Abstract
A more or less pronounced resistance to superinfection by a second strain of the infecting virus has been observed in many lentivirus-infected hosts. We used a chimeric feline immunodeficiency virus (FIV), designated FIVχ, containing a large part of the env gene of a clade B virus (strain M2) and all the rest of the genome of a clade A virus (a p34TF10 molecular clone of the Petaluma strain modified to grow in lymphoid cells), to gain insights into such resistance. FIVχ was infectious and moderately pathogenic for cats and in vitro exhibited the neutralization specificity of the env donor. The experiments performed were bidirectional, in that cats preinfected with either parental virus were challenged with FIVχ and vice versa. The preinfected animals were partially or completely protected relative to what was observed in naïve control animals, most likely due, at least in part, to the circumstance that in all the preinfecting/challenge virus combinations examined, the first and the second virus shared significant viral components. Based on the proportions of complete protection observed, the role of a strongly matched viral envelope appeared to be modest and possibly dependent on the time interval between the first and the second infection. Furthermore, complete protection and the presence of measurable neutralizing antibodies capable of blocking the second virus in vitro were not associated.
Recent reportsman immunodeficiency virus type 1 (HIV-1)-infected individuals can become superinfected with a second strain of the virus more frequently than previously estimated (1, 9, 26, 28, 46, 50). On the other hand, there is also convincing evidence that an established HIV-1 infection may confer on the host the ability to ward off acquisition of another HIV-1, especially if it belongs to the same clade as the initial virus and if exposure occurs after a sufficient time interval from the first infection (reviewed in references 8 and 50). The latter evidence is corroborated by studies with several animal lentiviruses, including simian immunodeficiency virus (SIV) in macaques, equine infectious anemia virus in ponies, and feline immunodeficiency virus (FIV) in cats, showing that prior infection with live attenuated and even wild-type viruses can prevent subsequent infection by fully virulent strains of the same viruses or at least afford substantial protection against their pathological effects (10, 11, 13, 18, 38 reviewed in reference 17). The mechanisms mediating such protection are, however, unresolved in both the primate and nonprimate systems (4, 27, 29, 32, 33, 55). The issue is important, since a clear understanding of the nature of these mechanisms might help in identifying immune correlates of protection against lentiviruses. That such correlates have so far remained elusive is considered a major obstacle to development and testing of candidate AIDS vaccines (2, 7, 21, 22, 37).
FIV is both a significant pathogen of domestic cats (42) and a widely used model to investigate HIV pathogenesis and approaches to AIDS vaccination (14, 16, 51, 54). In the present study, we developed a chimeric FIV, designated FIVχ, having most of the env gene of a clade B virus and the rest of the genome of a clade A virus and used it in an attempt to gain insight into the variables that may affect the resistance to superinfection by a second strain of virus in this system, including the neutralization specificity of the viral envelope (Env).
MATERIALS AND METHODS
Animals, cells, and viruses.
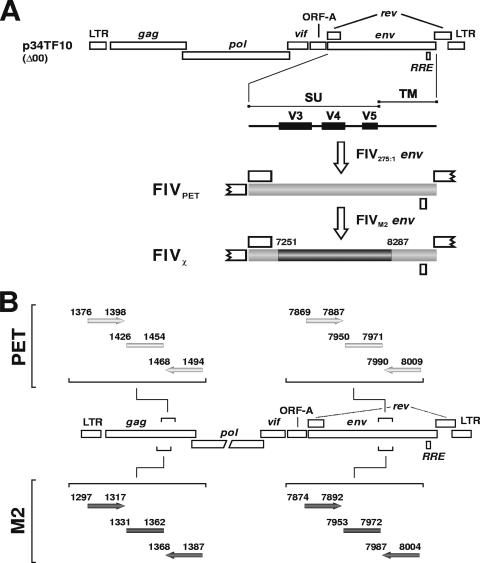
Specific-pathogen-free (SPF) female domestic cats, purchased from Iffa Credo (L'Arbresle, France) when they were 7 months old, were housed individually in our climate-controlled animal facility and had ad libitum access to fresh water and a proprietary brand of cat food in accordance with European Community guidelines. They ranged between 36 and 72 months of age when enrolled in the experiments. MBM cells are an interleukin 2 (IL-2)-dependent line of T lymphocytes originally established from the peripheral blood mononuclear cells (PBMC) of an FIV- and feline leukemia virus-negative cat. They are routinely grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 5 μg of concanavalin A (Sigma-Aldrich, Milan, Italy), and 20 U of human recombinant IL-2 (Roche Diagnostics, Monza, Italy) per ml. Preinfection of the cats was carried out by intravenous (i.v.) administration of 1 ml of the indicated dilution of virus. The stocks of FIVPET and FIVM2 used for this purpose were pooled cell-free plasma from acutely infected cats titrated in 50% cat infectious doses (CID50) as previously described (36, 43). The chimeric virus, designated FIVχ (Fig. 1A), was obtained starting from clone p34TF10, which carries the whole genome of FIVPET (GenBank accession no. NC_001482) (53). First, the stop codon in the open reading frame A accessory gene of p34TF10, which impairs the capability of this clone to grow in lymphoid cells, was removed by site-specific mutagenesis (43). Second, in an attempt to increase in vivo fitness, the entire env gene was replaced with that of an FIVPET reisolated from an experimentally infected cat (FIV275:1 [3]). Third, most of the env of the latter clone (amino acid positions 329 to 673) was replaced with the corresponding sequence amplified from ex vivo FIVM2. The viral stock was finally obtained by transfecting Crandell feline kidney cells and expanding the progeny virus in feline T-lymphoid MBM cells (3). Proper insertion and the absence of unwanted mutations were checked by sequencing the entire env gene exactly as described previously (44). Upon characterization, FIVχ proved readily infectious for SPF cats, where it produced a florid acute-phase infection and a clearly evident depletion of circulating CD4+ T lymphocytes (data not shown; see below).
FIG. 1.
Schematic representation of the chimeric virus FIVχ and of how it was prepared, starting from the parental viruses FIVPET and FIVM2. The locations of the primers used for the PCR assays in Fig. 2 are also shown.
Virus challenges.
The viral stocks used for the challenges consisted of the same frozen pooled plasma preparations from acutely infected SPF cats (FIVM2 and FIVPET) and supernatant of acutely infected MBM cells (FIVχ) used for the preinfections. The challenges were performed under slight anesthesia. The i.v. challenge was carried out by injecting 1 ml of the virus preparations diluted to contain the indicated dose. The mucosal inoculum was deposited onto the anterior vagina in 100 μl pyrogen-free saline using smooth pipette tips. No discharge from the vagina was observed after the inoculum was injected.
Quantitation and discrimination of the viruses replicating in infected cats.
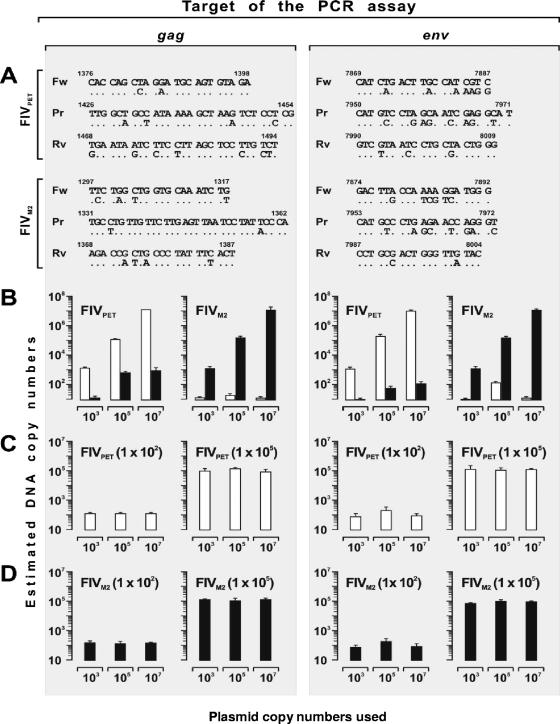
To quantitate and genotype the FIV(s) growing in the study cats, four gag- and env-based real-time PCR and reverse transcription (RT)-PCR assays were developed, which made use of the primers and probes shown in Fig. 1B. After preliminary experiments using plasmid DNA as standards, the following assay conditions were adopted: reaction mixture, 25-μl final volume containing 5 μl of either genomic DNA (200 to 500 ng) or cDNA, 100 nM probe, and 300 to 900 nM primers. The thermal cycling profile was as follows: 50°C, 2 min; 95°C, 10 min; 95°C, 15 s; and 60°C, 1 min (50 cycles) on the ABI Prism 7700 Sequence Detection System instrument (Applied Biosystems, Monza, Italy). The selectivity of these assays for the specific FIV targeted, albeit not absolute, was considered sufficient for the purposes of the study. Indeed, as shown by Fig. 2, the proviral and viral loads detected by measuring DNA or RNA of the homologous FIV with the gag-based assays were at least 2 log units higher than those detected by measuring DNA of the heterologous virus, regardless of the ratio between the two DNAs, and this differential was at least 3 log units with the env-based assays, which exploited the greater diversity existing between FIVPET and FIVM2 in this gene relative to gag (23.8%, with peaks of 36.4% in the variable regions targeted, versus 17.7%). For proviral-load quantitation, genomic DNA was extracted from PBMC or lymphoid tissues using the QIAamp DNA Blood Mini Kit (QIAGEN, Milan, Italy). The extracted DNA was then amplified in parallel with serial 10-fold dilutions (101 to 107) of the corresponding DNA plasmid standards diluted in 1 μg of genomic DNA. The sensitivity of the PCR assays was 50 copies per μg of genomic DNA, regardless of the target. For plasma viral-load quantitation, RNA was extracted from EDTA-collected blood with the QIAamp Viral RNA kit (QIAGEN) and reverse transcribed with reverse primer, and the cDNA was amplified in parallel with serial 10-fold dilutions (102 to 107) of the corresponding RNA transcripts. As evaluated by extracting and amplifying FIV-negative plasma spiked with serial 10-fold dilutions of RNA transcripts, the sensitivity of RT-PCR assays was 200 copies per ml, regardless of the target. Precautions to avoid misquantitation and false-positive and -negative results have been previously described (44).
FIG. 2.
Features of the PETgag-, PETenv-, M2gag-, and M2env-specific real-time PCR assays used in the study. (A) Forward primers (Fw), probes (Pr), and reverse primers (Rv) were designed based upon appropriately studied segments of gag or env of FIVPET or FIVM2 using Primer Express software (version 1.5; Applied Biosystems, Monza, Italy) and differed from the heterologous virus, as indicated under each oligonucleotide (nucleotide positions are based on FIVPET clone p34TF10, GenBank accession number NC_001482) (53). Note that in order to improve selectivity and fulfill real-time probe requirements, the M2gag primers and probe were designed from the antisense strand. (B to D) Selectivity of the PET-specific (open bars) and M2-specific (closed bars) assays, as determined by quantitating FIVPET and FIVM2 plasmids; the DNAs examined were FIVPET plasmids alone or FIVM2 plasmids alone (indicated at the tops of the graphs) at the copy numbers shown in the abscissa (B), 1 × 102 or 1 × 105 FIVPET plasmids (indicated at the tops of the graphs) mixed with the numbers of FIVM2 plasmids shown in the abscissa (C), and 1 × 102 or 1 × 105 FIVM2 plasmids (indicated at the tops of the graphs) mixed with the numbers of FIVPET plasmids shown in the abscissa (D). These degrees of selectivity were conserved in the RT-PCR assays, as determined by performing similar experiments with RNA transcribed on the viral plasmids (data not shown). The error bars represent standard deviations from three independent experiments.
Virus isolation and infectious-cell enumeration.
For FIV reisolation from infected cells, 106 Ficoll-Paque gradient-purified PBMC were stimulated with concanavalin A and cocultured with 106 MBM cells in RPMI 1640 medium containing 10% fetal bovine serum and 20 U/ml human recombinant IL-2. The culture supernatants were monitored biweekly for p25 production by enzyme-linked immunosorbent assay (ELISA) for up to 5 weeks. Quantitation of circulating infectious units was performed exactly as described above, except that the cocultures were established with 10-fold serial dilutions of the PBMC under scrutiny.
Serology.
Plasma was tested for antibodies against whole FIVPET antigen by ELISA as described previously (36). Plasma specimens found to be reactive at dilutions of 1:100 or greater were considered antibody positive. Neutralizing antibodies (NA) were determined against 10 50% tissue culture infective doses (TCID50) of the appropriate FIV by using MBM cells as indicator cells and quantitation of reverse transcriptase activity in the supernatant as an end point readout, exactly as described previously (19).
Hematology and lymphocyte subset analyses.
Complete blood counts and differential leukocyte counts were performed by standard methods. CD4+ and CD8+ T-cell counts were determined by flow cytometry using a FACScan (BD Biosciences-Life Science Research, Milan, Italy).
RESULTS
FIVχ challenge of cats preinfected with the parental viruses.
Two groups of five 72- and 57-month-old cats preinfected with 5 to 10 CID50 of FIVPET or FIVM2 34 and 36 months earlier, respectively, were used in this part of the study. Consistent with what is known about long-term steady-state FIV infections (51), the animals had moderate (FIVM2-preinfected) to low (FIVPET-preinfected) viral and proviral loads and markedly reduced circulating CD4+ T-cell counts (Table 1). Of note, the results of prechallenge monitoring corroborated the exquisite selectivity demonstrated by the assays used to differentiate/quantitate the infecting viruses during characterization (see Materials and Methods), since the animals consistently tested positive only if they had been infected with the FIV strain targeted by the assays (Fig. 3B and C).
TABLE 1.
Parameters of infection in study cats at times of challenge
| Preinfecting virus | Cat no. | Age at challenge (mo) | RNA copies/ml of plasmaa | DNA copies/mg of PBMC DNAb | Infectious units in 106 PBMC | CD4+ T lymphocytes/ml of bloodc |
|---|---|---|---|---|---|---|
| FIVPET | 2682 | 72 | 2,000 | 3,000 | 1 | 690 |
| 3552 | 72 | 6,100 | 4,200 | 10 | 200 | |
| 3608 | 72 | 3,300 | 7,600 | 1 | 300 | |
| 3633 | 72 | 11,300 | 1,900 | 10 | 440 | |
| 3646 | 72 | 17,900 | 10,000 | 1 | 398 | |
| FIVM2 | 2646 | 57 | 2,100 | 34,100 | 10 | 250 |
| 2647 | 57 | 5,000 | 62,000 | 100 | 210 | |
| 2658 | 57 | 9,000 | 84,700 | 100 | 326 | |
| 2664 | 57 | 20,000 | 87,600 | 100 | 230 | |
| 2668 | 57 | 28,000 | 98,300 | 100 | 408 | |
| FIVχd | 2839 | 36 | 6,000 | 2,600 | 10 | 800 |
| 2841 | 36 | 700 | 900 | 1 | 910 | |
| 2893 | 36 | 3,000 | 800 | 10 | 870 | |
| 3335 | 36 | 3,200 | 1,000 | 1 | 980 | |
| 3437 | 36 | 900 | 1,900 | 1 | 700 | |
| 2731 | 36 | 1,400 | 800 | 10 | 700 | |
| 2881 | 36 | 800 | 700 | 1 | 700 | |
| 2884 | 36 | 6,500 | 5,100 | 1 | 800 | |
| 2897 | 36 | 800 | 2,000 | 10 | 500 | |
| 3316 | 36 | 2,000 | 3,000 | 1 | 760 |
RNA copies determined with the appropriate RT-PCR-specific assay.
DNA copies determined with the appropriate PCR-specific assay.
Normal range, 1,000 to 3,500.
The first five animals in this group were challenged with FIVPET, the others with FIVM2.
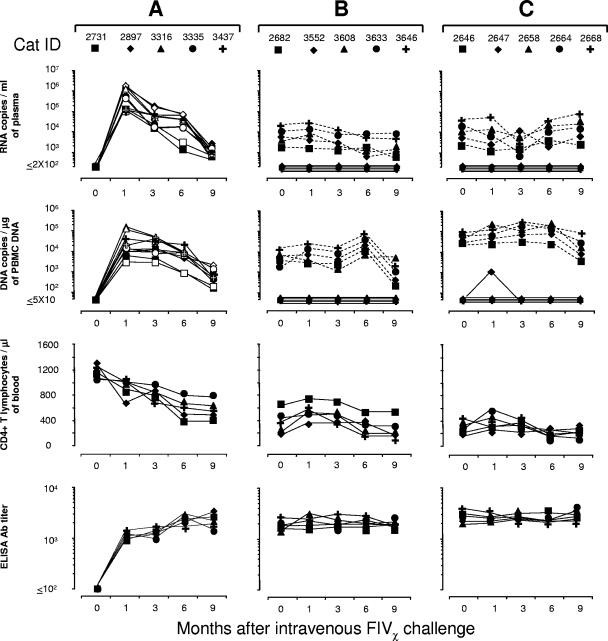
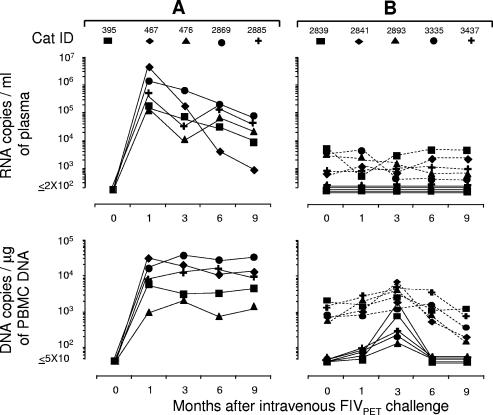
FIG. 3.
Set points in FIVPET- or FIVM2-preinfected cats and naïve controls at various times after i.v. challenge with FIVχ. (A) Naïve cats. FIVχ plasma viremia and proviral loads were determined with both the PETgag-specific assay (empty symbols) and the M2env-specific assay (solid symbols). Note that cats in this age range (27 months at challenge) showed no major spontaneous CD4+ T-lymphocyte count changes. (B) FIVPET-preinfected cats. FIVPET (dashed lines) and FIVχ (continuous lines) plasma viremia and proviral loads determined with the PETenv- and M2env-specific assays, respectively. (C) FIVM2-preinfected cats. FIVM2 (dashed lines) and FIVχ (continuous lines) plasma viremia and proviral loads determined with the M2gag- and PETgag-specific assays, respectively. Anti-FIV antibodies were determined by ELISA using whole FIV antigen.
(i) Systemic challenge.
The above-mentioned animals and five uninfected cats were injected i.v. with 20 TCID50 of FIVχ and then monitored for 9 months. Postchallenge (PC), all the controls developed sustained infections by all the parameters measured, including a progressive decline in CD4+ T-lymphocyte counts, which by the end of follow-up were approximately halved. Importantly, the kinetics and levels of plasma viremia and PBMC proviral loads of these animals were similar independently of the PETgag or M2env specificity of the assay used to monitor FIVχ infection, confirming the comparable performance characteristics of these assays (Fig. 3A).
In the preinfected cats (Fig. 3B and C), FIVχ challenge produced no appreciable changes in the preexisting infection set points. Furthermore, only one preinfected animal showed traces of the chimeric virus in the bloodstream. This was cat no. 2647, preinfected with FIVM2, which, although constantly negative for FIVχ in plasma, reacted transiently positive for the chimeric provirus in the PBMC 1 month PC. The other nine preinfected animals presented no evidence of FIVχ in the circulation, as shown by uniformly negative FIVM2- and FIVPET-specific PCR and RT-PCR assays in the FIVPET-preinfected cats and in the FIVM2-preinfected cats, respectively, throughout the follow-up. Also, preexisting anti-FIV ELISA antibody titers, CD4+ T-cell counts (Fig. 3B and C), and infectious-cell loads in the PBMC (data not shown) underwent no appreciable variations PC.
(ii) Mucosal challenge.
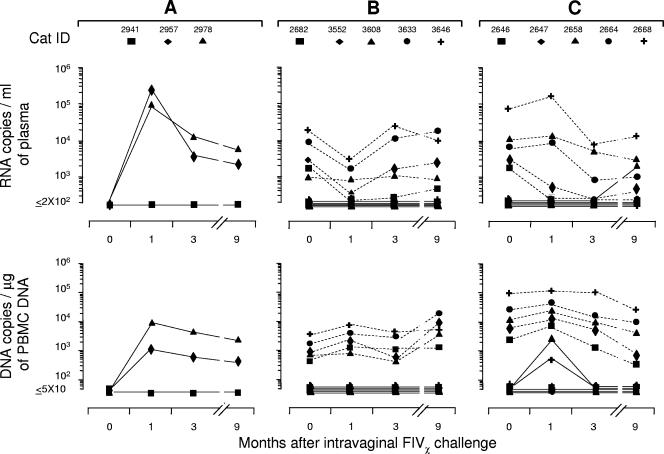
Compared to systemic challenge, mucosal FIV infections may be more difficult to control immunologically (5, 6, 39, 40, 44). To assess how the animals preinfected with the parental viruses dealt with mucosal challenge with the chimera, after follow-up of the systemic challenge described above was terminated, all the FIVPET- and FIVM2-preinfected cats were exposed intravaginally to 100 TCID50 of FIVχ and monitored for an additional 9 months. While two of three age-matched uninfected cats used as controls became readily FIVχ infected (Fig. 4A), none of the FIVPET-preinfected cats yielded FIVχ RNA or provirus in peripheral blood throughout the follow-up (Fig. 4B). In contrast, among the FIVM2-preinfected cats, two (no. 2658 and 2668) yielded the FIVχ provirus in PBMC at month 1 PC, and one of these (no. 2658) was also positive for FIVχ RNA in plasma at month 9 PC (Fig. 4C).
FIG. 4.
Viral set points in FIVPET- or FIVM2-preinfected cats and naïve control cats at various times after intravaginal challenge with FIVχ. (A) Naïve cats. FIVχ plasma viremia and proviral loads determined with the M2env-specific assay. (B) FIVPET-preinfected cats. FIVPET (dashed lines) and FIVχ (continuous lines) plasma viremia and proviral loads determined with the PETenv- and M2env-specific assays, respectively. (C) FIVM2-preinfected cats. FIVM2 (dashed lines) and FIVχ (continuous lines) plasma viremia and proviral loads determined with the M2gag- and PETgag-specific assays, respectively.
(iii) Detection of the challenge virus in the lymphoid organs.
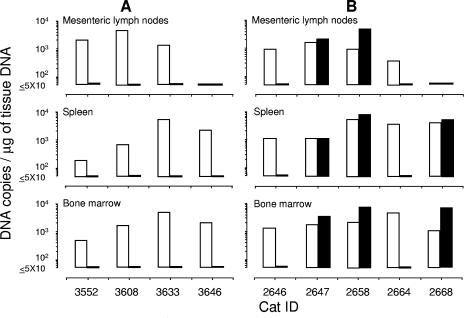
At the end of the mucosal challenge experiment described above, four cats in the FIVPET-preinfected group (one animal in this group could not be examined because it was mistakenly given for adoption to a volunteer) and all five FIVM2-preinfected cats were euthanized, and their mesenteric lymph nodes, spleen, and bone marrow were tested with the appropriate PCR assays. As shown by Fig. 5A, the animals preinfected with FIVPET showed generally high levels of the preinfecting virus in all or most tissues examined but no evidence of FIVχ provirus. On the other hand, three animals preinfected with FIVM2 yielded both the preinfecting virus and the FIVχ provirus at comparable copy numbers per μg of extracted tissue DNA (Fig. 5B). Of these, one (no. 2647) had tested transiently FIVχ positive in the circulation after the first challenge and two after the second challenge (no. 2658 and 2668).
FIG. 5.
Proviral loads of preinfecting viruses and challenge FIVχ in selected tissues of cats at the end of the experiment shown in Fig. 4. (A) FIVPET-preinfected cats examined with the M2env- and PETenv-specific assays. (B) FIVM2-preinfected cats examined with the M2gag- and PETgag-specific assays. Open bars, preinfecting viruses; closed bars, FIVχ.
(iv) NA at the challenges.
Sera taken at the time of systemic challenge, from the FIVPET- and FIVM2-preinfected cats were tested for NA to the chimera, as well as the parental viruses, in standard neutralization assays using lymphoid cells as a substrate. FIVχ-neutralizing activity was detected in three FIVM2-preinfected cats, two of which also possessed NA for the homologous virus, and in one FIVPET-preinfected animal, who instead invariably had NA for the homologous virus. Consistent with previous findings (12), essentially no cross-neutralization of FIVPET and FIVM2 was observed (Table 2). The tests were repeated at mucosal challenge to assess possible changes relative to the above-mentioned NA status resulting from the prior systemic challenge, but no changes were detected (data not shown).
TABLE 2.
Virus NA in day-of-challenge sera of the preinfected cats
| Preinfecting virus | Cat no. | NA titer against indicated virus
|
Challenge virus | Complete protectiona | ||
|---|---|---|---|---|---|---|
| FIVPET | FIVM2 | FIVχ | ||||
| FIVPET | 2682 | 64 | — | — | FIVχ | Yes |
| 3552 | 64 | — | — | Yes | ||
| 3608 | 128 | — | — | Yes | ||
| 3633 | 128 | — | — | Yes | ||
| 3646 | 128 | — | 16 | Yes | ||
| FIVM2 | 2646 | — | — | 16 | FIVχ | Yes |
| 2647 | — | — | — | No | ||
| 2658 | — | 32 | 128 | No | ||
| 2664 | — | — | — | Yes | ||
| 2668 | — | 32 | 256 | No | ||
| FIVχ | 2839 | — | 16 | 128 | FIVPET | No |
| 2841 | — | 16 | 32 | No | ||
| 2893 | — | 16 | 16 | No | ||
| 3335 | — | 32 | 64 | No | ||
| 3437 | — | 16 | 16 | No | ||
| 2731 | — | 32 | 32 | FIVM2 | Yes | |
| 2881 | — | 16 | 64 | Yes | ||
| 2884 | — | 64 | 128 | No | ||
| 2897 | — | 16 | 16 | No | ||
| 3316 | — | 16 | 16 | Yes | ||
As determined from the overall results obtained pre- and posteuthanasia. The efficiencies of the challenges were as follows: only 1 of 25 total naïve controls escaped infection. This was cat no. 2941 in the FIVχ mucosal challenge study shown in Fig. 4. —, <8.
Challenge of cats preinfected with FIVχ with the parental viruses.
The animals used in this part of the study were 36-month old cats that had been infected with FIVχ 10 months earlier. Five were the ones used as controls for systemic FIVχ challenge in the study described above, and five had been infected contemporaneously but had been left untreated. As expected, they were all positive by the PETgag- and PETenv- but not by the M2gag- and M2env-specific assays and had viral set points typical of a postacute FIV infection of moderate severity (Table 1 and Fig. 6B and 7B). These animals were sorted into two groups of five with comparable viral set points and used as reported below.
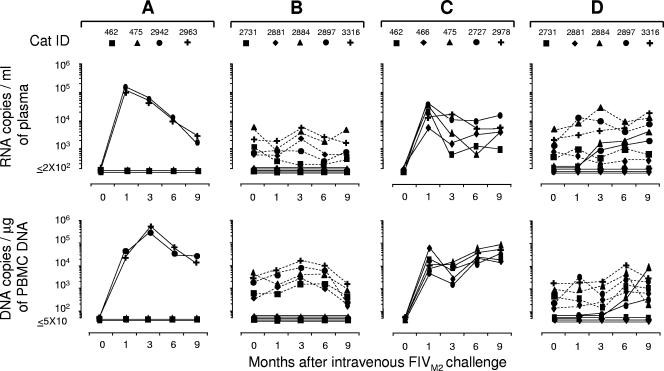
FIG. 6.
Viral set points in FIVχ-preinfected cats and naïve control cats after i.v. challenge with FIVPET. (A) Naïve cats. FIVPET plasma viremia and proviral loads determined with the PETenv-specific assay. (B) FIVχ-preinfected cats. FIVχ (dashed lines) and FIVPET (continuous lines) plasma viremia and proviral loads determined with the M2env- and PETenv-specific assays, respectively.
FIG. 7.
Viral set points in FIVχ-preinfected and naïve control cats after i.v. challenge with FIVM2. (A and B) First challenge with 10 CID50. (C and D) Second challenge with 30 CID50. (A and C) Naïve cats. FIVM2 plasma viremia and proviral loads determined with the M2gag-specific assay. (B and D) FIVχ-preinfected cats. FIVχ (dashed lines) and FIVM2 (continuous lines) plasma viremia and proviral loads determined with the M2gag- and PETgag-specific assays, respectively.
(i) Challenge with FIVPET.
In the first experiment, five FIVχ-preinfected cats and five virus-naïve age-matched controls were challenged i.v. with 10 CID50 of FIVPET and monitored for 9 months. PC, all the controls readily developed the expected plasma viremia and PBMC provirus loads (Fig. 6A). In the FIVχ-preinfected cats, the challenge failed to modify the preexisting plasma viremia with regard to both the viral-RNA load and the FIV strain detected throughout the follow-up. The numbers of infectious units found in the PBMC and FIV binding antibody titers also remained unchanged relative to what was observed prechallenge (data not shown); however, when observations were limited to 1 and 3 months PC, these cats underwent moderate to low elevations in the preexisting levels of FIVχ provirus in PBMC and showed clear evidence that the PBMC also harbored FIVPET (Fig. 6B).
(ii) Challenge with FIVM2.
The second group of FIVχ-preinfected cats and a group of four age-matched uninfected controls were inoculated i.v. with 10 CID50 of FIVM2. For 9 months PC, none of the FIVχ-preinfected cats showed evidence that FIVM2 was circulating in the blood, as indicated by consistently negative M2gag-specific PCR and RT-PCR (Fig. 7B). However, although a similar dose of virus had been fully effective at infecting younger cats in the previous experiment, the challenge was only partially successful, since two of four controls escaped infection, as revealed by persistently negative virological (Fig. 7A) and serological (data not shown) tests. For this reason, all the FIVχ-preinfected cats, along with the two controls that had escaped the infection, as described above, and three additional naïve cats, were inoculated i.v. with 30 CID50 of FIVM2. As shown by Fig. 7C, this second challenge with a higher viral dose readily infected all the controls. Conversely, the FIVχ-preinfected cats showed no substantial changes in preexisting plasma viremia and PBMC provirus loads, even after this robust challenge. However, two cats in the group (no. 2884 and 2897) yielded the challenge virus in plasma from month 3 PC onward, as well as the challenge provirus in PBMC starting from month 6 PC.
(iii) Detection of the challenge viruses in the lymphoid organs.
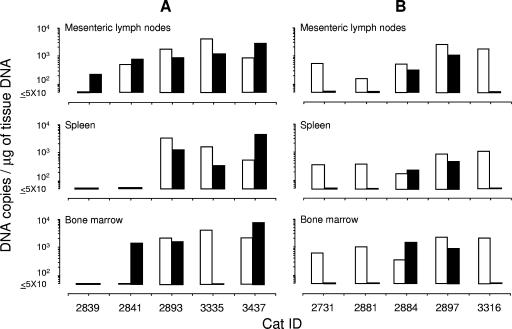
At the end of the follow-up, all of the cats preinfected with the chimera and challenged with FIVPET or FIVM2 were euthanized, and the viral contents in their mesenteric lymph nodes, spleens, and bone marrow were characterized. As shown by Fig. 8A, five of five FIVPET-challenged cats were found to carry the challenge virus in some or all the tissues examined, and in one (no. 2839), this was the only virus detected. Among the FIVM2-challenged cats, two of five tested positive for the challenge virus, and these were the same animals whose peripheral blood had tested positive (Fig. 8B). Interestingly, in a few instances, the proviral loads of the challenge viruses exceeded or even replaced those of FIVχ.
FIG. 8.
Proviral loads of the preinfecting FIVχ and of the challenge viruses in selected tissues of cats at the ends of the experiments shown in Fig. 6 and 7. (A) FIVPET-challenged cats examined with the M2env- and PETenv-specific assays. (B) FIVM2-challenged cats examined with the M2gag- and PETgag-specific assays. Open bars, FIVχ; closed bars, challenge viruses.
(iv) NA at the challenges.
Sera obtained at challenge from the FIVχ-preinfected cats were examined for NA. All had moderate to low titers of NA for both FIVχ and FIVM2, but none blocked FIVPET (Table 2).
DISCUSSION
The chimeric virus FIVχ was generated by replacing a large part of the Env of FIVPET (clade A), specifically, the part including the domains known to be involved in antibody-mediated neutralization (3, 35, 48, 49), with the corresponding region of FIVM2 (clade B). In previous superinfection studies, preinfection with FIVPET had exerted long-term beneficial effects against fully virulent FIVM2 (45) and significantly protected against a heterologous intraclade challenge given systemically or mucosally (44). Importantly, FIVχ was effectively neutralized by the sera of FIVM2-infected cats but very poorly or not at all by the sera of FIVPET-infected cats and elicited NA that neutralized itself and FIVM2 but not FIVPET, showing that in the new setting the transferred Env had conserved a great part, and possibly all, of its neutralization specificity. Furthermore, FIVχ readily infected naïve cats and, as judged by the depletion of circulating CD4+ T cells produced, was also moderately pathogenic.
FIVχ was used to investigate the role of Env compared to the other viral gene products in the resistance of lentivirus-infected hosts to superinfection by a second strain of the virus (8, 50) in two sets of experiments. In the first set, it served as a challenge for cats that had been infected with either parental virus approximately 3 years in advance, while in the second, it served to preinfect cats that 10 months later were challenged with either parental virus. The outcomes of the challenges were determined by monitoring the animals for 9 months by using appositely developed real-time PCR and RT-PCR assays in appropriate combinations that permitted the independent detection and quantitation of FIVχ and the parental viruses. Although we did not specifically look for the emergence of recombinant viruses in the cats that became superinfected, the results obtained in the assays targeting gag or env of the same viral strain were uniformly highly concordant, making it unlikely that recombinants were a major fraction of the total viral burden in such animals.
The overall results clearly demonstrated a general tendency of the preinfected cats to contain the challenge viruses: indeed, they were undetectable in the peripheral blood of many preinfected cats (Table 3) and, when detected, exhibited much reduced viral RNA and proviral DNA loads compared with those in naïve animals challenged in parallel as controls. Although this aspect was not specifically addressed, the fact that this extent of protection had not been observed in a previous superinfection study involving the same viral strains (45) suggests that the presence of identical components in the two viruses significantly facilitated containment of the second virus. Since plasma and PBMC were often found to be negative for the challenge viruses in cats that had clearly positive lymphoid organs when euthanized at the end of the experiments, the overall results also showed that characterizing the virus contents of a few blood samples may not suffice to rule out infection by a second virus. Similar discrepancies between the results of examining peripheral blood and lymphoid organs have been reported in several superinfection studies with wild-type and attenuated lentiviruses (15, 24, 30, 47), suggesting that containment of a second virus can occur not only at the level of establishment of the infection, but also at the level of how freely it may circulate in blood.
TABLE 3.
Summary of the outcomes of challenging preinfected cats
| Preinfecting virus | Challenge virus | Route of challenge | Detection of the challenge virus in:
|
No. of cats completely protected from the challenge | ||
|---|---|---|---|---|---|---|
| Plasmaa | PBMCb | Lymphoid organsc | ||||
| FIVPET | FIVχ | I.v. | 0/5 | 0/5 | ||
| Vagina | 0/5 | 0/5 | 0/4d | 5/5 | ||
| FIVM2 | FIVχ | I.v. | 0/5 | 1/5 (transient) | ||
| Vagina | 1/5 | 2/5 (transient) | 3/5 | 2/5 | ||
| FIVχ | FIVPET | I.v. | 0/5 | 5/5 (transient) | 5/5 | 0/5 |
| FIVM2 | I.v. | 2/5e | 2/5e | 2/5e | 3/5 | |
Virus positive cats/examined cats, as determined by RT-PCRs at selected times PC.
Provirus-positive cats/examined cats, as determined by PCRs at selected times PC.
Provirus- positive cats/examined cats, as determined by PCRs at the end of the experiment.
Cat 2682 was not available for autopsy.
Cumulative results after the second challenge.
Another bit of clear evidence that emerged from the overall results is that the presence at challenge of NA capable of blocking the second virus in vitro was not a major determinant of resistance to superinfection. Indeed, the proportions of preinfected subjects who possessed such antibodies were approximately the same among the cats who scored as completely protected (defined as negative for the challenge virus in blood at all times and in the lymphoid organs at the end of the experiment) and among those who became superinfected (5/10 and 4/10, respectively). On the other hand, in no instance did the NA reach the titer of 1/512, which was previously shown to correlate with protection in FIV-vaccinated cats (19) and to be rarely, if ever, detected in infected cats (12). However, it is paradoxical that the group of preinfected animals that resisted the challenge best was also the one in which NA to the challenge virus were the least frequent (Table 2).
If we examine the relative importance of a matched Env versus a matched non-Env component in resistance to superinfection by comparing the numbers of cases of complete protection that occurred, the results of the first part of the study fully support the above contentions, as well as previous SIV data showing that complete protection can be independent of a completely matched Env (20). Indeed, there were fewer cats completely protected against FIVχ in the group preinfected with FIVM2, which shared only Env with the challenge virus, than in the one preinfected with FIVPET, which shared all of the virion except Env with FIVχ (two of five versus five of five). Of note, no cat in the latter group allowed FIVχ infection despite the fact that challenge was carried out twice, first systemically and then intravaginally. This might indicate that resistance to superinfection was particularly robust in this preinfection-challenge combination, although the fact that FIVχ was molecularly cloned and grown in vitro, two circumstances known to weaken FIV challenge (14, 23, 25), may have contributed significantly.
The indications of the second set of experiments were less straightforward. First, all or a large fraction of the FIVχ-preinfected cats became superinfected with FIVPET or with FIVM2, respectively, possibly due to the circumstances that these viruses, being ex vivo derived, had a more complex quasispesies than the FIVχ used as a challenge in the experiment described above (14, 23) and that the time elapsed after preinfection was shorter than in the first part of the study. That the duration of this interval can influence the outcomes of superinfections has been observed in numerous studies with live attenuated SIV vaccines (reviewed in reference 32), as well as in an experiment with HIV-2 in which no macaques became superinfected when challenged 8 weeks after initial infection versus four of four and one of four challenged at weeks 4 and 2, respectively (41). Second, and most interestingly, three of five cats following challenge with FIVM2 and none following challenge with FIVPET proved to be completely protected, suggesting that under the experimental conditions used in this part of the study, effector mechanisms targeting the viral Env but distinct from NA played an important role in blocking the second virus. The implicated mechanisms might include antibody-dependent cell cytotoxicity mediated by Env-specific nonneutralizing antibodies, Env-specific cell-mediated immune responses, and viral interference (24, 34). Thus, the possibility exists that not only the strength of resistance to superinfection, but also the effectors implicated, evolved with time after the first infection (52).
In summary, this investigation of the resistance of FIV-preinfected cats to superinfection by a second strain of the virus indicated that (i) in long-term-infected hosts, resistance is mainly mediated by effector mechanisms that do not target strain-specific sites of Env; (ii) if these sites play a dominant role, it is only for a limited time after the first infection; and (iii) in any case, the role of NA is minimal or nonexistent. Many efforts are being done to identify correlates of protection from lentiviruses. As recently discussed (22, 31), the findings with primate lentiviruses have emphasized the significance of the mediators elicited by internal proteins of the virion, while NA are thought to play an ancillary function, if any. By and large, the present results with a nonprimate lentivirus fully agree with this picture.
Acknowledgments
This work was supported by Ministero della Salute-Istituto Superiore di Sanità, Programma per l'AIDS, and Ministero dell'Istruzione, dell'Università e della Ricerca, Rome, Italy.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. [DOI] [PubMed] [Google Scholar]
- 3.Bendinelli, M., M. Pistello, D. Del Mauro, G. Cammarota, F. Maggi, A. Leonildi, S. Giannecchini, C. Bergamini, and D. Matteucci. 2001. During readaptation in vivo, a tissue culture-adapted strain of feline immunodeficiency virus reverts to broad neutralization resistance at different times in individual hosts but through changes at the same position of the surface glycoprotein. J. Virol. 75:4584-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broche-Pierre, S., J. Richardson, A. Moraillon, and P. Sonigo. 2005. Evaluation of live feline immunodeficiency virus vaccines with modified antigenic properties. J. Gen. Virol. 86:2495-2506. [DOI] [PubMed] [Google Scholar]
- 5.Burkhard, M. J., and G. A. Dean. 2003. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr. HIV Res. 1:15-29. [DOI] [PubMed] [Google Scholar]
- 6.Burkhard, M. J., C. K. Mathiason, K. O'Halloran, and E. A. Hoover. 2002. Kinetics of early FIV infection in cats exposed via the vaginal versus intravenous route. AIDS Res. Hum. Retrovir. 18:217-226. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 8.Cheonis, N. 2006. Dual HIV infection. BETA 18:36-40. [PubMed] [Google Scholar]
- 9.Chohan, B., L. Lavreys, S. M. J. Rainwater, and J. Overbaugh. 2005. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J. Virol. 79:10701-10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor, R., D. Montefiori, J. Binley, J. Moore, S. Bonhoeffer, A. Gettie, E. Fenamore, K. Sheridan, D. Ho, P. Dailey, and P. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 12.Del Mauro, D., D. Matteucci, S. Giannecchini, F. Maggi, M. Pistello, and M. Bendinelli. 1998. Autologous and heterologous neutralization analyses of primary feline immunodeficiency virus isolates. J. Virol. 72:2199-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunham, S., and O. Jarrett. 2006. FIV as a model for AIDS vaccine studies, p. 293-332. In H. Friedman, M. Bendinelli, and S. Specter (ed.), Animal models of HIV infection and control, Springer, New York, NY.
- 15.Dunham, S. P., J. Bruce, D. Klein, J. N. Flynn, M. C. Golder, S. MacDonald, O. Jarrett, and J. C. Neil. 2006. Prime-boost vaccination using DNA and whole inactivated virus vaccines provides limited protection against virulent feline immunodeficiency virus. Vaccine 24:7095-7108. [DOI] [PubMed] [Google Scholar]
- 16.Elder, J. H., G. A. Dean, E. A. Hoover, J. A. Hoxie, M. H. Malim, L. Mathes, J. C. Neil, T. W. North, E. E. Sparger, M. B. Tompkins, W. A. Tompkins, J. Yamamoto, N. Yuhki, N. C. Pedersen, and R. H. Miller. 1998. Lessons from the cat: feline immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 14:797-801. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, H., M. Bendinelli, and S. Specter (ed.). 2006. Animal models of HIV infection and control, p. 436. Springer, New York, NY.
- 18.Fultz, P. N., A. Srinivasan, C. R. Greene, D. Butler, R. B. Swenson, and H. M. McClure. 1987. Superinfection of a chimpanzee with a second strain of human immunodeficiency virus. J. Virol. 61:4026-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannecchini, S., D. Del Mauro, D. Matteucci, and M. Bendinelli. 2001. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: reevaluation of neutralizing antibody levels elicited by a protective and a nonprotective vaccine after removal of antisubstrate cell antibodies. J. Virol. 75:4424-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundlach, B. R., S. Reiprich, S. Sopper, R. E. Means, U. Dittmer, K. Matz-Rensing, C. Stahl-Hennig, and K. Uberla. 1998. Env-independent protection induced by live, attenuated simian immunodeficiency virus vaccines. J. Virol. 72:7846-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeney, J. L. 2004. Requirement of diverse T-helper responses elicited by HIV vaccines: induction of highly targeted humoral and CTL responses. Exp. Rev. Vaccines 3:S53-S64. [DOI] [PubMed] [Google Scholar]
- 22.Heeney, J. L., and S. A. Plotkin. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7:1281-1284. [DOI] [PubMed] [Google Scholar]
- 23.Hosie, M. J., and J. A. Beatty. 2007. Vaccine protection against feline immunodeficiency virus: setting the challenge. Aust. Vet. J. 85:5-12. [DOI] [PubMed] [Google Scholar]
- 24.Hosie, M. J., and J. N. Flynn. 1996. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J. Virol. 70:7561-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosie, M. J., B. J. Willett, D. Klein, T. H. Dunsford, C. Cannon, M. Shimojima, J. C. Neil, and O. Jarrett. 2002. Evolution of replication efficiency following infection with a molecularly cloned feline immunodeficiency virus of low virulence. J. Virol. 76:6062-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, D. J., S. Subbarao, S. Vanichseni, P. A. Mock, A. Ramos, L. Nguyen, T. Chaowanachan, F. van Griensven, K. Choopanya, T. D. Mastro, and J. W. Tappero. 2005. Frequency of HIV-1 dual subtype infections, including intersubtype superinfections, among injection drug users in Bangkok, Thailand. AIDS 19:303-308. [PubMed] [Google Scholar]
- 27.Johnson, R. P. 2002. Mechanisms of protection against simian immunodeficiency virus infection. Vaccine 20:1985-1987. [DOI] [PubMed] [Google Scholar]
- 28.Jost, S., M. C. Bernard, L. Kaiser, S. Yerly, B. Hirschel, A. Samri, B. Autran, L. E. Goh, and L. Perrin. 2002. A patient with HIV-1 superinfection. N. Engl. J. Med. 347:731-736. [DOI] [PubMed] [Google Scholar]
- 29.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatissian, E., V. Monceaux, M. C. Cumont, M. P. Kieny, A. M. Aubertin, and B. Hurtrel. 2001. Persistence of pathogenic challenge virus in macaques protected by simian immunodeficiency virus SIVmacΔnef. J. Virol. 75:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 32.Koff, W. C., P. R. Johnson, D. I. Watkins, D. R. Burton, J. D. Lifson, K. J. Hasnkrug, A. B. McDermott, A. Schultz, T. J. Zamb, R. Boyle, and R. C. Desrosiers. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 7:19-23. [DOI] [PubMed] [Google Scholar]
- 33.Letvin, N. J., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 34.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi, S., C. Garzelli, C. La Rosa, L. Zaccaro, S. Specter, G. Malvaldi, F. Tozzini, and M. Bendinelli. 1993. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J. Virol. 67:4742-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, D. Del Mauro, I. Lonetti, L. Zaccaro, C. Pollera, S. Specter, and M. Bendinelli. 1997. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J. Virol. 71:8368-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227-255. [DOI] [PubMed] [Google Scholar]
- 38.Mills, J., R. Desrosiers, E. Rud, and N. Almond. 2000. Live attenuated HIV vaccines: a proposal for further research and development. AIDS Res. Hum. Retrovir. 16:1453-1461. [DOI] [PubMed] [Google Scholar]
- 39.Obert, L. A., and E. A. Hoover. 2000. Relationship of lymphoid lesions to disease course in mucosal feline immunodeficiency virus type C infection. Vet. Pathol. 37:386-401. [DOI] [PubMed] [Google Scholar]
- 40.Obert, L. A., and E. A. Hoover. 2002. Early pathogenesis of transmucosal feline immunodeficiency virus infection. J. Virol. 76:6311-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otten, R. A., D. L. Ellenberger, D. R. Adams, C. A. Fridlund, E. Jackson, D. Pieniazek, and M. A. Rayfield. 1999. Identification of a window period for susceptibility to dual infection with two distinct human immunodeficiency virus type 2 isolates in a Macaca nemestrina (pig-tailed macaque) model. J. Infect. Dis. 180:673-684. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 43.Pistello, M., F. Bonci, P. Isola, P. Mazzetti, A. Merico, L. Zaccaro, D. Matteucci, and M. Bendinelli. 2005. Evaluation of feline immunodeficiency virus ORF-A mutants as candidate attenuated vaccine. Virology 332:676-690. [DOI] [PubMed] [Google Scholar]
- 44.Pistello, M., D. Matteucci, F. Bonci, P. Isola, P. Mazzetti, L. Zaccaro, A. Merico, D. Del Mauro, N. Flynn, and M. Bendinelli. 2003. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: protection from an intraclade challenge administered systemically or mucosally by an attenuated vaccine. J. Virol. 77:10740-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pistello, M., D. Matteucci, G. Cammarota, P. Mazzetti, S. Giannecchini, D. Del Mauro, S. Macchi, L. Zaccaro, and M. Bendinelli. 1999. Kinetics of replication of a partially attenuated virus and of the challenge virus during a three-year intersubtype feline immunodeficiency virus superinfection experiment in cats. J. Virol. 73:1518-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos, A., D. J. Hu, L. Nguyen, K.-O. Phan, S. Vanichseni, N. Promadej, K. Choopanya, M. Callahan, N. L. Young, J. McNicholl, T. D. Mastro, T. M. Folks, and S. Subbarao. 2002. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J. Virol. 76:7444-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibata, R., C. Siemon, M. W. Cho, L. O. Arthur, S. M. Nigida, Jr., T. Matthews, L. A. Sawyer, A. Schultz, K. K. Murthy, Z. Israel, A. Javadian, P. Frost, R. C. Kennedy, H. C. Lane, and M. A. Martin. 1996. Resistance of previously infected chimpanzees to successive challenges with a heterologous intraclade B strain of human immunodeficiency virus type 1. J. Virol. 70:4361-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebelink, K. H., W. Huisman, J. A. Karlas, G. F. Rimmelzwaan, M. L. Bosch, and A. D. M. E. Osterhaus. 1995. Neutralization of feline immunodeficiency virus by polyclonal feline antibody: simultaneous involvement of hypervariable regions 4 and 5 of the surface glycoprotein. J. Virol. 69:5124-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siebelink, K. H., G. F. Rimmelzwaan, M. L. Bosch, R. H. Meloen, and A. D. M. E. Osterhaus. 1993. A single amino acid substitution in hypervariable region 5 of the envelope protein of feline immunodeficiency virus allows escape from virus neutralization. J. Virol. 67:2202-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, D. M., D. D. Richman, and S. J. Little. 2005. HIV superinfection. J. Infect. Dis. 192:438-444. [DOI] [PubMed] [Google Scholar]
- 51.Sparger, E. E. 2006. FIV as a model for HIV: an overview, p. 149-237. In H. Friedman, M. Bendinelli, and S. Specter (ed.), Animal models of HIV infection and control. Springer, New York, NY.
- 52.Stebbings, R., N. Berry, H. Waldmann, P. Bird, G. Hale, J. Stott, D. North, R. Hull, J. Hall, J. Lines, S. Brown, N. D'Arcy, L. Davis, W. Elsley, C. Edwards, D. Ferguson, J. Allen, and N. Almond. 2005. CD8+ lymphocytes do not mediate protection against acute superinfection 20 days after vaccination with a live attenuated simian immunodeficiency virus. J. Virol. 79:12264-12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talbott, R. L., E. E. Sparger, K. M. Lovelace, W. M. Fitch, N. C. Pedersen, P. A. Luciw, and J. H. Elder. 1989. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA 86:5743-5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vahlenkamp, T. W., M. B. Tompkins, and W. A. F. Tompkins. 2006: FIV as a model for AIDS pathogenesis studies, p. 239-273. In Friedman, H., M. Bendinelli, and S. Specter (ed.), Animal models of HIV infection and control. Springer, New York, NY.
- 55.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]