Abstract
Unlike prototypical lentiviruses like visna and caprine arthritis-encephalitis viruses, which are mainly macrophage tropic (M-tropic), primate lentiviruses primarily target CD4+ T lymphocytes. We previously reported that during the late phase of highly pathogenic chimeric simian/human immunodeficiency virus (SHIV) infections of rhesus macaques, when CD4+ T cells have been systemically eliminated, high levels of viremia are maintained from productively infected macrophages. The availability of several different M-tropic SHIVs from such late-stage immunocompromised animals provided the opportunity to assess whether they might contribute to the immune deficiency induced by their T-cell-tropic parental viruses or possibly cause a distinct disease based on their capacity to infect macrophages. Pairs of rhesus monkeys were therefore inoculated intravenously with six different M-tropic SHIV preparations, and their plasma viral RNA loads, circulating lymphocyte subset numbers, and eventual disease outcomes were monitored. Only one of these six M-tropic SHIVs induced any disease; the disease phenotype observed was the typical rapid, complete, and irreversible depletion of CD4+ T cells induced by pathogenic SHIVs. An analysis of two asymptomatic monkeys, previously inoculated with an M-tropic SHIV recovered directly from alveolar macrophages, revealed that this inoculum targeted alveolar macrophages in vivo, compared to a T-cell-tropic virus, yet no clinical disease occurred. Although one isolate did, in fact, induce the prototypical rapid, irreversible, and complete loss of CD4+ T cells, indicating that M-tropism and pathogenicity may not be inversely related, the majority of M-tropic SHIVs induced no clinical disease in immunocompetent macaques.
Human immunodeficiency virus type 1 (HIV-1) shares the property of macrophage tropism (M-tropism) with prototypical lentiviruses, such as equine infectious anemia virus, visna-maedi virus, and caprine arthritis-encephalitis virus, which are all mainly M-tropic lentiviruses (3, 11, 36, 45). Although the clinical/immunopathological abnormalities attributable to primate lentiviruses such as HIV-1 and simian immunodeficiency virus (SIV) primarily involve CD4+ T lymphocytes, tissue macrophages and microglia in the central nervous system (CNS) of patients with AIDS dementia complex were identified as some of the first non-lymphocyte-cell lineages that also supported virus replication in vivo (21, 42). Numerous studies have reported that tissue macrophages become infected during the early phase of HIV-1 infections (1, 8, 38). Because of their reported resistance to viral cytopathic effects and long life span, tissue macrophages are considered to be an important HIV-1 reservoir, able to produce and disseminate progeny virions systemically (12, 20). In asymptomatic individuals, the number of virus-producing macrophages, relative to infected CD4+ T lymphocytes, is probably quite low, but over time, the fraction of infected macrophages gradually increases. An analysis of HIV-1 decay in patients with symptomatic disease (mean CD4+ T-cell counts of 212/μl) indicated that 1 to 7% of the plasma virus was being produced by long-lived infected cells, assumed to be tissue macrophages (30). In AIDS patients with very low numbers of circulating CD4+ T lymphocytes (<50 cells/μl), high levels of cell-associated HIV-1 DNA become detectable in lung, colon, brain, liver, and kidney, presumably reflecting the presence of productively infected tissue macrophages in these organs (4, 9). It has also been suggested that the coexistence of opportunistic infections can greatly augment the number of productively HIV-1-infected cells of the monocyte/macrophage lineage (29).
SIV/HIV-1 chimeric viruses (SHIVs) have been extensively used for investigations of primate lentiviral pathogenesis and vaccine induced immunity. The highly pathogenic CXCR4-utilizing SHIVDH12R reproducibly causes rapid, systemic, and irreversible CD4+ T-lymphocyte depletion in infected rhesus macaques within weeks of infection and generates high levels of plasma viremia (5 × 106 to 1 × 108 RNA copies/ml) for several months thereafter (10, 16). These infected animals experience profound depletions of both naïve and memory CD4+ T-cell subsets and an inability to produce virus-specific antibodies (both binding and neutralizing), and they must eventually be euthanized because of intractable diarrhea, marked anorexia/weight loss, and opportunistic infections (16). During the late phase of the SHIV infection, when virtually no CD4+ T lymphocytes remain, virus production is maintained at high levels by macrophages residing in multiple tissues (the “macrophage phase” of infection) (14). We previously reported that virus recovered from infected monkeys during the macrophage phase had acquired amino acid changes (substitutions, deletions, and loss of glycosylation sites) principally affecting the V1 and V2 regions of gp120; some of these alterations conferred M-tropism (15, 18).
The availability of M-tropic SHIVs, isolated from late-stage immunocompromised animals (15, 18), provided the opportunity to ascertain whether these viruses might induce disease in naïve macaques with competent immune systems. Accordingly, pairs of rhesus monkeys were inoculated intravenously with six different M-tropic SHIV preparations (uncloned virus stocks/swarms and molecularly cloned viruses carrying specific gp120 V1/V2 changes known to confer M-tropism). In each case, plasma viral RNA loads, circulating lymphocyte subsets, and eventual disease outcomes were monitored for more than 4 years. Our results indicate that only one of the six M-tropic SHIVs induced clinical disease in immunocompetent macaques, and in that case, the disease observed was attributable to the global elimination of CD4+ T lymphocytes, as is observed with highly pathogenic T-cell-tropic SHIVs.
MATERIALS AND METHODS
Viruses.
The molecularly cloned SHIVM3 and SHIVM11 contained env gene sequences present in viruses circulating in rhesus monkeys infected with SHIVDH12R-PS1 and SHIVDH12R, respectively, during the macrophage phase of infection, as previously described (18). Both of these env sequences, when introduced into the highly pathogenic molecular clone SHIVDH12R-CL-7 (34), conferred M-tropism (18). SHIVWBJ and SHIV891631 were recovered from a mixture of mesenteric and colonic (SHIVWBJ) or colonic (SHIV891631) lymph node cells collected from animals infected with SHIVDH12R-CL-7 and SHIVDH12R, respectively (15). SHIVλ3-3 was cloned from Hirt fractionated DNA, prepared from SHIVWBJ-infected rhesus peripheral blood mononuclear cells (rh-PBMC) using the EMBL3 lambda phage cloning vector (Stratagene, La Jolla, CA) as previously described (40). Infectious molecular clones, SIVmac239, and SIVmac316 were generous gifts of Ronald C. Desrosiers, Harvard University. All SHIV and SIV molecular clones were transfected into HeLa cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's instruction, and culture supernatants were harvested at 48 h posttransfection. Virus stocks were prepared by infecting rh-PBMC with transfection supernatants. Uncloned virus was recovered from animals during the macrophage phase of SHIV infections by cocultivating lymph node suspensions with concanavalin A-stimulated rh-PBMC (15). The infectious titers of all SIV and SHIV inocula were determined by end-point dilution in human T-lymphoid MT-4 cells (13) and calculated by the method described by Reed and Muench (32). The virus inocula used in this study were normalized by infectious titer; 5,000 50% tissue culture infective dose (TCID50) of each virus stock was inoculated into animals intravenously. This amount of parental T-cell-tropic SHIVDH12R-CL-7 consistently caused the typical highly pathogenic SHIV disease (34).
Animal experiments.
Rhesus macaques were maintained in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals (Guide for the Care and Use of Laboratory Animals [26a]) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Phlebotomies and virus inoculations were performed with animals anesthetized with tiletamine-HCl and zolazepam-HCl (Telazol; Fort Dodge Laboratories, Fort Dodge, IA). Lymphocyte subset analyses and plasma viral load determinations were performed as previously described (10).
Assay of virus replication in rh-PBMC and AM.
The preparation and infection of macaque PBMC and alveolar macrophage (AM) have been previously described (15, 18). Briefly, PBMC stimulated with concanavalin A and cultured in the presence of recombinant human interleukin-2 were spinoculated (1,200 × g for 1 h) (28) with virus stocks at a multiplicity of infection (MOI) of 5 × 10−3. For infections of AM, 3 × 105 cells were plated in chamber slides and test viruses, normalized for infectious titer, were absorbed for 16 h at an MOI of 3 × 10−2. Culture supernatants were collected daily from PBMC and every other day from AM cultures to assess virus replication by virion-associated reverse transcriptase (RT) assay (44).
Direct virus isolation from AM and infectious center assay of infected BAL cells.
AM were collected by bronchoalveolar lavage (BAL) at the time of euthanasia from rhesus macaque RhBF37 previously inoculated with a mixture (1,000 TCID50 each) of five uncloned M-tropic SHIVs (SHIV891631, SHIVAP47, SHIVAG18, SHIVBD83, and SHIVWBJ) (15). The BAL cells were resuspended in Dulbecco's modified Eagle's medium supplemented with 10% human AB serum, 5% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 μg/ml gentamicin (macrophage medium) at 1 × 106 cells/ml and plated on tissue culture flasks (75 cm2). Floating cells were removed at 16 h by replacing the medium, and adherent cells were collected from the flasks at 48 h and mixed with freshly prepared rh-PBMC in a new flask (25 cm2). The coculture was maintained for 9 days, and supernatant medium was collected daily. The RT activity released into the medium was assessed, and fractions containing high levels of RT activity were combined, filtered through a 0.45-μm-pore filter, dispensed, and stored in liquid nitrogen until use. The infectious titer of the AM-derived M-tropic SHIVBF37AM stock was 4.0 × 105 TCID50/ml, as measured in MT-4 cells.
For infectious center assays, BAL cells from infected macaques were suspended in RPMI 1640 medium containing 10% fetal bovine serum and 20 U IL-2/ml (1 × 106 cells/ml), serially diluted (threefold), and dispensed in duplicate into 24-well tissue culture plate (1 ml/well). Freshly prepared, concanavalin A-stimulated uninfected rh-PBMC were immediately added to each well (4 × 106 cells/well) and cultured for 3 weeks. Half of the cells in each well were removed every 7 days and replaced with newly prepared rh-PBMC (4 × 106 cells/well), and RT activity released into the culture supernatant was assayed. The minimal number of BAL cells yielding detectable RT activity on day 7, 14, or 21 postplating was determined. The reciprocal numbers of virus-producing cells per 106 BAL cells at any time during the 3-week assay were calculated by the method described by Reed and Muench (32).
Virus neutralization assay.
Polyclonal neutralizing antisera, collected from macaques inoculated with SHIVDH12R-CL7 (low MOI) or the attenuated SHIVDH12R-CL8 (34), were used in neutralization assays as previously described (10, 16, 43). These molecularly cloned SHIVs are isogenic in their gp120 coding regions. SHIVDH12R-CL8-infected animals generate neutralizing antibodies (NAbs) that suppress both SHIVDH12R-CL7 and SHIVDH12R-CL8 with similar efficiencies (T. Igarashi and O. K. Donau, unpublished). Serial threefold dilutions of test plasma samples were incubated with test virus for 1 h at room temperature. Virus-antibody mixtures were then added to MT-4 cells in quadruplicate in 96-well culture plates for each antibody dilution. Virion-associated RT activity in the culture supernatant was measured at 2 weeks postinfection (p.i.), and the reciprocal of the dilution that inhibited virus replication in 50% of the replicate cultures was calculated by the method described by Reed and Muench (32).
RESULTS
Derivation of M-tropic SHIVs.
The M-tropic SHIVs used in these experiments (Table 1) were either uncloned virus stocks, prepared from specimens recovered from rhesus monkeys during the macrophage phase of pathogenic SHIV infections, or molecularly cloned viruses, bearing gp120 V1/V2 env gene sequences previously shown to confer M-tropism (M3 and M11) or a full-length viral DNA clone (λ3-3) from SHIVWBJ-infected cells (15, 18). These M-tropic SHIVs originated from two parental viruses (SHIVDH12R-PS1 [week 52] and SHIVDH12R [week 68]), both of which were recovered from a single infected rhesus macaque (Rh565Z) during the emergence of a highly pathogenic SHIV (16). Both parental SHIVs induced rapid, systemic, and nearly complete depletions of CD4+ T cells within weeks of infection and generated M-tropic SHIV variants during the later phases of their in vivo infections (10, 14, 15, 18). We also previously reported that the acquisition of M-tropism by these CXCR4-utilizing SHIVs was not accompanied by a change in coreceptor usage (15).
TABLE 1.
Macrophage-tropic SHIVs
| Virus | Origin | Clone or swarm | Derivation (reference) |
|---|---|---|---|
| WBJ | SHIVDH12R-PS1 | Swarm | Macrophage-phase virus recovered from RhWBJ at 26 wk p.i. following inoculation with SHIVDH12R-CL-7 (15) |
| λ3-3 | SHIVDH12R-PS1 | Clone | Full-length infectious molecular clone generated from SHIVWBJ-infected rh-PBMC |
| M3 | SHIVDH12R-PS1 | Clone | Molecular clone containing gp120 sequences known to confer M-tropism (from macrophage-phase SHIVDH12R-PS1 swarm) and inserted into SHIVDH12R-CL-7 (18) |
| M11 | SHIVDH12R | Clone | Molecular clone containing gp120 sequences known to confer M-tropism (from macrophage-phase SHIVDH12R swarm) and inserted into SHIVDH12R-CL-7 (18) |
| 891631 | SHIVDH12R | Swarm | Macrophage-phase virus recovered from Rh891631 at 7.4 wk p.i. following inoculation with SHIVDH12R (15) |
| BF37AM | SHIVDH12R-PS1 and SHIVDH12R | Swarm | Macrophage-phase virus isolated from AM prepared from RhBF37 at 111 wk p.i. following inoculation with 5 M-tropic SHIVs: SHIV891631, SHIVAP47, SHIVAG18, SHIVBD83, and SHIVWBJ (15) |
Envelope sequences present in M-tropic SHIVs.
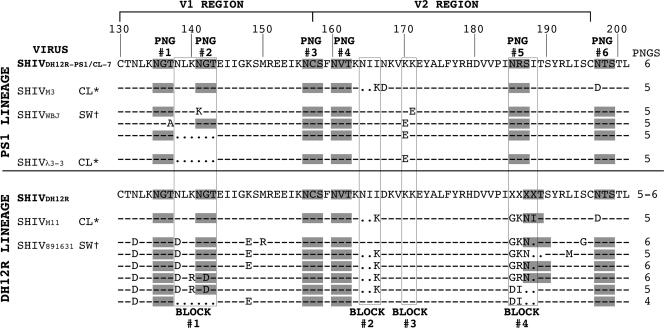
Sequence analyses of env genes in viruses recovered during the macrophage phase of SHIV infections demonstrated the presence of specific amino acid substitutions and deletions that primarily affected the V1/V2 regions of gp120 (15, 18). This segment of the Env glycoproteins from five of the M-tropic SHIVs used in this study has been aligned with the consensus sequence of the parental T-cell-tropic SHIVDH12R-PS1 or SHIVDH12R (Fig. 1). Four discrete regions of V1/V2, designated blocks 1 to 4, sustained alterations, which were associated with the acquisition of M-tropism, and some of these changes affected the number or location of potential N-linked glycosylation sites (PNGs). The parental SHIVDH12R-PS1 has six potential PNGs, which have been designated PNG 1 to 6 (at positions 135, 141, 156, 160, 185, and 197, respectively) in Fig. 1. Changes in block 1, including a six-amino-acid deletion of the duplicated NLKNGT motif, eliminated PNG 2. The block 2 alteration introduced a two-amino-acid deletion at positions 164 and 165 in combination with an I166K substitution. Block 3 changes (only observed in the SHIVDH12R-PS1 family of M-tropic viruses) were associated with K170E or K171E substitutions. Block 4 alterations, observed exclusively in SHIVDH12R-derived M-tropic SHIVs, targeted residues 185 to 188. Block 4 comprises the most genetically diverse portion of the parental SHIVDH12R gp120 V1/V2 region and is depicted as “XXXX” in its consensus sequence (Fig. 1). In addition to a plethora of different amino acid substitutions, block 4 changes included one- and two-amino-acid residue deletions, which, in some instances, preserved but shifted the location of PNG 5.
FIG. 1.
Alignment of Env V1/V2 amino acid sequences from M- and T-tropic SHIVs. The V1/V2 gp120 regions of the two M-tropic SHIV lineages (PS1 and DH12R) have been aligned with comparable regions of their T-cell-tropic parental viruses (SHIVDH12R-PS1/CL-7 and SHIVDH12R). The numbering is based on the Env amino acid sequence of HIV-1DH12 (37). PNGs are shaded and numbered (PNGs 1 to 6). The total number of PNGs within each V1/V2 segment is shown on the right. Common regions of amino acid substitutions and deletions are boxed and numbered (blocks 1 to 4). *, molecular clone; †, virus swarm; -, identical sequence; ., deletion; X, variable or deleted amino acid residue.
Three of the M-tropic SHIVs analyzed in this study were derived from molecular clones (SHIVM3, SHIVλ3-3, and SHIVM11), and two were uncloned “swarms” (SHIVWBJ and SHIV891631) generated from virus collected from animals during the macrophage stage of their infections. The cloned SHIVM3 and SHIVM11 were constructed by introducing the indicated V2 changes into the genetic background of SHIVDH12R-PS1/CL-7 by site-directed mutagenesis (18) and were otherwise isogenic. SHIVλ3-3 is a full-length molecular clone obtained from rh-PBMC infected with the M-tropic SHIVWBJ (see Table 1) using a lambda phage vector. The SHIVλ3-3 gp120 contains only the indicated V1/V2 changes and an E320K substitution in the V3 loop (15), compared to the sequence of the T-cell-tropic SHIVDH12R-PS1/CL-7.
It is worth noting from the V1/V2 sequence alignment presented in Fig. 1 that M-tropic SHIV891631, derived during the macrophage phase from an animal inoculated with the SHIVDH12R uncloned swarm, is itself extremely genetically heterogeneous. SHIV891631 contains individual component virus populations that carry V1/V2 changes present in some of the individual molecularly cloned M-tropic SHIVs, including (i) the six-amino-acid deletion in block 1, (ii) the two-residue deletion in block 2, and (iii) unique amino acid substitutions in block 4. It also should be pointed out that SHIVM3 and SHIVM11 are genomically isogenic except for three amino acids in block 4. As will be described below, this difference conferred unique pathogenic phenotypes in vivo, which were not discernible in vitro.
Replication of M-tropic SHIVs in cultured cells.
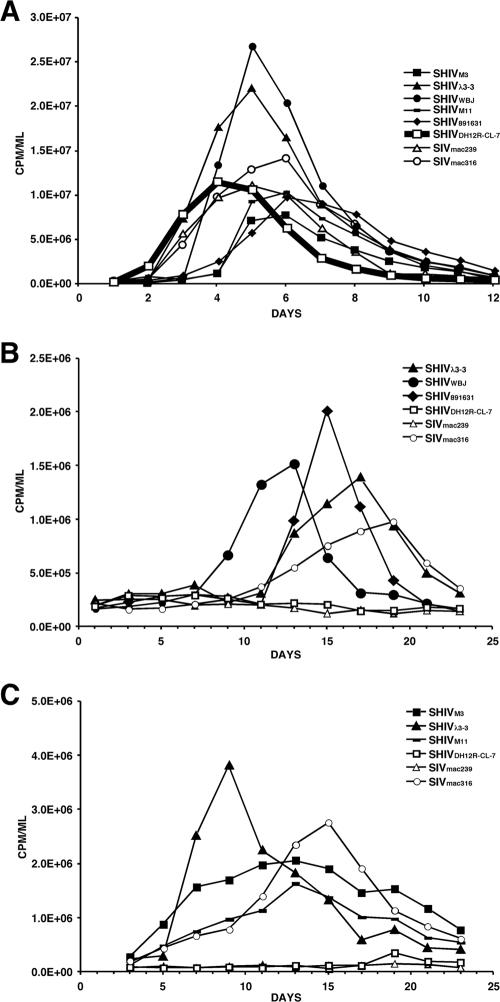
To evaluate the replication properties of the M-tropic SHIVs in rh-PBMC, virus inocula were normalized for infectivity (2.5 × 104 TCID50) and spinoculated onto concanavalin A-treated cells. As shown in Fig. 2A, the parental T-cell-tropic SHIVDH12R-CL7 produced peak levels of virus on day 4 p.i.; the T-cell-tropic SIVmac239 and M-tropic SIVmac316 controls exhibited slightly delayed replication profiles in these cells. While the M-tropic SHIVs reached peak virus production in rh-PBMC in the same time frame, SHIVλ3-3 and SHIVWBJ consistently replicated to higher levels than the three other M-tropic SHIVs. Three of the M-tropic SHIVs (SHIVM3, SHIVM11, and SHIV891631) induced marked syncytia in macaque PBMC, while SHIVWBJ and SHIVλ3-3 did not (data not shown). Thus, the higher levels of virus production directed by SHIVλ3-3 and SHIVWBJ could reflect their lower cytopathicity, resulting in better preservation of infected cells and prolonging the release of the progeny virions into the culture supernatant, as detected by RT assay.
FIG. 2.
Replication of M-tropic SHIVs in rhesus PBMC (A) or AM (B and C). Each SHIV preparation was spinoculated onto 5 × 106 cells of monkey PBMC (MOI, 5 × 10−3), and virus production was monitored by RT activity released into the medium (A). The replication of the parental T-cell-tropic SHIVDH12R-CL-7 is indicated by the thick line. AM (3 × 105) collected from the same donor animal on two different occasions were infected with the indicated viruses at an MOI of 3 × 10−2, and virus replication was assayed as described for PBMC (B and C).
The infectivity of the M-tropic SHIVs and control viruses was also evaluated in AM, collected from foamy virus-negative rhesus monkeys as previously described (18). Because it was logistically difficult to prepare sufficient numbers of AM to evaluate all of the viruses in a single experiment, cells collected from the same donor animal on two different occasions were used, as shown in Fig. 2B and C. As expected, the two T-cell-tropic viruses (SHIVDH12R-CL7 and SIVmac239) failed to replicate in AM, whereas the M-tropic SIVmac316 produced peak amounts of progeny virions on day 15 or 20 p.i., depending on the AM preparation. The M-tropic SHIVs replicated in the macaque AM system with kinetics that were somewhat faster than those of SIVmac316. Taken together, these results show that M-tropic SHIVs, like M-tropic SIVmac316, are able to establish productive infections in both rhesus monkey PBMC and AM cultures.
Infection of rhesus monkeys with M-tropic SHIVs.
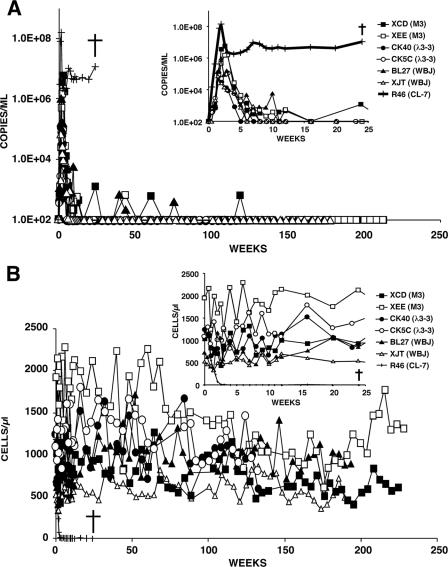
Pairs of macaques were inoculated intravenously with 5,000 TCID50 of each M-tropic SHIV to ascertain whether they were able to induce disease in animals with competent immune systems. As noted earlier, this inoculum size consistently induced a rapid and irreversible loss of CD4+ T lymphocytes in animals inoculated with the T-cell-tropic SHIVDH12R and SHIVDH12R-CL7. Three different patterns of in vivo infection were observed, as manifested by differences in the levels of plasma viremia, numbers of circulating CD4+ T lymphocytes, and clinical outcomes. The monkeys inoculated with M-tropic SHIVs containing SHIVDH12R-PS1-derived env genes (SHIVM3, SHIVλ3-3, and SHIVWBJ) all exhibited lower peak plasma virus loads (6.1 × 104 to 5.7 × 106 RNA copies/ml) compared to an animal (RhR46) that was infected with the T-cell-tropic SHIVDH12R-CL7 control (1.5 × 108 RNA copies/ml) (inset in Fig. 3A). Following peak virus production at week 2 p.i., plasma viremia declined to background levels between weeks 5 and 12 in this group of M-tropic SHIV-infected macaques, and while the numbers of circulating CD4+ T lymphocytes fluctuated somewhat, they remained relatively unchanged compared to prechallenge values (insets in Fig. 3A and B). In contrast to monkey RhR46, which had to be euthanized at week 24 because of systemic elimination of its CD4+ T cells, marked weight loss, and deteriorating clinical status, the animals inoculated with the three SHIVDH12R-PS1-derived M-tropic SHIVs have remained asymptomatic for more than 4 years, with relatively unchanged and stable numbers of CD4+ T cells and undetectable plasma viral RNA.
FIG. 3.
Profiles of plasma viral RNA loads (A) and circulating CD4+ T lymphocytes (B) in pairs of macaques infected with M-tropic SHIVM3, SHIVWBJ, or SHIVλ3-3 (5,000 TCID50). One rhesus monkey (R46) was infected with the highly pathogenic T-cell-tropic SHIVDH12R-CL-7 (5,000 TCID50). The insets show measurements made during the first 25 weeks of the infections. The detection limit of our RT-PCR assay is 200 RNA copies/ml, and undetectable values are plotted as 100 RNA copies/ml of plasma. †, time of euthanasia.
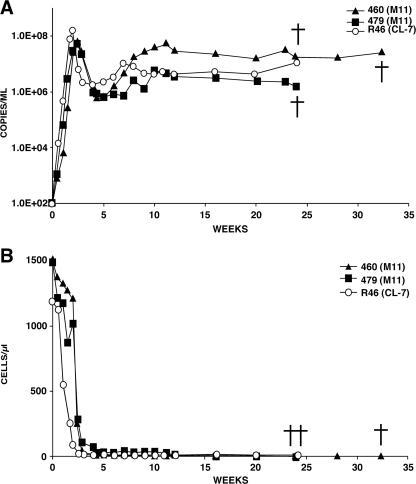
A completely different result was obtained with the two animals (Rh460 and Rh479) inoculated with M-tropic SHIVM11. Both exhibited profiles of plasma viremia and circulating CD4+ T cells similar to those experienced by the macaque infected with the highly pathogenic T-cell-tropic SHIVDH12R-CL7 (Fig. 4). The CD4+ T-lymphocyte numbers fell to 6 cells/μl of blood at week 4.3 in monkey 460, while animal 479 was able to preserve its cells slightly longer (72 cells/μl at week 4.0) but eventually experienced a decline to 8 cells/μl at week 16. Macaques 460 and 479 required euthanasia at weeks 33 and 26, respectively, due to anorexia and marked weight loss.
FIG. 4.
Profiles of plasma viral RNA loads (A) and circulating CD4+ T lymphocytes (B) in two macaques infected with M-tropic SHIVM11 (5,000 TCID50). One rhesus monkey (R46) was infected with the highly pathogenic T-cell-tropic SHIVDH12R-CL-7 (5,000 TCID50). The detection limit of our RT-PCR assay is 200 RNA copies/ml, and undetectable values are plotted as 100 RNA copies/ml of plasma. †, time of euthanasia.
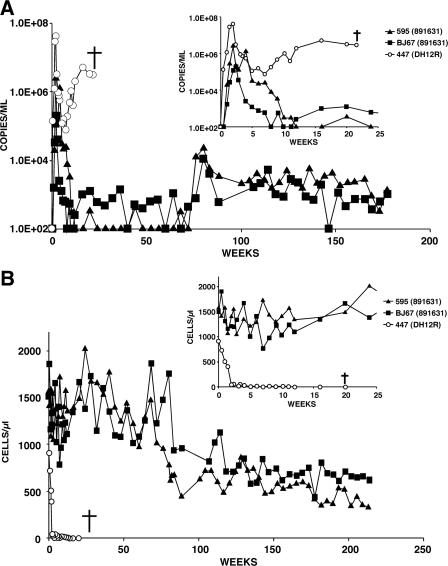
The final M-tropic SHIV (SHIV891631), a genetically heterogeneous virus swarm (see Fig. 1), induced an unusual and intermediate pathogenic phenotype in two infected animals (Fig. 5). The uncloned SHIV891631 generated lower levels of peak viremia (1.9 × 105 and 2.5 × 106 RNA copies/ml) than that observed in monkey Rh447 (4.1 × 107 RNA copies/ml), which had been inoculated with the parental T-cell-tropic SHIVDH12R; virus replication was effectively controlled in both of the SHIV891631-infected macaques by week 12 p.i. Nonetheless, viral RNA again became detectable in animal RhBJ67 at week 15 p.i. and was maintained at levels ranging from 4.0 × 102 to 5.2 × 103 RNA copies/ml for more than 4 years (Fig. 5A). SHIV891631 also reemerged in the second monkey (Rh595) at week 76 p.i. and sustained itself at similar low levels for an additional 2.5 years. The persistent viremia in both animals was accompanied by a gradual decline of circulating CD4+ T cells beginning 1 year p.i. In monkey Rh595, CD4+ T lymphocytes fell from 1,536 cells/μl at the time of virus inoculation to 328 cells/μl at week 235 p.i.; the decline in macaque RhBJ67 during the same time interval was from 1,467 to 621 cells/μl blood. Nonetheless, both infected animals have remained clinically asymptomatic.
FIG. 5.
Profiles of plasma viral RNA loads (A) and circulating CD4+ T lymphocytes (B) in two macaques infected with M-tropic SHIV891631 (5,000 TCID50). One rhesus monkey (R447) was infected with the highly pathogenic T-cell-tropic SHIVDH12R (5,000 TCID50). The insets show measurements made during the first 25 weeks of the infections. The detection limit of our RT-PCR assay is 200 RNA copies/ml, and undetectable values are plotted as 100 RNA copies/ml of plasma. †, time of euthanasia.
Sensitivity of M-tropic SHIVs to neutralizing antibodies.
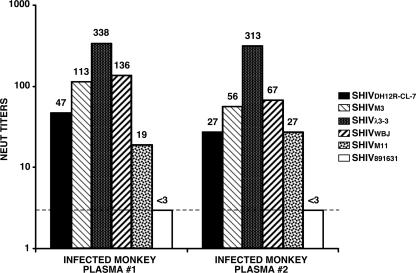
Previous studies have reported that M-tropic SIVmac316 is significantly more (800-fold) sensitive to NAbs than its T-cell-tropic progenitor SIVmac239 (24, 31). We therefore examined the neutralization sensitivities of the M-tropic SHIVs to ascertain whether they also exhibited a similar phenotype relative to their parental T-cell-tropic SHIVDH12R-CL7. Two anti-SHIV polyclonal Nab preparations were used in these experiments. One was plasma collected at week 11 p.i. from a macaque inoculated with a low dose (100 TCID50) of SHIVDH12R-CL7, which did not develop disease (34) but did generate high titers of anti- SHIVDH12R-CL7 NAbs. The second source of anti-SHIV NAb was plasma obtained from an animal inoculated with the molecularly cloned SHIVDH12R-CL-8 (34). SHIVDH12R-CL8 carries a gp120 that is identical to that of SHIVDH12R-CL7 but is nonpathogenic (23, 34). As shown in Fig. 6, the two plasma preparations gave similar patterns of neutralization against the panel of five M-tropic SHIVs. The three bearing the SHIVDH12R-PS1-derived env genes (SHIVM3, SHIVλ3-3, and SHIVWBJ) were somewhat more sensitive to both preparations of NAbs than the T-cell-tropic SHIVDH12R-CL7. The most sensitive of these three M-tropic SHIVs, SHIVλ3-3, was 7.2- and 11.6-fold more neutralizable than the T-cell-tropic SHIVDH12R-CL7 in the two independent assays shown. This modest increase in neutralization sensitivity is far less than that reported for the M-tropic SIV prototype, SIVmac316 (800-fold), and suggests that there may be no correlation between M-tropism and neutralizability among closely related primate lentiviruses. The striking resistance of SHIV891631 to neutralization shown in Fig. 6 is clearly not representative of the other M-tropic SHIVs; it is more likely due to the remarkable genetic heterogeneity of its Env glycoprotein. In this regard, it is worth noting that the polyclonal NAb preparations used in these neutralization experiments were generated in rhesus monkeys inoculated with molecularly cloned viruses bearing the gp120 V1/V2 sequence of SHIVDH12R-CL7 (Fig. 1, top line), which is markedly different from the collection of several env gene sequences comprising the SHIV891631 swarm (Fig. 1, bottom).
FIG. 6.
Sensitivities of M-tropic SHIVs to neutralizing antibodies. Plasma samples from an animal inoculated with SHIVDH12R-CL-7 (plasma 1) or SHIVDH12R-CL-8 (plasma 2) were used in neutralization assays described in Materials and Methods. These two SHIVs carry identical gp120 coding sequences (34). The neutralization (NEUT) titers against each virus are shown above the bars. The dashed line indicates the detection limit of the assay (<1:3).
An M-tropic SHIV recovered directly from AM targets AM in vivo but is not pathogenic in immunocompetent macaques.
To ascertain whether any of the M-tropic SHIVs described earlier specifically targeted macrophages in vivo, one macaque (Rh4462) was inoculated intravenously with 5,000 TCID50 of SHIVM3; blood was analyzed for viral RNA levels, and AM were collected at week 4 p.i. by BAL for infectious center assays. The peak plasma viremia reached a level (3.8 × 105 RNA copies/ml) similar to that previously observed for SHIVM3-infected animals (see Fig. 3A). As a control for the infectious center assay, AM were also collected from two rhesus monkeys (95D139 and H589) previously inoculated with the T-cell-tropic SIVmac239 (27). The recovered BAL samples from all three animals were serially diluted and then cocultivated with activated rh-PBMC for 21 days, and the frequency of virus-infected AM was determined by infectious center assay. As shown in Table 2, 50- to 100-fold more AM, from the macaque inoculated with M-tropic SHIVM3, were releasing virus at week 4 p.i. compared to samples collected from the two animals infected with the T-cell-tropic SIVmac239.
TABLE 2.
Infectious centers in the pulmonary space of SHIV- or SIV-infected animals
| Animal | Virus inoculum | No. of virus-producing cells/106 cells
|
|
|---|---|---|---|
| 4 wk p.i. | 8 wk p.i. | ||
| 95D139 | SIVmac239 | 5.2 | <0.1 |
| H589 | SIVmac239 | 2.3 | <0.1 |
| 4462 | SHIVM3 | 243.0 | NDa |
| P12 | SHIVBF37AM | 60.9 | 243.0 |
| P25 | SHIVBF37AM | 2187.0 | 46.8 |
ND, not done.
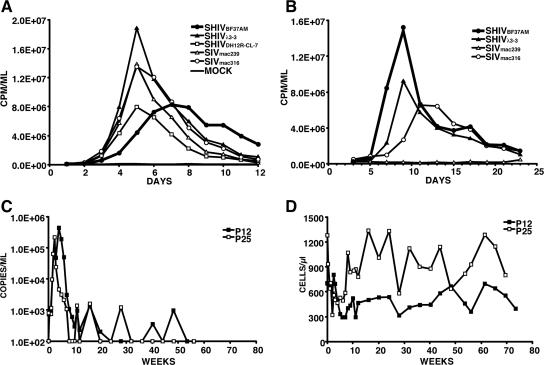
We considered the possibility that the failure of four of five M-tropic SHIVs to induce clinical disease in immunocompetent monkeys might reflect their isolation from blood or lymph nodes rather than from nonlymphoid organs known to harbor productively infected macrophages, such as lung or the CNS. Accordingly, AM were collected by BAL during the macrophage phase of infection of rhesus macaque RhBF37 that had been inoculated with a mixture (1,000 TCID50 each) of five uncloned M-tropic SHIVs (SHIV891631, SHIVAP47, SHIVAG18, SHIVBD83, and SHIVWBJ (15). This mixed uncloned SHIV inoculum was used to increase the possible recovery of an M-tropic SHIV with augmented pathogenic potential. Forty-eight hours following the plating of the AM from macaque RhBF37, adherent cells were collected and cocultivated with concanavalin A-stimulated rh-PBMC. The virus released into the supernatant medium during 9 days of coculture was collected as a virus stock for in vitro and in vivo experiments. As shown in Fig. 7A, this AM-derived virus (SHIVBF37AM) replicated with delayed kinetics in macaque PBMC compared to T-cell- and M-tropic SHIVs and SIVs. Nonetheless, SHIVBF37AM exhibited robust infection kinetics in AM cultures, generating higher levels of RT activity than either the M-tropic SHIVλ3-3 or SIVmac316 (Fig. 7B), indicating that it was, in fact, M-tropic. However, intravenous inoculation of two monkeys with SHIVBF37AM resulted in modest levels of peak plasma viremia (4.4 × 105 and 2.2 × 105 RNA copies/ml), which was rapidly and durably controlled (Fig. 7C). The numbers of circulating CD4+ T lymphocytes did not change appreciably in monkey P12 or P25, and neither developed clinical symptoms (Fig. 7D).
FIG. 7.
Infectivity of AM-derived M-tropic SHIVB37AM in vitro and in vivo. SHIVB37AM and the indicated viruses were spinoculated onto 5 × 106 cells of monkey PBMC (MOI, 5 × 10−3), and virus production was monitored by RT activity released into the medium (A). AM (3 × 105) were infected with the indicated viruses at an MOI of 3 × 10−2, and virus replication was assayed as described for PBMC (B). The replication curves for SHIVB37AM are indicated by thick lines. (C and D) Profiles of plasma viral RNA loads (C) and circulating CD4+ T lymphocytes (D) in rhesus macaques P12 and P25 infected with M-tropic SHIVB37AM (5,000 TCID50). The detection limit of the RT-PCR assay is 200 RNA copies/ml, and undetectable values are plotted as 100 RNA copies/ml of plasma.
The capacity of the M-tropic SHIVBF37AM to specifically target macrophages in infected animals was also evaluated by infectious center assay using AM collected by BAL from monkeys P12 and P25 at weeks 4 and 8 p.i. Table 2 shows that considerably more AM from both SHIVBF37AM-infected monkeys produced progeny virus compared to the two animals inoculated with the T-tropic SIVmac239 at both time points. In fact, at week 8 p.i., virus-producing AM were only detected in the monkeys inoculated with the M-tropic SHIVBF37AM. Thus, despite its derivation from tissue macrophages, robust replication properties in AM in vitro, and capacity to target AM in vivo, M-tropic SHIVBF37AM, like most other M-tropic SHIVs, was nonpathogenic following its inoculation into monkeys with competent immune systems.
DISCUSSION
When this study began, we considered the possibility that M-tropic viral variants emerging shortly after the inoculation of macaques with highly pathogenic T-cell-tropic SHIVs might contribute to the rapid and complete depletion of CD4+ T cells observed in these animals. In fact, one of the M-tropic SHIVs evaluated in this study, SHIV891631, was recovered at week 7.4 p.i. from an animal experiencing the typical massive elimination of CD4+ T lymphocytes. However, since five of six M-tropic SHIVs evaluated in this study failed to induce any disease in animals with competent immune systems, we concluded that M-tropism, per se, contributed very little to the rapid, unremitting, and ultimately fatal X4-SHIV immunodeficiency syndrome. A more likely explanation is that M-tropic SHIVs simply emerged following the irreversible depletion of CD4+ T cells as the viral infection was redirected to the only remaining alternative CD4-expressing cell in vivo: those of the monocyte/macrophage lineage. This retargeting of the SHIV infection required the acquisition of specific changes affecting gp120, including altered glycosylation sites and shortened V1/V2 regions (15, 18) (see Fig. 1), to facilitate entry into cells of the macrophage lineage, known to express lower levels of CD4 compared to T lymphocytes (2, 25, 26, 41). These Env glycoprotein alterations, while conferring macrophage tropism (and preserving the preexisting T-cell tropism), had occurred in monkeys lacking a functional immune system and very likely resulted in the range of pathogenic phenotypes observed in the M-tropic SHIVs that subsequently emerged.
Only one of the six M-tropic SHIVs evaluated in this study, SHIVM11, induced clinical disease. Moreover, the disease phenotype observed with SHIVM11 was indistinguishable from the immunodeficiency induced by prototypical highly pathogenic T-cell-tropic SHIVs. Since M-tropism, as defined in in vitro systems, is inseparable from T-cell tropism for all known primate lentiviruses, it was not totally unexpected that the M-tropic SHIVM11 might only induce a “T-cell disease” rather than a distinct pathological entity related to its ability to infect macrophages. Prototypical lentiviruses, such as visna-maedi virus, caprine arthritis-encephalitis virus, and equine infectious anemia virus, are primarily M-tropic, do not target T lymphocytes, and do not induce immunodeficiency. Instead, the diseases induced by the ungulate lentiviruses are characterized by intense inflammatory responses in affected tissues (lung, brain, and joints) with marked infiltrations of macrophages and lymphocytes. Thus, visna-maedi virus causes pulmonary and neurological manifestations in selected breeds of sheep such as Corridale (39). Although caprine arthritis-encephalitis virus can induce debilitating arthritis, this occurs in less than 10% of infected goats (6, 7, 22). In the case of equine infectious anemia virus, approximately 90% of experimentally inoculated horses control the acute febrile phase of the infection and become chronic carriers with no recognizable clinical symptoms (35). It would appear that these M-tropic prototypical lentiviruses establish symptomatic infections in only a fraction of infected animals, which become recognized because they may be less vigorous or produce products of economic importance in smaller amounts or of poorer quality.
Our results would suggest that M-tropism per se is not predictive of loss of pathogenic potential based on the results obtained with SHIVM11, which induced prototypical disease in two of two inoculated animals. Side-by-side pathogenicity comparisons using T-cell-tropic SHIVs, also recovered from late-stage-infected macaques, were not conducted, although previous reports from our laboratory and other investigators have shown that unfractionated SHIV preparations, isolated from animals with marked depletions of CD4+ T cells, are highly pathogenic (16, 33). It is possible that the forced acquisition of M-tropism due to loss of CD4+ T-cell targets or the appearance of M-tropic SHIVs following the collapse of the immune system, both characteristic of the SHIV system in vivo, contributes to the emergence of attenuated nonpathogenic variants.
As noted above, only SHIVM11 of the six M-tropic SHIVs evaluated induced immunodeficiency. It is interesting that the entire 9.2-kb genome of SHIVM11 is isogenic with the nonpathogenic M-tropic SHIVM3, except for three adjacent amino acids (positions 185 to 187) located in the V2 region of gp120 (see Fig. 1). This relatively minor difference in env could affect a myriad of functions that could be examined biochemically. However, because SHIVM3 and SHIVM11 exhibited indistinguishable replication profiles in cultured PBMC and AM (Fig. 2), we concluded that the attenuation of SHIVM3 is due to a presently unknown property of its gp120 V2 region manifested only during replication in vivo. Because disease induction by pathogenic X4-SHIVs is dependent on exceeding a threshold level of viral replication during the initial 2 weeks of the acute infection (10, 17), the relatively modest increase (two- to sixfold) in neutralization sensitivity exhibited by SHIVM3 compared to SHIVM11 (Fig. 6) associated with the somewhat lower levels of peak viremia (5.6 × 106 and 1.6 × 106 RNA copies/ml [Fig. 3A]) could be functionally significant and tip the balance in favor of the host, resulting in effective suppression of SHIVM3 replication and the abrogation of any clinical disease.
In the present study, we observed relatively minor differences in neutralization sensitivities between the SHIVDH12R family of M-tropic viruses and their T-cell-tropic progenitor, SHIVDH12R-CL-7. This result contrasts with reports of SIVmac239 and its M-tropic derivatives, which in general are exquisitely sensitive to neutralizing antibodies compared to their T-cell-tropic parent (24, 31). The difference in neutralization sensitivities exhibited by the two families of M-tropic primate lentiviruses undoubtedly reflects the unique biological and biochemical properties of each, including (i) coreceptor usage (SIV, CCR5; SHIV, CXCR4), (ii) relative neutralizing sensitivities of parental T-cell-tropic viruses (SIVmac239, resistant; SHIVDH12R-Cl-7, relatively sensitive), (iii) CD4 dependency of M-tropic derivatives (SIV, independent [31]; SHIV, dependent [H. Imamichi and J. Reeves, unpublished]), and (iv) env genetic determinants of M-tropism (SIV, distributed throughout gp120 and gp41 [25]; SHIV, mapping primarily to V1/V2 [15, 18]). Irrespective of these differences, both families of M-tropic nonhuman primate lentiviruses exhibit a common biological property: they are attenuated in vivo following inoculation of immunocompetent rhesus monkeys (5, 19, 31).
One might ask what role do M-tropic primate lentiviruses play during infections of monkeys and humans? In the case of HIV-1, M-tropic strains have been detected during all phases of the infection but only become relatively abundant in individuals with functionally impaired immune systems (1, 8, 29, 38). In this regard, we have previously reported the presence of virus in multinucleated giant cells in the brains of SHIV-infected macaques exhibiting no apparent neurological symptoms (17a). It is quite likely that the immune system in these monkeys was sufficiently intact to minimize virus-induced CNS injury and associated clinical symptoms. Taken together with our failure to observe any disease during de novo infections of rhesus monkeys with functional immune systems, initiated with five of six M-tropic SHIVs of diverse origins and clonalities, we surmise that a majority of M-tropic primate lentiviruses are intrinsically attenuated and only become “dangerous” in immunologically compromised hosts. During the asymptomatic phase of their infections, M-tropic HIV-1, SIV, and SHIV strains comprise an important virus reservoir in tissue macrophages systemically but do not induce a unique disease based on their M-tropic properties. Rather, by continuously releasing progeny virions capable of infecting and destroying CD4+ T cells, these M-tropic viruses contribute to the unrelenting impairment of the immune system and only cause symptomatic “macrophage disease” (e.g., affecting the CNS) during the very late stages of infection.
Acknowledgments
We are indebted to Liz Scanlon, Devorah Crown, Jennifer Post, Shelley Lower, Frances Banks, Sekou Savane, L. “Dusty” Rhodes, Joel Beren, and Liza Murray for their diligence and assistance in the care and maintenance of our animals; Anthony F. Suffredini for training us to use the BAL procedure; Robert E. Means and Ronald C. Desrosiers for providing molecular clones of SIVmac239 and SIVmac316 and advice for preparation and cultivation of monkey AM; Mark Bryant, Lauren Brinster, Victoria Hoffmann, and Michael Eckhaus for interpreting histopathological specimens; and Ronald L. Willey, Bernard A. P. Lafont, and Vanessa M. Hirsch for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Appleman, M. E., D. W. Marshall, R. L. Brey, R. W. Houk, D. C. Beatty, R. E. Winn, G. P. Melcher, M. G. Wise, C. V. Sumaya, and R. N. Boswell. 1988. Cerebrospinal fluid abnormalities in patients without AIDS who are seropositive for the human immunodeficiency virus. J. Infect. Dis. 158:193-199. [DOI] [PubMed] [Google Scholar]
- 2.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie, S. J., L. D. Pearson, M. C. Zink, H. M. Bickle, B. C. Anderson, K. A. Marcom, and J. C. DeMartini. 1995. Ovine lentivirus expression and disease. Virus replication, but not entry, is restricted to macrophages of specific tissues. Am. J. Pathol. 146:250-263. [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, Y. Z., D. Dieterich, P. A. Thomas, Y. X. Huang, M. Mirabile, and D. D. Ho. 1992. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 6:65-70. [DOI] [PubMed] [Google Scholar]
- 5.Clements, J. E., R. C. Montelaro, M. C. Zink, A. M. Amedee, S. Miller, A. M. Trichel, B. Jagerski, D. Hauer, L. N. Martin, R. P. Bohm, and M. Murphey-Corb. 1995. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J. Virol. 69:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford, T. B., and D. S. Adams. 1981. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. J. Am. Vet. Med. Assoc. 178:713-719. [PubMed] [Google Scholar]
- 7.Cutlip, R. C., H. D. Lehmkuhl, J. M. Sacks, and A. L. Weaver. 1992. Prevalence of antibody to caprine arthritis-encephalitis virus in goats in the United States. J. Am. Vet. Med. Assoc. 200:802-805. [PubMed] [Google Scholar]
- 8.Davis, L. E., B. L. Hjelle, V. E. Miller, D. L. Palmer, A. L. Llewellyn, T. L. Merlin, S. A. Young, R. G. Mills, W. Wachsman, and C. A. Wiley. 1992. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42:1736-1739. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson, Y. K., J. E. Bell, J. W. Ironside, R. P. Brettle, J. R. Robertson, A. Busuttil, and P. Simmonds. 1994. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet 343:382-385. [DOI] [PubMed] [Google Scholar]
- 10.Endo, Y., T. Igarashi, Y. Nishimura, C. Buckler, A. Buckler-White, R. Plishka, D. S. Dimitrov, and M. A. Martin. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendelman, H. E., O. Narayan, S. Molineaux, J. E. Clements, and Z. Ghotbi. 1985. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc. Natl. Acad. Sci. USA 82:7086-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorry, P. R., M. Churchill, S. M. Crowe, A. L. Cunningham, and D. Gabuzda. 2005. Pathogenesis of macrophage tropic HIV-1. Curr. HIV Res. 3:53-60. [DOI] [PubMed] [Google Scholar]
- 13.Harada, S., Y. Koyanagi, and N. Yamamoto. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563-566. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igarashi, T., O. K. Donau, H. Imamichi, M.-J. Dumaurier, R. Sadjadpour, R. J. Plishka, A. Buckler-White, C. Buckler, A. F. Suffredini, H. C. Lane, J. P. Moore, and M. A. Martin. 2003. Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages. J. Virol. 77:13042-13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi, T., Y. Endo, G. Englund, R. Sadjadpour, T. Matano, C. Buckler, A. Buckler-White, R. Plishka, T. Theodore, R. Shibata, and M. Martin. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA 96:14049-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi, T., Y. Endo, Y. Nishimura, C. Buckler, R. Sadjadpour, O. K. Donau, M.-J. Dumaurier, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Early control of highly pathogenic simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys usually results in long-lasting asymptomatic clinical outcomes. J. Virol. 77:10829-10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Igarashi, T., H. Imamichi, C. R. Brown, V. M. Hirsch, and M. A. Martin. 2003. The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J. Leukoc. Biol. 74:772-780. [DOI] [PubMed] [Google Scholar]
- 18.Imamichi, H., T. Igarashi, T. Imamichi, O. K. Donau, Y. Endo, Y. Nishimura, R. L. Willey, A. F. Suffredini, H. C. Lane, and M. A. Martin. 2002. Amino acid deletions are introduced into the V2 region of gp120 during independent pathogenic simian immunodeficiency virus/HIV chimeric virus (SHIV) infections of rhesus monkeys generating variants that are macrophage tropic. Proc. Natl. Acad. Sci. USA 99:13813-13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, W. E., J. D. Lifson, S. M. Lang, R. P. Johnson, and R. C. Desrosiers. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J. Virol. 77:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedzierska, K., and S. M. Crowe. 2002. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr. Med. Chem. 9:1893-1903. [DOI] [PubMed] [Google Scholar]
- 21.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. Dal Canto, G. H. Pezeshkpour, M. Yungbluth, F. Janotta, A. Aksamit, M. A. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089-1093. [DOI] [PubMed] [Google Scholar]
- 22.Konishi, M., S. Tsuduku, M. Haritani, K. Murakami, T. Tsuboi, C. Kobayashi, K. Yoshikawa, K. M. Kimura, and H. Sentsui. 2004. An epidemic of caprine arthritis encephalitis in Japan: isolation of the virus. J. Vet. Med. Sci. 66:911-917. [DOI] [PubMed] [Google Scholar]
- 23.Mao, H., B. A. P. Lafont, T. Igarashi, Y. Nishimura, C. Brown, V. Hirsch, A. Buckler-White, R. Sadjadpour, and M. A. Martin. 2005. CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype. J. Virol. 79:14887-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori, K., D. J. Ringler, T. Kodama, and R. C. Desrosiers. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J. Virol. 66:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori, K., M. Rosenzweig, and R. C. Desrosiers. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J. Virol. 74:10852-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.National Research Council. 1985. Guide for the care and use of laboratory animals (NIH 85-23). National Academy Press, Washington, DC.
- 27.Nishimura, Y., T. Igarashi, A. Buckler-White, C. Buckler, H. Imamichi, R. M. Goeken, W. R. Lee, B. A. P. Lafont, R. Byrum, H. C. Lane, V. M. Hirsch, and M. A. Martin. 2007. Loss of naïve cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in simian immunodeficiency virus-infected macaques. J. Virol. 81:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 30.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 31.Puffer, B. A., S. Pöhlmann, A. L. Edinger, D. Carlin, M. D. Sanchez, J. Reitter, D. D. Watry, H. S. Fox, R. C. Desrosiers, and R. W. Doms. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J. Virol. 76:2595-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, L., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadjadpour, R., T. S. Theodore, T. Igarashi, O. K. Donau, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2004. Induction of disease by a molecularly cloned highly pathogenic simian immunodeficiency virus/human immunodeficiency virus chimera is multigenic. J. Virol. 78:5513-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellon, D. C., F. J. Fuller, and T. C. McGuire. 1994. The immunopathogenesis of equine infectious anemia virus. Virus Res. 32:111-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellon, D. C., S. T. Perry, L. Coggins, and F. J. Fuller. 1992. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J. Virol. 66:5906-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata, R., M. D. Hoggan, C. Broscius, G. Englund, T. S. Theodore, A. Buckler-White, L. O. Arthur, Z. Israel, A. Schultz, H. C. Lane, and M. A. Martin. 1995. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 69:4453-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidtis, J. J., and R. W. Price. 1990. Early HIV-1 infection and the AIDS dementia complex. Neurology 40:323-326. [DOI] [PubMed] [Google Scholar]
- 39.Straub, O. C. 2004. Maedi-Visna virus infection in sheep. History and present knowledge. Comp. Immunol. Microbiol. Infect. Dis. 27:1-5. [DOI] [PubMed] [Google Scholar]
- 40.Theodore, T. S., G. Englund, A. Buckler-White, C. E. Buckler, M. A. Martin, and K. W. C. Peden. 1996. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retrovir. 12:191-194. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, E. R., R. L. Dunfee, J. Stanton, D. Bogdan, J. Taylor, K. Kunstman, J. E. Bell, S. M. Wolinsky, and D. Gabuzda. 2007. Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology 360:105-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiley, C. A., R. D. Schrier, J. A. Nelson, P. W. Lampert, and M. B. A. Oldstone. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83:7089-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willey, R. L., R. Shibata, E. O. Freed, M. W. Cho, and M. A. Martin. 1996. Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J. Virol. 70:6431-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zink, M. C., J. A. Yager, and J. D. Myers. 1990. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am. J. Pathol. 136:843-854. [PMC free article] [PubMed] [Google Scholar]