Abstract
The Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen (LANA) protein is functionally pleiotropic. LANA contributes to KSHV-associated pathogenesis, in part, by increasing entry of cells into S phase through a process that is driven by LANA interaction with the serine-threonine kinase glycogen synthase kinase 3 (GSK-3) and stabilization of β-catenin. We now show that LANA affects the activity of another protein involved in cell cycle regulation, c-Myc. Sequencing of c-Myc coding sequences revealed that c-Myc in KSHV-positive primary effusion lymphoma (PEL) cell lines is wild type in the N-terminal region that regulates c-Myc protein stability. Despite this, c-Myc in PEL cells is stabilized. In LANA-expressing cells, inactivation of nuclear GSK-3 reduced phosphorylation of c-Myc at Thr58 and contributed to c-Myc stabilization by decreasing c-Myc ubiquitination. Phosphorylation of c-Myc on Ser62 also affects c-Myc stability and function. We now show that LANA increases the level of phosphorylated extracellular signal-regulated kinase 1 (ERK1) and increases ERK phosphorylation of c-Myc on Ser62. LANA also interacted with c-Myc, and c-Myc amino acids 147 to 220 were required for this interaction. LANA (L1006P) retained the ability to bind to c-Myc and activate ERK1, indicating that these events did not require LANA interaction with GSK-3. Thus, LANA stabilizes c-Myc; prevents the phosphorylation of c-Myc at Thr58, an event that promotes Myc-induced apoptosis; and independently stimulates phosphorylation of c-Myc at Ser62, an event that transcriptionally activates c-Myc. LANA-mediated manipulation of c-Myc function is likely to contribute to KSHV-associated tumorigenesis through the induction of c-Myc regulated cellular genes, as well as by the stimulation of cell cycle progression.
Kaposi's sarcoma-associated herpesvirus (KSHV) was discovered in lesions of Kaposi's sarcoma using differential display (12) and was subsequently recognized to also be associated with primary effusion lymphoma and multicentric Castleman's disease (10, 18, 52, 59). The KSHV latency-associated nuclear antigen (LANA) is one of a limited number of KSHV genes consistently expressed in latently infected cells and in KSHV-associated malignancies (47). LANA is encoded by KSHV ORF73 and has unique N-terminal and C-terminal domains separated by three sets of repeated sequences that represent approximately half of the total protein sequence. These repeats function similarly to the central repeat region of the Epstein-Barr virus EBNA-1 protein by inhibiting antigen presentation and allowing tumor cells expressing LANA to escape immune surveillance (2, 16, 70).
LANA is a multifunctional protein that is essential for the replication (5, 20, 29, 34) and maintenance (4) of KSHV episomal DNA during latent infection. LANA binds to the terminal repeats of the KSHV genome (14, 25); links the genomes to the cell chromosomes through interactions with chromatin-associated proteins such as the core histones H2A and H2B, DEK, HP1, Brd4, and MeCP2 (6, 28, 37, 69); and recruits cellular DNA replication machinery to the terminal repeats (45, 60, 62, 64). Expression of LANA in a transgenic mouse generated activated, hyperproliferative B cells, and mice developed lymphoma with a long latency (19). LANA has multiple properties that could contribute to tumorigenesis. These include inhibition of p53-mediated apoptosis (9, 21), stimulation of S-phase entry through stabilization of β-catenin and upregulation of cyclin D1 (24) and through induction of Rb/E2F-regulated genes (1, 49), and overcoming G1 cell cycle arrest mediated by p16 (1) and BRD4 and BRD2 (46).
LANA is also responsible for promoting KSHV latency gene expression at the expense of lytic induction and for some of the reprogramming of cell gene expression that occurs in KSHV-infected cells (1, 57, 65, 66). Targeting of LANA to DNA either through the use of Gal4-LANA fusion proteins (38, 53) through binding of LANA to the KSHV terminal repeats (25) or through LANA recruitment to cell (57) or viral promoters (39, 42) leads to transcriptional repression. LANA binds to histone deacetylase-associated corepressors (38) and is also capable of recruiting de novo DNA methyltransferases and the histone methyl transferase SUV39H1 to downregulate targeted cell promoters through CpG methylation (50, 57).
LANA has also been reported to increase expression of genes regulated by a variety of transcription factors (40, 44, 61, 63). A source of indirect transcriptional reprogramming is the interaction between LANA and glycogen synthase kinase 3 (GSK-3). LANA mediates a cell cycle-regulated nuclear relocalization of GSK-3 that depletes GSK-3 from the cytoplasmic β-catenin destruction complex, stabilizing β-catenin and making β-catenin available for transcriptional activation of target genes (24). In addition, the LANA-GSK-3 interaction leads to an overall inactivation of nuclear GSK-3. This inactivation is mediated by phosphorylation of GSK-3β on serine 9 by the coordinated activity of the kinases extracellular signal-regulated kinase 1/2 (ERK1/2) and ribosomal S6 kinase 1 (RSK1), which coprecipitate with LANA (41). ERK1/2 and RSK1 form a complex, and ERK1/2 also binds to GSK-3. ERK1/2 priming phosphorylation promotes serine 9 phosphorylation of GSK-3β by RSK1 (17). LANA-mediated inactivation of nuclear GSK-3 opens up the possibility that decreased GSK-3 activity may modify the function of transcription factors that are normally GSK-3 substrates. This was shown to be the case for the C/EBP proteins C/EBPα and C/EBPβ, where diminished GSK-3 phosphorylation translated into a LANA-mediated block in terminal differentiation (41).
Another known GSK-3 substrate is Myc, whose activity is also regulated by ERK1/2 (31). The interactions between LANA and the GSK-3 and ERK1/2 kinases led us to evaluate the effects of LANA expression on Myc phosphorylation and activity. We show that LANA both decreases Myc phosphorylation on threonine 58 and increases phosphorylation on serine 62. This combination affects Myc stability, transcriptional activity, and apoptotic functions and is likely to account for the high proportion of Myc-regulated genes present in a previous gene array analysis documenting LANA modulation of cell gene expression. While this work was being prepared for publication, another study was published that also found reduced threonine 58 phosphorylation of Myc in LANA-expressing cells (8).
MATERIALS AND METHODS
Plasmids and antibodies.
Flag-LANA and HA-GSK-3β expression plasmids were previously described (22). The wild-type c-Myc expression vector was obtained from C. Dang, Johns Hopkins (30). c-Myc deletion mutants were obtained from W. Tansey, Cold Spring Harbor Laboratory (51). S62A and T58A c-Myc mutants were from S. Hann, Vanderbilt University (27). Anti-c-Myc, anti-pT58-c-Myc, and anti-pS62-c-Myc monoclonal antibodies were purchased from Abcam, and the anti-hemagglutinin (HA) and anti-Flag antibodies used for immunoblotting and immunoprecipitation were obtained from Sigma.
Cell culture and transfection.
BCBL1, BC2, BC3, and JSC1 PEL cells and Daudi, Raji, and Akata 4E3 lymphoma B cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum. HeLa cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum and transfected by using calcium phosphate precipitation. For protein stability experiments, cells were treated with 25 μM cycloheximide for the indicated period before harvesting.
Immunoprecipitation and immunoblotting.
HeLa cells seeded at 106 per 10-cm dish were transfected by using calcium phosphate precipitation with HA-c-Myc (7 μg), Flag-LANA (7 μg), and/or S-GSK-3β (7 μg). Cells were harvested 48 h posttransfection, resuspended in 2 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.8], 0.2% Nonidet P-40, 5% glycerol, 1 mM dithiothreitol, 0.5 mM EDTA, 50 mM NaCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin per ml, 5 μg of aprotinin per ml, 0.5 mM NaF, and 1 mM sodium pyrophosphate) and lysed with rotation for 30 min. Lysates were precleared by mixing with Sepharose beads (50 μl), followed by centrifugation at 5,000 rpm for 5 min. The supernatant was subjected to a second centrifugation at 15,000 rpm for 15 min. The supernatant was then incubated with anti-HA monoclonal antibody (1 μg) or anti-Flag M2 affinity gel for 2 h at 4°C, followed by incubation with protein G-Sepharose beads (50 μl, swollen volume) for 2 h at 4°C. Beads were washed four times with ice-cold lysis buffer, resuspended in sample buffer (50 μl), and boiled prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Metabolic labeling with [32P]orthophosphate.
Two days after transfection, HeLa cells were rinsed with 1× phosphate-buffered saline (pH 7.4), washed with phosphate-free DMEM, incubated in phosphate-free DMEM plus 10% dialyzed fetal bovine serum for 1 h, and then incubated for 4 h with the same medium containing [32P]orthophosphate (carrier-free [Amersham; 4 mCi/10-cm dish, 250 μCi/ml), with or without 25 mM LiCl. Labeling was stopped by washing the the cells twice with ice-cold PBS. Labeled cells were lysed and subjected to immunoprecipitation with anti-c-Myc antibody as described above. Immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by autoradiography and Western blotting.
RESULTS
c-Myc is wild-type but stabilized in PEL cells.
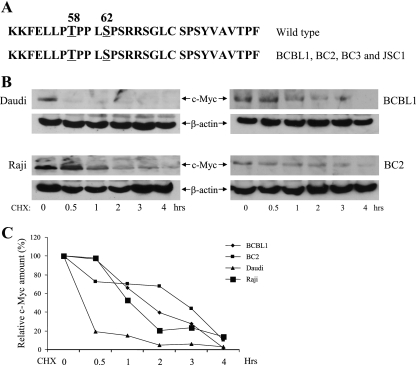
The N-terminal domains of c-Myc are frequently mutated in human lymphomas (3). To ascertain the status of c-Myc in primary effusion lymphoma (PEL) cell lines, c-Myc transcripts were amplified by reverse transcription-PCR, and codons 1 to 152 were directly sequenced. The BCBL1, BC2, BC3, and JSC1 PEL cell lines were all found to contain wild-type c-Myc in the region encoding amino acids (aa) 1 to 152 (Fig. 1A). The stability of c-Myc in the BCBL1 and BC2 PEL cell lines was next compared to that of c-Myc in Epstein-Barr virus (EBV) lymphoma cell lines carrying wild-type c-Myc (Daudi) or mutated c-Myc (Raji). Cell extracts were prepared at the indicated times after a cycloheximide block, and c-Myc protein was detected by immunoblotting. As expected for N-terminally mutated c-Myc versus wild-type c-Myc, the half-life of c-Myc in Raji cells was much longer than that in Daudi B cells (Fig. 1B and C). Surprisingly, the half-life of c-Myc in the BCBL1 and BC2 PEL lines was also extended, despite the fact that no mutations were present in the c-Myc N terminus (Fig. 1B and C).
FIG. 1.
c-Myc is wild-type and stabilized in PEL cells. (A) Comparison of the amino acid sequence surrounding the phospho-regulatory region of wild-type c-Myc and the sequence predicted from RT-PCR sequencing of c-Myc transcripts in BCBL1, BC2, BC3, and JSC1 PEL cells. (B) c-Myc stability. Western blots of extracts of B cells (Daudi [wt c-Myc] and Raji [mutant c-Myc]) and PEL cells (BCBL1 and BC2) probed with anti-c-Myc or anti-β-actin antibodies are shown. Cells were harvested after treatment for the indicated times with cycloheximide (25 μg/ml). (C) Quantitation of the immunoblot signal in panel B. c-Myc was normalized to the β-actin signal and then plotted against the signal obtained at 0 h of cycloheximide treatment. CHX, cycloheximide.
LANA stabilizes wild-type c-Myc but not S62A c-Myc.
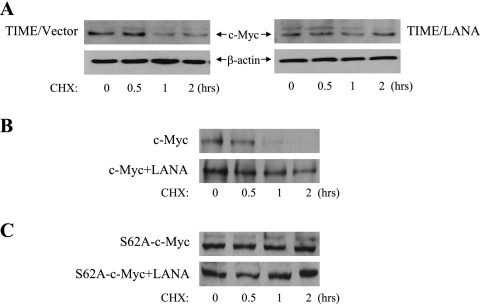
To determine whether LANA was responsible for the enhanced stability of c-Myc in PEL cells, endogenous c-Myc stability was examined using cycloheximide treatment in vector-transduced telomerase-immortalized microvascular endothelial (TIME) cells versus LANA-transduced TIME cells. Western blotting revealed that c-Myc half-life was extended in the LANA-expressing TIME cells (Fig. 2A). LANA was also able to stabilize wild-type c-Myc in transfected cells. Western blotting of extracts from HeLa cells transfected with wild-type c-Myc alone (Fig. 2B, upper) or plus LANA (Fig. 2B, lower) and treated with cycloheximide showed that the transfected c-Myc was stabilized in the presence of LANA. c-Myc is phosphorylated by GSK-3 on Thr58, an event that targets c-Myc for degradation and is primed by phosphorylation of Ser62 by ERK. In the absence of Ser62 phosphorylation, GSK-3 cannot phosphorylate Thr58. The stability of an S62A c-Myc protein was then examined in transfected HeLa cells. Cotransfection of LANA with S62A c-Myc had no effect on c-Myc stability (Fig. 2C). The data suggested that LANA-mediated inactivation of nuclear GSK-3 plays a role in stabilization of c-Myc.
FIG. 2.
LANA stabilizes c-Myc. (A) Stabilization of c-Myc in TIME cells. Western blots of extracts of LANA- or vector-transduced TIME cells probed with anti-c-Myc or anti-β-actin antibodies. Cells were harvested after treatment for the indicated times with cycloheximide (25 μg/ml). (B and C) Western blots of extracts from HeLa cells transfected with wild-type c-Myc (B) or S62A c-Myc mutated in the ERK priming site (C). Cells were transfected with c-Myc alone (upper panel) or c-Myc plus LANA (lower panel) and harvested after treatment for the indicated times with cycloheximide. Membranes were probed with anti-c-Myc antibody. CHX, cycloheximide.
LANA blocks Thr58 phosphorylation by GSK-3 and c-Myc ubiquitination.
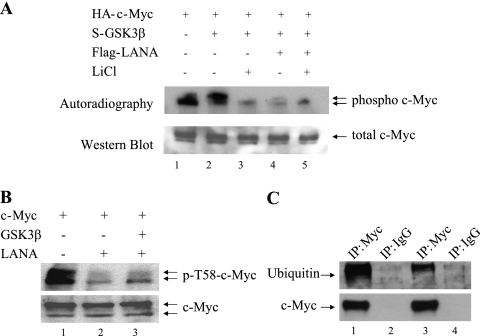
The effect of LANA on GSK-3 phosphorylation of c-Myc was examined in transfected HeLa cells that were incubated with [32P]orthophosphate for 4 h prior to harvesting to metabolically label phosphorylated proteins. HA-c-Myc was immunoprecipitated from the transfected cell extracts with anti-HA antibody, and the immunoprecipitates were subjected to Western blotting with anti-c-Myc antibody (Fig. 3A, lower) and autoradiography to visualize phosphorylated HA-c-Myc (Fig. 3A, upper). Cotransfection of GSK-3β led to an increase in a slower-migrating, presumably doubly Thr58/Ser62 phosphorylated, form of c-Myc (Fig. 3A, lane 2). Incubation of the cells with LiCl, a GSK-3 inhibitor, reduced the presence of the faster-migrating phosphorylated form and eliminated detection of the slower-migrating phosphorylated form of c-Myc (Fig. 3A, lane 3), indicating that GSK-3 phosphorylation at Thr58 was required for the formation of the slower-migrating form and also contributed to the faster-migrating phosphorylated form of c-Myc. Cotransfection of the cells with LANA also led to a decrease in the faster-migrating phosphorylated form of c-Myc and to a dramatic reduction in the presence of the slower migrating phosphorylated form (Fig. 3A, lane 4 versus lane 2). The addition of LANA to cells treated with LiCl resulted in the same pattern of c-Myc phosphorylation, as was seen in cells treated with LiCl in the absence of LANA (Fig. 3A, lanes 3 and 5). This result implies that LANA and LiCl are functioning similarly and is consistent with LANA blocking the GSK-3 phosphorylation of c-Myc.
FIG. 3.
LANA blocks GSK-3 phosphorylation and ubiquitination of c-Myc. (A) HeLa cells transfected with the indicated plasmids were metabolically labeled with [32P]orthophosphate for 4 h. Extracts were immunoprecipitated with anti-HA antibody and subjected to autoradiography (upper panel) or Western blotting with anti-HA antibody to detect HA-c-Myc (lower panel). LiCl is a GSK-3 inhibitor. (B) Western blots of extracts of HeLa cells transfected with the indicated plasmids and probed with T58 phospho-specific c-Myc antibody (upper panel) or anti-c-Myc antibody (lower panel). (C) Extracts of HeLa cells transfected with HA-c-Myc and GSK-3β alone (lanes 1 and 2) or plus LANA (lanes 3 and 4) were immunoprecipitated with anti-HA or control anti-body as indicated. Western blots were probed with anti-ubiquitin antibody (upper panel) or anti-c-Myc antibody (lower panel).
The concept that LANA-mediated inactivation of GSK-3 was preventing GSK-3 phosphorylation of c-Myc on Thr58 was directly tested by probing Western blots of transfected HeLa cell lysates with phospho-Thr58 specific anti-c-Myc antibody. In cells transfected with c-Myc alone, the anti-phospho-Thr58 antibody detected two c-Myc bands (Fig. 3B, lane 1) that resembled the two-band pattern seen with metabolic labeling of c-Myc in Fig. 3A. The two forms of c-Myc detected by the anti-phospho-Thr58 antibody are the dually S62 and Thr58 phosphorylated protein and the singly Thr58 phosphorylated protein. Cotransfection with LANA reduced the levels of both bands (Fig. 3B, lane 2), and LANA mediated this effect even in the presence of cotransfected GSK-3β (Fig. 3B, lane 3). Western blots of the same samples were probed with anti-c-Myc antibody to show equivalent c-Myc protein loading (Fig. 3B, lower). GSK-3 phosphorylation of c-Myc on Thr58 targets c-Myc for ubiquitination and proteasomal degradation. The effect of LANA on c-Myc ubiquitination was also examined. HeLa cells were transfected with HA-c-Myc, alone or in the presence of LANA, and cell extracts were immunoprecipitated with anti-c-Myc antibody and subjected to immunoblotting with anti-ubiquitin antibody. The c-Myc immunoprecipitated from the transfected cells was heavily ubiquitinated (Fig. 3C, lane 1), and cotransfection with LANA significantly reduced the amount of ubiquitination (Fig. 3C, lane 3). Equal amounts of total c-Myc were present in the samples examined, as shown by probing a parallel immunoblot with c-Myc antibody (Fig. 3C, lower). The anti-immunoglobulin G immunoprecipitations (Fig. 3C, lanes 2 and 4) served as controls for the specificity of the anti-c-Myc immunoprecipitations. Thus, LANA stabilizes c-Myc in part by blocking GSK-3 phosphorylation of c-Myc on Thr58 and limiting the subsequent ubiquitination of c-Myc.
c-Myc interacts with LANA.
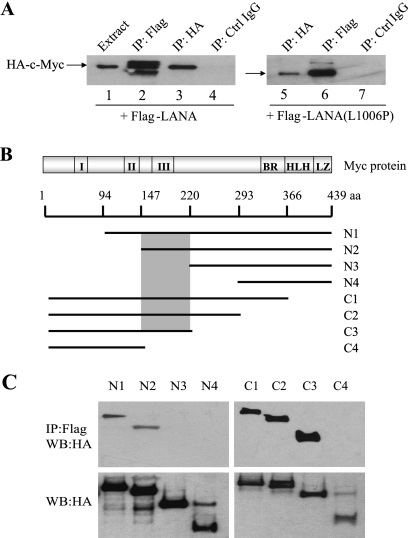
LANA-mediated blocking of c-Myc phosphorylation at Thr58 is an indirect effect of LANA's interaction with, and inactivation of, nuclear GSK-3. We wondered whether LANA might also manipulate c-Myc function in other ways and tested for an interaction between LANA and c-Myc. HeLa cells were cotransfected with HA-c-Myc and Flag-LANA and immunoprecipitates of the cell extracts were Western blotted and probed with anti-HA antibody to detect HA-c-Myc. HA-c-Myc was present in the cell extract (Fig. 4A, lane 1), in the direct anti-HA immunoprecipitate (Fig. 4A, lane 3), but not in the immunoprecipitate generated with control immunoglobulin G (Fig. 3A, lane 4). HA-c-Myc was also detected in the indirect immunoprecipitate generated with anti-Flag antibody (Fig. 4A, lane 2), indicating that LANA interacts with c-Myc.
FIG. 4.
LANA interacts with c-Myc. (A) Western blots of extracts of HeLa cells transfected with HA-c-Myc and either wild-type Flag-LANA or Flag-LANA(L1006P). Membranes were probed with anti-HA antibody to detect c-Myc. (B) Schematic of c-Myc N-terminal and C-terminal deletions. I, II, III, Myc conserved domains; BR, basic region; HLH, helix-loop-helix; LZ, leucine zipper. The shaded box indicates LANA-binding domain. (C) Western blots of extracts of HeLa cells cotransfected with Flag-LANA and the HA-c-Myc variants. Extracts were immunoprecipitated with anti-Flag antibody (upper panel) and probed with anti-HA antibody. Expression of the HA-c-Myc variants is detected with anti-HA antibody (lower panel).
Both LANA and c-Myc are substrates for GSK-3, and LANA interacts with GSK-3 (23). It was therefore possible that the apparent interaction between LANA and c-Myc was an indirect interaction mediated through GSK-3. To test for a GSK-3 mediated interaction, the experiment was repeated with either wild-type Flag-LANA or a LANA mutant (LANA-L1006P) that has been shown to be incapable of binding GSK-3 (23). Western blotting of extracts from cells cotransfected with Flag-LANA(L1006P) detected HA-c-Myc in both direct anti-HA and indirect anti-Flag immunoprecipitates (Fig. 4A, lanes 5 and 6). This result indicates that the interaction between LANA and c-Myc is not mediated through a common GSK-3 interaction and suggests that the interaction may be direct.
c-Myc aa 147 to 220 are required for LANA interaction.
A series of N-terminal and C-terminal deletions of HA-c-Myc (51) were used to map the region of c-Myc that was required for interaction with LANA (Fig. 4B). HeLa cells were cotransfected with Flag-LANA and the HA-c-Myc variants. Cell extracts were immunoprecipitated with anti-Flag antibody, and Western blots were probed with anti-HA antibody to detect coprecipitating HA-c-Myc. The N-terminal deletion variants N1 and N2 retained the ability to coprecipitate with Flag-LANA, but N3 and N4 did not coprecipitate, indicating that c-Myc sequences between aa 147 and 220 were necessary for interaction with LANA (Fig. 4C, upper section). The same region of c-Myc was identified by using the C-terminal c-Myc deletions. HA-c-Myc variants C1, C2, and C3 coprecipitated with Flag-LANA, whereas C4 did not coprecipitate (Fig. 4C, upper section). The C3 variant terminates at aa 220, and the C4 variant terminates at aa 147. The expression of the HA-c-Myc variants is demonstrated in the Western blot of the transfected cell extracts (Fig. 4C, lower section).
LANA activates ERK1 and increases S62 c-Myc phosphorylation.
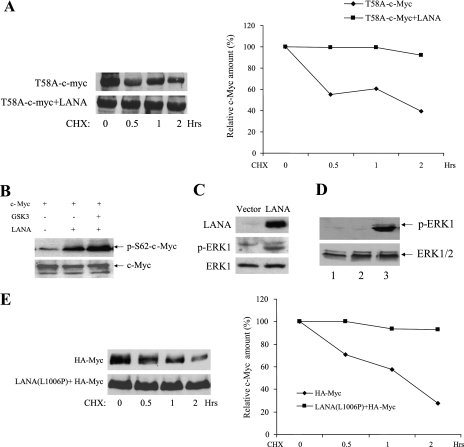
We have recently shown that LANA is a substrate for ERK1 and that ERK1 is present in anti-LANA immunoprecipitates generated using PEL cell extracts (41). Singly S62 phosphorylated c-Myc is also stabilized and activated in settings in which S62 phosphorylation does not prime for subsequent GSK-3 phosphorylation. The ability of LANA to associate with both ERK1 and c-Myc suggested that LANA might also increase S62 phosphorylation of c-Myc. To examine this question, we first tested the ability of LANA to stabilize the T58A c-Myc mutant that is unable to be phosphorylated by GSK-3. A Western blot of extracts from T58A c-Myc transfected and T58 c-Myc plus LANA cotransfected HeLa cells treated with cycloheximide was probed with anti-c-Myc antibody (Fig. 5A, left panel). The stability of T58A-c-Myc was significantly increased by cotransfection of LANA (Fig. 5A, right panel).
FIG. 5.
LANA increases S62 c-Myc phosphorylation and activates endogenous ERK1 independently of the LANA-GSK-3 interaction. (A) The left panel shows an immunoblot of extracts from HeLa cells transfected in the absence (upper) or presence of LANA (lower) with T58A c-Myc mutated in the GSK-3 phosphorylation site and harvested at the indicated times after treatment with cycloheximide (25 μg/ml). The right panel shows quantitation of the immunoblot signal. (B) Immunoblot of transfected HeLa cell extracts probed with phospho-S62 specific c-Myc antibody (upper) or c-Myc antibody (lower). (C) Immunoblot of LANA-transfected HeLa cell extracts probed with anti-LANA antibody (upper section), anti-phospho-ERK1 antibody (middle section), or anti-ERK1 antibody (lower section). (D) Immunoblots of extracts from HeLa cells that were untransfected (lane 1) or transfected with vector plasmid (lane 2) or the non-GSK-3 interacting mutant LANA(L1006P) (lane 3) and probed with anti-phospho-ERK1 antibody (upper section) or anti-ERK1 antibody (lower section). (E) The left panel shows immunoblots of extracts from HeLa cells transfected with HA-Myc alone (upper section) or in the presence of LANA(L1006P) (lower section). Cells were harvested at the indicated times after treatment with CHX (25 mg/ml). The right panel shows quantitation of the immunoblot signal. CHX, cycloheximide.
To demonstrate that the increased stability of T58A c-Myc was linked to increased S62 c-Myc phosphorylation, S62 phosphorylation of wild-type c-Myc was monitored by using anti-phospho S62 specific antibody. Western blotting of transfected cell extracts revealed that cotransfection with LANA significantly increased S62 phosphorylation of c-Myc, and this increase was not overcome by including GSK-3 in the cotransfection (Fig. 5B). Equal loading of total c-Myc was monitored by probing a parallel Western blot with c-Myc antibody (Fig. 5B, lower).
ERK1 is activated by phosphorylation (36). The strong increase in ERK phosphorylation of c-Myc led to an investigation of the effect of LANA on the phosphorylation status of ERK itself. Western blotting of extracts from LANA-transfected HeLa cells revealed that, compared to the vector-transfected control, phosphorylation of endogenous ERK1 was increased in LANA-expressing cells (Fig. 5C). Total ERK1 levels were not affected by LANA, as shown by probing the extracts with anti-ERK1 antibody (Fig. 5C, lower). To determine whether the increase in ERK1 phosphorylation was a downstream consequence of the LANA-GSK-3 interaction, the effect of a non-GSK-3 interacting mutant (23) on endogenous ERK1 phosphorylation was examined in LANA(L1006P)-transfected HeLa cells. Increased ERK1 phosphorylation was observed in cells transfected with LANA(L1006P) (Fig. 5D, lane 3) compared to untransfected or vector-transfected cells (Fig. 5D, lanes 1 and 2), indicating that the LANA-mediated increase in ERK1 phosphorylation was independent of signaling elicited as a consequence of the LANA-GSK-3 interaction.
If the component of c-Myc stabilization that is mediated by S62 phosphorylation is a consequence of increased ERK1 phosphorylation, then LANA(L1006P) should also be capable of stabilizing c-Myc. This point was examined in HeLa cells transfected with HA-Myc alone or in the presence of LANA(L1006P) (Fig. 5E, left panel). LANA(L1006P) efficiently stabilized HA-Myc (Fig. 5E, right panel). Thus, LANA stabilizes c-Myc through two mechanisms, only one of which is dependent on the LANA-GSK-3 interaction.
LANA increases Ser62 phosphorylation and decreases Thr58 phosphorylation to generate stabilized, activated c-Myc.
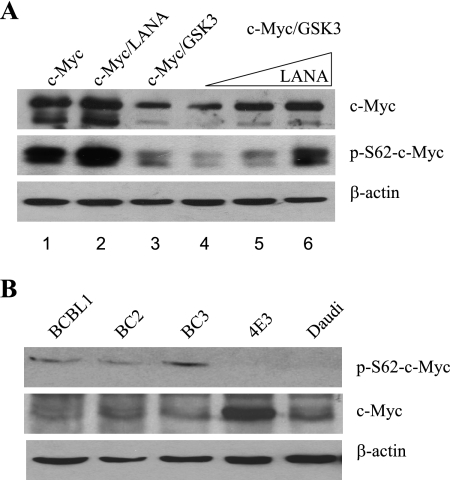
The correlation between increased S62-c-Myc phosphorylation, decreased GSK-3 (Thr58) phosphorylation, and wild-type c-Myc stabilization was examined in transfected HeLa cells. Western blots of transfected cell extracts probed with anti-c-Myc antibody (Fig. 6A, upper) showed that, as expected, cotransfection of GSK-3 with wild-type c-Myc reduced the steady-state level of c-Myc (Fig. 6A, upper section, lane 3 versus lane 1). Cotransfection of LANA with c-Myc and GSK-3 led to a dose-dependent increase in the steady-state level of c-Myc (Fig. 6A, upper section, lanes 4 to 6), demonstrating that LANA can overcome the destabilizing effect of Thr58 phosphorylation of c-Myc by GSK-3. A Western blot of the same samples was probed with phospho S62-c-Myc antibody (Fig. 6A, middle). Cotransfection of GSK-3 with wild-type c-Myc also decreased the level of phospho S62-c-Myc as dual S62/T58 phosphorylation increases proteasomal targeting of c-Myc (Fig. 6A, middle section, lane 3 versus lane 1). In cells receiving LANA plus GSK-3 there was a dose-responsive increase in the S62 phosphorylated form of c-Myc (Fig. 6A, middle section, lanes 4 to 6). The Western blot was also probed with anti-β-actin antibody to monitor protein loading (Fig. 6A, lower section). The data indicate that LANA leads to an accumulation of the S62 phosphorylated form of activated, stabilized c-Myc.
FIG. 6.
LANA increases active, steady-state c-Myc. (A) Immunoblot of transfected HeLa cell extract probed with anti-c-Myc antibody (upper section), anti-phospho-S62 c-Myc antibody (middle section), or anti-β-actin antibody (lower section). (B) Immunoblot of extracts from PEL cells (BCBL1, BC2, and BC3) and lymphoma B cells (4E3, Daudi) probed with anti-phospho-S62, anti-c-Myc, or anti-β-actin antibodies as indicated.
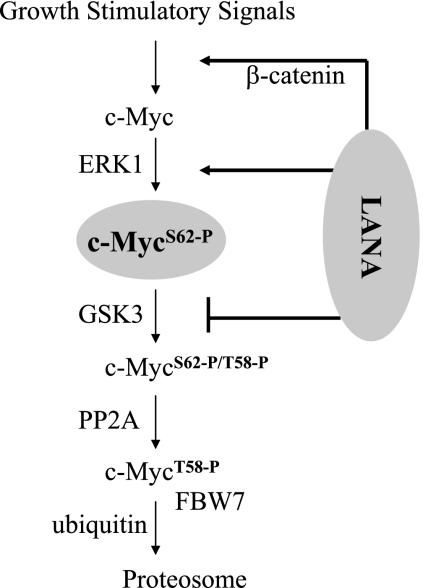
The phosphorylation status of c-Myc was also examined in PEL and EBV-positive and -negative B-cell lines by Western blotting. Phospho-S62-c-Myc was not detectable in extracts from the EBV-negative (4E3) or EBV-positive (Daudi) lymphoma cell lines but was present in extracts of the BCBL1, BC2, and BC3 PEL cells. Blots were also probed with anti-c-Myc antibody and anti-β-actin antibody to show c-Myc and total protein loading (Fig. 6B, middle and lower panels). Thus, the accumulation of S62 stabilized c-Myc seen in transfected cells is also a feature of KSHV-positive, LANA-expressing PEL cells. LANA interactions with the c-Myc pathway that lead to the induction, stabilization, and transcriptional activation of c-Myc are summarized in Fig. 7.
FIG. 7.
Summary of LANA interactions with the c-Myc pathway. LANA increases c-Myc transcription through the previously described accumulation of β-catenin (24) and stabilizes S62 phosphorylated c-Myc by the combination of ERK activation and GSK-3 inhibition.
DISCUSSION
Myc expression is frequently dysregulated in cancers. Translocation of the myc locus such that Myc expression is driven by the immunoglobulin enhancer occurs in Burkitt's lymphoma but the Myc in Burkitt's lymphoma cell lines also contains mutations, particularly in the N-terminal region of Myc. This observation suggests that upregulated transcription of Myc alone is insufficient to drive the oncogenic process and that additional changes that affect Myc protein stability, transcriptional activity or Myc-induced apoptotic activity are also required. PEL cells do not contain Myc translocations but the LANA that is expressed in latently KSHV-infected cells would be expected to upregulate Myc transcriptionally through LANA-mediated induction of β-catenin which is a known transactivator of Myc expression. The N terminus of the Myc gene amplified from KSHV-infected PEL cell lines in our study was found to have a wild-type sequence, and other investigators have also recently described Myc to be wild type in PEL (8). We have now demonstrated that LANA also interacts with Myc and regulates Myc function posttranscriptionally by altering the phosphorylation state of Myc at both T58 and S62.
The interaction between LANA and GSK-3 leads to a reduction in overall nuclear GSK-3 enzymatic activity. The GSK-3 bound to LANA is capable of phosphorylating the LANA N terminus but does not phosphorylate an exogenous substrate in vitro (23). Further, introduction of LANA blocks GSK-3 phosphorylation of nuclear substrates such as C/EBPα or C/EBPβ in cotransfected cells (41). We now show that another substrate, Myc, also shows a reduction in GSK-3 phosphorylation in the presence of LANA. GSK-3 phosphorylates c-Myc on T58. The doubly phosphorylated T58/S62 Myc is bound by Fbw7, a substrate recognition protein of the ubiquitin ligase complex. GSK-3 phosphorylation of Myc on T58 is essential for Fbw7 binding and increases ubiquitination and proteasomal degradation of Myc. T58A Myc was found to be more stable and less ubiquitinated than wild-type Myc (26, 51, 67). An increase in nuclear GSK-3 mediated by RhoB was also found to decrease the stability of Myc (35). However, the half-life of T58A Myc is less than that of Myc in Burkitt's lymphoma cells, suggesting that there are other mechanisms that contribute to Myc stability. Known mechanisms include S62 phosphorylation, which is discussed below, and regulation through the aa 226 to 270 PEST sequence. The PEST motif regulates protein stability by calpain-mediated proteolysis, and Myc is cleaved after calpain activation (58).
Mutation of T58 increases Myc transforming ability in a variety of systems. MycT58A can cooperate with Ras and telomerase to cause anchorage-independent growth of human fibroblasts (68), and mice reconstituted with stem cells expressing T58A Myc developed tumors with decreased latency and increased penetrance compared to that seen with wild-type Myc (32). Although there is evidence that the T58 mutation affects Myc stability, the contribution to tumorigenesis is predominantly through an effect on apoptosis. Deregulation of Myc induces apoptosis (43), but the T58A mutation discriminates between the apoptotic and cell proliferative functions of Myc (13). In the mouse hematopoietic stem cell reconstitution experiments (32), coexpression of Bcl-2 eliminated the differences in pre-B cell tumor formation seen with T58A and wild-type Myc. The explanation appears to be that phosphorylation of T58 regulates expression of the proapoptotic Myc target Bim (32). Thus, the LANA-associated reduction in T58 phosphorylation would both increase c-Myc stability and decrease Myc-mediated apoptosis.
LANA affected Myc phosphorylation at a second position, S62. In this case, LANA had the opposite effect, and Myc S62 phosphorylation was increased. The role of S62 phosphorylation is complex because the phosphorylation of S62 primes Myc for GSK-3 phosphorylation at T58, and this should increase T58-dependent Myc apoptotic activity and/or Myc degradation. However, there is evidence that S62 phosphorylation contributes to transformation. The Myc mutants S62A, S62P, and T58I (which also prevents S62 phosphorylation) are less oncogenic than wild-type Myc (11, 48). S62 is the site of ERK-induced stabilization of Myc (55, 68), and enhanced S62 phosphorylation increases Myc transcriptional activation of Gal4-Myc and of the E2F2 promoter (54, 56). One study found a role for S62 phosphorylation in the specific transcriptional upregulation of gamma-glutamyl-cysteine synthetase, a key enzyme in the protective response to oxidative stress (7). In a study of BJAB cells converted to express LANA in an inducible manner, 186 cell genes demonstrated LANA-modulated expression (1). A comparison of these genes with the myc gene target library (www.myccancergenes.org) revealed that 51 of 186 (27%) were Myc regulated. If the LANA-modulated genes are restricted to those in the categories of Rb/E2F pathway, cell cycle regulation, apoptosis, signal transduction, transcription, tumorigenesis, and metabolism, then the numbers are 38 of 119 (31.9%). In either case, the numbers are significantly higher than the 10 to 15% of total cellular genes that are estimated to be regulated by c-Myc (15).
LANA could not stabilize S62A Myc. Since the mutation of S62A would prevent priming of Myc for GSK-3 phosphorylation, this result does not discriminate between the contributions of S62 and T58 to Myc protein stability. However, LANA was able to increase S62 phosphorylation and stabilize the T58A Myc mutant. Increased S62-phosphorylated Myc was also detectable in PEL cells compared to KSHV-negative 4E3 and Daudi B-cell lines. Thus, LANA stabilization of Myc arises from two sources: (i) LANA-mediated interference with GSK-3 phosphorylation of T58 and (ii) LANA-induced phosphorylation of S62. We have previously shown that ERK1 coprecipitates with LANA and that the LANA associated ERK1 is enzymatically active (41). We now show that the proportion of ERK1 present in the phosphorylated, activated state is increased in LANA-transfected cells. It is currently unclear by what means LANA facilitates increased nuclear ERK1 phosphorylation. However, we found that the phosphorylation of ERK1 was independent of the LANA-GSK-3 interaction.
We also found Myc interacting with LANA. The interaction with Myc was similarly independent of the GSK-3 interaction since Myc coprecipitated with a LANA mutant that has lost GSK-3 interaction. It is possible that LANA is serving as a platform to bring ERK1 and Myc into apposition to facilitate S62 phosphorylation of Myc. The Myc region from aa 147 to 220 was required for the binding of LANA. This region of Myc lies between the conserved Myc Box II (MB II) and the PEST region and covers the conserved region MB III. The interaction between LANA and Myc thus has the potential to modify the activity of MB III. However, MB III is not well characterized, making the outcome of the interaction difficult to predict. Deletion of MB III did not affect the S-phase-promoting activity of Myc and had a modest effect on the transcriptional activation of target genes but produced a more significant loss in transcriptional repression of the Myc targets gadd45, p21, and p15, with a corresponding increase in Myc-induced apoptosis (33). Since loss of T62 phosphorylation ameliorates the apoptotic activity of Myc, this particular function may not be affected by LANA binding to MB III, but the possibility that LANA might modify Myc transcriptional repression activity is interesting.
In summary, LANA blocks Myc phosphorylation at T58 and stimulates phosphorylation at S62. The posttranscriptional regulation at T58 increases Myc stability and decreases Myc-induced apoptosis, whereas phosphorylation at S62 increases Myc stability and enhances Myc transcriptional activity. Both T58 and S62 modifications enhance Myc oncogenic activity. Reanalysis of LANA modulated cellular genes from a published study indicates that ∼31% of genes in cell growth regulatory categories are regulated indirectly by LANA through c-Myc. In addition, LANA may modify Myc transcriptional repression activity through interaction with MB III and increase Myc expression at the transcriptional level through the upregulation of β-catenin. The multiple facets of Myc function targeted by LANA highlight both the critical importance of Myc for cell proliferation and the complexities of Myc regulation.
Acknowledgments
We thank C. V. Dang (Johns Hopkins School of Medicine), W. P. Tansey (Cold Spring Harbor Laboratory), and S. R. Hann (Vanderbilt University School of Medicine) for providing c-Myc expression plasmids.
This study was supported by National Cancer Institute grant PO1 CA113239 to S.D.H.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from P16INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. [DOI] [PubMed] [Google Scholar]
- 2.Apcher, S., R. Fahraeus, and B. Manoury. 2004. Epstein-Barr virus: exploiting the immune system by interfering with defective ribosomal products. Microbes Infect. 6:1212-1218. [DOI] [PubMed] [Google Scholar]
- 3.Bahram, F., N. von der Lehr, C. Cetinkaya, and L. G. Larsson. 2000. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 95:2104-2110. [PubMed] [Google Scholar]
- 4.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 5.Barbera, A. J., M. E. Ballestas, and K. M. Kaye. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856-861. [DOI] [PubMed] [Google Scholar]
- 7.Benassi, B., M. Fanciulli, F. Fiorentino, A. Porrello, G. Chiorino, M. Loda, G. Zupi, and A. Biroccio. 2006. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol. Cell 21:509-519. [DOI] [PubMed] [Google Scholar]
- 8.Bubman, D., I. Guasparri, and E. Cesarman. 2007. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 26:4979-4986. [DOI] [PubMed] [Google Scholar]
- 9.Cai, Q. L., J. S. Knight, S. C. Verma, P. Zald, and E. S. Robertson. 2006. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 11.Chang, D. W., G. F. Claassen, S. R. Hann, and M. D. Cole. 2000. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol. Cell. Biol. 20:4309-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Conzen, S. D., K. Gottlob, E. S. Kandel, P. Khanduri, A. J. Wagner, M. O'Leary, and N. Hay. 2000. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol. Cell. Biol. 20:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter, M. A., II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 15.Dang, C. V., K. A. O'Donnell, K. I. Zeller, T. Nguyen, R. C. Osthus, and F. Li. 2006. The c-Myc target gene network. Semin. Cancer Biol. 16:253-264. [DOI] [PubMed] [Google Scholar]
- 16.Dantuma, N. P., and M. G. Masucci. 2003. The ubiquitin/proteasome system in Epstein-Barr virus latency and associated malignancies. Semin. Cancer Biol. 13:69-76. [DOI] [PubMed] [Google Scholar]
- 17.Ding, Q., W. Xia, J. C. Liu, J. Y. Yang, D. F. Lee, J. Xia, G. Bartholomeusz, Y. Li, Y. Pan, Z. Li, R. C. Bargou, J. Qin, C. C. Lai, F. J. Tsai, C. H. Tsai, and M. C. Hung. 2005. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 19:159-170. [DOI] [PubMed] [Google Scholar]
- 18.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 19.Fakhari, F. D., J. H. Jeong, Y. Kanan, and D. P. Dittmer. 2006. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B-cell hyperplasia and lymphoma. J. Clin. Investig. 116:735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fejer, G., M. M. Medveczky, E. Horvath, B. Lane, Y. Chang, and P. G. Medveczky. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84:1451-1462. [DOI] [PubMed] [Google Scholar]
- 21.Friborg, J. J., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 22.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 77:8019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimuro, M., J. Liu, J. Zhu, H. Yokosawa, and S. D. Hayward. 2005. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Virol. 79:10429-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimuro, M., F. Y. Wu, C. apRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 25.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 26.Gregory, M. A., and S. R. Hann. 2000. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt's lymphoma cells. Mol. Cell. Biol. 20:2423-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory, M. A., Y. Qi, and S. R. Hann. 2003. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J. Biol. Chem. 278:51606-51612. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths, R., and A. Whitehouse. 2007. Herpesvirus saimiri episomal persistence is maintained via an interaction between open reading frame 73 and the cellular chromosome associated protein MeCP2. J. Virol. 81:4021-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haggerty, T. J., K. I. Zeller, R. C. Osthus, D. R. Wonsey, and C. V. Dang. 2003. A strategy for identifying transcription factor binding sites reveals two classes of genomic c-Myc target sites. Proc. Natl. Acad. Sci. USA 100:5313-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hann, S. R. 2006. Role of posttranslational modifications in regulating c-Myc proteolysis, transcriptional activity, and biological function. Semin. Cancer Biol. 16:288-302. [DOI] [PubMed] [Google Scholar]
- 32.Hemann, M. T., A. Bric, J. Teruya-Feldstein, A. Herbst, J. A. Nilsson, C. Cordon-Cardo, J. L. Cleveland, W. P. Tansey, and S. W. Lowe. 2005. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 436:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst, A., M. T. Hemann, K. A. Tworkowski, S. E. Salghetti, S. W. Lowe, and W. P. Tansey. 2005. A conserved element in Myc that negatively regulates its proapoptotic activity. EMBO Rep. 6:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang, M., U. Kamasani, and G. C. Prendergast. 2006. RhoB facilitates c-Myc turnover by supporting efficient nuclear accumulation of GSK-3. Oncogene 25:1281-1289. [DOI] [PubMed] [Google Scholar]
- 36.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 37.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan, K., D. A. Kuppers, S. C. Verma, and E. S. Robertson. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 78:6585-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 41.Liu, J., H. Martin, M. Shamay, C. Woodard, Q. Q. Tang, and S. D. Hayward. 2007. The Kaposi's sarcoma-associated herpesvirus LANA protein down-regulates nuclear GSK-3 activity and consequently blocks differentiation. J. Virol. 81:4722-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, F., L. Day, S. J. Gao, and P. M. Lieberman. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 80:5273-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer, N., S. S. Kim, and L. Z. Penn. 2006. The Oscar-worthy role of Myc in apoptosis. Semin. Cancer Biol. 16:275-287. [DOI] [PubMed] [Google Scholar]
- 44.Murakami, Y., S. Yamagoe, K. Noguchi, Y. Takebe, N. Takahashi, Y. Uehara, and H. Fukazawa. 2006. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem. 281:28113-28121. [DOI] [PubMed] [Google Scholar]
- 45.Ohsaki, E., K. Ueda, S. Sakakibara, E. Do, K. Yada, and K. Yamanishi. 2004. Poly(ADP-ribose) polymerase 1 binds to Kaposi's sarcoma-associated herpesvirus (KSHV) terminal repeat sequence and modulates KSHV replication in latency. J. Virol. 78:9936-9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottinger, M., T. Christalla, K. Nathan, M. M. Brinkmann, A. Viejo-Borbolla, and T. F. Schulz. 2006. The Kaposi's Sarcoma-Associated Herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80:10772-10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pulverer, B. J., C. Fisher, K. Vousden, T. Littlewood, G. Evan, and J. R. Woodgett. 1994. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene 9:59-70. [PubMed] [Google Scholar]
- 49.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 50.Sakakibara, S., K. Ueda, K. Nishimura, E. Do, E. Ohsaki, T. Okuno, and K. Yamanishi. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78:7299-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salghetti, S. E., S. Y. Kim, and W. P. Tansey. 1999. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 18:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz, T. F. 2006. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J. Pathol. 208:187-198. [DOI] [PubMed] [Google Scholar]
- 53.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sears, R., G. Leone, J. DeGregori, and J. R. Nevins. 1999. Ras enhances Myc protein stability. Mol. Cell 3:169-179. [DOI] [PubMed] [Google Scholar]
- 55.Sears, R., F. Nuckolls, E. Haura, Y. Taya, K. Tamai, and J. R. Nevins. 2000. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seth, A., E. Alvarez, S. Gupta, and R. J. Davis. 1991. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J. Biol. Chem. 266:23521-23524. [PubMed] [Google Scholar]
- 57.Shamay, M., A. Krithivas, J. Zhang, and S. D. Hayward. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. USA 103:14554-14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Small, G. W., T. Y. Chou, C. V. Dang, and R. Z. Orlowski. 2002. Evidence for involvement of calpain in c-Myc proteolysis in vivo. Arch. Biochem. Biophys. 400:151-161. [DOI] [PubMed] [Google Scholar]
- 59.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 60.Stedman, W., Z. Deng, F. Lu, and P. M. Lieberman. 2004. ORC, MCM, and histone hyperacetylation at the Kaposi's sarcoma-associated herpesvirus latent replication origin. J. Virol. 78:12566-12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang, J., G. M. Gordon, M. G. Muller, M. Dahiya, and K. E. Foreman. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77:5975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma, S. C., B. G. Bajaj, Q. Cai, H. Si, T. Seelhammer, and E. S. Robertson. 2006. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus recruits uracil DNA glycosylase 2 at the terminal repeats and is important for latent persistence of the virus. J. Virol. 80:11178-11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma, S. C., S. Borah, and E. S. Robertson. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 78:10348-10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma, S. C., T. Choudhuri, R. Kaul, and E. S. Robertson. 2006. Latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 80:2243-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe, T., M. Sugaya, A. M. Atkins, E. A. Aquilino, A. Yang, D. L. Borris, J. Brady, and A. Blauvelt. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 77:6188-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welcker, M., A. Orian, J. Jin, J. E. Grim, J. W. Harper, R. N. Eisenman, and B. E. Clurman. 2004. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA 101:9085-9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh, E., M. Cunningham, H. Arnold, D. Chasse, T. Monteith, G. Ivaldi, W. C. Hahn, P. T. Stukenberg, S. Shenolikar, T. Uchida, C. M. Counter, J. R. Nevins, A. R. Means, and R. Sears. 2004. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 6:308-318. [DOI] [PubMed] [Google Scholar]
- 69.You, J., V. Srinivasan, G. V. Denis, W. J. Harrington, Jr., M. E. Ballestas, K. M. Kaye, and P. M. Howley. 2006. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80:8909-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaldumbide, A., M. Ossevoort, E. J. Wiertz, and R. C. Hoeben. 2007. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma herpesvirus. Mol. Immunol. 44:1352-1360. [DOI] [PubMed] [Google Scholar]