were induced after the prime (F-NEUT50 = 92 ± 116). After the second dose, the titers increased considerably (F-NEUT50 = 5,120 ± 8,022). It is important to note that, in spite of the antivector response after the prime, a booster response to the DENV proteins was not hampered (Fig. 2B).
DENV2 VRP immunization protects mice from lethal challenge.
The DENV challenge model is based on the development of lethal encephalitis in young mice (6 weeks or younger) upon i.c. inoculation with a lethal dose of the mouse-adapted NGC strain of DENV2. This model has been used to screen DENV vaccine candidates in mice before testing them in nonhuman primates (9).
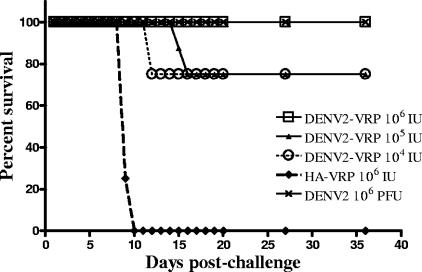
Three-week-old BALB/c mice (eight per group) were immunized with DENV2 VRP (106, 105, or 104 IU s.c.). Control mice were immunized with HA VRP (106 IU s.c.) and DENV2 NGC (106 PFU i.p.). The mice received a single immunization at 3 weeks of age (experiment 1) (data not shown) or two immunizations 2 weeks apart at 3 weeks and 5 weeks of age (experiment 2) (Fig. 4). All mice were challenged in week 6 by i.c. injection of 1 × 104 PFU of DENV2 NGC. The animals were monitored daily for 3 weeks for weight loss, morbidity, and mortality after challenge.
FIG. 4.
Survival of immunized mice following DENV2 NGC lethal i.c. challenge. BALB/c mice were immunized at 3 and 5 weeks of age with the indicated formulation and doses (n = 8 per group). In week 6, all mice were challenged by i.c. inoculation with 104 PFU of DENV2 NGC. The mice were monitored for clinical signs and weight loss for 21 days after challenge.
All mice immunized with either one (data not shown) or two doses of 106 IU of DENV2-VRP were completely protected from DENV2 challenge (Fig. 4). These mice survived the infection without showing signs of disease, and weight loss did not exceed 10% of the initial weight. All mice immunized i.p. with the DENV2 NGC were protected from challenge, as well. On the other hand, all the mice in the control group immunized with HA VRP succumbed to lethal encephalitis (Fig. 4).
Lower doses of DENV2 VRP induced high levels of protection, as well. Mice immunized with two doses of 105 and 104 IU of DENV2 VRP showed significant (75%) but not complete protection from challenge (Fig. 4). In another experiment, all mice immunized with a single dose of 105 IU of DENV2 VRP were protected from challenge (experiment 1) (data not shown). A single dose of 104 IU was not tested.
VRP-vaccinated dams transfer protective antibodies to their offspring.
To further explore the role of neutralizing antibodies induced by the VRP vaccine in protection, we evaluated whether passively transferred anti-DENV antibodies from DENV2 VRP-immune mothers would protect their offspring. Female BALB/c mice that had received two doses of DENV2 VRP s.c. (106 IU) or two doses of control HA VRP s.c. (106 IU) or DENV2 NGC i.p. (106 PFU) were mated 3 weeks after the boost with naïve BALB/c males. The pups were allowed to nurse for 15 and 21 days before they were challenged i.c. with a lethal dose of DENV2 NGC. The pups were monitored daily for 3 weeks for weight loss, morbidity, and mortality after the i.c. challenge. We found that 10 of 11 pups (91%) born to DENV2 VRP-immune dams (the F-NEUT50 GMT was 160 when they became pregnant) and challenged at 21 days were protected from a lethal challenge with 103 PFU DENV2 NGC. Nine out of nine pups born to DENV2 NGC-immune dams were also protected from the lethal challenge, while eight out of eight pups from HA VRP-immunized dams succumbed to the challenge. Pups nursed on DENV2 VRP-immunized dams for 15 days (n = 7) and then challenged also showed significant protection (86%) (data not shown). Taken together, these results demonstrate protection in neonates suckled on VRP-immunized dams and that anti-DENV antibodies transferred from dam to pup significantly inhibit replication of DENV upon challenge.
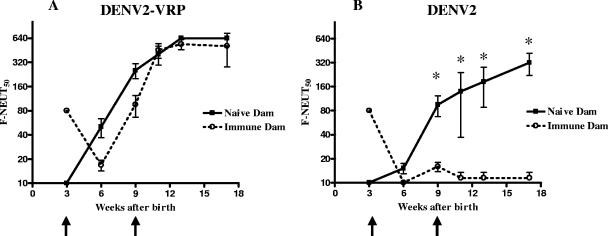
DENV2 VRP induces neutralizing antibodies in the presence of maternal antibodies.
The presence of placentally transferred DENV-specific maternal antibodies in human newborns in DENV-endemic areas is a major obstacle for early-life immunization with live attenuated DENV vaccines. To model maternal-antibody interference in mice, the responses to VRP and live-virus vaccines in pups from DENV-immune dams were compared to responses in pups born to and suckled on DENV-naïve dams. Adult female BALB/c mice (n = 6) were immunized twice with 106 PFU of DENV2 NGC by i.p. injections to mimic natural exposure to replicating virus, and their subsequent DENV2-immune status was confirmed (F-NEUT50 GMT = 160). A DENV naïve control group of six mice received PBS. Females were mated with naïve adult BALB/c males, and pups born to both groups of dams were allowed to nurse for 21 days, during which period murine maternal IgG continues to be transferred from dams to their suckling pups through the milk, in addition to the transplacentally transferred IgG (59). Weanlings (four to six pups per group) were immunized at 3 and 9 weeks of age with DENV2 VRP (105 IU) s.c. or live DENV2 NGC (105 PFU) i.p. One group (four pups) from DENV-immune dams was left unvaccinated to determine the rates of accumulation and decay of maternal antibodies in the nursing and weanling pups. In these animals, neutralizing-antibody titers (F-NEUT50) of 80 to 160 at the time of weaning (21 days) had dropped to 20 or lower by 6 weeks of age (data not shown). The pups were bled the day before each immunization; at 3 and 6 weeks postprime; and at 2, 4, and 8 weeks postboost. The time courses of the neutralizing-antibody responses in the presence and absence of maternal antibodies are shown in Fig. 5. Mice immunized with DENV2 VRP (Fig. 5A) in the absence of maternal antibodies developed an F-NEUT50 of 254 at 6 weeks after the prime and an F-NEUT50 of 538 at 8 weeks after the boost. Maternal F-NEUT50 titers of 80 at the time of pup immunization resulted in only a minor delay in the titers induced by the DENV2 VRP vaccine at 3 and 6 weeks postprime. Furthermore, after the boost, there was no difference in the titers induced by DENV2 VRP in the presence or absence of maternal antibodies (Fig. 5A). On the other hand, mice vaccinated i.p. with DENV2 NGC (Fig. 5B) in the absence of maternal antibodies developed an F-NEUT50 of 80 at 6 weeks after the prime and F-NEUT50 of 360 at 9 weeks after the boost. In the presence of maternal antibodies (the F-NEUT50 was 80 at the time of first immunization), the ability of the DENV2 live-virus vaccine to induce an endogenous neutralizing-antibody response was significantly reduced (P < 0.05), even when measured 8 weeks after a booster. Although we were surprised that the maternal-antibody block continued after the boost, low levels of maternal antibodies, below the level of detection of the F-NEUT50 assay, present at the time of the boost may explain this observation. These results suggest that the DENV2 VRP vaccine is able to induce long-lived neutralizing-antibody responses in young mice, even in the presence of maternally transferred anti-DENV antibodies that otherwise interfere with the immunogenicity of a live-virus vaccine.
FIG. 5.
Induction of DENV2 neutralizing antibodies in the presence and absence of maternal antibodies. DENV-specific neutralizing-antibody titers in mice born to DENV-naïve or DENV-immune dams after vaccination with 105 IU of DENV2 VRP (A) or 105 PFU of DENV2 NGC (B). Weanling mice were immunized at 3 and 9 weeks after birth. The immunization times are indicated by arrows. At the indicated times, the mice were bled, and DENV2-specific serum neutralizing-antibody titers were determined by flow cytometry-based neutralization tests on Vero cells (F-NEUT). The data represent the F-NEUT50 GMTs. The error bars represent standard errors. *, significantly different at a P value of <0.05 by Mann-Whitney U test. n = 4 to 6 mice per group.
DISCUSSION
This study shows the feasibility of a second-generation DENV vaccine candidate based on VRP vectors as a promising platform to overcome the drawbacks of the current slate of candidate live attenuated DENV vaccines. The novel contribution of this report is the demonstration that a VRP-based vaccine is potentially useful, not only in DENV-naïve, but also in DENV-immune populations, especially in infants born to DENV-immune mothers whose maternal antibodies may interfere with the endogenous response to a live attenuated virus immunization administered early in life.
The feasibility of the VRP approach for a DENV vaccine was demonstrated using VRP expressing prM and E membrane protein genes from DENV2 strain NGC. These proteins play major roles in the induction of protective neutralizing antibodies when expressed in the context of viral vectors, plasmid DNA vectors, or chimeric flavivirus vaccines (9, 29, 37, 38, 50, 60, 71).
The humoral immune response elicited by the DENV2 VRP vaccine in weanling mice shows a number of attributes that make it a promising candidate DENV vaccine. (i) Expression of appropriate DENV immunogens.
The form of the immunizing antigen expressed from VRP has not been rigorously established. However, the proper folding and antigenicity of the expressed proteins was strongly inferred by their reactivity with (i) MAbs 4G2 and 3H5, which bind to conformational epitopes on E; (ii) anti-DENV2 NGC mouse hyperimmune sera; and (iii) human convalescent-phase sera to DENV2. The biologically relevant conformation of the VRP-expressed proteins was further confirmed by the induction of antibodies that neutralized infection by DENV2 NGC in vitro (PRNT and F-NEUT assays) and in vivo (protection from i.c. challenge).
(ii) Rapid induction of a strong and long-lasting immune response.
The kinetics of induction and the duration of the immune response represent important attributes of this vaccine platform that will likely contribute to a safe and efficacious DENV vaccine. Neutralizing antibodies were detected as soon as 1 week after primary immunization with 106 IU of DEN2 VRP in all mice (n = 8), with F-NEUT50 titers ranging from 20 to 40. A rapid induction of neutralizing-antibody titers is important for a pediatric vaccine in areas of endemicity, where the risk of infection is high as protective maternal antibodies wane.
Consistent with other VRP vaccines, a sustained neutralizing-antibody response was observed after the prime and the boost. Twelve weeks after a single dose, the titers were comparable to those in week 3 postprime. Although later times after a single dose were not examined, the neutralization titers were sustained for 30 weeks after a second immunization. Based on these results, we hypothesize that in a tetravalent VRP cocktail expressing prM/E proteins from the four DENV serotypes, a long-lasting antibody response to each serotype will be induced. This would be a necessary safety attribute of a VRP-based tetravalent DENV vaccine to avoid sensitizing the vaccine recipient to a more severe disease if homologous titers drop to subprotective levels while in the presence of nonprotective heterologous enhancing antibodies.
The quality of the antibody response to DENV antigens delivered by VRP in infant mice was assessed by IgG subclass distributions as markers for Th1/Th2-type responses. The DENV2 VRP vaccine induced predominantly IgG2a in 3-week-old infant mice after the first dose, indicating a Th1-biased response, which became more balanced after the boost. A VRP-based vaccine may be able to enhance Th1 responses early in life, which could be a desirable attribute for an early-life vaccine, since other neonatal immunizations in mice and humans have been associated with limited Th1 responses (54). In our study, immunization of weanling mice with live attenuated virus showed much lower levels of IgG2a after the prime, indicating an overall Th2-biased response.
(iii) The VRP-induced immune response is protective in a mouse challenge model.
In the absence of an adequate animal model to reproduce the disease caused by DENV infection in humans, and in the absence of precise correlates of protection for DENV vaccines, we assessed here the protective efficacy of the DENV2 VRP vaccine in a mouse model of DENV-induced encephalitis after an i.c. challenge. Antibodies induced by DENV2 VRP immunization were protective in pups that (i) had been actively immunized with the VRP vaccine or (ii) had been passively immunized by IgG transfer from their DENV2 VRP-immunized mothers through the placenta and milk. Because precise correlates of protection have not been identified for DENV vaccines, it is unclear whether the neutralizing-antibody titers that correlate with protection in mice are similar to those in macaques and humans (30).
(iv) The immune response induced by VRP vaccine is effective in the presence of maternal anti-DENV antibodies.
We used a murine model of early-life immunization and maternal-antibody interference to model conditions in dengue-endemic areas. In such environments, most adult individuals are immune to one or more serotypes, and these antibodies are transferred passively from mothers to their infant children (45, 63). Mice immunized as weanlings with two doses of DENV2 VRP 6 weeks apart showed similar titers regardless of maternal-antibody status (DENV naïve versus DENV immune). In contrast, control mice immunized with two doses of live DENV2 NGC virus i.p. failed to overcome maternal-antibody inhibition. We conclude that for the maternal-antibody titers and vaccine doses tested in this model, VRP-based immunization shows a promising advantage over immunization based on live replicating viruses.
The inhibitory effect of maternal antibodies on live attenuated vaccines, killed vaccines, and subunit vaccines has been documented for the past 25 years in clinical and preclinical studies (1, 7, 20, 32, 44, 54, 55). For example, maternal-antibody interference is the primary cause of live attenuated measles vaccine failure. Proposed mechanisms of interference include neutralization of the live-virus vaccine and epitope-specific B-cell masking (53). We hypothesize that because the DEN antigens are not exposed on the VRP surface, and because VRP undergo only a single round of infection, preexisting neutralizing antibodies to DENV have little effect on VRP delivery to and expression of the DENV proteins in the VRP target cells in the draining lymph node.
The ability of DENV2 VRP to overcome maternal-antibody interference needs to be further characterized to determine how changes in the ratio between vaccine antigen dosage and maternal antibodies affect the ability to overcome interference and how interference is affected by the nature of the immunogen. The VRP platform can be used for other pediatric vaccines, like measles and respiratory syncytial virus, where maternal antibodies represent a barrier to effective early immunization.
Other vaccines and antigen delivery systems have been tested for the ability to overcome maternal-antibody interference (6, 42, 53, 54). Preclinical studies of neonatal DNA vaccination highlight potential advantages of this vector for early-life immunizations (6). Immunization of neonatal mice with a Sindbis virus DNA measles vaccine induced adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies (8). Human adenovirus 5 recombinant viruses expressing the HA and the nucleoprotein of swine influenza virus successfully defeated the suppressive effects of maternal-antibody interference (64), and replication-defective adenovirus expressing rabies glycoproteins in mice resulted in the induction of an endogenous immune response not impaired by maternal immunity (62).
(v) Antivector immunity does not prevent booster immunization.
One of the major limitations of recombinant viral-gene delivery vectors is antivector immunity. It has been shown that preexisting antivector immunity suppresses the immunogenicity of recombinant adenovirus type 5 vector-based vaccines and that the generation of antivector immunity after priming limits the efficiency of homologous boost immunization (3). To assess whether immunity to VEE induced during successive VRP immunizations would limit the effectiveness of the booster immunization, we measured VEE-specific neutralizing antibodies in the sera of mice after one and two immunizations with DENV2 VRP. We found that VRP immunization elicits significant levels of antibody against VEE envelope proteins capable of neutralizing infection in vitro. However, the presence of anti-VEE neutralizing antibodies did not hamper the antibody response to DENV immunogens.
Other recombinant alphaviruses, Sindbis and Semliki Forest viruses, have been used as vaccine vectors expressing prM and E proteins from the flaviviruses Japanese encephalitis virus, Murray Valley encephalitis virus, and louping ill virus (13, 18, 47). These studies showed variable levels of protection, and none of them addressed the effect of preexisting immunity to the transgene on the vaccine-induced immune response. Under such circumstances, the specific targeting of VRP vectors to the lymph node (35), their ability to induce cytokines of the innate immune response in the initial target cells (40, 65; J. L. Konopka and R. E. Johnston, unpublished data), their adjuvant activity (57), and their relative resistance to type I interferons may represent advantages over other alphavirus vaccine vectors.
Although a general strategy for DENV VRP vaccination in countries where DENV is endemic would be to immunize infants before the age of 6 months, this vaccine will also be useful in older children and adults regardless of their immune status. Protection against all four DENV serotypes is typically induced after natural infection with any one of the four. However, this heterologous protection wanes, so that after approximately 1 year, the individual is left monotypically immune. If a tetravalent live attenuated or chimeric vaccine were administered during this period, induction of endogenous immunity to three of the four serotypes would be inhibited by the transiently protective heterologous antibodies. As with maternal antibodies in infants, such individuals would remain susceptible to the most serious complications of DENV infection.
In summary, these results demonstrate that a DENV VRP vaccine can induce protective and long-lasting antibodies to DENV, even in the face of preexisting antibodies, such as those transferred to infants from DENV-immune mothers. This implies that such vaccines administered early in life could protect infants against the most severe aspects of DENV disease. We feel that these experiments justify the construction of a VRP-based tetravalent DENV vaccine and its characterization in infant macaques.
Acknowledgments
This work was supported by Public Health Service grant R21-AI063061 from the National Institute of Allergy and Infectious Diseases.
We gratefully thank Nancy Davis, Mark Heise, and Joseph Thompson for critical reading of the manuscript. We also acknowledge the expert assistance of Martha Collier in packaging of VRP, Bianca Trollinger and Wrennie Edwards for cell culture maintenance, and Amy Partington and Krista Johnson for excellent animal care.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Albrecht, P., F. A. Ennis, E. J. Saltzman, and S. Krugman. 1977. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91:715-718. [DOI] [PubMed] [Google Scholar]
- 2.Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. David Wilson, I. K. Liu, and N. James MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 4.Bhamarapravati, N., and Y. Sutee. 2000. Live attenuated tetravalent dengue vaccine. Vaccine 18(Suppl. 2):44-47. [DOI] [PubMed] [Google Scholar]
- 5.Blaney, J. E., Jr., A. P. Durbin, B. R. Murphy, and S. S. Whitehead. 2006. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 19:10-32. [DOI] [PubMed] [Google Scholar]
- 6.Bot, A., and C. Bona. 2002. Genetic immunization of neonates. Microbes Infect. 4:511-520. [DOI] [PubMed] [Google Scholar]
- 7.Burstyn, D. G., L. J. Baraff, M. S. Peppler, R. D. Leake, J. St Geme, Jr., and C. R. Manclark. 1983. Serological response to filamentous hemagglutinin and lymphocytosis-promoting toxin of Bordetella pertussis. Infect. Immun. 41:1150-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capozzo, A. V. E., K. Ramirez, J. M. Polo, J. Ulmer, E. M. Barry, M. M. Levine, and M. F. Pasetti. 2006. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J. Immunol. 176:5671-5681. [DOI] [PubMed] [Google Scholar]
- 9.Chambers, T. J., Y. Liang, D. A. Droll, J. J. Schlesinger, A. D. Davidson, P. J. Wright, and X. Jiang. 2003. Yellow fever virus/dengue-2 virus and yellow fever virus/dengue-4 virus chimeras: biological characterization, immunogenicity, and protection against dengue encephalitis in the mouse model. J. Virol. 77:3655-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi, U. C., R. Shrivastava, and R. Nagar. 2005. Dengue vaccines: problems and prospects. Indian J. Med. Res. 121:639-652. [PubMed] [Google Scholar]
- 11.Chen, S., M. Yu, T. Jiang, Y. Deng, C. Qin, and E. Qin. 2007. Induction of tetravalent protective immunity against four dengue serotypes by the tandem domain III of the envelope protein. DNA Cell Biol. 26:361-367. [DOI] [PubMed] [Google Scholar]
- 12.Chulay, J., D. Burke, S. Karim, N. Russel, M. Wecker, M. Allen, G. Ferrari, and P. Gilbert. 2006. Safety and immunogenicity of an alphavirus replicon HIV gag vaccine (AVX101) in healthy HIV-uninfected adults. Abst. Antivir. Ther. 11(Suppl. 2):196. [Google Scholar]
- 13.Colombage, G., R. Hall, M. Pavy, and M. Lobigs. 1998. DNA-based and alphavirus-vectored immunisation with PrM and E proteins elicits long-lived and protective immunity against the flavivirus, Murray Valley encephalitis virus. Virology 250:151-163. [DOI] [PubMed] [Google Scholar]
- 14.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin, A. P., R. A. Karron, W. Sun, D. W. Vaughn, M. J. Reynolds, J. R. Perreault, B. Thumar, R. Men, C. J. Lai, W. R. Elkins, R. M. Chanock, B. R. Murphy, and S. S. Whitehead. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 65:405-413. [DOI] [PubMed] [Google Scholar]
- 16.Eckels, K. H., and R. Putnak. 2003. Formalin-inactivated whole virus and recombinant subunit flavivirus vaccines. Adv. Virus Res. 61:395-418. [DOI] [PubMed] [Google Scholar]
- 17.Edelman, R. 2005. Dengue and dengue vaccines. J. Infect. Dis. 191:650-653. [DOI] [PubMed] [Google Scholar]
- 18.Fleeton, M. N., B. J. Sheahan, E. A. Gould, G. J. Atkins, and P. Liljestrom. 1999. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J. Gen. Virol. 80:1189-1198. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca, B. A., S. Pincus, R. E. Shope, E. Paoletti, and P. W. Mason. 1994. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine 12:279-285. [DOI] [PubMed] [Google Scholar]
- 20.Glezen, W. P. 2003. Effect of maternal antibodies on the infant immune response. Vaccine 21:3389-3392. [DOI] [PubMed] [Google Scholar]
- 21.Gubler, D. J., and M. Meltzer. 1999. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 53:35-70. [DOI] [PubMed] [Google Scholar]
- 22.Guirakhoo, F., J. Arroyo, K. V. Pugachev, C. Miller, Z. X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Soike, M. Ratterree, and T. P. Monath. 2001. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 75:7290-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzman, M. G., G. Kouri, L. Valdes, J. Bravo, M. Alvarez, S. Vazques, I. Delgado, and S. B. Halstead. 2000. Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am. J. Epidemiol. 152:793-799, 804. [DOI] [PubMed] [Google Scholar]
- 24.Guzman, M. G., G. P. Kouri, J. Bravo, M. Soler, S. Vazquez, and L. Morier. 1990. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42:179-184. [DOI] [PubMed] [Google Scholar]
- 25.Halstead, S. B. 1974. Etiologies of the experimental dengue in Siler and Simmons. Am. J. Trop.Med. Hyg. 23:974-982. [DOI] [PubMed] [Google Scholar]
- 26.Halstead, S. B., N. T. Lan, T. T. Myint, T. N. Shwe, A. Nisalak, S. Kalyanarooj, S. Nimmannitya, S. Soegijanto, D. W. Vaughn, and T. P. Endy. 2002. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg. Infect. Dis. 8:1474-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halstead, S. B., S. Nimmannitya, and S. N. Cohen. 1970. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 42:311-328. [PMC free article] [PubMed] [Google Scholar]
- 28.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 29.Holman, D. H., D. Wang, K. Raviprakash, N. U. Raja, M. Luo, J. Zhang, K. R. Porter, and J. Y. Dong. 2007. Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes. Clin. Vaccine Immunol. 14:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hombach, J. 2007. Scientific consultation on immunological correlates of protection induced by dengue vaccines. Vaccine 25:4130-4139. [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal, S., N. Khanna, and S. Swaminathan. 2003. Replication-defective adenoviral vaccine vector for the induction of immune responses to dengue virus type 2. J. Virol. 77:12907-12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanra, G., S. S. Yalcin, M. Ceyhan, and K. Yurdakok. 2000. Clinical trial to evaluate immunogenicity and safety of inactivated hepatitis A vaccination starting at 2-month-old children. Turk. J. Pediatr. 42:105-108. [PubMed] [Google Scholar]
- 33.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38:411-419. [DOI] [PubMed] [Google Scholar]
- 34.Lambeth, C. R., L. J. White, R. E. Johnston, and A. M. de Silva. 2005. Flow cytometry-based assay for titrating dengue virus. J. Clin. Microbiol. 43:3267-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackenzie, J. S., D. J. Gubler, and L. R. Petersen. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 10:S98-S109. [DOI] [PubMed] [Google Scholar]
- 37.Mason, P. W., S. Pincus, M. J. Fournier, T. L. Mason, R. E. Shope, and E. Paoletti. 1991. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology 180:294-305. [DOI] [PubMed] [Google Scholar]
- 38.Minke, J. M., L. Siger, K. Karaca, L. Austgen, P. Gordy, R. Bowen, R. W. Renshaw, S. Loosmore, J. C. Audonnet, and B. Nordgren. 2004. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch. Virol. Suppl. 18:221-230. [DOI] [PubMed] [Google Scholar]
- 39.Monath, T. P., K. McCarthy, P. Bedford, C. T. Johnson, R. Nichols, S. Yoksan, R. Marchesani, M. Knauber, K. H. Wells, J. Arroyo, and F. Guirakhoo. 2002. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 20:1004-1018. [DOI] [PubMed] [Google Scholar]
- 40.Moran, T. P., M. Collier, K. P. McKinnon, N. L. Davis, R. E. Johnston, and J. S. Serody. 2005. A novel viral system for generating antigen-Specific T cells. J. Immunol. 175:3431-3438. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen, T. H., H. Y. Lei, T. L. Nguyen, Y. S. Lin, K. J. Huang, B. L. Le, C. F. Lin, T. M. Yeh, Q. H. Do, T. Q. Vu, L. C. Chen, J. H. Huang, T. M. Lam, C. C. Liu, and S. B. Halstead. 2004. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J. Infect. Dis. 189:221-232. [DOI] [PubMed] [Google Scholar]
- 42.Osterhaus, A., G. van Amerongen, and R. van Binnendijk. 1998. Vaccine strategies to overcome maternal antibody mediated inhibition of measles vaccine. Vaccine 16:1479-1481. [DOI] [PubMed] [Google Scholar]
- 43.Pancharoen, C., and U. Thisyakorn. 2001. Dengue virus infection during infancy. Trans. R. Soc. Trop. Med. Hyg. 95:307-308. [DOI] [PubMed] [Google Scholar]
- 44.Perkins, F. T., R. Yetts, and W. Gaisford. 1959. A comparison of the responses of 100 infants to primary poliomyelitis immunization with two and with three doses of vaccine. Br. Med. J. 1:1083-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perret, C., P. Chanthavanich, K. Pengsaa, K. Limkittikul, P. Hutajaroen, J. E. G. Bunn, and B. J. Brabin. 2005. Dengue infection during pregnancy and transplacental antibody transfer in Thai mothers. J. Infect. 51:287-293. [DOI] [PubMed] [Google Scholar]
- 46.Pugachev, K. V., F. Guirakhoo, D. W. Trent, and T. P. Monath. 2003. Traditional and novel approaches to flavivirus vaccines. Int. J. Parasitol. 33:567-582. [DOI] [PubMed] [Google Scholar]
- 47.Pugachev, K. V., P. W. Mason, R. E. Shope, and T. K. Frey. 1995. Double-subgenomic Sindbis virus recombinants expressing immunogenic proteins of Japanese encephalitis virus induce significant protection in mice against lethal JEV infection. Virology 212:587-594. [DOI] [PubMed] [Google Scholar]
- 48.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 49.Raviprakash, K., D. Ewing, M. Simmons, K. R. Porter, T. R. Jones, C. G. Hayes, R. Stout, and G. S. Murphy. 2003. Needle-free Biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology 315:345-352. [DOI] [PubMed] [Google Scholar]
- 50.Raviprakash, K., T. J. Kochel, D. Ewing, M. Simmons, I. Phillips, C. G. Hayes, and K. R. Porter. 2000. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine 18:2426-2434. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez, V., S. Gimenez, B. Tomlinson, P. K. Chan, G. N. Thomas, R. Forrat, L. Chambonneau, F. Deauvieau, J. Lang, and B. Guy. 2006. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3). Vaccine 24:4914-4926. [DOI] [PubMed] [Google Scholar]
- 52.Schultz-Cherry, S., J. K. Dybing, N. L. Davis, C. Williamson, D. L. Suarez, R. Johnston, and M. L. Perdue. 2000. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology 278:55-59. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist, C. A. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406-3412. [DOI] [PubMed] [Google Scholar]
- 54.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 55.Siegrist, C. A., C. Barrios, X. Martinez, C. Brandt, M. Berney, M. Cordova, J. Kovarik, and P. H. Lambert. 1998. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 28:4138-4148. [DOI] [PubMed] [Google Scholar]
- 56.Simmons, C. P., T. N. Chau, T. T. Thuy, N. M. Tuan, D. M. Hoang, N. T. Thien, B. Lien Le, N. T. Quy, N. T. Hieu, T. T. Hien, C. McElnea, P. Young, S. Whitehead, N. T. Hung, and J. Farrar. 2007. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J. Infect. Dis. 196:416-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson, J. M., A. C. Whitmore, J. L. Konopka, M. L. Collier, E. M. B. Richmond, N. L. Davis, H. F. Staats, and R. E. Johnston. 2006. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc. Natl. Acad. Sci. USA 103:3722-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thu, H. M., K. Lowry, T. T. Myint, T. N. Shwe, A. M. Han, K. K. Khin, K. Z. Thant, S. Thein, and J. Aaskov. 2004. Myanmar dengue outbreak associated with displacement of serotypes 2, 3, and 4 by dengue 1. Emerg. Infect. Dis. 10:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van de Perre, P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374-3376. [DOI] [PubMed] [Google Scholar]
- 60.van Der Most, R. G., K. Murali-Krishna, R. Ahmed, and J. H. Strauss. 2000. Chimeric yellow fever/dengue virus as a candidate dengue vaccine: quantitation of the dengue virus-specific CD8 T-cell response. J. Virol. 74:8094-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Y., Z. Xiang, S. Pasquini, and H. C. Ertl. 1997. The use of an E1-deleted, replication-defective adenovirus recombinant expressing the rabies virus glycoprotein for early vaccination of mice against rabies virus. J. Virol. 71:3677-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanaveeradej, V., T. P. Endy, R. Samakoses, A. Kerdpanich, S. Simasathien, N. Polprasert, C. Aree, D. W. Vaughn, C. Ho, and A. Nisalak. 2003. Transplacentally transferred maternal-infant antibodies to dengue virus. Am. J. Trop. Med. Hyg. 69:123-128. [PubMed] [Google Scholar]
- 64.Wesley, R. D., and K. M. Lager. 2006. Overcoming maternal antibody interference by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of swine influenza virus. Vet. Microbiol. 118:67-75. [DOI] [PubMed] [Google Scholar]
- 65.White, L. J., J.-G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitehead, S. S., J. E. Blaney, A. P. Durbin, and B. R. Murphy. 2007. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 5:518-528. [DOI] [PubMed] [Google Scholar]
- 67.WHO. 2002. Dengue and dengue hemorrhagic fever. Fact sheet N. 117. WHO, Geneva, Switzerland.
- 68.WHO. 1997. Dengue hemorrhagic fever, diagnosis, treatment, prevention and control, 2nd ed. WHO, Geneva, Switzerland.
- 69.Wilson, J. A., M. Bray, R. Bakken, and M. K. Hart. 2001. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology 286:384-390. [DOI] [PubMed] [Google Scholar]
- 70.Witayathawornwong, P. 2001. Dengue hemorrhagic fever in infancy at Petchabun Hospital, Thailand. Southeast Asian J. Trop. Med. Public Health 32:481-487. [PubMed] [Google Scholar]
- 71.Wu, Y., F. Zhang, W. Ma, J. Song, Q. Huang, and H. Zhang. 2004. A plasmid encoding Japanese encephalitis virus PrM and E proteins elicits protective immunity in suckling mice. Microbiol. Immunol. 48:585-590. [DOI] [PubMed] [Google Scholar]