Abstract
The roles of cellular proteases in Moloney murine leukemia virus (MLV) infection were investigated using MLV particles pseudotyped with vesicular stomatitis virus (VSV) G glycoprotein as a control for effects on core MLV particles versus effects specific to Moloney MLV envelope protein (Env). The broad-spectrum inhibitors cathepsin inhibitor III and E-64d gave comparable dose-dependent inhibition of Moloney MLV Env and VSV G pseudotypes, suggesting that the decrease did not involve the envelope protein. Whereas, CA-074 Me gave a biphasic response that differentiated between Moloney MLV Env and VSV G at low concentrations, at which the drug is highly selective for cathepsin B, but was similar for both glycoproteins at higher concentrations, at which CA-074 Me inhibits other cathepsins. Moloney MLV infection was lower on cathepsin B knockout fibroblasts than wild-type cells, whereas VSV G infection was not reduced on the B−/− cells. Taken together, these results support the notion that cathepsin B acts at an envelope-dependent step while another cathepsin acts at an envelope-independent step, such as uncoating or viral-DNA synthesis. Virus binding was not affected by CA-074 Me, whereas syncytium induction was inhibited in a dose-dependent manner, consistent with cathepsin B involvement in membrane fusion. Western blot analysis revealed specific cathepsin B cleavage of SU in vitro, while TM and CA remained intact. Infection could be enhanced by preincubation of Moloney MLV with cathepsin B, consistent with SU cleavage potentiating infection. These data suggested that during infection of NIH 3T3 cells, endocytosis brings Moloney MLV to early lysosomes, where the virus encounters cellular proteases, including cathepsin B, that cleave SU.
Moloney murine leukemia virus (MLV) has been widely used as the prototype ecotropic MLV in studies of retroviral replication. Its genome, structural and enzymatic proteins, and envelope protein (Env) also serve as the bases for many recombinant retroviral vectors. Infection begins with binding of virion Env to the high-affinity ecotropic virus receptor, which is thought to produce conformation changes in the envelope protein that lead to fusion of the viral and cellular membranes for penetration of the virion core.
Studies of the pH dependence of Moloney MLV infection suggested that entry events may be more complex than a straightforward scenario of receptor triggering of Env. The seemingly paradoxical observations gave rise to the idea that a host cell protease might be involved in ecotropic MLV infection. The initial finding was Klaus Andersen's report that a protease inhibitor reduced infection (2). That same year, Anderson and Nexo reported that ecotropic MLV infection was inhibited by the weak base NH4Cl in SC-1 cells (3), a finding later confirmed by McClure and coworkers in NIH 3T3 and other normal mouse and rat cells (17). Paradoxically, McClure and coworkers found that low pH did not trigger Moloney MLV-induced cell-cell fusion; rather, the fusion occurred at neutral pH (17), suggesting that acidic pH was not involved directly.
More puzzling still was their finding that unlike in other host cells, NH4Cl did not inhibit infection in rat XC cells (17). They reconciled these unusual findings by proposing that XC cells express a cell surface protease normally found in intracellular compartments that is important to infection and so are able to escape NH4Cl inhibition (17). In line with this possibility, limited trypsin exposure increased cell-cell fusion (4), and it was found that by 6 h postinfection, some of the surface subunit (SU) of the viral glycoprotein was cleaved into smaller fragments (1). However, the fate of SU at times less than 6 h postinfection was not determined, nor was it shown if the cleavage at 6 h was postentry degradation of the glycoprotein versus a beneficial event to the virus.
To date, the question of cellular protease involvement in ecotropic MLV infection remains unanswered. No protease has been identified, and its role in infection has not been determined. We considered the suggestion of McClure et al. that XC cells may express the critical proteases inappropriately on their surfaces and searched the literature for clues as to what proteases these cells might overexpress. Originally derived from a sarcoma induced by Rous sarcoma virus, XC cells are highly transformed as a result of viral src gene expression (23, 26, 27). Notably viral-src-transformed cells secrete cellular proteases, particularly cathepsins (13, 25), suggesting that they comprise at least one type of protease that is secreted by XC cells and thus is accessible at the cell surface.
Cathepsins are a large family of lysosomal proteases and include aspartyl, serinyl, and cysteinyl proteases that show the highest activity at pH 5 to 6 (29). This property and their location in the lysosomal compartment suggest that cathespins would be less active within NH4Cl- and other weak-base-treated cells but might remain highly active on the cell surface, where the weak bases have no effect. Thus, we examined the possibility that host cell cathepsins are involved in Moloney MLV infection.
Here, we report results identifying cathepsin B as a host cell protease involved in the membrane fusion step of ecotropic Moloney MLV infection. Cathepsin B appears to act on the Moloney MLV envelope protein by cleaving SU, but not TM or CA, into specific fragments. Evidence is also presented that another as-yet-unidentified cathepsin is involved at a different step in infection that is envelope independent. This step is likely to be a postpenetration step, such as uncoating or viral-DNA synthesis.
MATERIALS AND METHODS
Cell lines and viruses.
Mouse NIH 3T3 fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 8% donor calf serum. The cathepsin B−/− murine embryonic fibroblasts (MEFs), control wild-type parental cathepsin B+/+ MEFs (a gift of T. S. Dermody), and rat XC sarcoma cells (ATCC) were maintained in DMEM supplemented with 10% fetal bovine serum. Replication-defective pseudotype MLVs were produced as previously described by transient cotransfection of a Moloney MLV gag-pol plus Moloney MLV env (31) or vesicular stomatitis virus (VSV) G protein (a gift of S. R. Ross)-encoding plasmids into H1BAG cells harboring a lacZ-transducing MLV-based genome. Replication-competent wild-type ecotropic MLV production was initiated by transfection of NIH 3T3 cells with plasmid p63.2, an infectious molecular clone of Moloney MLV (a gift of H. Fan) (5). Thereafter, virus was produced by infecting NIH 3T3 cells or NIH 3T3 BAG clone C8 (a clonal NIH 3T3 cell line stably expressing the pBAG genome containing the Escherichia coli β-galactosidase gene). All virus-containing supernatants were filtered through a 0.45-μm filter. Except where noted, Polybrene (10 μg/ml) was added to Moloney MLV Env pseudovirions prior to exposure to cells.
Protease inhibitor studies.
Stock solutions of protease inhibitors were 2.5 mg/ml leupeptin hemisulfate (Calbiochem) or 5 mg/ml leupeptin hydrochloride (Sigma) in water, 1 mM pepstatin A, 1 mM cathepsin inhibitor III, 6 mM E-64d, and 5.4 mM CA-074 Me (a membrane-permeable methyl ester form of CA-074) (Calbiochem), all in dimethyl sulfoxide (DMSO) (Sigma). For infection experiments, naïve NIH 3T3 cells seeded in 24-well plates were pretreated with medium containing the specified concentration of inhibitor for 30 min (pepstatin A, cathepsin inhibitor III, E-64d, CA-074Me, and CA-074) or 1 h (leupeptin) at 37°C. Quadruplicate wells were then exposed to inhibitor plus lacZ-transducing replication-defective virus for an additional 2 h at 37°C. Independent virus productions ranging in titer from 0.4 × 106 to 1 × 106 lacZ-tranducing units/ml were used in each replication of these experiments. Forty-eight hours later, the cells were fixed with 0.5% glutaraldehyde and stained with the chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) for β-galactosidase activity. Infected foci were counted by light microscopy. In all experiments, the control wells lacking inhibitor and representing 100% infection gave a minimum of 300, and more typically 500 to 600, foci per well. The values are the mean percent infection ± standard deviation calculated as follows: (mean number of blue foci in drug-treated wells/mean number of blue foci in DMSO-treated wells) × 100.
For studies of the effect of CA-074 Me on infectious virus, NIH 3T3 BAG clone C8 was incubated in the presence or absence of 75 μM CA-074 Me for 4 h prior to exposure to replication-competent wild-type ecotropic MLV for 1 h, during which time the inhibitor was maintained. The inhibitor and unbound virions were then removed by gentle washing of the cells, and the supernatant was collected every 24 h up to 96 h. A portion of each supernatant was 10-fold serially diluted and applied to naïve NIH 3T3 cells for end point titration. Another portion was subjected to ultracentrifugation for 45 min at 30,000 rpm in an SW41 rotor. The pellets were resuspended in 40 μl of phosphate-buffered saline for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described below.
Virus binding assay and syncytium induction.
The virus binding assays were performed as described previously (15), except that some cells were incubated in medium containing 100 μM CA-074 Me at 37°C for 30 min prior to detachment of cells and during the 1-h incubation of replication-competent wild-type Moloney MLV with NIH 3T3 cells. To quantify virus-induced cell-cell fusion, XC or NIH 3T3 cells seeded at 50% confluence in 24-well tissue culture plates were preincubated in the presence or absence of the indicated amounts of CA-074Me for 2 h to inhibit endogenous cathepsin B, the inhibitor was washed out once with regular culture medium, and then replicate wells (n = 3; in some cases, n = 4) were exposed to replication-defective MLV particles pseudotyped with Moloney MLV Env. In some cases, purified cathepsin B (Calbiochem) was added to the virus prior to its application to cells. After 6 h, the cells were fixed with absolute ethanol and stained with 0.5% methylene blue (Sigma) in ethanol. Light micrographs were taken of 10 consecutive 100× fields of each well using a Zeiss Axiocam digital camera, and the number of nuclei in syncytia, the number of syncytia, and the total number of nuclei were determined. A total of at least 1,500 nuclei were scored for each well. The fusion index was calculated as follows: (number of nuclei in syncytia/total number of nuclei) × 100.
Immunoblot analysis.
For immunoblot analysis, the proteins in 10 μl of virus pellet or 10 μl of protease reaction mixture were separated by SDS-PAGE (10 to 20% Tris glycine gradient gels; Invitrogen), transferred to nitrocellulose membranes (Protran; Schleicher and Schuell), and immunoblotted with goat anti-Rauscher MLV SU antiserum (Quality Biotech no. 80S000018; 1:100). Immediately after detection of the anti-SU reactive proteins, the blots were stripped and reprobed with goat anti-capsid p30 antiserum (Quality Biotech no. 81S000263; 1:10,000).
In vitro protease cleavage of intact wild-type Moloney MLV particles and its effect on infection.
Replication-competent wild-type Moloney MLV was harvested from the medium of chronically infected NIH 3T3 BAG clone C8, filtered through a 0.45-μm filter to remove the cells, and then centrifuged in a Beckman SW41 rotor at 30,000 rpm for 45 or 90 min at 4°C. The virions sedimented for 45 min retained infectivity, whereas those sedimented for 90 min were noninfectious. Others have shown that shortening the duration of high-speed centrifugation can preserve infectivity (9). The virus pellets were resuspended in phosphate-buffered saline and stored at −80°C until they were used. For analysis of cathepsin B cleavage, aliquots of pelleted virus were incubated at 37°C for 1 h in acetate buffer (pH 5.5) plus or minus the specified amount of cathepsin B (Calbiochem). In some cases, 100 μM CA-074 was added along with the cathepsin B, and in some cases, reaction mixtures were incubated for longer periods, up to 4 h at 37°C. The digestions were terminated on ice by the addition of stop solution (100 μM CA-074, 100 mM HEPES, pH 7.4. and protease inhibitor cocktail [Sigma; 1:50 dilution]). Ten microliters of each reaction mixture was analyzed by SDS-PAGE, immunoblotted to anti-SU, and then reprobed with anti-CA as described above. The remainder of each reaction mixture was diluted back to its original volume (1×) in DMEM plus 8% fetal bovine serum, serially diluted, and applied to NIH 3T3 cells for end point titration. The cells were fixed and stained after 48 h for the number of lacZ-transducing units/ml as a relative measure of the infectivity present.
RESULTS
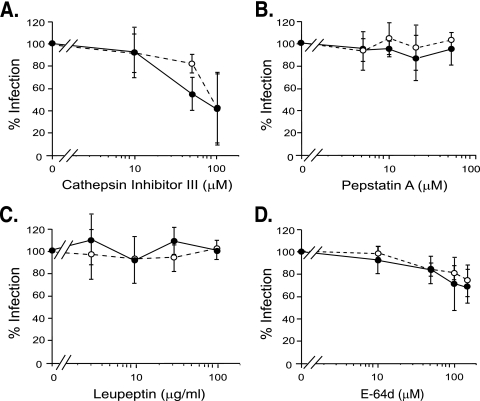
Cathepsins are a large, diverse family that includes aspartyl, serinyl, and cysteinyl proteases, although most are cysteinyl proteases (29). Cathepsin inhibitor III, a broad-spectrum cathepsin inhibitor, markedly reduced Moloney MLV infection in NIH 3T3 cells in a dose-dependent manner (Fig. 1A). The reduction was not due to effects on cell division, since NIH 3T3 cells in untreated and inhibitor-treated samples underwent comparable numbers of doublings (data not shown). VSV G pseudotype MLV infection was inhibited similarly to Moloney MLV (Fig. 1A), suggesting that the reduction was envelope independent.
FIG. 1.
Effects of protease inhibitors on infection of NIH 3T3 cells. Naïve NIH 3T3 cells were exposed to lacZ-transducing, replication-defective pseudovirions for 2 h in the presence of the indicated amounts of inhibitor. Closed circles, Moloney MLV Env-pseudotyped MLV. Open circles, VSV G-pseudotyped MLV. (A) Cathepsin inhibitor III, a broad-spectrum inhibitor of cathepsins, was used at 10, 50, 100, and 150 μM. (B) Pepstatin A, an inhibitor of aspartyl proteases, was used at 5, 10, 20, and 50 μM. (C) Leupeptin, an inhibitor of serinyl and cysteinyl proteases, was used at 3, 10, 30, and 100 μg/ml. (D) E-64d, an inhibitor of cysteinyl cathepsins (all known cathepsins except D, E, and G), was used at 10, 50, 100, and 150 μM. The values shown are the mean percent infection ± standard deviation of three independent experiments for cathepsin inhibitor III, pepstatin A, and E-64d in which cells (quadruplicate samples) were preincubated with inhibitor for 30 min prior to virus exposure. For leupeptin, the values shown are the mean percent infection ± standard deviation of three independent experiments performed without preincubation of host cells with inhibitor; a fourth experiment was performed with leupeptin preincubation for 1 h. Similar results were observed in all experiments.
Pepstatin A, an inhibitor of aspartyl proteases, including cathepsins D and E, did not affect infection, suggesting that neither of the two is involved (Fig. 1B). We then examined the effect of leupeptin, a general inhibitor of serinyl and cysteinyl proteases, including many cathepsins. Moloney MLV Env infection was not reduced by leupeptin; instead, it was typically slightly increased (Fig. 1C). Preincubation of host cells for an hour prior to virus exposure, use of leupeptin hydrochloride instead of the hemisulfate form, and omitting Polybrene gave similar results (data not shown).
The lack of leupeptin inhibition is consistent with Simmons and coworkers’ finding that leupeptin does not inhibit infection by amphotropic 4070A Env pseudotypes of MLV (24). Instead, infection by MLV pseudotyped with amphotropic Env was slightly increased at the highest concentration of leupeptin (24). However, these results contrast with Andersen's report that leupeptin inhibited Moloney MLV infection of mouse fibroblasts (2). We do not know why our results differed from Andersen's, but we suspect that the pleiotropic effects of leupeptin on cellular proteases other than cathepsins lie behind it.
Next, we examined E-64d, an inhibitor of cysteinyl proteases, including all the cathepsins except D, E, and G. E-64d decreased Moloney MLV infection in a dose-dependent manner (Fig. 1D). Similar inhibition was seen in VSV G pseudotype infection, consistent with the inhibitor acting at an envelope-independent step, as had been suggested by the cathepsin inhibitor III response (Fig. 1D).
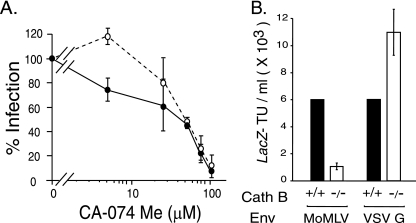
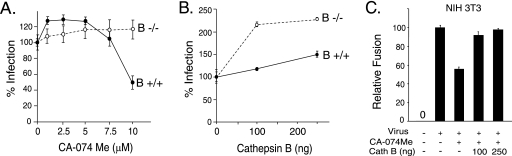
Since cathepsin B is the predominant cysteinyl protease in most cell types (14, 28), we examined the effect of CA-074 Me, which at 1 to 10 μM is a specific dead-end inhibitor of cathepsin B (7, 19). At higher concentrations, CA-074 Me is less specific and inhibits other cathepsins (19). Moloney MLV infection was inhibited in a dose-dependent manner, with greater than 90% inhibition of Moloney pseudovirions at 100 μM CA-074 Me (Fig. 2A). The reduction was not due to an effect on cell division (data not shown). Interestingly, VSV G pseudovirions showed a biphasic response as the dose of inhibitor increased. VSV G-mediated infection was slightly increased at 5 μM but markedly reduced at 50 μM and greater, concentrations that correlate well with the specificity profile of the inhibitor.
FIG. 2.
Host cell cathepsin B is important to ecotropic Moloney MLV infection. (A) Quadruplicate wells of naïve NIH 3T3 cells were preincubated with the indicated amounts of CA-074Me (5, 25, 50, 75, and 100 μM) for 30 min prior to the addition of Moloney MLV Env-pseudotyped MLV (closed circles) or VSV G-pseudotyped MLV (open circles) for 2 h. The inhibitor was maintained during virus exposure. The values shown are the mean percent infection ± standard deviation of three independent experiments. (B) Cathepsin B knockout cells are less susceptible to Moloney MLV (MoMLV) but not to control VSV G pseudovirions. Quadruplicate wells of naïve cathepsin B−/− MEFs were exposed to serially diluted Moloney MLV pseudovirions or to serially diluted VSV G-pseudotyped MLV, and the lacZ-transducing titer was calculated from the end point dilution 48 h later. For comparison, aliquots of the serial virus dilutions were applied to parental wild-type cathepsin MEFs (B+/+). The values shown are the mean LacZ-transducing units (TU)/ml ± standard deviations of three independent experiments.
If cathepsin B is important to infection, then MEFs derived from a cathepsin knockout (B−/−) mouse should be less susceptible than parental B+/+ MEFs from wild-type mice (11). Infection of cathepsin B−/− cells by Moloney MLV was reduced by up to 7.5-fold less than that of the control MEFs, whereas VSV G pseudotype MLV infection was not reduced (Fig. 2B). These results indicate that cathepsin B acts exclusively on the envelope-dependent step in MLV infection and suggest that a different cathepsin acts on the step that is independent of the envelope glycoprotein.
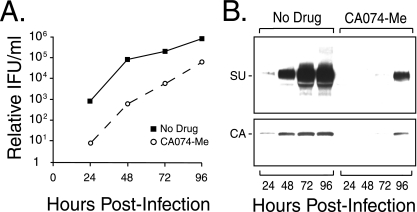
We then examined the ability of this cathepsin B inhibitor to block replication of wild-type Moloney MLV versus a single round of pseudovirion infection. Cells were incubated with 75 μM CA-074 Me for 4 h to inactivate the endogenous cathepsin B. This concentration was used because it gave an 80% reduction in infection in the dose-response studies. We reasoned that the initial infection would be reduced 80% and that virus spread should also be inhibited during the first hour of virus exposure, since the inhibitor was present during that time, as well. The infectious-MLV yield (Fig. 3) and expression of envelope protein SU and CA were markedly reduced in inhibitor-treated cells (Fig. 3), indicating that wild-type Moloney MLV replication is sensitive to inhibition of host cell cathepsin B. These results are similar to those reported for wild-type Ebola virus inhibition by this inhibitor (8).
FIG. 3.
Replication-competent Moloney MLV is sensitive to inhibition. Naïve NIH 3T3 cells were preincubated in the presence or absence of 75 μM CA-074 Me for 4 h prior to the addition of replication-competent Moloney MLV. After a 1-h incubation, the unbound virus and inhibitor were washed from the cells. The culture medium was harvested every 24 h for 4 days and analyzed by end point dilution titration for infectivity. (B) Immunoblot analysis of SU and CA expression in culture medium samples from panel A. The results are representative of four independent experiments. IFU, infectious units.
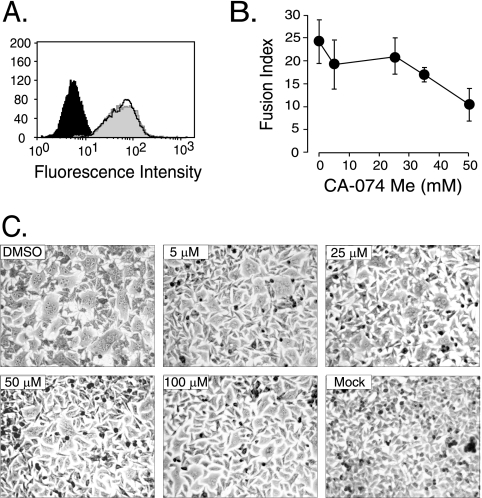
In considering the mechanism by which cathepsin B influences infection, at this point, we could not rule out the possibility that CA-074 Me reduced infection by reducing the number of virus binding sites (e.g., acting on the receptor). To determine if the inhibitor down-regulated receptors, a standard virus binding assay was performed using NIH 3T3 cells incubated with CA-074 Me at the maximal concentration used in the infection studies. The drug was present prior to and during incubation with wild-type Moloney MLV. For comparison, wild-type virus was incubated with untreated NIH 3T3 cells. The mean fluorescence intensity for virus-bound NIH 3T3 cells treated with the inhibitor was comparable to that of untreated cells (Fig. 4A), indicating that the inhibitor does not act on the receptor.
FIG. 4.
Inhibition of cathepsin B influences membrane fusion. (A) CA-074Me does not down-regulate virus binding sites. The mean fluorescence intensity was 63.1 for 99% of NIH 3T3 cells preincubated in the vehicle DMSO (white peak and solid black line) compared to 63.7 for 99% of NIH 3T3 cells treated with the inhibitor (gray peak), 5.6 for 99% of NIH 3T3 cells with mock virus plus primary and secondary antibodies (solid black peak) and receptor-negative human 293 cells, and 3.6 for 100% of NIH 3T3 cells without antibodies. Similar results were obtained in a second independent experiment. (B) Quadruplicate samples of XC cells were preincubated for 30 min with CA-074 Me (0, 5, 10, 25, 35, and 50 μM) prior to the addition of replication-defective Moloney MLV pseudovirions. Cell-cell fusion was scored by light microscopy. The values shown are the mean fusion index ± standard deviation (n = 4) calculated as follows: (number of nuclei in syncytium/total number of nuclei) × 100. Similar results were obtained in three additional experiments. (C) Inhibition of cathepsin B markedly reduced the number of nuclei per syncytium. Light micrographs of XC cells exposed to the indicated concentrations of CA-074 Me and then to Moloney MLV (DMSO, 5 μM, 25 μM, 50 μM, and 100 μM) or mock virus (no virus). Micrographs taken at a magnification of ×80.
Since the inhibitor did not act at virus binding, we examined the possibility that it acted at membrane fusion by determining the effect of CA-074 Me on cell-cell fusion. Rat XC cells were preincubated with increasing amounts of the inhibitor and then exposed to high-titer replication-defective Moloney MLV pseudovirions in the presence of the drug. Syncytium induction was monitored by light microscopy and quantified 4 to 8 h later, when substantial syncytia were visible in the control untreated cells. The fusion index decreased as the concentration of inhibitor increased, with greater than 50% inhibition of cell-cell fusion at 50 μM CA-074 Me (Fig. 4B). The reduction was due to a decrease in size at 5 μM and then to a decrease in the number and size of syncytia at higher drug levels (Fig. 4C).
Several additional studies were performed as further tests of the involvement of cathepsin B in virus infection and envelope-induced membrane fusion. One prediction was that infection of cathepsin B−/− cells should not be altered by CA-075 Me at 1 to 10 μM, the range that inhibits cathepsin B and not other cellular cathepsins. Indeed, while 10 μM CA-074 Me reduced infection of cathepsin B+/+ control cells, infection of B−/− cells was not changed by the inhibitor (Fig. 5A). Providing an exogenous supply of cathepsin B by adding the protease to the virus supernatant prior to exposure increased infection of cathepsin B−/− cells by over twofold (Fig. 5B). Infection of control B+/+ cells was also increased somewhat, suggesting that access to endogenous cathepsin B may be somewhat limiting in the control MEFs. Lastly, we asked if the addition of cathepsin B would increase cell-cell fusion. Fusion of NIH 3T3 cells was inhibited by preincubation with 50 μM CA-074 Me (Fig. 5C), similar to the inhibition seen in XC cells. Addition of exogenous cathepsin B restored virus-induced NIH 3T3 cell-cell fusion (Fig. 5C).
FIG. 5.
Further evidence supporting a role for cathepsin B in the membrane fusion step of infection. (A) Cathepsin B−/− MEFs are not sensitive to inhibition by CA-074 Me in the range that is selective for inhibition of cellular cathepsin B. Quadruplicate samples of B−/− and control B+/+ MEFs were preincubated with 0, 1, 2.5, 5, 7.5, and 10 μM CA-074 Me for 1 h prior to the addition of replication-defective virus plus the inhibitor; 6 h later, the cells were fixed, stained, and then scored by light microscopy. Similar results were obtained in two additional experiments. One hundred percent infection (no CA-074 Me) was 1 × 103 ± 0.1 × 103 for B+/+ cells and 4.8 × 102 ± 0.1 × 102 LacZ TU/ml for the poorly susceptible B−/− cells. The error bars indicate standard deviations. (B) Addition of purified exogenous cathepsin B increased infection of B−/− MEFs. Triplicate samples of B−/− and B+/+ cells were exposed to virus mixed with the indicated amounts of purified cathepsin B. Similar results were observed in two additional independent experiments. One hundred percent infection (no cathepsin B added) was 3.8% ± 0.8% of B+/+ cells infected and 0.8% ± 0.2% of B−/− cells. The standard deviations are not visible for the 100-ng B+/+ and 250-ng B−/− samples because their error bars were smaller than the symbol for the data point. (C) Addition of exogenous cathepsin B overcame the inhibition of fusion resulting from CA-074 Me. Quadruplicate wells of NIH 3T3 cells were preincubated with 50 μM CA-074 Me for 2 h to irreversibly inhibit endogenous cathepsin, and then the cells were rinsed once in DMEM, and virus plus the indicated amounts of purified cathepsin B were applied to the cells for 6 h. A similar restoration of syncytium induction was seen in an independent experiment.
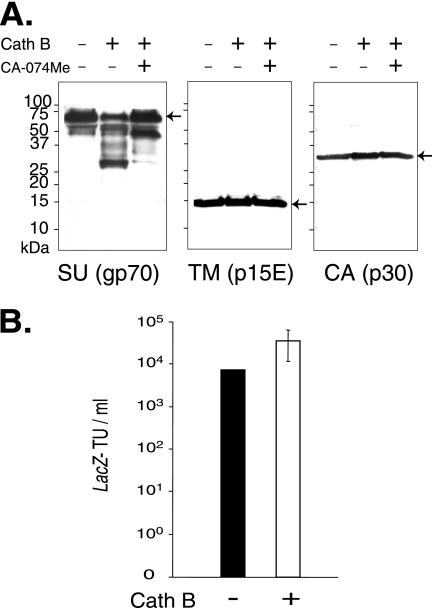
Next, we asked what virion component cathepsin B might act upon. Intact wild-type Moloney MLV particles were isolated by high-speed centrifugation and incubated in the absence of enzyme (mock) or with increasing amounts of purified cathepsin B, and samples were analyzed by immunoblotting them using polyclonal anti-SU antiserum. Here, the inhibitor was used to determine if any in vitro cleavage of a virion component was a specific cleavage, so the highest dose of the drug was used. CA-074 was used instead of the methyl ester form because no cellular esterases were present in the in vitro reactions to activate the drug by hydrolyzing the ester bond. The surface subunit of the envelope protein was cleaved into discrete fragments, but neither the transmembrane subunit nor the capsid protein was digested (Fig. 6A). The absence of the major cleavage products when CA-074 was present during the digestion demonstrated that the cleavage was specific.
FIG. 6.
Cathepsin B (Cath B) cleaves the envelope SU on virions. Aliquots of Moloney MLV pseudovirions purified by high-speed ultracentrifugation for 45 min were incubated with or without purified cathepsin B (68 μg/ml) for 1 h in acetate buffer, pH 5.5, in the presence or absence of 100 μM CA-074Me. (A) Viral proteins in 10 microliters of each reaction mixture analyzed by separation on 10 to 20% Tris-glycine gradient gels (Invitrogen) were immunoblotted to anti-SU antiserum, and the immunoblot was stripped and reprobed with anti-TM antibody (a gift of Alan Rein) and then stripped a second time and reprobed with anti-CA antiserum. Similar results were obtained in a second independent experiment. (B) Effect of preincubation with cathepsin B on virus infection. The values shown are the mean lacZ-transducing units (TU)/ml ± standard deviations from six independent experiments. Virus was prepared and incubated with cathepsin B as for panel A, except that after incubation with the protease, or with acetate reaction buffer alone, the reaction mixtures were diluted to their original volumes (1× with respect to the virus concentration in the original virus stock) in regular growth medium and then serially diluted and applied to quadruplicate wells of naïve NIH 3T3 cells.
We then asked if preincubation with purified cathepsin B affects infection. Aliquots of Moloney MLV purified by ultracentrifugation for 45 min were incubated with or without cathepsin B and then used immediately in end point dilution titration to quantify infection. The protease did not reduce infection, indicating that it does not inactivate the envelope protein. Instead, in vitro incubation with cathepsin B increased infection by 2.5-, 5-, 6.7-, 10-, 2.5-, and 1.3-fold in six independent experiments, suggesting that the protease can enhance the infectivity of virions (Fig. 6B). In three additional experiments using virus sedimented for 30, 60, or 75 min, no stimulation was observed (data not shown). We do not know why these conditions did not result in an increase in infection.
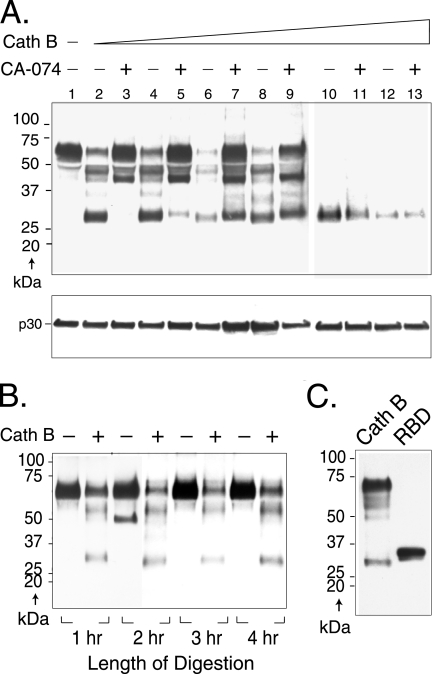
Cleavage of SU was dependent on the dose of the protease in the range of 1.25 to 375 μg/ml (Fig. 7A). Even at the lowest concentration of protease, intact SU was substantially diminished and bands of approximately 52, 42, 37, and 28 kDa appeared (lane 2). As evidence that these new bands represented specific cleavage products of cathepsin B, all but the 42-kDa fragment were lost when 100 μM CA-074 was present, along with 1.25 μg/ml of cathepsin B (Fig. 7A, lane 3). In addition, as the enzyme concentration was increased while the inhibitor was held constant, these cleavage products reappeared (lanes 5, 7, and 9). At higher concentrations, only the 28-kDa fragment was visible, indicating that this fragment is highly resistant to further cleavage. Reprobing of the immunoblot with anti-CA showed that the capsid protein was not cleaved, suggesting that virions remained intact even at the highest concentrations of cathepsin B (Fig. 7A, bottom).
FIG. 7.
Cathepsin B (Cath B) cleaves virion SU at specific sites in a dose-dependent manner. (A) Aliquots of replication-competent Moloney MLV purified by high-speed ultracentrifugation for 90 min were incubated with increasing amounts of cathepsin B for 1 h in acetate buffer, pH 5.5, in the presence or absence of 100 μM CA-074Me and then immunoblotted to anti-SU antiserum (top), and the immunoblot was subsequently stripped and reprobed with anti-CA antiserum (bottom). Lane 1, no cathepsin B; lanes 2 and 3, 1.25 μg/ml; lanes 4 and 5, 12.5 μg/ml; lanes 6 and 7, 68 μg/ml; lanes 8 and 9, 125 μg/ml; lanes 10 and 11, 250 μg/ml; lanes 12 and 13, 375 μg/ml cathepsin B. Similar results were obtained in a second independent experiment. (B) Virus was incubated in the presence or absence of cathepsin B in buffer, pH 5.5. Aliquots were removed at hourly intervals through 4 h, and digestion was terminated as described in Materials and Methods. Samples were analyzed by immunoblot analysis to anti-SU antiserum. (C) The RBD of SU is similar in antiserum reactivity and molecular mass to the 28-kDa fragment. Purified recombinant RBD (residues 1 to 236 plus 6 additional residues remaining from an affinity purification tag) from the closely related Friend MLV was immunoblotted to the anti-SU antiserum. For comparison, a sample of Moloney MLV incubated with cathepsin B (68 μg/ml for 1 h) as described above was included.
During infection, might the cathepsin B that the virus encountered in the lysosomes degrade SU with time, or would cleavage be specific? To begin to address this question, we asked if specific digestion proceeded to degradation over time as it had when the enzyme concentration was increased to 250 μg/ml. No substantive differences were observed in SU cleavage for periods up to 4 h of digestion (Fig. 7B).
DISCUSSION
Here, we identify the cathepsin family of cellular proteases as important to early steps in Moloney MLV infection. The broad-spectrum inhibitors cathepsin inhibitor III and E-64d gave comparable dose-dependent inhibition of Moloney MLV Env and VSV G pseudotypes of MLV, suggesting that the decrease did not involve the envelope protein. Presumably, the drugs affected an envelope-independent step, that is, one downstream of penetration, such as uncoating of the viral core or completion of reverse transcription. Although the steps in the uncoating of retroviruses and most other enveloped viruses are largely unknown, some insight can be drawn from reovirus infection (11). Host cell cathepsins B and L cleave the reovirus capsid protein at specific sites, and this cleavage appears to initiate membrane penetration and uncoating of the internalized reovirus particles (11, 30).
Interestingly, Simmons et al. reported that lentivirus pseudotyped with VSV G was not inhibited by up to 10 μM E-64d, suggesting that lentivirus uncoating does not involve cathepsins (24). Since these authors did not examine higher doses of E-64d or any dose of cathepsin inhibitor III, further comparisons of the broad-spectrum cathepsin inhibitors cannot be made. If host cell cathepsin is involved in MLV uncoating but not in lentivirus uncoating, the difference could reflect the physical state of maturity of lentiviral particles versus MLV particles, particularly the degree to which the viral protease has processed the Gag precursor during assembly and after budding. Lentiviral Gag is essentially completely cleaved by the viral protease into its component structural proteins shortly after budding, whereas substantial amounts of MLV Gag remain partially cleaved (18). Perhaps MLV Gag cleavage must be completed after penetration of a host cell and a host cell cathepsin other than cathepsin B is involved in facilitating such a step.
Alternatively, a cellular cathepsin may be involved in viral-DNA synthesis; the two possibilities are not mutually exclusive. For example, further cleavage of Gag-Pol polyproteins to release additional molecules of mature polymerase could be a stimulus for completion of reverse transcription. Future studies will need to identify the specific cathepsin involved and determine if it acts directly through specific cleavage of MLV Gag, Gag-Pol, or any of their products.
In the present study, VSV G-pseudotyped MLV particles showed a biphasic response to CA-074 Me: control VSV G infection increased at 5 μM and decreased at higher doses of the drug, whereas the drug inhibited Moloney MLV at all concentrations examined. CA-074 Me is a derivative of E-64d that is selective for cathepsin B in the range of 1 to 10 μM (7) but is less specific and inhibits other cathepsins at 50 to 100 μM (7, 19). Thus, the biphasic response seen in VSV G but not Moloney MLV Env pseudotypes was the first indication that cathepsins may be involved in more than one step of infection: an envelope-dependent step, revealed at low concentration where CA-075 Me is specific to cathepsin B, and an envelope-independent step, revealed by the broad-spectrum cathepsin inhibitors and seen again at high concentrations of CA-074 Me, where the drug loses specificity for cathepsin B.
Clear evidence that cathepsin B is involved in an Env-dependent step of infection and not in a postpenetration event came from studies of cathepsin B knockout MEFs. Virus containing Moloney MLV Env was much less infectious on cathepsin B−/− cells than on B+/+ cells. In contrast, control VSV G pseudotype infection was not reduced on the knockout cells, indicating a Moloney MLV Env-specific role for the protease. The increase in VSV G-mediated infection of B−/− cells was consistent with the increased infection of NIH 3T3 cells seen for VSV G pseudovirions at 5 μM CA-074 Me, a concentration at which the drug selectively inhibits cathepsin B. Further addition of exogenous cathepsin B to the B−/− cells increased their infection, and 10 μM CA-074 Me did not decrease B−/− cell infection.
Taken together, the results reported here suggest that cathepsin B potentiates Moloney MLV Env-mediated membrane fusion by specific cleavage of SU. First, CA-074 Me did not affect virus binding or down-regulate receptors. Instead, it influenced membrane fusion, a function associated with the envelope protein. The drug caused a dose-dependent decrease in the number and size of syncytia induced in XC cells by replication-defective Moloney MLV. Virus-induced fusion of NIH 3T3 cells was also decreased by preincubation with the drug, but addition of exogenous cathepsin B overcame that inhibition. Second, in vitro incubation of intact Moloney MLV particles with purified cathepsin B stimulated infection by a mean increase of 6-fold ± 3.3-fold. Lastly, cathepsin B cleaved the SU subunit of Env into specific fragments but did not digest TM or the capsid protein. Given that cathepsin B−/− MEFs retained some susceptibility to Moloney MLV infection, cathepsin B could be one of several lysosomal proteases that aid in infection by cleaving SU.
These data are consistent with endocytosis of Moloney MLV bringing virus particles to early lysosomes, where they encounter cellular proteases, including cathepsin B, that cleave SU and enhance membrane fusion. Recent studies have shown an involvement of host cell cathepsins in Ebola virus, severe acute respiratory syndrome coronavirus, and Hendra virus infections. Chandran et al. and Schornberg et al. reported that host cell cathepsins B and L are involved in Ebola virus GP glycoprotein-mediated infection (8, 22). Chandran et al. showed that the cathepsins cleaved GP in vitro into a 19-kDa fragment and that preincubation with cathepsins B and L gave a fourfold increase in infection (8). This level of increase is comparable to the 6-fold ± 3.3-fold increase in infection we report here for Moloney MLV. Simmons et al. and Huang et al. demonstrated that host cell cathepsin L influences severe acute respiratory syndrome coronavirus spike infection (12, 24). Although Simmons et al. did not show specific cleavage of the spike protein by the protease, these authors obtained evidence that cathepsin L is important to virus-cell membrane fusion. Similarly, Pager and Dutch reported that Hendra virus F (fusion) protein is activated by cathepsin B as it enters a new host cell (21).
XC cells are known to acidify the medium in high-confluence cultures, but infection and cell fusion experiments are typically performed at much lower confluence. The pH of XC cell culture medium at the confluence of our experiments was pH 7. While this is not its optimal pH, cathepsin B should be moderately active because the protease is active in the range of pH 4 to 7. Thus, it is possible that the paradox of the resistance of XC cell infection to inhibition by weak bases and the exceptional fusogenic phenotype of this cell line may be explained by the presence of secreted cathepsin B. However, additional studies will be required to determine if this is the case.
The prominent 28-kDa cleavage product of Moloney MLV uniquely resisted further degradation at higher concentrations of cathepsin B. Although detailed analysis of the 28-kDa cleavage product is required for a positive identification of its origin, it is intriguing that its apparent molecular mass is similar to that of the receptor binding domain (RBD) of SU (6). Consequently, recombinant RBD from the closely related ecotropic Friend 57 MLV (a gift of Jim Cunningham) was submitted to Western blot analysis using the anti-SU antiserum. This fragment contains residues 1 to 236 of Friend RBD plus 6 additional residues used in its purification (a total of 242 residues). For comparison, a sample of virions incubated with cathepsin B was included. The anti-SU reacted strongly with the purified RBD fragment, which was clearly visible as a major and minor species migrating at 32 kDa and 30 kDa, respectively (Fig. 7C). The ability of the anti-SU antiserum to recognize the RBD is consistent with Niman and Elder's report that SU contains two dominant antigenic hot spots, one within the first 19 residues in the RBD and the other downstream in the proline-rich region (20). Purified RBD retains receptor binding function (10) and can act as a modular unit during virus entry, specifically when rescuing the membrane fusion defects resulting from mutation of histidine 8 in SU (16). It is tempting to speculate that protease cleavage of SU may generate modular RBD units during infection. Identifying the four major cleavage products and the locations of the cleavage sites in future studies should shed light on this issue.
Acknowledgments
We thank Terrance Dermody for the cathepsin B knockout and wild-type MEFs, Hung Fan for plasmid p63.2 encoding an infectious molecular clone of Moloney MLV, Jim Cunningham for the purified receptor binding domain fragment, Alan Rein for the anti-TM antibody, Susan Ross for the VSV G expression plasmid, Adrienne Allen for the NIH 3T3 BAG C8 cells, and Krish Kizhatil for urging pursuit of the identity of the cellular protease.
This work was supported by PHS NIH grant AI33410 (to L.M.A.).
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Andersen, K. B. 1987. Cleavage fragments of the retrovirus surface protein gp70 during virus entry. J. Gen. Virol. 68:2193-2202. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, K. B. 1983. Leupeptin inhibits retrovirus infection in mouse fibroblasts. J. Virol. 48:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, K. B., and B. A. Nexo. 1983. Entry of murine retrovirus into mouse fibroblasts. Virology 125:85-98. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, K. B., and H. Skov. 1989. Retrovirus-induced cell fusion is enhanced by protease treatment. J. Gen. Virol. 70:1921-1927. [DOI] [PubMed] [Google Scholar]
- 5.Bacheler, L., and H. Fan. 1981. Isolation of recombinant DNA clones carrying complete integrated proviruses of Moloney murine leukemia virus. J. Virol. 37:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battini, J. L., O. Danos, and J. M. Heard. 1995. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 69:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttle, D. J., M. Murata, C. G. Knight, and A. J. Barrett. 1992. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 299:377-380. [DOI] [PubMed] [Google Scholar]
- 8.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, T. H., and R. Dornburg. 1997. Toward highly efficient cell-type-specific gene transfer with retroviral vectors displaying single-chain antibodies. J. Virol. 71:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey, R. A., C. A. Hamson, J. J. Healey, and J. M. Cunningham. 1997. In vitro binding of purified murine ecotropic retrovirus envelope surface protein to its receptor, MCAT-1. J. Virol. 71:8096-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609-24617. [DOI] [PubMed] [Google Scholar]
- 12.Huang, I. C., B. J. Bosch, F. Li, W. Li, K. H. Lee, S. Ghiran, N. Vasilieva, T. S. Dermody, S. C. Harrison, P. R. Dormitzer, M. Farzan, P. J. Rottier, and H. Choe. 2006. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 281:3198-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishidoh, K., S. Taniguchi, and E. Kominami. 1997. Egr family member proteins are involved in the activation of the cathepsin L gene in v-src-transformed cells. Biochem. Biophys. Res. Commun. 238:665-669. [DOI] [PubMed] [Google Scholar]
- 14.Kirschke, H., A. J. Barrett, and N. D. Rawlings. 1995. Proteinases 1: lysosomal cysteine proteinases. Protein Profile 2:1581-1643. [PubMed] [Google Scholar]
- 15.Kizhatil, K., and L. M. Albritton. 1997. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 71:7145-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavillette, D., A. Ruggieri, S. J. Russell, and F. L. Cosset. 2000. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J. Virol. 74:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767-773. [DOI] [PubMed] [Google Scholar]
- 18.Menendez-Arias, L., I. T. Weber, and S. Oroszlan. 1995. Mutational analysis of the substrate binding pocket of murine leukemia virus protease and comparison with human immunodeficiency virus proteases. J. Biol. Chem. 270:29162-29168. [DOI] [PubMed] [Google Scholar]
- 19.Montase, M., G. Lalmanach, and L. Mach. 2002. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol. Chem. 383:1305-1308. [DOI] [PubMed] [Google Scholar]
- 20.Niman, H. L., and J. H. Elder. 1982. Structural analysis of Rauscher virus Gp70 using monoclonal antibodies: sites of antigenicity and P15(E) linkage. Virology 123:187-205. [DOI] [PubMed] [Google Scholar]
- 21.Pager, C. T., and R. E. Dutch. 2005. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 79:12714-12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schornberg, K., S. Matsuyama, K. Kabsch, S. Delos, A. Bouton, and J. White. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80:4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simkovic, D., N. Valentova, and V. Thurzo. 1962. An in vitro system for the detection of the Rous sarcoma virus in the cells of the rat tumour XC. Neoplasma 9:104-106. [PubMed] [Google Scholar]
- 24.Simmons, G., D. N. Gosalia, A. J. Rennekamp, J. D. Reeves, S. L. Diamond, and P. Bates. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 102:11876-11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearns, N. A., J. M. Dong, J. X. Pan, D. A. Brenner, and G. G. Sahagian. 1990. Comparison of cathepsin L synthesized by normal and transformed cells at the gene, message, protein, and oligosaccharide levels. Arch. Biochem. Biophys. 283:447-457. [DOI] [PubMed] [Google Scholar]
- 26.Stetina, R., J. Svoboda, and O. Mach. 1975. Long-term preservation of transfecting activity of DNA isolated from rat virogenic XC cells transformed by Prague strain of Rous sarcoma virus. Folia Biol. 21:334-339. [PubMed] [Google Scholar]
- 27.Svoboda, J., P. Chyle, D. Simkovic, and I. Hilgert. 1963. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol. 9:77-81. [PubMed] [Google Scholar]
- 28.Turk, B., V. Turk, and D. Turk. 1997. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol. Chem. 378:141-150. [PubMed] [Google Scholar]
- 29.Turk, V., B. Turk, and D. Turk. 2001. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20:4629-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, G. J., E. L. Nason, C. S. Hardy, D. H. Ebert, J. D. Wetzel, B. V. V. Prasad, and T. S. Dermody. 2002. A single mutation in the carboxy terminus of reovirus outer-capsid protein sigma 3 confers enhanced kinetics of sigma 3 proteolysis, resistance to inhibitors of viral disassembly, and alterations in sigma 3 structure. J. Virol. 76:9832-9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavorotinskaya, T., and L. M. Albritton. 1999. Failure To cleave murine leukemia virus envelope protein does not preclude its incorporation in virions and productive virus-receptor interaction. J. Virol. 73:5621-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]