Abstract
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus associated with the development of both lymphoid and epithelial tumors. The EBV critical latent antigens EBNA1 and EBNA3C interact with Nm23-H1, a known suppressor of cell migration and tumor metastasis. This interaction is critical for the regulation of downstream cellular genes involved in tumorigenesis and cell migration. The significance of these interactions was determined in nude mice using cancer cells expressing both EBV antigens and Nm23-H1. The EBV antigens promoted the growth of transformed cells in vivo, but their expression was less critical during the later stage of tumor development. The expression of Nm23-H1 affected the growth of cancer cells and suppressed their metastatic potential. This effect was effectively rescued by the expression of both EBV antigens. Interestingly, the prometastatic potential of EBNA3C was greater than that of EBNA1, which triggered a dramatic immune response, as indicated by increased spleen size and development of ascites in the mice. These studies now bridge the expression of the EBV antigens with tumorigenesis and metastasis and widen the range of potential targets for development of therapies for EBV-associated malignancies.
Nm23-H1 was initially proposed to be a metastatic suppressor because of its reduced expression in highly metastatic murine melanoma cell lines (43). In human tissues, the two most abundantly expressed nm23 genes are nm23-H1 (39) and nm23-H2 (42). These genes encode the A and B subunits, respectively, of nucleoside diphosphate kinase (11, 58). The murine orthologs, nm23-M1 and nm23-M2, encode proteins that share 94 and 98% identity, respectively, with their human counterparts (43, 52). The nm23 gene family is highly conserved among a wide variety of eukaryotic species. Eight genes have been identified in humans: nm23-H1 (39), nm23-H2 (42), nm23-H3 (55), nm23-H4 (26), nm23-H5 (27), nm23-H6 (25, 51), nm23-H7 (53), and nm23-H8 (33). Members of the nm23 gene family are structurally and functionally conserved, consisting of four to six identically folded subunits, enclosing a large (25-Å) central cavity (59). The expression of nm23 genes have also been linked to suppression of differentiation, apoptosis, proliferation, and DNA mutation rate (10) and is also associated with nucleoside diphosphate kinase activity (5, 29, 58), serine phosphorylation (6, 14, 23, 28), and histidine protein kinase activities (57), as well as transcriptional stimulatory activities (1, 3, 36, 37).
The human nm23-H1 gene product is the best-characterized member of this family of proteins. Some studies have reported an inverse association between Nm23-H1 expression at the mRNA or protein level and the metastatic potential of human solid tumors such as melanoma and breast, liver, and colon carcinomas (13, 32). However, other studies on these and other tumor types have failed to show such a relationship. Indeed, neuroblastoma aggressiveness was found to be directly associated with the expression of nm23-H1 (8, 54). Nevertheless, in agreement with studies showing an inverse association between Nm23 expression and metastasis, aggressive tumor cell lines that were engineered to overexpress nm23-H1 displayed reduced metastatic potential in experimental animal models of metastasis (2, 4, 20, 21).
Epstein-Barr virus (EBV) is a gammaherpesvirus and one of the most common human viruses, which predominantly targets B cells and epithelial cells and is associated with a number of human cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma (NPC), and Hodgkin's disease, as well as AIDS-associated and transplant-associated immunoblastic lymphoma and, controversially, invasive breast carcinoma (7, 19, 38). Although the EBV genome encodes approximately 90 genes, only 11 of these are expressed in EBV-immortalized lymphoblastoid cell lines (60). Nine of these eleven genes encode the EBV latent proteins: the Epstein-Barr nuclear antigens (EBNAs) 1, 2, 3A, 3B, and 3C and leader protein (LP) and the latent membrane proteins 1, 2A, and 2B and two encode early RNAs (EBERs) (38). EBNA1 is one of the essential nuclear antigens that is expressed in all forms of latency.
EBNA1 is expressed in all forms of EBV latency and known to be required for maintenance of the EBV episome, a finding consistent with the central role of this protein in the maintenance and segregation of the episomal latent EBV genome (16, 38). EBNA1 is a DNA-binding phosphoprotein that through binding to the plasmid origin of viral replication, oriP, is responsible for tethering the virus episome to chromosomes (24, 62). EBNA1 is also capable of transactivating the Wp/Cp promoters responsible for initiation of transcription of the six EBNAs in type III latency (16, 38) and negatively regulates the Qp promoter during type I latency in the absence of expression of the other EBNAs (9). In addition, studies indicate that EBNA1 antisense oligodeoxyribonucleotides inhibited the proliferation of EBV-immortalized cells and that EBNA1 expression in transgenic mice can induce lymphomas (61). Moreover, EBNA1 increased the tumorigenic potential and metastatic capability of an NPC cell line (41). These studies suggest that EBNA1 may have a direct role in contributing to the tumorigenic potential of EBV. However, the mechanisms and relevance to the pathogenesis of EBV-associated tumors remain unknown. Expression of EBNA1 has been demonstrated in all EBV-associated tumors, including EBV-positive NPCs and gastric adenocarcinomas (16, 38). The contribution of EBNA1 expression to the oncogenic process is thought to relate to the role of the protein in maintaining the extrachromosomal integrity of the EBV genome (15, 31). However, it is evident that additional functions are associated with EBNA1.
Another EBV latent nuclear antigen, EBNA3C, was shown to interact with the suppressor of cell migration and metastasis Nm23-H1 (47). This interaction between Nm23-H1 and EBNA3C was shown to result in an increase in transcriptional activity on a responsive promoter (48). Nm23-H1 tethered to DNA by a Gal4 DNA-binding domain can activate transcription from a basal promoter at relatively low levels. However, when EBNA3C was expressed, a substantial increase in transactivation activity was observed (48). These results suggest that Nm23-H1 may possess transcriptional regulatory activities independent of a possible role in directly binding to DNA but through interaction with EBNA3C or other transcription factors (47). Interestingly, the presence of EBNA3C mediates the cellular translocation of Nm23-H1 from a mostly cytoplasmic to a predominantly nuclear signal and reverses the antimigratory effects of Nm23-H1 in vitro (47).
We present here evidence to suggest that the EBV latent nuclear antigens EBNA3C and EBNA1 promote metastasis and can overcome the metastatic suppressor effects of Nm23-H1 in the nude mouse model. These results strongly support a role for EBV proteins EBNA3C and EBNA1 in promoting cell migration and metastasis in an in vivo setting.
MATERIALS AND METHODS
Constructs and cell lines.
A subclone of the human breast carcinoma MDA-MB-231 cell line designated MDA-MB-231T cells (obtained from Patricia S. Steeg, Women's Cancers Section, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute) was chosen for its reliable in vivo experimental metastatic potential. The expression constructs of Nm23-H1 (pA3M-Nm23-H1), EBNA1 (pCDNA-EBNA1), and EBNA3C (pA3M-EBNA3C) used in the present study have been described earlier (30, 47).
Generation of cells stably expressing Nm23-H1 and the EBNAs.
MDA-MB-231T cells were transfected by electroporation with a Bio-Rad Gene Pulser II electroporator. Ten million cells were harvested in exponential phase, collected and washed in phosphate-buffered saline, and then resuspended in 400 μl of Dulbecco modified Eagle medium with DNA for transfection. Resuspended cells were transferred to a 0.4-cm cuvette and electroporated at 975 μF and 220 V. The electroporated cells were then transferred to 10 ml of complete medium, followed by incubation at 37°C in 5% CO2. The cells were then subjected to G418 selection (0.8 mg/ml). After an 8-week selection, vector control cells and cells stably transfected with Nm23-H1, EBNA1, and EBNA3C from four independently derived clones were examined for the level of Nm23-H1, EBNA1, and EBNA3C expression through analysis of their mRNA levels by reverse transcription (RT-PCR) and protein levels by Western blotting and immunofluorescence. Mice were randomized into nine groups—i.e., control mice, MDA-MA-231T vector control, MDA-MA-231T Nm23-H1, MDA-MA-231T EBNA3C, MDA-MA-231T EBNA3C+Nm23-H1, MDA-MA-231T EBNA1, MDA-MA-231T EBNA1+Nm23-H1, MDA-MA-231T EBNA3C+EBNA1, and MDA-MA-231T EBNA3C+EBNA1+Nm23H1—of 10 mice each. Each group received MDA-MB-231T-cell clones stably expressing EBNA1 or EBNA3C or metastasis suppressor Nm23-H1 or a combination of these proteins. One group of 10 mice was injected with MDA-MB-231T cells stably transfected with vector control. The animal study was approved by Institutional Biosafety Committee via approval dated 12 December 2005.
Western blot analysis.
Protein was extracted from cultured cells or primary tumor tissues and concentration was calculated. Tissues were homogenized by using tissue tearer (Biospec Products, Inc., Bartlesville, OK) prior to processing for protein extraction. Portions (100 μg) of cultured cell lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Equal loading of samples was confirmed with Ponceau-S staining of the membrane in all cases. The EBNA1 and Myc-tagged Nm23-H1 or EBNA3C were analyzed as reported with the use of anti-EBV human serum and anti-Myc antibody, respectively (56).
Immunofluorescence assays.
The MDA-MB-231T cells were grown on chamber slides overnight and washed with phosphate-buffered saline (PBS). The cells were fixed with a 1:1 ratio of methanol-acetone solution for 10 min at -20°C, dried, and rehydrated with PBS. For blocking, cells were incubated with PBS containing 20% goat serum for 30 min. Cells were then cross-reacted with appropriate antibodies (human EBV serum, a 1:100 dilution of anti-Myc ascites antibody). Slides were washed three times in PBS and further incubated with a 1:1,000 dilution of goat anti-human or goat anti-mouse immunoglobulin-fluorescein isothiocyanate-conjugated secondary antibodies. Slides were examined with an Olympus IX70 fluorescence microscope, and images were captured with a PixelFly digital camera (Cooke, Inc., Auburn Hills, MI).
RT-PCR analysis for levels of viral and cellular gene transcripts.
Total RNA was isolated from cells or tissues by using TRIZOL reagent (Life Technologies, Gaithersburg, MD). Tissues were homogenized by using a tissue tearer (Biospec Products, Inc., Bartlesville, OK) prior to processing for RNA isolation. RT was carried out with SuperScript II RNase H-reverse transcriptase (Life Technologies, Gaithersburg, MD). Briefly, 5 μg of total RNA was reverse transcribed by using 200 U of Moloney murine leukemia virus reverse transcriptase in a total volume of 20 μl containing 125 μM deoxynucleoside triphosphate, 20 U of RNasin, and 50 ng of random hexanucleotide primers. After incubation at 25°C for 10 min, followed by 50°C for 50 min, the reaction was stopped by heating the mixture to 95°C for 5 min. PCR was then performed in a total volume of 25 μl using specific primers. The Nm23-H1-Myc transcript was amplified for 30 cycles (30 s at 94°C, 1 min at 50°C, and 1 min at 72°C) using the forward primer 5′-GATTACACGAGCTGTGCTCA-3′ and the reverse primer 5′-TTCGCTAGCCAAGTCTTCTT-3′. designed to amplify junction sequence between Nm23-H1 and Myc tag. The EBNA3C-Myc transcript was amplified for 30 cycles (30 s at 94°C, 1 min at 56°C, and 1 min at 72°C) using the forward primer 5′-CGGGATCCGGAAGGAACCATGGCCA-3′ and the reverse primer 5′-GAATTCTCCTGTCATTTCATAGATCCA-3′. The EBNA1 transcript was amplified for 30 cycles (30 s at 94°C, 1 min at 60°C, and 1 min at 72°C) using the forward primer 5′-CGGGATCCGGAAGGAACCATGGCCA-3′ and the reverse primer 5′-GAATTCTCCTGTCATTTCATAGATCCA-3′. Amplification products were also resolved in 1.5% agarose gels.
Cell motility assays.
MDA-MB-231T cells stable clones were used for motility assays. Cell motility was determined by using 96-well Boyden chemotaxis chambers (NeuroProbe, Inc.). The chemoattractant used was fetal bovine serum at concentrations of 0.2 and 0.5%. Dilution of the chemoattractant was carried out in Dulbecco modified Eagle medium containing 0.1% bovine serum albumin, 10 mM HEPES, and 100 U of penicillin-streptomycin/ml, and serial dilutions were placed in the lower part of the chamber. An 8-μm-pore-size polycarbonate polyvinylpyrrolidone-free membrane was sandwiched between the upper and lower chambers. Cells were harvested at 72 h after transfection, washed in PBS, and then resuspended in Dulbecco modified Eagle medium containing 0.1% bovine serum albumin, 10 mM HEPES, and 100 U of penicillin-streptomycin/ml. A total of 7.5 × 104 selected cells were added to the upper wells of the chamber, and the chamber was incubated for 2 h at 37°C in a humidifying CO2 incubator. After the removal of cells from the upper side of the membrane, it was stained with Gill's hematoxylin stain, and the migrated cells were counted at ×40 magnification with an Olympus BX40 light microscope. The data presented for each concentration represent the mean of three separate experiments.
Tumorigenicity and metastasis assay.
Six-week-old Nu/Nu mice (n = 90) were acclimatized for 2 weeks and randomized to nine groups (10 mice per group) as described above. All mice from each group received the injection of stable MDA-MB-231T cells at the site of the fourth mammary fat pad. Each mouse was anesthetized by intraperitoneal administration of a ketamine (100 mg/kg) and xylazine (10 mg/kg) mixture. Once anesthetized, the fourth mammary pad of the mouse was cleaned with ethanol to provide a sterile area before incision. A small incision was made above the site of injection with a sterile scalpel, and 5 × 105 viable cells of each cell line in 10 μl of sterile PBS solution were injected into the fat pad by using a Hamilton needle and syringe. The incision was closed with the use of auto clips, which were removed 1 week later. Four weeks later, five randomly selected mice from each group were also injected intravenously with 5 × 105 viable cells of the corresponding stable cell line.
Throughout the experiment, mice were weighed twice weekly and monitored for signs of ill health and labored breathing. The mice were observed for the appearance of tumor foci at the site of injection, the number of tumor foci, and also for signs of general illness related to tumor metastasis (e.g., ascites). The tumor sizes were measured by using electronic Vernier calipers and recorded. Mice were euthanized if pathological conditions unrelated to the study (e.g., breathing difficulties) developed. Mice from all groups were euthanized when the tumor size grew to more than 15 mm in size or after 93 days. All mice were necropsied to quantitate gross metastases. Lungs, spleens, mammary fat pads, livers, and local lymph nodes were preserved in 10% formalin for histopathology and in 50% buffered glycerol for mRNA extraction and detection.
Histopathology.
The lung tissue, spleen, liver, and lymph node tissue obtained at necropsy from each of the four mice per group that had multiple metastases was fixed in formalin, embedded in paraffin, and sectioned (6-μm sections). Paraffin-embedded tissues were sectioned at the New Bolton Center, Animal Pathology Service, University of Pennsylvania School of Veterinary Medicine. One randomly chosen section from each of the five mice per treatment group was stained for cytokeratin 19 protein using a specific antibody (Dako North America, Inc., Carpinteria, CA). In brief, sections were deparaffinized in xylene, dehydrated in 100% ethanol, rehydrated in 95% ethanol and PBS, and blocked sequentially with hydrogen peroxide and goat serum. Antigen retrieval was accomplished by incubating the slides in 10 mM citrate buffer (pH 3.0) for 30 min at 37°C. Slides were incubated with primary antibody (diluted 1:5 in PBS with 1% goat serum) in a humidified chamber overnight at room temperature. Staining was visualized by using the Dako LSAB+System. Stained sections were examined under a microscope, and every visible metastasis in the tissue section was counted.
RESULTS
MDA-MB-231T stable clones express Nm23-H1/EBNA1/EBNA3C.
We generated clones from the breast carcinoma cell line MDA-MB-231T. These were tested for expression of Nm23-H1, EBNA1 and EBNA3C by Western blot analysis and immunofluorescence assays. The clones with relatively higher levels of expression of desired proteins were selected for use in metastasis experiments in mice. RT-PCR confirmed the expression of transcript at the mRNA levels (Fig. 1b), and the immunofluorescence and Western blot assays of the clones showed expression of all of the proteins (Fig. 1a). All cell lines generated had similar growth properties, as determined by propagation in an in vitro culture.
FIG. 1.
EBNA1 and EBNA3C can rescue the inhibition of MDA-MB-231T cell migration by the suppressor of metastasis Nm23-H1. MDA-MB-231T stable clones showed expression of Nm23-H1, EBNA1, and EBNA3C proteins by immunofluorescence (a), RT-PCR for mRNA detection (b), and Western blot for expression levels (c). The expression of Nm23-H1, EBNA3C, and Nm23-H1 was tested by Western blots in clones selected to be used in the experiment. The clones were then tested by cell migration assay to determine the effect of expressed proteins on cell motility in an in vitro assay (d). In each case the number of cells migrating was reduced in the presence of Nm23-H1. This was effectively reversed when Nm23-H1 was coexpressed with the EBV latent antigens.
EBNA1 and EBNA3C can rescue the inhibition of MDA-MB-231T cell migration by the suppressor of metastasis Nm23-H1.
The inhibition of cell migration and metastasis by Nm23-H1 has been established in in vitro assays and in animal studies (40, 45). We have previously shown that the EBV latent antigen EBNA3C, as well as EBNA1, can reverse the ability of Nm23-H1 to suppress cell migration (30, 47). This was also was confirmed with MDA-MB-231T cell clones stably expressing the desired proteins before using them in an in vivo study (Fig. 1d). The mechanism of action, however, is yet to be understood. In our previous report we showed that EBNA3C reversed the effects of Nm23-H1 in its ability to suppress cell migration (47) but had no effect on migration if expressed alone. Moreover, when EBNA1 or Nm23-H1 was expressed alone, the number of cells migrating was dramatically reduced, but when both were coexpressed the migratory potential of these cells was restored to levels similar to that seen with the control (30). Similar results were observed when the assays were done with MDA-MB-231T clones in these studies (Fig. 1d). In each case the number of cells migrating was reduced in the presence of Nm23-H1, but this was effectively reversed when Nm23-H1 was coexpressed with either of the EBV latent antigens. The MDA-MB-231T clones expressing EBNA1 alone did not show any reduction in cell migration, as seen in the MDA-MB-435 clone expressing EBNA1 (30); however, the results are consistent with the in vivo enhancement of metastasis.
EBV latent antigens induce tumor growth in nude mice.
The ability to form tumors in the mouse formations is known as tumorigenic potential or tumorigenicity. Although the loss of anchorage dependence for growth is an important indicator for the tumorigenic, as well as the metastatic, potential of cells, the ability of cells to form tumors in nude mice is considered the best indicator of the tumorigenic potential or tumorigenicity of cancer cells (17, 46). We decided to investigate the effect of EBV latent antigens and Nm23-H1 on the tumorigenicity of cancer cells in nude mice by studying different parameters. These included studying the proportion of mice out of those injected that developed primary tumors, i.e., the “tumor take”; the rate of primary tumor growth during initial phase measured in terms of the number of days it took for mice to develop a detectable primary tumor; and the growth of primary tumor subsequent to that measured as size in diameter of primary tumor foci at the time of mouse death or sacrifice. EBNA3C and EBNA1 have been reported to be essential for EBV immortalization of cells (18, 49). Here we tested whether the presence of these proteins in cancer cells affected the growth of tumors in vivo. The presence of EBNA1, EBNA3C, or both in cancer cells resulted in detectable primary tumor growth by 45 days postinoculation, whereas the mice injected with vector control MDA-MB-231T cells grew to detectable tumors only after 75 to 80 days postinjection. This suggested that the presence of EBV latent antigens can contribute to the accelerated growth of transformed cells in nude mice, especially during the initial phase leading to the generation of primary tumors at the site of injection. The number of mice that developed primary tumor was also higher in the group injected with cells expressing both EBNA1 and EBNA3C. Primary “tumor take” was 80% in EBNA1+EBNA3C group compared to 40% in EBNA3C group and 60% in EBNA1 group (Fig. 2a). It should be noted, however, that either EBNA1 or EBNA3C expressed with Nm23-H1 consistently showed higher tumorigenicity in the initial phase measured in terms of number of days after injection it took for appearance of detectable primary tumor. All 10 mice grew tumors within 45 days postinoculation compared to the vector control, which took 80 days after injection to show detectable primary tumor and was similar to that seen when either antigen expressed was alone, where it took 45 to 50 days to show detectable primary tumor (Fig. 2c). Interestingly, when both EBNA1 and EBNA3C were expressed individually with Nm23-H1 the tumor take went up to 100%, whereas when both of these proteins were coexpressed together with Nm23-H1 the tumor take decreased to the level of vector control (60%). This suggests that Nm23-H1 had an inhibitory effect on tumor development from cancer cells during the initial stages if both viral antigens are coexpressed (Fig. 2a). A surprising finding in this series of experiments was that the cells expressing EBNA1 under any combination resulted in an increase in the number of mice developing ascites in their body cavities (Fig. 2b). This suggests that EBNA1 may trigger a dramatic immune response in nude mice. To confirm expression of the related antigens in the tumors, RT-PCR analysis was performed with specific primers and Western blot analysis (Fig. 3). The results showed that the tumors did express the antigens that were heterologously expressed in the original cell lines generated.
FIG. 2.
EBV latent antigens induce tumor growth in nude mice. Development of primary tumor at the site of injection in different groups that were inoculated with MDA-MB-231T cell clones stably expressing EBV nuclear antigens or Nm23-H1 or both. (a) The data shown represent tumor development after 90 days postinjection or at time of death, whichever was earlier. The number of mice that showed significant primary tumor growth was also higher in mice injected with cancer cells expressing both EBNA1 and EBNA3C compared to those with either of the two. (b) The number of mice that developed ascites resulting in the accumulation of fluid in their body cavity is shown. The mice injected with cells expressing EBNA1 in any combination were more likely to develop ascites. Mice that developed ascites invariably died within 2 to 3 days. (c) Mice injected with cells expressing EBV antigens with or without Nm23-H1 developed primary tumor much earlier than those injected with vector control cells. (d) Mice with ascites and primary tumor at site of injection.
FIG. 3.
Analysis of tumor samples for expression of antigens from stable cell lines confirmed expression in the tumors. (a) Protein was extracted from primary tumor tissues, and the concentration was calculated. Tissues were homogenized by using a tissue tearer prior to processing for protein extraction. Portions (100 μg) of lysate were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Equal loading of samples was confirmed with Ponceau-S staining of the membrane in all cases. The EBNA1 and Myc-tagged Nm23-H1 or EBNA3C were analyzed as reported with the use of anti-EBV human serum and anti-Myc antibody, respectively. (b) Total RNA was isolated from tissues by using TRIZOL reagent. Tissues were homogenized by using a tissue tearer prior to processing for RNA isolation. RT was carried performed with SuperScript II RNase H reverse transcriptase. followed by PCR with specific primers to detect the desired transcript. The Nm23-H1-Myc transcript was amplified by using the forward primer 5′-GATTACACGAGCTGTGCTCA-3′ and the reverse primer 5′-TTCGCTAGCCAAGTCTTCTT-3′ designed to amplify the junction sequence between Nm23-H1 and Myc tag. The EBNA3C-Myc transcript was amplified by using the forward primer 5′-CGGGATCCGGAAGGAACCATGGCCA-3′ and the reverse primer 5′-GAATTCTCCTGTCATTTCATAGATCCA-3′. The EBNA1 transcript was amplified by using the forward primer 5′-CGGGATCCGGAAGGAACCATGGCCA-3′ and the reverse primer 5′-GAATTCTCCTGTCATTTCATAGATCCA-3′. Amplification products were resolved in 1.5% agarose gels.
Cells expressing Nm23-H1 and the essential EBV antigens dramatically increased tumor mass and size in the nude mouse model.
Nm23-H1 has been known to function as a suppressor of metastasis (43, 45). Here we tested whether Nm23-H1 might also play some role in affecting the tumorigenic potential of cancer cells in terms of the size of the primary tumor. We found that the sizes of the primary tumors developed at the site of injection were greater (about 8 mm in diameter) in mice injected with cells expressing Nm23-H1 than in mice injected with vector control cells (about 4 mm in diameter) (Fig. 4a). When Nm23-H1 was coexpressed with either EBV latent nuclear antigen EBNA1 or EBNA3C, this resulted in a synergistic effect of tumor growth as it relates to all of the measured growth parameters of the primary tumor in the experimental mice (Fig. 4a and b). The mice in which cells expressing Nm23-H1 with either of EBNA1 or EBNA3C were injected developed much larger primary tumors that were 14 mm in diameter for the EBNA3C+Nm23-H1 group and 18 mm in diameter in EBNA1+Nm23-H1 group than mice in which cells expressing any of the three proteins were injected, resulting in tumors 4 mm in diameter in the EBNA3C group, 5.5 mm in diameter in the EBNA1 group, and 8 mm in diameter in the Nm23-H1 group. Since the detectable primary tumor had appeared at similar times around 45 days postinjection in all of these groups, it is clear that the tumors subsequently grew more rapidly in mice injected with cells expressing either of EBNAs with Nm23-H1 and led to the development of larger tumor mass in these mice compared to mice in which the cells expressed any one of these proteins. When Nm23-H1 was coexpressed with both EBNA1 and EBNA3C, similar results were seen (Fig. 4a). In most of the mice the injection of cancer cells resulted in the development of a primary tumor at the site of injection. However, in some mice, the resultant tumor developed as multiple subcutaneous outgrowths (see Fig. 2d), which appeared to originate from the same primary injection site. This was confirmed at the time of postmortem examination after the mice either died or were sacrificed. We counted these multiple outgrowths of primary tumors as “primary tumor foci” and found that the presence of Nm23-H1 also led to an increase in number of primary tumor foci; the effect was more profound in particular when EBNA1 was expressed (Fig. 4b and c).
FIG. 4.
Cells expressing Nm23-H1 and the essential EBV antigens dramatically increased tumor mass and size in the nude mouse model. (a) The primary tumor sizes (diameters in millimeters) developed in mice injected with MDA-MB-231T cell clones stably expressing EBV nuclear antigens or Nm23-H1 or both were determined. The data represent the measurement of the total tumor mass in mice of each group at the time of death or after 90 days postinjection. The bar represents the median value. The median mass of the primary tumor in mice injected with cancer cells expressing both EBNA1 and EBNA3C was greater than in mice injected with cells expressing EBNA3C or EBNA1 alone. The mice in which cells expressing Nm23-H1 with either of EBNA1 or EBNA3C were injected developed larger primary tumors than those in which cells expressing only one of the three proteins were injected. (b) Total numbers of primary tumor foci developed in mice injected with MDA-MB-231T cell clones stably expressing EBV nuclear antigens or Nm23-H1 or both. The data represent the numbers of total tumor foci in all mice in each group after 90 days postinjection or at the time of death, whichever was earlier. The total numbers of tumor foci were greater than the numbers of mice injected with tumor cells expressing both EBNA1 and EBNA3C compared to those expressing these alone or with Nm23-H1. (c) Average number of primary tumor foci per mouse. Mice injected with cells expressing EBNA1 with Nm23-H1 developed a greater number of primary tumor foci.
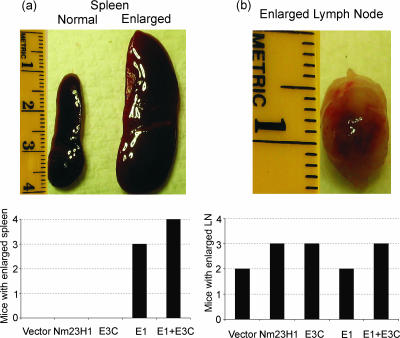
Cells expressing EBNA1 result in spleen enlargement.
On examination of the internal organs of mice after death or at the end of the experiment when the surviving mice were sacrificed, we found that the majority of mice injected with cells expressing EBNA1 developed splenomegaly (Fig. 5a). The other most common gross lesion was the enlargement of lymph nodes that was seen in all groups of mice, including the mice injected with vector control cells (Fig. 5b). Thus, EBNA1 is likely to trigger a dramatic immune response compared to EBNA3C in this mouse model system.
FIG. 5.
Cells expressing EBNA1 result in spleen enlargement. The majority of mice injected with cells expressing EBNA1 developed splenomegaly. (a) A photographs show the enlarged spleen seen in mice that died due to tumor metastasis resulting from cells expressing EBNA1 alone or with EBNA3C. (b) The other most common gross lesion was the enlargement of lymph nodes that was seen in all groups of mice, including the mice injected with vector control cells.
Cells expressing viral antigens had a propensity for increased metastases in the lung.
To study the role of EBV latent nuclear antigens in metastasis, we examined hematoxylin-and-eosin (H&E)-stained sections of harvested lungs obtained from mice injected with MDA-MB-231T cells expressing Nm23-H1, EBNA1, EBNA3C, or a combination of the different antigens for the presence of micrometastasis (Fig. 6). Mice injected with cancer cells expressing EBNA1 alone or both EBNA1 and EBNA3C developed threefold more metastases than the vector control (Fig. 6j and k). This finding is in agreement with the in vitro cell migration assay data above, which showed that both EBNA1 and EBNA3C increased cell motility. Strikingly, the mice injected with MDA-MB-231T cells expressing EBNA3C alone developed 14-fold more metastases than in the vector control group (Fig. 6i). This suggests that the EBV latent nuclear antigens can contribute to the promotion of cancer cell metastasis in vivo. As expected, cancer cells overexpressing the metastasis suppressor Nm23-H1 did show a lower number of metastatic foci in the lungs than the vector control cell lines. The expression of EBNA3C with Nm23-H1 did result in a dramatic rescue of cells from the antimetastatic effect of Nm23-H1, suggesting that prometastatic effects of EBNA3C can effectively override the antimetastatic effects of Nm23-H1. However, the presence of EBNA1 was only able to rescue the cells from the antimetastatic effects of Nm23-H1 up to the level similar to the vector alone control (Fig. 6i).
FIG. 6.
Cells expressing viral antigens had a propensity for increased metastases in the lung. (a to f) H&E-stained lung sections show tumor foci formed due to metastasis of cancer cells from site of primary injection. The sections of lungs stained with H&E are shown for each experimental group. (g and h) Lung sections were stained with anti-cytokeratin 19 antibody to detect micrometastases. (i to k) The metastasis potential of cancer cells, as represented by the relative count of metastasis foci in lungs of mice observed after histopathology in different groups of mice, is shown in the bottom panels. The differences in metastatic foci count in mice injected with EBV antigens were significantly higher than in those injected with vector control, as shown by P values above the bars.
DISCUSSION
We have established an in vivo experimental model to assay for the effects of EBV latent antigens on metastatic colonization by analyzing the effect of MDA-MB-231T breast carcinoma cells in terms of growth, metastasis, arrest, extravasation, and proliferation in the lung of nude mice for up to 12 weeks. Our goal was to examine the effects of the EBV latent nuclear antigens in this model system to provide evidence for the role of these proteins in promoting metastasis in vivo. Moreover, we also explored the role of the EBV latent nuclear antigens in affecting the growth of cancer cells at the site of primary tumor during the initial stages leading to the detection of primary tumor and subsequent growth of the tumor at the primary site. We had earlier reported that interaction with the suppressor of metastasis, Nm23-H1, may be a part of a complex mechanism by which these molecules can regulate cell signaling pathways to exhibit phenotypic changes in cell migration and metastasis (30, 47, 48). In the present study we attempted to test this hypothesis using an in vivo experimental nude mouse model by generating cell lines stably expressing the EBV latent antigens with or without Nm23-H1 expression, injecting these cells in nude mice, and observing the development of primary tumors at the site of injection, as well as the development of tumor foci due to metastasis in lungs. The MDA-MB-231T cell line used in the present study is a subclone of the MDA-MB-231 cells and was chosen because of their reported reliability in in vivo experimental metastatic assays (35).
EBV is associated with a number of human lymphoid and epithelial tumors (34). The EBV antigen EBNA3C has been shown to interact with the known metastasis suppressor Nm23-H1, and the binding domain for Nm23-H1 has been mapped to a glutamine-rich domain of EBNA3C (48). The interaction between EBNA3C and Nm23-H1 is of interest since the interaction between these two molecules, the essential viral oncoprotein, and the known cellular metastasis suppressor and regulator of cell proliferation can lead to the reversion of cell migration in vitro and so affect cell proliferation and metastasis in EBV-positive tumors (47). Nm23-H1 has been shown to localize to the cytoplasm. However, in the presence of EBNA3C, its localization signals were exclusively nuclear (47). This change in localization strengthens the possibility that Nm23-H1 may function as a transcriptional regulator. Moreover, it has been demonstrated earlier that EBNA1, which is known to be expressed in all EBV-positive tumors, interacts with Nm23-H1 in vitro and in EBV-transformed cells (30). EBNA3C has not been detected in all EBV-positive tumors and is typically seen in lymphoproliferative disorder-associated lymphomas in immunocompromised patients due to AIDS or after organ transplantation (38). However, EBNA1 has been consistently detected in all EBV-associated tumors, including lymphoproliferative diseases (12). These studies suggest an important role for these viral antigens in EBV-mediated cancers.
The function of EBNA1 has been extensively studied in B cells, where in vitro infection with EBV is efficient and results in B-cell transformation (38, 63). Its role with regard to epithelial EBV infection is, however, less clear. Although EBV infection of epithelial cells obviously occurs in the context of virus-associated carcinomas, the requirements for this process have not been extensively examined. In addition, EBV is associated with a wide variety of human malignances, including NPCs, gastric carcinomas, Hodgkin's lymphoma, and Burkitt's lymphoma (38). EBNA1 is expressed in all EBV-infected tumor cells and plays a role in the segregation and maintenance of the episomal EBV genome (16, 38).
In our in vivo assay using the nude mouse model, we observed that cancer cells expressing the EBV latent antigens showed a distinctly different phenotype compared to vector control cells after injection in mice. There was a clear difference in the growth characteristics of cancer cells evident from the fact that the mice injected with cancer cells expressing EBV latent antigens showed visible primary tumor growth at least 4 weeks prior to those seen in mice injected with vector alone control cancer cells. This clearly suggested that EBV latent antigens can contribute to promoting the growth rate of transformed cells in vivo during the initial stages of tumor development. However, when the total tumor masses in mice were compared, we found that there were no major differences between EBV latent antigen groups and the vector alone control group. This is evident from the fact that although the primary tumor appeared after day 80 in the vector control group compared to days 45 to 50 in the EBNA3C and EBNA1 groups, the average sizes of the primary tumors that developed in mice in these groups were similar (4 mm in the vector control and EBNA3C group and 5.5 mm in the EBNA1 group). Indeed, the differences were almost negligible after day 93, suggesting that EBNA3C and EBNA1 might have a role in initiation of tumor progression but that their significance or contribution during the later stage of tumor development was likely to be less critical. We also examined whether or not the presence of both EBV nuclear antigens in cancer cells resulted in a similar effect. Interestingly, in a group of mice that were injected with cell lines expressing both of the EBV latent antigens, the primary tumor could be similarly detected as early as 7 weeks (day 42 postinjection) compared to the other experimental groups (day 45 in the EBNA1 and EBNA3C groups and day 80 in the vector control group) with subsequent progression of the tumors showing a dramatic increase in growth rate after about 9 weeks into experiment (7-mm tumor size). This observation clearly suggests that although EBNA1 and EBNA3C may not have a significant effect on the tumorigenicity of cancer cells during later stages of tumor progression when present individually, it was highly probable that they have collaborative functions that can result in a dramatic increase in the tumorigenic potential of the virus-infected cells. It should be noted that EBNA1 is expressed in all forms of latency, whereas EBNA3C is predominantly expressed only in type III latency (64). Therefore, the observation described above may have some biological significance in the context of the different stages of latency.
A widely accepted definition of a metastasis suppressor gene states that such a gene when expressed can inhibit the spread of cancer cells to secondary sites without affecting tumorigenicity (22). Our results, however, appear to suggest that the expression of the metastasis suppressor Nm23-H1 may in fact affect the growth rate of the cancer cells. It must also be noted that there was a clear difference between detectable primary tumors expressing the EBV latent antigens at week 7 postinjection and those expressing Nm23-H1 at week 8 postinjection. Indeed, the comparison of subsequent progression in the growth of primary tumor in terms of the total mass of the primary tumor over time showed that the growth rate of the primary tumor in mice injected with cell lines expressing EBV latent antigen was similar to those of tumors in mice injected with Nm23-H1-expressing or vector-alone control cell lines. When cancer cells stably coexpressing EBV latent antigens and Nm23-H1 were tested, the primary tumor could be detected almost as early as in mice injected with cell lines expressing Nm23-H1 alone or the EBV latent antigens alone. However, the subsequent growth of tumors at the primary site occurred more rapidly and was larger (minimum of 14 mm on average). Does this finding imply that Nm23-H1 has a role in affecting the tumorigenicity of cancer cells? In another study MDA-MB-435 cells expressing Nm23-H1 were injected in the left hind flanks of nude mice and were observed for tumor growth (50). In the present study, Nm23-H1-expressing cells produced tumors that were both smaller and fewer in number in injected mice than did vector control cells. The difference in results may merely be related to the difference in the type of cells used (MDA-MB-231T compared to MDA-MB-435) or the site of injection (fourth mammary fat pad compared to the left hind flank) considering that both of these are human breast carcinoma cell lines. In our experiment, we confirmed the expression of Nm23-H1 both at RNA levels by RT-PCR and at protein levels by Western blotting in tumor tissues collected from all groups of mice at autopsy or at the end of experiment when all mice still alive were sacrificed. The expression level was found to be comparable to that of the MDA-MB-231T clones used in the experiment.
It has been shown that higher Nm23-H1 levels correlate with a reduced metastasis potential of several human solid tumors (13, 32). To study the effect of EBV latent antigens and their interaction with Nm23-H1 on the metastasis potential of cancer cells, we compared the number of micrometastatic foci in the lungs of mice injected with different stable cell lines. Lung sections from the mice were stained with H&E and analyzed for the presence of micrometastatic foci derived from the cell lines. Our results showed that the metastasis potential of Nm23-H1-expressing cells was ∼2-fold less than the vector control cell line, thus confirming the antimetastatic potential of Nm23-H1 (44). The mice injected with cancer cells expressing EBNA3C developed metastasis foci in lungs that were 14-fold greater in number than in the vector control group of mice, suggesting a strong prometastatic potential of EBNA3C. EBNA1 was less potent with an ∼3-fold increase, suggesting that there was a difference in the prometastatic potential associated with the two EBV latent nuclear antigens, with EBNA3C being about 5-fold more prometastatic than EBNA1. The presence of both EBNA1 and EBNA3C resulted in prometastasis effects that were threefold greater than in vector-alone control cells. This finding suggests that EBNA1 and EBNA3C act through similar pathways and have regulatory effects on each other in terms of their metastatic potential in EBV-transformed cells in vivo. In EBV latently infected lymphoblastoid cells, EBNA1 and EBNA3C are both expressed (49). Therefore, it appears that EBNA1 may have a dominant effect on EBNA3C molecular functions as it relates to the metastasis potential of the viral infected cell. Considering that these proteins when expressed together result in very high tumorigenicity compared to when they are expressed individually, it is quite possible that these EBV latent nuclear antigens contribute to the tumorigenicity potential of EBV-infected cells of the type III latency associated with AIDS-associated lymphomas, posttransplantation lymphoproliferative disorders, and lympho-epithelioma-like adenocarcinoma. It is very likely that type I and II latency where EBNA1 is predominantly expressed as the viral nuclear antigen can also have dramatic consequences on the tumorigenic and metastasis potential of these virus-infected cells.
We also examined the effect of EBV latent nuclear antigens on the antimetastatic effects due to Nm23-H1. The mice injected with cancer cell lines expressing EBNA3C and Nm23-H1 developed four times the number of metastatic foci in their lungs compared to the Nm23-H1 group. This strongly suggests that EBNA3C can rescue the cancer cells from the antimetastatic effects of Nm23-H1. Similarly, EBNA1 could rescue the cancer cells from the antimetastatic effects of Nm23-H1 to the levels of vector control alone. These studies provide in vivo evidence to show that one of the critical mechanism by which EBV antigens can promote metastasis is through their interaction with Nm23-H1 (30, 47, 48). The fact that EBNA3C alone is able to produce greater effects compared to EBNA1 alone or both EBNA3C and EBNA1 stresses a more complex interaction inside the cell in which other cellular interacting proteins are involved.
In summary, using a nude mouse model to test the metastatic and tumorigenic potential of EBV latent nuclear antigens not explored previously, we have been able to demonstrate a role for EBNA1 and EBNA3C and their interaction with Nm23-H1 in promoting metastasis and affecting the tumorigenic potential of EBV-positive cancer cells.
Acknowledgments
This study was supported by grants from the Leukemia and Lymphoma Society of America and Public Health Service grants CA072510, CA108461, and CA091792 from the NCI and DE014136 from the NIDCR (to E.S.R.). E.S.R. is a scholar of the Leukemia and Lymphoma Society of America.
We thank Patricia S. Steeg (Women's Cancers Section, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute) for providing the MDA-MB-231T cells. We also thank Perry Habecker (New Bolton Center, Animal Pathology Service, University of Pennsylvania School of Veterinary Medicine) for preparing the histology slides.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Arcinas, M., and L. M. Boxer. 1994. Differential protein binding to the c-myc promoter during differentiation of hematopoietic cell lines. Oncogene 9:2699-2706. [PubMed] [Google Scholar]
- 2.Baba, H., T. Urano, K. Okada, K. Furukawa, E. Nakayama, H. Tanaka, K. Iwasaki, and H. Shiku. 1995. Two isotypes of murine nm23/nucleoside diphosphate kinase, nm23-M1 and nm23-M2, are involved in metastatic suppression of a murine melanoma line. Cancer Res. 55:1977-1981. [PubMed] [Google Scholar]
- 3.Berberich, S. J., and E. H. Postel. 1995. PuF/NM23-H2/NDPK-B transactivates a human c-myc promoter-CAT gene via a functional nuclease hypersensitive element. Oncogene 10:2343-2347. [PubMed] [Google Scholar]
- 4.Bhujwalla, Z. M., E. O. Aboagye, R. J. Gillies, V. P. Chacko, C. E. Mendola, and J. M. Backer. 1999. Nm23-transfected MDA-MB-435 human breast carcinoma cells form tumors with altered phospholipid metabolism and pH: a 31P nuclear magnetic resonance study in vivo and in vitro. Magn. Reson. Med. 41:897-903. [DOI] [PubMed] [Google Scholar]
- 5.Biggs, J., E. Hersperger, P. S. Steeg, L. A. Liotta, and A. Shearn. 1990. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell 63:933-940. [DOI] [PubMed] [Google Scholar]
- 6.Bominaar, A. A., A. D. Tepper, and M. Veron. 1994. Autophosphorylation of nucleoside diphosphate kinase on non-histidine residues. FEBS Lett. 353:5-8. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet, M., J. M. Guinebretiere, E. Kremmer, V. Grunewald, E. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. [DOI] [PubMed] [Google Scholar]
- 8.Chang, C. L., X. X. Zhu, D. H. Thoraval, D. Ungar, J. Rawwas, N. Hora, J. R. Strahler, S. M. Hanash, and E. Radany. 1994. Nm23-H1 mutation in neuroblastoma. Nature 370:335-336. [DOI] [PubMed] [Google Scholar]
- 9.Davenport, M. G., and J. S. Pagano. 1999. Expression of EBNA-1 mRNA is regulated by cell cycle during Epstein-Barr virus type I latency. J. Virol. 73:3154-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Rosa, A., R. L. Williams, and P. S. Steeg. 1995. Nm23/nucleoside diphosphate kinase: toward a structural and biochemical understanding of its biological functions. Bioessays 17:53-62. [DOI] [PubMed] [Google Scholar]
- 11.Gilles, A. M., E. Presecan, A. Vonica, and I. Lascu. 1991. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J. Biol. Chem. 266:8784-8789. [PubMed] [Google Scholar]
- 12.Hammerschmidt, W., and B. Sugden. 2004. Epstein-Barr virus sustains Burkitt's lymphomas and Hodgkin's disease. Trends Mol. Med. 10:331-336. [DOI] [PubMed] [Google Scholar]
- 13.Hartsough, M. T., and P. S. Steeg. 2000. Nm23/nucleoside diphosphate kinase in human cancers. J. Bioenerg. Biomembr. 32:301-308. [DOI] [PubMed] [Google Scholar]
- 14.Hemmerich, S., and I. Pecht. 1992. Oligomeric structure and autophosphorylation of nucleoside diphosphate kinase from rat mucosal mast cells. Biochemistry 31:4580-4587. [DOI] [PubMed] [Google Scholar]
- 15.Imai, S., S. Koizumi, M. Sugiura, M. Tokunaga, Y. Uemura, N. Yamamoto, S. Tanaka, E. Sato, and T. Osato. 1994. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 91:9131-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn, P., and S. I. Shin. 1979. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J. Cell Biol. 82:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2395. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 19.Knight, J. S., M. A. Cotter II, and E. S. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 276:22971-22978. [DOI] [PubMed] [Google Scholar]
- 20.Leone, A., U. Flatow, C. R. King, M. A. Sandeen, I. M. Margulies, L. A. Liotta, and P. S. Steeg. 1991. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell 65:25-35. [DOI] [PubMed] [Google Scholar]
- 21.Leone, A., U. Flatow, K. VanHoutte, and P. S. Steeg. 1993. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene 8:2325-2333. [PubMed] [Google Scholar]
- 22.Lombardi, D. 2006. Commentary: nm23, a metastasis suppressor gene with a tumor suppressor gene aptitude? J. Bioenerg. Biomembr. 38:177-180. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald, N. J., A. De la Rosa, M. A. Benedict, J. M. Freije, H. Krutsch, and P. S. Steeg. 1993. A serine phosphorylation of Nm23, and not its nucleoside diphosphate kinase activity, correlates with suppression of tumor metastatic potential. J. Biol. Chem. 268:25780-25789. [PubMed] [Google Scholar]
- 24.Marechal, V., A. Dehee, R. Chikhi-Brachet, T. Piolot, M. Coppey-Moisan, and J. C. Nicolas. 1999. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. J. Virol. 73:4385-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehus, J. G., P. Deloukas, and D. O. Lambeth. 1999. NME6: a new member of the nm23/nucleoside diphosphate kinase gene family located on human chromosome 3p21.3. Human Genet. 104:454-459. [DOI] [PubMed] [Google Scholar]
- 26.Milon, L., M. F. Rousseau-Merck, A. Munier, M. Erent, I. Lascu, J. Capeau, and M. L. Lacombe. 1997. nm23-H4, a new member of the family of human nm23/nucleoside diphosphate kinase genes localized on chromosome 16p13. Human Genet. 99:550-557. [DOI] [PubMed] [Google Scholar]
- 27.Munier, A., C. Feral, L. Milon, V. P. Pinon, G. Gyapay, J. Capeau, G. Guellaen, and M. L. Lacombe. 1998. A new human nm23 homologue (nm23-H5) specifically expressed in testis germinal cells. FEBS Lett. 434:289-294. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Dorado, J., S. Inouye, and M. Inouye. 1993. Eukaryotic-like protein serine/threonine kinases in Myxococcus xanthus, a developmental bacterium exhibiting social behavior. J. Cell Biochem. 51:29-33. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Dorado, J., S. Inouye, and M. Inouye. 1990. Nucleoside diphosphate kinase from Myxococcus xanthus. II. Biochemical characterization. J. Biol. Chem. 265:2707-2712. [PubMed] [Google Scholar]
- 30.Murakami, M., K. Lan, C. Subramanian, and E. S. Robertson. 2005. Epstein-Barr virus nuclear antigen 1 interacts with Nm23-H1 in lymphoblastoid cell lines and inhibits its ability to suppress cell migration. J. Virol. 79:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, P. G., G. Niedobitek, E. Kremmer, F. Grasser, G. M. Reynolds, A. Cruchley, D. M. Williams, N. Muller-Lantzsch, and L. S. Young. 1996. In situ detection of the Epstein-Barr virus-encoded nuclear antigen 1 in oral hairy leukoplakia and virus-associated carcinomas. J. Pathol. 178:44-47. [DOI] [PubMed] [Google Scholar]
- 32.Ouatas, T., M. Salerno, D. Palmieri, and P. S. Steeg. 2003. Basic and translational advances in cancer metastasis: Nm23. J. Bioenerg. Biomembr. 35:73-79. [DOI] [PubMed] [Google Scholar]
- 33.Padma, P., A. Hozumi, K. Ogawa, and K. Inaba. 2001. Molecular cloning and characterization of a thioredoxin/nucleoside diphosphate kinase related dynein intermediate chain from the ascidian, Ciona intestinalis. Gene 275:177-183. [DOI] [PubMed] [Google Scholar]
- 34.Pagano, J. S. 1999. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc. Assoc. Am. Physicians 111:573-580. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri, D., D. O. Halverson, T. Ouatas, C. E. Horak, M. Salerno, J. Johnson, W. D. Figg, M. Hollingshead, S. Hursting, D. Berrigan, S. M. Steinberg, M. J. Merino, and P. S. Steeg. 2005. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J. Natl. Cancer Inst. 97:632-642. [DOI] [PubMed] [Google Scholar]
- 36.Postel, E. H., S. J. Berberich, S. J. Flint, and C. A. Ferrone. 1993. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 261:478-480. [DOI] [PubMed] [Google Scholar]
- 37.Postel, E. H., and C. A. Ferrone. 1994. Nucleoside diphosphate kinase enzyme activity of NM23-H2/PuF is not required for its DNA binding and in vitro transcriptional functions. J. Biol. Chem. 269:8627-8630. [PubMed] [Google Scholar]
- 38.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 39.Rosengard, A. M., H. C. Krutzsch, A. Shearn, J. R. Biggs, E. Barker, I. M. Margulies, C. R. King, L. A. Liotta, and P. S. Steeg. 1989. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature 342:177-180. [DOI] [PubMed] [Google Scholar]
- 40.Russell, R. L., K. R. Geisinger, R. R. Mehta, W. L. White, B. Shelton, and T. E. Kute. 1997. nm23: relationship to the metastatic potential of breast carcinoma cell lines, primary human xenografts, and lymph node negative breast carcinoma patients. Cancer 79:1158-1165. [DOI] [PubMed] [Google Scholar]
- 41.Sheu, L. F., A. Chen, C. L. Meng, K. C. Ho, W. H. Lee, F. J. Leu, and C. F. Chao. 1996. Enhanced malignant progression of nasopharyngeal carcinoma cells mediated by the expression of Epstein-Barr nuclear antigen 1 in vivo. J. Pathol. 180:243-248. [DOI] [PubMed] [Google Scholar]
- 42.Stahl, J. A., A. Leone, A. M. Rosengard, L. Porter, C. R. King, and P. S. Steeg. 1991. Identification of a second human nm23 gene, nm23-H2. Cancer Res. 51:445-449. [PubMed] [Google Scholar]
- 43.Steeg, P. S., G. Bevilacqua, L. Kopper, U. P. Thorgeirsson, J. E. Talmadge, L. A. Liotta, and M. E. Sobel. 1988. Evidence for a novel gene associated with low tumor metastatic potential. J. Natl. Cancer Inst. 80:200-204. [DOI] [PubMed] [Google Scholar]
- 44.Steeg, P. S., G. Bevilacqua, R. Pozzatti, L. A. Liotta, and M. E. Sobel. 1988. Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res. 48:6550-6554. [PubMed] [Google Scholar]
- 45.Steeg, P. S., A. de la Rosa, U. Flatow, N. J. MacDonald, M. Benedict, and A. Leone. 1993. Nm23 and breast cancer metastasis. Breast Cancer Res. Treat. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 46.Stoker, M., C. O'Neill, S. Berryman, and V. Waxman. 1968. Anchorage and growth regulation in normal and virus-transformed cells. Int. J. Cancer 3:683-693. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian, C., M. A. Cotter II, and E. S. Robertson. 2001. Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-H1: a molecular link to cancer metastasis. Nat. Med. 7:350-355. [DOI] [PubMed] [Google Scholar]
- 48.Subramanian, C., and E. S. Robertson. 2002. The metastatic suppressor Nm23-H1 interacts with EBNA3C at sequences located between the glutamine- and proline-rich domains and can cooperate in activation of transcription. J. Virol. 76:8702-8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomkinson, B., E. Robertson, and E. Kieff. 1993. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 67:2014-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseng, Y. H., D. Vicent, J. Zhu, Y. Niu, A. Adeyinka, J. S. Moyers, P. H. Watson, and C. R. Kahn. 2001. Regulation of growth and tumorigenicity of breast cancer cells by the low molecular weight GTPase Rad and nm23. Cancer Res. 61:2071-2079. [PubMed] [Google Scholar]
- 51.Tsuiki, H., M. Nitta, A. Furuya, N. Hanai, T. Fujiwara, M. Inagaki, M. Kochi, Y. Ushio, H. Saya, and H. Nakamura. 1999. A novel human nucleoside diphosphate (NDP) kinase, Nm23-H6, localizes in mitochondria and affects cytokinesis. J. Cell Biochem. 76:254-269. [DOI] [PubMed] [Google Scholar]
- 52.Urano, T., K. Takamiya, K. Furukawa, and H. Shiku. 1992. Molecular cloning and functional expression of the second mouse nm23/NDP kinase gene, nm23-M2. FEBS Lett. 309:358-362. [DOI] [PubMed] [Google Scholar]
- 53.van Golen, K. L., S. Risin, A. Staroselsky, D. Berger, M. A. Tainsky, S. Pathak, and J. E. Price. 1996. Predominance of the metastatic phenotype in hybrids formed by fusion of mouse and human melanoma clones. Clin. Exp. Metastasis 14:95-106. [DOI] [PubMed] [Google Scholar]
- 54.van Noesel, M. M., and R. Versteeg. 2004. Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene 325:1-15. [DOI] [PubMed] [Google Scholar]
- 55.Venturelli, D., R. Martinez, P. Melotti, I. Casella, C. Peschle, C. Cucco, G. Spampinato, Z. Darzynkiewicz, and B. Calabretta. 1995. Overexpression of DR-nm23, a protein encoded by a member of the nm23 gene family, inhibits granulocyte differentiation and induces apoptosis in 32Dc13 myeloid cells. Proc. Natl. Acad. Sci. USA 92:7435-7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma, S. C., and E. S. Robertson. 2003. ORF73 of herpesvirus Saimiri strain C488 tethers the viral genome to metaphase chromosomes and binds to cis-acting DNA sequences in the terminal repeats. J. Virol. 77:12494-12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner, P. D., and N. D. Vu. 1995. Phosphorylation of ATP-citrate lyase by nucleoside diphosphate kinase. J. Biol. Chem. 270:21758-21764. [DOI] [PubMed] [Google Scholar]
- 58.Wallet, V., R. Mutzel, H. Troll, O. Barzu, B. Wurster, M. Veron, and M. L. Lacombe. 1990. Dictyostelium nucleoside diphosphate kinase highly homologous to Nm23 and Awd proteins involved in mammalian tumor metastasis and Drosophila development. J. Natl. Cancer Inst. 82:1199-1202. [DOI] [PubMed] [Google Scholar]
- 59.Webb, P. A., O. Perisic, C. E. Mendola, J. M. Backer, and R. L. Williams. 1995. The crystal structure of a human nucleoside diphosphate kinase, NM23-H2. J. Mol. Biol. 251:574-587. [DOI] [PubMed] [Google Scholar]
- 60.West, M. J. 2006. Structure and function of the Epstein-Barr virus transcription factor, EBNA 3C. Curr. Protein Peptide Sci. 7:123-136. [DOI] [PubMed] [Google Scholar]
- 61.Wilson, J. B., J. L. Bell, and A. J. Levine. 1996. Expression of Epstein-Barr virus nuclear antigen-1 induces B-cell neoplasia in transgenic mice. EMBO J. 15:3117-3126. [PMC free article] [PubMed] [Google Scholar]
- 62.Yates, J. L., S. M. Camiolo, S. Ali, and A. Ying. 1996. Comparison of the EBNA1 proteins of Epstein-Barr virus and herpesvirus papio in sequence and function. Virology 222:1-13. [DOI] [PubMed] [Google Scholar]
- 63.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]
- 64.Young, L. S., C. W. Dawson, and A. G. Eliopoulos. 2000. The expression and function of Epstein-Barr virus encoded latent genes. Mol. Pathol. 53:238-247. [DOI] [PMC free article] [PubMed] [Google Scholar]