Abstract
We have studied the interactions of exogenous prions with an epithelial cell line inducibly expressing PrPc protein and permissive to infection by a sheep scrapie agent. We demonstrate that abnormal PrP (PrPSc) and prion infectivity are efficiently internalized in Rov cells, whether or not PrPc is expressed. At odds with earlier studies implicating cellular heparan sulfates in PrPSc internalization, we failed to find any involvement of such molecules in Rov cells, indicating that prions can enter target cells by several routes. We further show that PrPSc taken up in the absence of PrPc was unable to promote efficient prion multiplication once PrPc expression was restored in the cells. This observation argues that interaction of PrPSc with PrPc has to occur early, in a specific subcellular compartment(s), and is consistent with the view that the first prion multiplication events may occur at the cell surface.
Prion diseases are fatal neurodegenerative disorders characterized by the accumulation in the infected tissues of a detergent-insoluble and protease-resistant isoform of the cellular PrPc protein (7, 43). The causative infectious agent, or prion, is thought to be an abnormal form of PrPc (PrPSc), or a precursor of it, and prions apparently multiply by triggering the conversion of the normal PrPc into the abnormal conformer (29). While prion multiplication does not occur in cells or tissues that do not express PrPc (5, 9), several lines of evidence indicate that PrPc is not sufficient to confer permissiveness. Mouse tissues expressing high levels of PrP do not necessarily accumulate prion infectivity (30), and very few PrPc-expressing cell lines are able to propagate prions (37). This indicates that prions require an additional cellular and/or molecular factor(s) to multiply efficiently. The fact that some strains seem refractory to multiplication in cultured cells further suggests that at least some of the required factors are strain specific. The initial interactions of exogenous infecting PrPSc with a target cell are poorly characterized. Although a variety of cultured cell types internalize prion-infected material (13, 16, 17, 22, 24, 25, 33, 35), only a few will become infected (37), indicating that other cellular processes must occur for the establishment of cell infection. Various aspects of prion infection can be studied with Rov cells (41), including cell-to-cell prion dissemination (26) and, through a reverse genetics approach, the effects of natural polymorphisms of ovine PrPc on prion multiplication (34). Several features make Rov cells an attractive model for the study of cell-prion interactions. First, they propagate a natural sheep scrapie strain. Second, the expression of the ovine PrPc protein in Rov cells, and thus permissiveness to prion multiplication, can be selectively induced through the addition of doxycycline (dox) to the cell culture medium. This makes it possible to rigorously assess the influence of the PrPc protein on the interaction of the cells with exogenous abnormal PrP and on the establishment of cell infection. In this study, we show that while the presence of the PrPc protein is not required for efficient entry of PrPSc and infectivity in Rov cells, it is required at a very early stage for infection of Rov cells.
MATERIALS AND METHODS
Reagents and antibodies.
Dextran sulfate 500 (DS500) was from Sigma, and the dextran-based heparan mimetic agents HM20602 and CR36 (10) were kindly provided by D. Papy-Garcia. DS500, HM2602, and CR36 were made at 5 μg ml−1 in phosphate-buffered saline (PBS) and were stored at −20°C. Aliquots diluted in cell culture medium were filter sterilized before being incubated with the cells.
4F2 monoclonal antibody (MAb) (19) was used to detect the expression of PrPc in Rov cells. Immunoblot analysis of abnormal PrP in proteinase K (PK)-digested cell lysates was performed with ICSM18 (3) or Sha31 MAb (12).
Inoculum.
The PG127 sheep isolate and experimental sheep bovine spongiform encephalopathy (BSE) agent were serially propagated onto tg338 transgenic mice (20) that express the VRQ allele of ovine PrPc on a mouse Prnp0/0 background (42) to obtain the 127S and BSEov strains used in this study. Infected brains were homogenized at 10% (wt/vol) in a sterile 5% glucose solution with a Ribolyser (Hybaid, Middlesex, United Kingdom) and were sonicated for 1 to 2 min before incubation with Rov cells.
When PrPSc uptake was analyzed by immunofluorescence, the inoculum consisted of Rov cultures persistently infected with 127S. These cultures were incubated for 2 days in culture medium without dox, rinsed, scraped into PBS, pelleted by centrifugation, and resuspended in a 5% glucose solution. After four freeze-thaw cycles, the cell suspensions were sonicated for 1 to 2 min in a cup-horn apparatus before being diluted in cell culture medium.
Cell culture and uptake of PrPSc.
Rov cells (the Rov9 clone) (41) were maintained at 37°C in 6% CO2 in alpha minimal essential medium supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin, and 10 μg ml−1 streptomycin. Rov cells were grown to confluence in 12-well plates. To induce the expression of ovine PrPc (the VRQ allele) in Rov cells, 1 μg ml−1 dox was added to the culture medium. Infected brain homogenates diluted in 0.5 ml of cell culture medium were incubated with the Rov monolayers for the indicated periods of time. The medium was removed, and the cultures were carefully rinsed three times with PBS. The cell monolayer of each well was solubilized in 500 μl of lysis buffer (50 mM Tris-HCl, pH 7.4, 0.5% Triton X-100, 0.5% sodium deoxycholate) for 10 min at 4°C. Lysates were clarified (5,000 rpm for 1 min in a microcentrifuge), and cellular proteins were quantified by bicinchoninic acid. The proteins from each culture well were digested with PK for 2 h at 37°C (2 μg of PK for 500 μg of proteins) in the presence of 0.02% bromophenol blue to better visualize the pellets obtained after centrifugation. Pefabloc (4 mM) was added to stop the reaction, and blue pellets containing aggregated, PK-resistant PrP (PrPres) were collected by centrifugation at 13,000 rpm in a microcentrifuge for 30 min at room temperature (27). PrPres from each well was analyzed by immunoblotting after electrophoresis on sodium dodecyl sulfate-12% polyacrylamide gels and transfer to nitrocellulose filters. The filters were developed with an ECL reagent kit (Amersham), and chemiluminescent signals corresponding to the PrP glycoforms were quantified after acquisition with a GeneGnome digital imager (Syngene, Frederick, MD).
Detection of PrPSc uptake by immunofluorescence microscopy.
To specifically visualize the entry of PrPSc in Rov cells, an inoculum depleted of PrPc was prepared (as described above) by using persistently infected Rov cell cultures that were incubated in cell culture medium without dox for 2 days, so as to downregulate the expression of PrPc. Under these conditions, normal PrP was no longer detectable, while PrPSc was still present in large amounts. Rov cells grown on coverslips were incubated for 24 h in the presence of this inoculum. Cell monolayers were washed thoroughly with PBS, fixed with 4% paraformaldehyde-4% sucrose for 10 min, and permeabilized with 0.2% Triton X-100 in PBS for 3 min. Cells then were treated for 5 min with 3 M guanidine thiocyanate in PBS to expose PrPSc epitopes (39). ICSM35 MAb (3) was used to detect PrPSc taken up by the cells. Bound MAbs were revealed with fluorescein isothiocyanate-coupled goat anti-mouse secondary antibody. Nuclei were stained with propidium iodide. Dual-immunofluorescence confocal images were acquired by using a confocal laser-scanning microscope (CLSM 310; Carl Zeiss) equipped with a Plan-Apochromat 63× oil immersion objective (1.4 numerical aperture). Images were processed using Zeiss software for overlays of red and green channels.
Uptake of prion infectivity.
Rov cells left untreated or treated with 1 μg ml−1 of dox were grown to confluence in a 75-cm2 flask. The cell monolayers then were incubated with the equivalent of 2.5 μl of 10% infectious brain homogenate for 6 h. The cultures were rinsed three times with PBS, scraped into PBS, and pelleted by centrifugation. The cells were resuspended in a sterile 5% glucose solution, frozen and thawed four times, sonicated, and inoculated intracerebrally (20 μl) into tg338 mice. Mice showing neurological signs were monitored almost daily and were euthanized in extremis.
Amiloride treatment.
Rov cells were left untreated or were treated with 3 mM amiloride for 1 h prior to incubation with 2.5% of infectious brain homogenate for 30 min. We verified that this treatment prevented the hepatocyte growth factor-mediated induction of ruffles, as assessed by microscopic examination of Rov cells stained with fluorochrome-conjugated phalloidin.
Infection of Rov cells.
dox-treated confluent Rov cultures in 12-well plates were incubated overnight with the infectious brain homogenate diluted to 0.25% in the cell culture medium. In some experiments, the cells were pretreated for 24 h with sulfated glycans, and incubation of the cells with the inoculum was performed in the presence of the molecules. The monolayers then were rinsed three times with PBS, and each well was passaged into a 25-cm2 flask. A few hours later, the dox-containing medium of each flask was changed and replaced with fresh medium. One week later, cell cultures were lysed in 1.5 ml of lysis buffer, and 500 μg cellular proteins was digested with PK to analyze PrPres by immunoblotting as described above.
RESULTS
Infection requires the presence of PrPc at the time of exposure to prions.
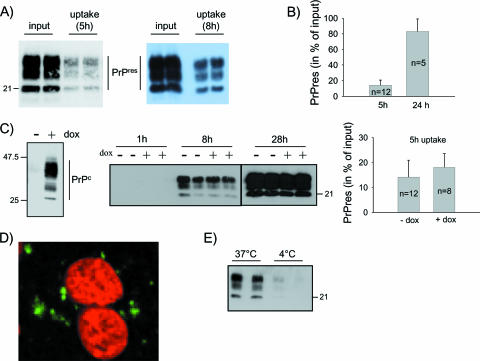
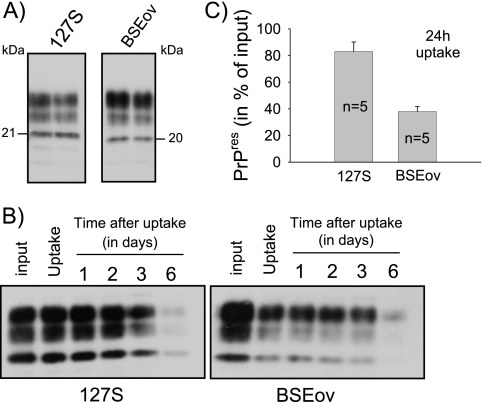
Although scrapie-associated fibrils represent the purest forms of prions known to date, they might be poorly relevant biologically, since such detergent-extracted, protease-treated, highly aggregated forms of abnormal PrP are unlikely to have counterparts in vivo. Thus, in this study we used homogenized brain tissue as a source of abnormal PrP and infectivity. Brain homogenate from ovine PrP tg338 mice infected with the 127S strain of sheep scrapie was diluted to 0.25% in cell culture medium and was incubated for 5 h with confluent Rov cells. The monolayers were rinsed thoroughly to remove the inoculum, and the cells were solubilized in detergent-containing lysis buffer. The cell-associated abnormal PrP was analyzed by Western blotting after PK digestion and centrifugation of the lysates. As shown in Fig. 1A, a substantial portion of exogenous PrPSc became associated with Rov cells. Quantitative measurements indicated that as much as 82% of the exogenously added PrPres became associated with Rov cells after a 24-h incubation period (Fig. 1B). To determine if this process depended on the presence of PrPc, we took advantage of the tight regulation of ovine PrPc expression in Rov cells: cell cultures grown in the presence of dox express high levels of PrPc, while no PrPc is detected in untreated Rov cells (Fig. 1C) (41). Kinetic experiments using dox-treated and untreated control Rov cells showed that the association of PrPSc with the cells was entirely independent of the expression of PrPc, as illustrated in Fig. 1C. The presence of PrPSc within individual cells could be visualized by immunostaining and confocal analysis of exposed Rov cultures (Fig. 1D). Moreover, blocking endocytosis by lowering the temperature during the exposure period strongly reduced the amount of abnormal PrP taken up by the cells. Quantification of experiments such as the one shown in Fig. 1E indicated that abnormal PrP taken up was 10- to 20-fold reduced at 4°C (n = 4 experiments). These findings confirmed that the observed phenomenon essentially involved an active internalization process. We then examined how long abnormal PrP would remain in the cells after internalization. After a 24-h exposure to infected brain homogenate, the cultures were rinsed. Some of them were lysed to quantify the uptake of abnormal PrP, while others were further incubated for up to 6 days to study its persistence in the cells. Figure 2B and C show that internalized abnormal PrP persisted for several days in the cells, implying that its degradation did not take place rapidly. We then examined the internalization of abnormal PrP from another prion strain, ovine BSE (BSEov). We chose BSEov because (i) at the terminal stage of the disease, tg338 mice expressing the VRQ allele of ovine PrP and infected with BSEov and 127S show similar amounts of PrPres in their brains (Fig. 2A), and (ii) in contrast to 127S, BSEov does not multiply in Rov cells (data not shown). Results shown in Fig. 2B and C clearly show that abnormal PrP from BSEov was taken up less efficiently than that from 127S (35% ± 5% for BSEov and 82% ± 7% for 127S). That PrPSc from different strains enters the cells with distinct efficiencies indicates some selectivity of the internalization process.
FIG. 1.
Exogenous abnormal PrP is taken up by Rov cells independently of PrPc expression. (A) Infectious brain homogenate was diluted to 0.25% in culture medium and was incubated with Rov cells (in duplicate). Five (left panel) or 8 (right panel) h later, the cultures were extensively washed and extracted, and lysates were treated with PK. Abnormal PrP taken up by the cells was analyzed by immunoblotting with anti-PrP MAb, and the level of PrPSc uptake was compared to that in the inoculum (input). (B) Rov cells were incubated for 5 or 24 h with infectious brain homogenate (0.25%), and abnormal PrP taken up by the cells was analyzed by immunoblotting as described in Materials and Methods. PrPres in the cells is expressed as mean percentages ± standard deviations of PrPres in the inoculum (input). (C) Uptake of abnormal PrP depending on PrPc expression. The left panel shows inducible expression of ovine PrPc in Rov cells. Equal amounts of methanol-precipitated proteins (20 μg) from Rov cultures treated with 1 μg ml−1 of dox (+) or left untreated (−) were analyzed by immunoblotting with 4F2 anti-PrP MAb. The middle panel shows duplicate wells of Rov cells grown in the presence (+) or in the absence (−) of dox and incubated for 1, 8, or 28 h in the presence of infectious brain homogenate. Immunoblotting of abnormal PrP taken up by Rov cells was performed as described in Materials and Methods. The right panel shows the quantification of abnormal PrP taken up for 5 h in Rov cells expressing (+dox) or not expressing (−dox) PrPc, expressed as mean percentages ± standard deviations of PrPres in the inoculum (input). (D) Confocal microscopic examination of PrPSc internalized in Rov cells. Rov cells exposed to the inoculum (see Materials and Methods) were fixed, permeabilized, treated with guanidine thiocyanate to expose PrPSc epitopes, and stained with ICSM35 anti-PrP antibody. The image shows abnormal PrP staining (in green) and nuclei (in red) in an x-y plane. No PrP staining was observed when Rov cells were incubated with a mock-infected inoculum. (E) Infectious brain homogenate was incubated in duplicate for 6 h at 37°C or at 4°C with Rov cells. Immunoblotting of abnormal PrP taken up by Rov cells was performed as described in Materials and Methods. The positions of the molecular mass marker proteins are indicated (in kilodaltons).
FIG. 2.
Internalization of abnormal PrP from different strains. (A) PrPres was isolated in duplicate from 0.6 mg of brain equivalent from terminally ill tg338 mice infected with strain 127S or BSEov; samples were analyzed on the same gel by immunoblotting with anti-PrP MAb. Note the distinct banding patterns of 127S and BSEov PrPres. (B) Uptake and persistence in Rov cells of abnormal PrP from 127S and BSEov. 127S or BSEov brain homogenate was diluted to 0.5% in cell culture medium (input) and incubated with Rov cells. The inoculum was removed 24 h later by washing, and the cultures were extracted either immediately (uptake) or after 1 to 6 days, as indicated. Cell lysates were treated with PK, and abnormal PrP taken up by the cells was analyzed by immunoblotting with anti-PrP MAb. (C) Uptake efficiency of abnormal PrP from two different strains. 127S or BSEov brain homogenate was diluted to 0.5% in cell culture medium and incubated with Rov cells for 24 h. Abnormal PrP taken up by the cells was analyzed by immunoblotting as described in Materials and Methods, and the level of uptake was compared to that in the inoculum (input). The data represent the means ± standard errors from five independent experiments.
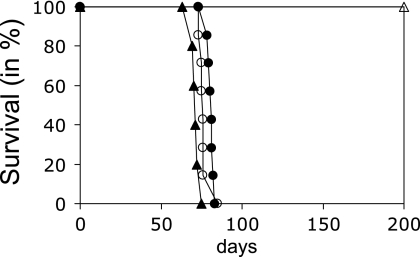
While providing a surrogate marker of prion multiplication in infected cells and tissues, the aggregated, PK-resistant form of PrP (PrPres) may not be the most infectious (36). To see whether the uptake of infectivity also was independent of the presence of PrPc, 10% infected brain homogenate was diluted 4,000-fold in culture medium and was incubated with Rov cells that either expressed or did not express ovine PrPc. Six hours later, the cells were rinsed, collected, and inoculated into susceptible ovine PrPc transgenic mice to quantify prion infectivity taken up by the cells. As a result, the two groups of mice (n = 7) showed similar survival times (77 ± 1.4 and 81 ± 0.7 days for untreated and dox-treated cells, respectively), establishing that Rov cells have taken up the infectivity with an efficiency that did not depend on the presence of PrPc at the cell surface (Fig. 3). Based on the dose-dependent incubation time of the 127S agent in tg338 mice, it was calculated that about 5 to 10% of input infectivity was taken up by the cells within 6 h of exposure.
FIG. 3.
Prion infectivity is taken up by Rov cells independently of PrPc expression. Infectious inoculum (2.5 μl of 10% brain homogenate) was incubated for 6 h with Rov cells expressing (open circles) or not expressing (filled circles) PrPc. The cultures then were harvested and inoculated intracerebrally in tg338 mice (n = 7), and the mice were monitored until death to determine the survival time. Rov cells not exposed to the inoculum were inoculated in parallel (open triangles). The survival time of tg338 mice inoculated with 20 μl of a 1,000-fold-diluted 10% brain homogenate (filled triangles) also is shown for comparison.
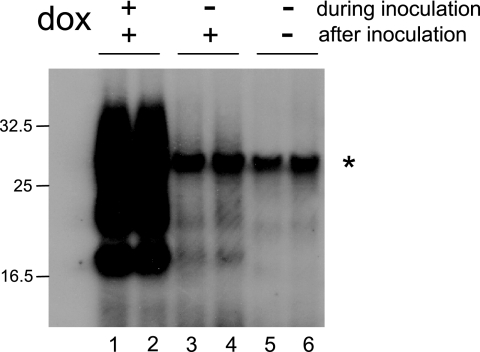
The regulatable expression of PrPc in Rov cells made it possible to investigate if abnormal PrP taken up in the absence of PrPc still would trigger infection once PrPc expression is restored. Control or dox-treated Rov cells were exposed overnight to infected brain homogenate to allow the uptake of abnormal PrP. The inoculum was removed, and then the cells were trypsinized and transferred to new flasks in the presence of dox and grown for 7 days to detect infection. PrPc-expressing Rov cells exposed to the inoculum produced PrPres (Fig. 4, lanes 1 and 2), while no PrPres was detected in cultures that did not express PrPc (Fig. 4, lanes 5 and 6). Remarkably, infection did not develop in cultures in which PrPc expression was induced after the time of exposure (Fig. 4, lanes 3 and 4).
FIG. 4.
PrPc is required at the time of inoculation for efficient cell infection. Duplicate Rov cell cultures were exposed for 18 h to infectious brain homogenate (0.25%) in the presence (lanes 1 and 2) or in the absence (lanes 3 and 4) of dox. After removal of the inoculum, the cultures were passaged and further grown for 7 days in dox-containing fresh medium. Cell lysates then were digested with PK and analyzed by immunoblotting for PrPres. Cultures expressing no PrPc at the moment of inoculation showed little or no PrPres (lanes 3 and 4). These data are representative of six experiments. The signal denoted by an asterisk is due to cross-reaction of the ICSM18 MAb with PK. The positions of molecular mass marker proteins are indicated (in kilodaltons).
The above data demonstrated that Rov cells are able to efficiently internalize abnormal PrP irrespective of whether (i) the agent will multiply or (ii) PrPc is expressed. Conversely, PrPc expression at the time of exposure to prions appeared to be a crucial requirement for the infection to proceed.
PrPSc infection, but not internalization, is inhibited by heparan sulfates.
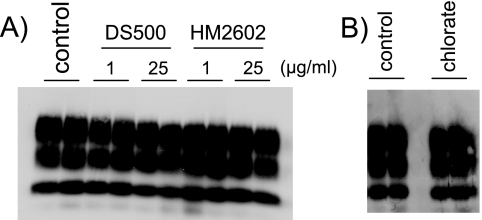
To explore if abnormal PrP has to be internalized to infect Rov cells, we studied further the uptake of PrPSc in these cells. A strong inhibition of the uptake of exogenous PrPsc by sulfated glycans (1 μg/ml), such as DS500 or HM2602, has been reported in several recent studies, pointing to cellular heparan sulfates as possible receptors for abnormal PrP (16, 17, 35). To test the effects of these molecules on Rov cells, cultures were preincubated for 24 h with DS500 or the heparan mimetic agents HM2602 and CR36, and inoculum was added to the culture medium for an additional 18 h in the presence of the tested molecules. Data shown in Fig. 5A indicate that none of the sulfated glycans (at up to 25 μg/ml) inhibited the uptake of abnormal PrP in the cells (data not shown for CR36). We also looked for an effect of Na chlorate on PrPSc uptake. Treatment with Na chlorate, a potent inhibitor of protein sulfation (1), including cellular heparan sulfates, was found to strongly inhibit PrPSc uptake (17). However, we found that treatment of Rov cells with Na chlorate did not affect PrPSc uptake in Rov cells (Fig. 5B). Therefore, unlike the results found for CHO, GT1, and N2a cells, we failed to find an involvement of heparan sulfates in the uptake of PrPSc by Rov cells.
FIG. 5.
Sulfated glycans have no detectable effect on the uptake of abnormal PrP by Rov cells. Immunoblots of abnormal PrP taken up by Rov cells are shown. (A) Duplicate wells were left untreated or were treated for 24 h with the indicated concentrations of DS500 or HM2602. Infectious brain homogenate (0.25%) was added to the cells, which were further incubated for 18 h. (B) Duplicate wells were incubated for 24 h with Na chlorate (30 mM) or were left untreated (control). Infectious brain homogenate (0.25%) was added to the cells, which were further incubated for 18 h.
Macropinocytosis, a major form of endocytosis in various cell types, is involved in cell invasion by various pathogens (8, 38). To test the possibility that this internalization route might contribute to the uptake of abnormal PrP, we performed the uptake assay in the presence of amiloride, an inhibitor of macropinocytosis (45). Rov cells were pretreated with amiloride (3 mM) for 1 h (see Materials and Methods). Infected brain homogenate then was added for 30 min, and the amount of PrPSc taken up was quantified by immunoblotting. As a result, amiloride treatment did not affect the uptake of abnormal PrP (data not shown). Therefore, the mode of internalization of PrPSc in Rov cells remains to be determined.
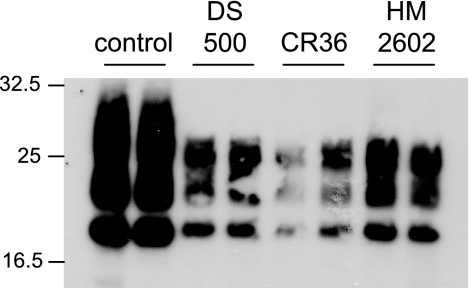
To determine if heparan sulfates could affect transmission of infection to Rov cells, cultures were exposed to the infectious inoculum in the presence of DS500, CR36, and HM20602 (25 μg/ml). After the inoculum and the heparan sulfate molecules were washed out, the treated cultures were grown in regular cell culture medium for 1 week to detect accumulation of cell-derived abnormal PrP. Figure 6 shows that the presence of DS500, CR36, and HM20602 at the time of exposure of the cells to prions strongly inhibits infection of Rov cells. From these data it was concluded that sulfated glycans do not have a detectable effect on the uptake of PrPsc but strongly inhibit subsequent infection of Rov cells.
FIG. 6.
Sulfated glycans inhibit Rov cell infection. Duplicate dox-treated Rov cultures were left untreated or were incubated with the indicated molecules (25 μg ml−1) for 24 h. Infectious brain homogenate (0.25%) was added to the cells, which were further incubated for 18 h in the presence of the molecules. After removal of the inoculum and of the tested molecules, the cultures were passaged and further grown for 7 days in dox-containing fresh medium. The cells then were lyzed, digested with PK, and analyzed by immunoblotting for PrPres. These data are representative of four experiments. The positions of molecular mass marker proteins are indicated (in kilodaltons).
DISCUSSION
Biochemical analyses performed in this study showed that acquisition of abnormal PrP by epithelial Rov cells is an active and a selective process, the efficiency of which can depend on the prion strain. Among the potential cellular receptors that could interact with and promote the entry of PrPSc, a prime candidate is the cellular form of PrPc (6), which is constitutively endocytosed from the cell surface to intracellular compartments (15, 28). The use of the PrPc-inducible Rov cell system allowed us to formally demonstrate that the presence of PrPc at the cell surface is not required for internalization of abnormal PrP. Similar conclusions were recently obtained with nonpermissive CHO cells (16) and primary cultured neurons (22), therefore indicating that PrPc is not an obligatory receptor for the entry of PrPSc in various cell types.
Recent studies have provided evidence that cellular heparan sulfates and/or the laminin receptor LRP/LR may be a cellular receptor for abnormal PrP. Cellular heparan sulfates are sulfated linear polysaccharides, typically linked through serine residues to proteins to form heparan sulfate proteoglycans located at the cell surface (40). Heparan sulfate proteoglycans bind numerous extracellular proteins and regulate a wide range of cellular functions (4), and they also have been shown to mediate the internalization of proteins and pathogens (21, 31, 32). The enzymatic removal of cell surface heparan sulfates (17), the inhibition of their sulfation by chlorate (17), or competition with heparin (16) and heparan mimetic agents (17, 35) prevented the binding and the uptake of detergent-extracted, aggregated forms of abnormal PrP in N2a, GT1, and CHO cells, providing evidence that cellular heparan sulfates might be involved in the entry of aggregated PrPSc in the cells. The laminin receptor LRP/LR also might mediate internalization of PrPSc, as PrPSc uptake can be partly inhibited in Caco-2/TC7 cells by W3 anti-LRP/LR antibody (25), while it is stimulated in LRP/LR-transfected BHK21 cells (13). LRP/LR is a heparan sulfate binding molecule (14, 18), and, interestingly, LRP/LR-mediated uptake of PrPSc in BHK21 cells is inhibited by sulfated glycans (13). This suggested that the LRP/LR and cellular heparan sulfates participate in PrPSc internalization through the same pathway. Our study, which failed to find evidence that the efficient entry of PrPSc in Rov cells is mediated by cellular heparan sulfates, suggests that additional mechanisms are involved in the entry of abnormal PrP in the cells. Consistent with this possibility, LRP/LR-independent uptake of PrPSc has been observed recently in BHK21 cells (13). One possible explanation is that internalization routes vary depending on the cell type. Alternatively, the utilization of heparan sulfates as opposed to that of other macromolecules as a receptor might depend on the biochemical state of abnormal PrP. Indeed, PrPres obtained by detergent extraction of infected tissues in combination with proteolysis (such as that used previously [17]) results in the formation of fibrils, while membrane-associated PrPSc (as in the brain homogenates prepared in physiological buffers used in the present study) forms amorphous aggregates (23). The finding that large aggregates of purified PrPres or abnormal PrP isolated by sodium phosphotungstic acid precipitation infect target SN56 cells much less efficiently than homogenized tissue (2) can be viewed as an indication that detergent-extracted, purified PrPres and membrane-associated forms of abnormal PrP interact differently with the cells.
We show that abnormal PrP taken up in Rov cells remained undegraded for days. Using a different experimental approach, similar conclusions recently were reported by Magalhães and coworkers (22), who showed that fluorescence-labeled aggregates of PrPres taken up by SN56 cells or primary neurons remained in a large set of intracellular vesicles, including late endosomes and lysosomes, for days. However, a salient finding in our study is that internalized PrPSc, while infectious for indicator mice (Fig. 3), does not necessarily initiate infection in the uptaking cells. Indeed, infection was impaired if PrPSc entered the cells, while PrPc was not expressed. In a previous study, we showed that exogenous prions preferentially infect Rov cells through the apical membrane, where PrPc is localized (27). Other studies on N2a neuroblastoma cells have shown that removal of PrPc from the plasma membrane precludes the initiation of infection (11). Taken together, these observations indicate that PrPc expression has to meet precise temporal and spatial requirements at the early steps for successful prion infection, and they strengthen the view that the presence of PrP at the cell surface is crucial for the establishment of infection. One possibility is that PrPc conversion occurs primarily or at least is initiated at the plasma membrane. The finding that prions bound to physical supports, such as stainless steel wires, can transmit infection to N2a cells (44) also supports the cell surface as a possible conversion site. However, other scenarios can be envisioned to explain why intracellular PrPSc taken up in the absence of PrPc is unable to infect Rov cells. The plasma membrane may be a meeting place required for the formation of complexes between PrPSc, PrPc, and other putative cofactors, such as cellular heparan sulfates (17), but complexes could be driven to subcellular compartments permissive to conversion. Further work will be necessary to fully characterize the different internalization routes of PrPSc and to clarify their biological significance regarding the initiation of cell infection.
Acknowledgments
We thank D. Papy-Garcia (CRRET, CNRS FRE24-12, Créteil, France) for providing us with the heparan mimetic agents. We also thank S. Hawke and J. Collinge (Imperial College, London, United Kingdom) for ICSM18 and ICSM35 MAbs and J. Grassi (Service de Pharmacologie et Immunologie, Commissariat à l'Energie Atomique, Saclay, France) for 4F2 and Sha31 MAbs.
S.P. was supported by a fellowship from INRA, the Ile de France region, and by the Fondation pour la Recherche Médicale. N.D. and M.-P.C. were partially supported by a grant from the European Union (QLRI 81833).
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Baeuerle, P. A., and W. B. Huttner. 1986. Chlorate—a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 141:870-877. [DOI] [PubMed] [Google Scholar]
- 2.Baron, G. S., A. C. Magalhaes, M. A. Prado, and B. Caughey. 2006. Mouse-adapted scrapie infection of SN56 cells: greater efficiency with microsome-associated versus purified PrP-res. J. Virol. 80:2106-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringue, V., G. Mallinson, M. Kaisar, M. Tayebi, Z. Sattar, G. Jackson, D. Anstee, J. Collinge, and S. Hawke. 2003. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain 126:2065-2073. [DOI] [PubMed] [Google Scholar]
- 4.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 5.Büeler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 6.Caughey, B., and G. S. Baron. 2006. Prions and their partners in crime. Nature 443:803-810. [DOI] [PubMed] [Google Scholar]
- 7.Collinge, J. 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24:519-550. [DOI] [PubMed] [Google Scholar]
- 8.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 9.Daude, N., M. Marella, and J. Chabry. 2003. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J. Cell Sci. 116:2775-2779. [DOI] [PubMed] [Google Scholar]
- 10.Dulce Papy-Garcia, V. B.-C., V. Rouet, M.-E. Kerros, C. Klochendler, M.-C. Tournaire, D. Barritault, J.-P. Caruelle, and E. Petit. 2005. Nondegradative sulfation of polysaccharides, synthesis and structure characterization of biologically active heparan sulfate mimetics. Macromolecules 38:4647-4654. [Google Scholar]
- 11.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feraudet, C., N. Morel, S. Simon, H. Volland, Y. F. Frobert, C. Creminon, D. Vilette, S. Lehmann, and J. Grassi. 2004. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247-11258. [DOI] [PubMed] [Google Scholar]
- 13.Gauczynski, S., D. Nikles, S. El-Gogo, D. Papy-Garcia, C. Rey, S. Alban, D. Barritault, C. I. Lasmezas, and S. Weiss. 2006. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycans. J. Infect. Dis. 194:702-709. [DOI] [PubMed] [Google Scholar]
- 14.Guo, N. H., H. C. Krutzsch, T. Vogel, and D. D. Roberts. 1992. Interactions of a laminin-binding peptide from a 33-kDa protein related to the 67-kDa laminin receptor with laminin and melanoma cells are heparin-dependent. J. Biol. Chem. 267:17743-17747. [PubMed] [Google Scholar]
- 15.Harris, D. A. 2003. Trafficking, turnover and membrane topology of PrP. Br. Med. Bull. 66:71-85. [DOI] [PubMed] [Google Scholar]
- 16.Hijazi, N., Z. Kariv-Inbal, M. Gasset, and R. Gabizon. 2005. PrPSc incorporation to cells requires endogenous glycosaminoglycan expression. J. Biol. Chem. 280:17057-17061. [DOI] [PubMed] [Google Scholar]
- 17.Horonchik, L., S. Tzaban, O. Ben-Zaken, Y. Yedidia, A. Rouvinski, D. Papy-Garcia, D. Barritault, I. Vlodavsky, and A. Taraboulos. 2005. Heparan sulfate is a cellular receptor for purified infectious prions. J. Biol. Chem. 280:17062-17067. [DOI] [PubMed] [Google Scholar]
- 18.Kazmin, D. A., T. R. Hoyt, L. Taubner, M. Teintze, and J. R. Starkey. 2000. Phage display mapping for peptide 11 sensitive sequences binding to laminin-1. J. Mol. Biol. 298:431-445. [DOI] [PubMed] [Google Scholar]
- 19.Krasemann, S., M. Groschup, G. Hunsmann, and W. Bodemer. 1996. Induction of antibodies against human prion proteins (PrP) by DNA-mediated immunization of PrP0/0 mice. J. Immunol. Methods 199:109-118. [DOI] [PubMed] [Google Scholar]
- 20.Le Dur, A., V. Beringue, O. Andreoletti, F. Reine, T. L. Lai, T. Baron, B. Bratberg, J. L. Vilotte, P. Sarradin, S. L. Benestad, and H. Laude. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc. Natl. Acad. Sci. USA 102:16031-16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, J., and S. C. Thorp. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1-25. [DOI] [PubMed] [Google Scholar]
- 22.Magalhães, A. C., G. S. Baron, K. S. Lee, O. Steele-Mortimer, D. Dorward, M. A. Prado, and B. Caughey. 2005. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 25:5207-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinley, M. P., R. K. Meyer, L. Kenaga, F. Rahbar, R. Cotter, A. Serban, and S. B. Prusiner. 1991. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J. Virol. 65:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra, R. S., S. Basu, Y. Gu, X. Luo, W. Q. Zou, R. Mishra, R. Li, S. G. Chen, P. Gambetti, H. Fujioka, and N. Singh. 2004. Protease-resistant human prion protein and ferritin are cotransported across Caco-2 epithelial cells: implications for species barrier in prion uptake from the intestine. J. Neurosci. 24:11280-11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morel, E., T. Andrieu, F. Casagrande, S. Gauczynski, S. Weiss, J. Grassi, M. Rousset, D. Dormont, and J. Chambaz. 2005. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am. J. Pathol. 167:1033-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paquet, S., C. Langevin, J. Chapuis, G. S. Jackson, H. Laude, and D. Vilette. 2007. Efficient dissemination of prions through preferential transmission to nearby cells. J. Gen. Virol. 88:706-713. [DOI] [PubMed] [Google Scholar]
- 27.Paquet, S., E. Sabuncu, J. L. Delaunay, H. Laude, and D. Vilette. 2004. Prion infection of epithelial Rov cells is a polarized event. J. Virol. 78:7148-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado, M. A., J. Alves-Silva, A. C. Magalhaes, V. F. Prado, R. Linden, V. R. Martins, and R. R. Brentani. 2004. PrPc on the road: trafficking of the cellular prion protein. J. Neurochem. 88:769-781. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 30.Raeber, A. J., A. Sailer, I. Hegyi, M. A. Klein, T. Rulicke, M. Fischer, S. Brandner, A. Aguzzi, and C. Weissmann. 1999. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication. Proc. Natl. Acad. Sci. USA 96:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiland, J., and A. C. Rapraeger. 1993. Heparan sulfate proteoglycan and FGF receptor target basic FGF to different intracellular destinations. J. Cell Sci. 105:1085-1093. [DOI] [PubMed] [Google Scholar]
- 32.Rostand, K. S., and J. D. Esko. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybner-Barnier, C., C. Jacquemot, C. Cuche, G. Dore, L. Majlessi, M. M. Gabellec, A. Moris, O. Schwartz, J. Di Santo, A. Cumano, C. Leclerc, and F. Lazarini. 2006. Processing of the bovine spongiform encephalopathy-specific prion protein by dendritic cells. J. Virol. 80:4656-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabuncu, E., S. Petit, A. Le Dur, T. Lan Lai, J. L. Vilotte, H. Laude, and D. Vilette. 2003. PrP polymorphisms tightly control sheep prion replication in cultured cells. J. Virol. 77:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schonberger, O., L. Horonchik, R. Gabizon, D. Papy-Garcia, D. Barritault, and A. Taraboulos. 2003. Novel heparan mimetics potently inhibit the scrapie prion protein and its endocytosis. Biochem. Biophys. Res. Commun. 312:473-479. [DOI] [PubMed] [Google Scholar]
- 36.Silveira, J. R., G. J. Raymond, A. G. Hughson, R. E. Race, V. L. Sim, S. F. Hayes, and B. Caughey. 2005. The most infectious prion protein particles. Nature 437:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solassol, J., C. Crozet, and S. Lehmann. 2003. Prion propagation in cultured cells. Br. Med. Bull. 66:87-97. [DOI] [PubMed] [Google Scholar]
- 38.Swanson, J. A., and C. Watts. 1995. Macropinocytosis. Trends Cell Biol. 5:424-428. [DOI] [PubMed] [Google Scholar]
- 39.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbull, J., A. Powell, and S. Guimond. 2001. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11:75-82. [DOI] [PubMed] [Google Scholar]
- 41.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilotte, J. L., S. Soulier, R. Essalmani, M. G. Stinnakre, D. Vaiman, L. Lepourry, J. C. Da Silva, N. Besnard, M. Dawson, A. Buschmann, M. Groschup, S. Petit, M. F. Madelaine, S. Rakatobe, A. Le Dur, D. Vilette, and H. Laude. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissmann, C. 2004. The state of the prion. Nat Rev. Microbiol. 2:861-871. [DOI] [PubMed] [Google Scholar]
- 44.Weissmann, C., M. Enari, P. C. Klohn, D. Rossi, and E. Flechsig. 2002. Transmission of prions. J. Infect. Dis. 186(Suppl. 2):S157-S165. [DOI] [PubMed] [Google Scholar]
- 45.West, M. A., M. S. Bretscher, and C. Watts. 1989. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]