Abstract
Varicella-zoster virus (VZV) glycoprotein E (gE) is essential for viral replication and is involved in cell-to-cell spread, secondary envelopment, and entry. We created a set of mutations in the gE promoter to investigate the role of viral and cellular transcriptional factors in regulation of the gE promoter. Deletion or point mutation of the two Sp1 sites in the gE promoter abolished Sp1 binding and IE62-mediated transactivation of the gE promoter in vitro. Incorporation of the deletion or the point mutations disrupting both of the Sp1 binding sites into the VZV genome was not compatible with viral replication. A point mutation altering the atypical Sp1 binding site was lethal, while altering the second site impaired VZV replication significantly, indicating functional differences between the two Sp1 binding sites. Deletions in the gE promoter that abolished putative binding sites for cellular transcriptional factors other than Sp1, identified by bioinformatics analysis, were inserted in the VZV genome. Replication of the viruses with mutations of the gE promoter did not differ from control recombinants in melanoma cells or primary human tonsil T cells in vitro. These deletions did not affect infection of human skin xenografts in SCIDhu mice. These results indicate that Sp1 is required for IE62-mediated transactivation of the gE promoter and that this transcriptional factor appears to be the only cellular factor essential for regulation of the gE promoter.
Varicella-zoster (VZV) is a human alphaherpesvirus that causes varicella (chickenpox) during primary infection; VZV establishes latency in the sensory ganglia and reactivation causes herpes zoster (shingles) (2). The VZV genome encodes for nine putative glycoproteins, which are involved in attachment and entry, secondary envelopment, cell-cell fusion, and egress. Among these, VZV glycoprotein E (gE), a 623-amino-acid type I membrane protein, was shown to be essential for viral replication and to be involved in cell-cell spread and secondary envelopment (9, 25-27, 30, 48). The extracellular domain of VZV gE has been recently shown to interact with the insulin-degrading enzyme, a possible cellular receptor for VZV (23), indicating that gE is involved in the VZV entry process. The importance of gE trafficking to the plasma membrane and the trans-Golgi network in infected cells is indicated by the lethal effect of deletion of the cytoplasmic C-terminal domain and mutation of the endocytosis motif located in this region (31). In addition, the N-terminal region of the VZV gE ectodomain was shown to be a unique domain essential for VZV replication and important for cell-cell spread and secondary envelopment in vitro and for skin infection in vivo (4).
The requirement for gE expression and correct trafficking during the VZV replication cycle and in VZV pathogenesis in vivo makes the mechanisms of gE promoter regulation a central issue. Different cellular transcriptional factors, together with the viral transactivators, might differentially regulate gE transcription in different cell types and affect VZV pathogenesis in vivo, as previously shown for the VZV gI promoter (17). Binding sites for cellular transcriptional factors have been identified in the putative promoters of different VZV genes (47); these cellular factors interact with the major viral transactivator IE62, the immediate-early protein encoded by the duplicated open reading frame 62/71 (ORF62/71) genes (8, 38, 40). For instance, the upstream stimulatory transcription factor (USF) (49) was shown to physically interact with IE62 (43) and to cooperate with IE62 in the regulation of the bidirectional viral promoter of ORF28/29 (28, 35, 53) and ORF10 (7). Activator protein 1 (AP-1), a member of the Jun and Fos family (1), was shown to be important for VZV gene regulation, and the consensus binding motif for this factor is present in several VZV promoters (45). A cis-acting element regulating the ORF67 (gI) promoter activity contains binding sites specific for AP-1 and USF (14). Finally, the GC-box binding factor, specificity protein 1 (Sp1) (22, 51), was shown to bind IE62 (37, 47) and to recruit IE62 to the gI promoter (37). In addition, possible GC-rich elements were identified in the ORF4 and ORF63 promoters (19).
The transcriptional regulation of the gE promoter was shown to be influenced by both cellular factors and viral transactivators in transient-expression experiments (16, 44, 46). Analysis of the ORF67-ORF68 intergenic region, a 246-bp fragment containing the putative gE promoter, showed the presence of consensus sites for cellular transcriptional factors and an atypical TATA-box that interacts with the TATA-binding protein TBP (44, 46). Two GC-rich elements were identified within this region and were shown to be binding sites for Sp1 (46, 47). While one of these two sites is a canonical Sp1 consensus sequence, the second one is an atypical binding site identical to the one identified in the VZV gI promoter (14, 47).
Deletion of the atypical TATA-box, with or without concomitant deletion of the two Sp1 binding sites, resulted in a decrease or complete block of the gE promoter activity in transfection experiments (46).
We define here the role of VZV transactivators and cellular transcriptional factors in regulating the gE promoter, and we analyze the possibility that cellular factors other than Sp1 might be responsible for differential gE promoter expression in different cell types. Stepwise deletions of the intergenic ORF67-ORF68 region containing the gE promoter were performed and analyzed in transfection experiments to identify regions in the gE promoter essential for transactivation mediated by the VZV proteins. Cooperation between Sp1 and IE62 was investigated in vitro, and the role of Sp1 was analyzed in the context of viral replication. Finally, the relevance of potential cellular factor binding sites identified by bioinformatics analysis in different regions of the gE promoter was evaluated in primary human tonsil T cells and in vivo in human skin xenografts in the SCIDhu mouse model (31-33).
MATERIALS AND METHODS
Plasmids and cosmids.
The complete genome of the VZV parental Oka strain (P-Oka) is contained in four overlapping cosmids: pPme2, pSpe14, pFsp73, and pSpe23 (34). ORF68 (gE) is located in the unique short region of the VZV genome, in the pSpe23 cosmid (nucleotides [nt] 115911 to 117782 in P-Oka; nt 115808 to 117679 in Dumas strain). A SacI fragment of about 6 kb that contains the ORF68 sequence was cloned in pBluescript II SK(+) plasmid (Stratagene) in which the KpnI site was eliminated (PBSKΔKpnI). In this plasmid, named PBSKΔKpnI-SacI, the KpnI and BglII sites, located 680 bp upstream and 480 bp downstream of the ORF68 ATG, respectively, are unique sites, and they were used to insert the gE promoter mutations. The ORF67-ORF68 intergenic region from VZV P-Oka (nt 115564 to 115910; nt 115561 to 115807 in the Dumas strain) was amplified by PCR from the P-Oka genome and cloned in the pGL3 basic vector (Promega). The ΔI, ΔII, and ΔIII deletions were amplified by PCR from the wild-type gE promoter and cloned in the pGL3 basic vector.
To introduce the gE promoter mutations into the VZV genome, ΔI, ΔII, and ΔIII deletions (Fig. 1A) were cloned into the PBSKΔKpnI-SacI plasmid from which the sequence of gI gene (ORF67) was deleted as previously described to make VZV ΔgI-N and its rescue (25). This plasmid is referred to as PBSKΔKpnI-SacI-ΔgI-N@AvrII. For this purpose, the three deletions were amplified by PCR: each deletion was amplified in two fragments, A and B. The A fragment was amplified by using the SalI-fw primer (5′-CAAATCTTCGTCGACAATACATTG-3′; nt 114101 to 114106 in P-Oka. Underlining indicates the SalI site), and the reverse primer 5′-phosphorylated (5′-CTATTTAACAAACGGGTTTACAAC-3′; nt 115536 to 115559); the B fragment was amplified by using the BglII-rev primer (5′-GGATTAAGATCTCCTTTAAACACG-3′; underlining indicates the BglII site) and the ΔI, ΔII, or ΔIII forward primer 5′-phosphorylated (ΔI, 5′-GCGTTTTGATTACGCGTTGTGA-3′; ΔII, 5′-TAACTATAAGTTAACACGCCCAC-3′; ΔIII, 5′-TGAAGCCTTAAAGGCCGAGCT-3′). The A and B fragments were digested with SalI and BglII, respectively, and ligated into the PBSKΔKpnI-SacI-ΔgI-N@AvrII. Each plasmid was analyzed by sequencing to confirm the mutation. The fragment AvrII-SgrAI was cut from PBSKΔKpnI-SacI-ΔgI-N@AvrII containing the ΔI, ΔII, or ΔIII deletion and cloned into the pSpe23ΔAvrII cosmid, substituting the wild-type AvrII-SgrAI fragment.
FIG. 1.
Elements of the gE promoter region required for basal activity. (A) Schematic representation of the gI-gE intergenic region. The three deletions ΔI, ΔII, ΔIII, the two Sp1 binding sites (Sp1-A and Sp1-B), and the putative TATA-box (in boldface and italics) are indicated. The arrow indicates the transcriptional start site, and the rectangle encloses the gE ATG. (B) Effect of stepwise deletions on the transcriptional activity of the gE promoter. The bars indicate the means ± the standard deviations of three independent transfections done in duplicate. ut, untransfected cells; pGL3, cells transfected with the luciferase vector without any promoter.
The SP1A and SP1B promoters contain two nucleotide substitutions in each of the two Sp1 binding sites in the gE promoter, while both Sp1 sites are mutated in the SP1AB construct. These constructs were amplified by PCR to introduce the two base mutations with the method previously described (3) and using the following primers: SP1A-fw, 5′-AGTTAACACGaaCACATTTGGGCGGGGATG-3′; SP1A-rev, 5′-GCCCAAATGTGttCGTGTTAACTTATAGTTAGGA-3′; SP1B-fw, 5′-GCCCACATTTGttCGGGGATGTTTTATGAAGCCT-3′; and SP1B-rev, 5′-AAACATCCCCGaaCAAATGTGGGCGTGTTA (underlining indicates the Sp1 binding sites; the two base mutations are indicated in lowercase letters). The SP1AB promoter was produced by amplification of the SP1B construct with the following primers: SP1AB-fw, 5′-AAGTTAACACGaaCACATTTGttCGGGGATGTT-3′; and SP1AB-rev, 5′-GaaCAAATGTGttCGTGTTAACTTATAGTTAGG-3′. Each forward primer was used in combination with the BglII-rev primer, and each reverse primer was used with the KpnI-fw primer (5′-GGTCCGGTACCTCCCTGTTT-3′; underlining indicates the KpnI site). The KpnI-BglII PCR fragment containing the Sp1 binding site mutation A, B, or AB was cloned in pCR4-TOPO plasmid (Invitrogen), and the sequence was verified by sequencing. This fragment was cloned in the PBSKΔKpnI-SacI plasmid.
The SP1A, SP1B, and SP1AB mutations were inserted in the VZV genome by constructing mutated pSpe23 cosmids. The fragment AvrII-SgrAI was cut from PBSKΔKpnI-SacISP1A, PBSKΔKpnI-SacISP1B, and PBSKΔKpnI-SacISP1AB and cloned in the pSpe23ΔAvrII cosmid, substituting the wild-type AvrII-SgrAI fragment. The SP1A, SP1B, and SP1AB promoters were also amplified by PCR and cloned in the pGL3 basic vector. For the gE promoter mutation rescue, a fragment containing the ORF68, as well as 271 bp upstream (the intact promoter region) and 183 bp downstream, was inserted in the AvrII restriction site in the pSpe23 cosmid. All of the mutated cosmids were sequenced to verify the gE promoter mutations and/or the insertion of the rescue fragment. Sequence analyses were performed by Biotech Core, Inc., Sunnyvale, CA, and Elim Biopharmaceuticals, Inc., Hayward, CA.
The pCMV-IE62, pCMV-ORF61, pCMV-IE63, and pCMV-ORF4 plasmids contain the IE62, ORF61, IE63, and IE4 coding sequences under the control of the cytomegalovirus (CMV) immediate-early (IE) promoter (a gift from P. Kinchington, University of Pittsburgh, Pittsburgh, PA).
Cells and recombinant viruses.
Human melanoma cells, MeWo (13), were grown in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, nonessential amino acids, and antibiotics. VZV recombinant P-Oka virus was obtained from transfection of MeWo cells with four overlapping cosmids containing the entire P-Oka genome (18, 25, 50).
Transfection and reporter assays.
MeWo cells were seeded at the density of 2 × 105 cells per well in 24-well plates and transfected with Lipofectamine 2000 (Invitrogen). A total of 1 μg of the reporter pGL3 constructs was used in each transfection; when the pGL3 constructs were cotransfected with the VZV transactivators (pCMV-IE62, pCMV-ORF61, pCMV-IE63, and pCMV-ORF4), 0.9 μg of the pGL3 constructs and 0.1 μg of the transactivators were used. The pGL3 basic vector was used as a control in all experiments. The cells were incubated with the transfection mix in Opti-MEM (Invitrogen); after 6 h the medium was replaced with complete culture medium. A plasmid containing the human β-globin promoter (54) driving the Renilla gene in the phRL plasmid (Promega) or the phRL plasmid without any promoter was used as internal control. In some experiments, the luciferase activity was normalized by total protein content. At 24 h after transfection the cells were lysed in 1× PBL buffer (Promega), and a dual luciferase assay was performed according to the manufacturer's recommendations (Promega). In the transfection and infection experiments, MeWo cells were transfected as described above. After 6 h, the transfected cells were overlaid with VZV-infected cells using a 1:8 ratio of infected to transfected cells. Cells were harvested 24 h after transfection/infection and assayed for luciferase reporter gene expression as described above. Differences in the luciferase expression were calculated with the Student's t test and considered statistically significant at a P value of <0.05.
Electrophoretic mobility shift assay (EMSA).
Four sets of oligonucleotides were designed for targeting the VZV ORF67-ORF68 intergenic region containing the wild-type and the single or double mutated consensus sequence for the two Sp1 binding sites (wtSP1-fw, 5′-TAAGTTAACACGCCCACATTTGGGCGGGGATGTTTT-3′; wtSP1-rev, 5′-AAAACATCCCCGCCCAAATGTGGGCGTGTTA-3′; SP1A-fw, 5′-TAAGTTAACACGAACACATTTGGGCGGGGATGTTTT-3′; SP1A-rev, 5′-AAAACATCCCCGCCCAAATGTGTTCGTGTTA-3′; SP1B-fw, 5′-TAAGTTAACACGCCCACATTTGTTCGGGGATGTTTT-3′; SP1B-rev, 5′-AAAACATCCCCGAACAAATGTGGGCGTGTTA-3′; SP1AB-fw, 5′-TAAGTTAACACGAACACATTTGTTCGGGGATGTTTT-3′; SP1AB-rev, 5′-AAAACATCCCCGAACAAATGTGTTCGTGTTA-3′; underlining indicates the Sp1 consensus sequences; mutated nucleotides are indicated in boldface). For probe labeling, oligonucleotides were annealed, and 100 μg of the 36-nt double-stranded oligonucleotide probes were labeled with 30 μCi of [α32P]dCTP (Perkin-Elmer) by using the Klenow enzyme (Promega). EMSA was performed in a final volume of 10 μl with 30 ng of the human recombinant Sp1 protein (Promega) and 35 fmol of [α-32P]dCTP-labeled double-stranded oligonucleotide probe incubated in 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 50 mg/μl of poly(dI-dC) (Roche) as nonspecific competitor. Samples were incubated at 23°C for 20 min. For competition, 5- and 50-fold molar excesses of unlabeled wild-type double-stranded oligonucleotide was preincubated with the protein for 5 min at 23°C and then incubated with the wild-type probe for another 15 min. DNA-protein complexes were resolved on a 6% polyacrylamide gel in 0.5× Tris-borate-EDTA and detected by autoradiography.
Cosmid transfections, DNA isolation, and confirmation of mutations.
Cosmid DNA preparation and transfection procedures were done as previously described (25, 50). DNA was recovered from transfected cells by using the DNAzol (Gibco-BRL). PCR and sequencing of the PCR products were performed to confirm the expected gE promoter mutations. The sequence analysis was performed by Biotech Core, Inc., Sunnyvale, CA, and Elim Biopharmaceuticals, Inc., Hayward, CA.
Western blotting.
MeWo cells were infected with rOka-gE promoter mutant viruses or the rOka control, and cell lysates were collected in radioimmunoprecipitation assay buffer at 24 and 72 h postinfection. Proteins were separated on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Mouse anti-gE antibody 3B3 (48) was used at a dilution of 1:8,000 to 1:15,000. Rabbit anti-IE4 (kindly provided by Paul Kinchington, University of Pittsburgh) was used at a dilution of 1:500 to 1:2,500, and mouse anti-α-tubulin monoclonal antibody (Sigma) was used at a 1:10,000 dilution. Antibodies were detected with sheep anti-mouse and sheep anti-rabbit antibody horseradish peroxidase conjugated (Amersham) at a dilution of 1:2,000 to 1:10,000.
Primary human tonsil cell preparation and fluorescence-activated cell sorting (FACS) analysis.
Primary tonsil T cells were prepared from human tonsils obtained from the Department of Pathology, Stanford University Medical Center, according to a protocol approved by the Stanford University Committee on Human Subjects in Research. Suspensions of T cells were prepared as previously described (20). Human embryonic lung fibroblasts (HELF) infected with rOka or the rOka mutants were overlaid with 107 tonsil cells; uninfected fibroblasts were overlaid with tonsil cells were used as negative control. At 48 h postinfection, the infected fibroblasts were titrated onto melanoma cells, while tonsil cells were processed for FACS staining. A total of 2 × 105 tonsil cells were resuspended in 100 μl of FACS buffer (1% fetal bovine serum in 1× phosphate-buffered saline). The cells were incubated with human VZV immune or nonimmune polyclonal immunoglobulin G (32) on ice for 40 min, washed twice in FACS buffer, and stained with goat anti-human fluorescein isothiocyanate-conjugated (Caltag) and mouse anti-human-CD3-phycoerythrin (Caltag) on ice for 40 min. Cells were incubated with the appropriate isotype control antibody as a control. Samples were analyzed on a FACSCalibur apparatus (Becton Dickinson, Inc.).
Infection of skin xenografts in SCIDhu mice.
Skin xenografts were made in homozygous CB-17scid/scid mice, using human fetal tissue obtained according to federal and state regulations (32, 33). Animal use was in accordance with the Animal Welfare Act and approved by the Stanford University Administrative Panel on Laboratory Animal Care. rOka and gE promoter mutant viruses were passed three times in primary HELF before inoculation of the xenografts. Infectious virus titers were determined for each inoculum at the time of inoculation. Skin xenografts were harvested 10 and 21 days postinfection, and virus recovered from each implant was analyzed by infectious focus assay and immunohistochemistry with polyclonal anti-VZV human immune serum (32). Virus recovered from the tissues was tested by PCR and sequencing to confirm the expected mutations.
RESULTS
Effect of stepwise deletions on gE promoter basal activity.
Three stepwise deletions (ΔI, ΔII, and ΔIII) were produced in the ORF67-ORF68 intergenic region (Fig. 1A). Melanoma cells (MeWo) were transfected with constructs that had the intact gE promoter or the mutated promoter elements ΔI, ΔII, and ΔIII, and the level of luciferase reporter gene expression was evaluated. As shown in Fig. 1B, the ΔI deletion did not cause a significant decrease in the activity of the gE promoter, while the ΔII deletion, which contains the TATA-box and the two Sp1 sites, the atypical Sp1-A binding site, and the canonical consensus Sp1-B (47), showed a consistent reduction in luciferase expression of about 75%. The construct with the ΔIII promoter mutation, containing the TATA-box but not the two Sp1 sites, showed a level of luciferase expression similar to the control vector. These results indicated that the regions deleted in the ΔII and ΔIII promoter mutants contain cis-elements important for the basal activity of the gE promoter in vitro.
Effect of gE promoter deletions on viral transactivator activity.
The VZV transactivators IE62 and IE4 are known to transactivate the gE promoter in vitro (16). We used the gE promoter constructs to identify regions in the gE promoter that are required for transactivation mediated by these VZV proteins and IE63 and ORF61. Cotransfection of plasmids containing each of the four transactivators with the intact gE promoter element showed that IE62 was able to significantly transactivate the gE promoter (250-fold increase) as expected, while the effect of IE4 alone was modest (5-fold increase) (Fig. 2A). In addition, both ORF61 and IE63 had only a minimal effect on the gE promoter (4- and 1.2-fold increase, respectively) (Fig. 2A). Cotransfection of IE62 with each of the other three transactivators—ORF61, IE63, and IE4—showed a significant increase in the luciferase reporter expression when IE62 was transfected with either IE63 or IE4 (290- or 320-fold increases), indicating an enhancement of IE62 activation by IE63, in addition to the previously described enhancement by IE4 (16) (Fig. 2A). Since IE62 showed the strongest effect on the gE promoter expression compared to the minimal effect of the other VZV transactivators IE4, ORF61, and IE63, we focused on the mechanism of IE62 regulation of the gE promoter.
FIG. 2.
Elements of the gE promoter required for VZV transactivator activity. Melanoma cells were transfected with the gE promoter construct and the plasmids expressing the VZV transactivators IE62, ORF61, IE63, and IE4, either individually or in combination with IE62 (A) and the ΔI, ΔII, and ΔIII gE promoter deletion constructs and pCMV-IE62 (B). Cells were harvested 24 h after transfection, and the luciferase assay was performed. Transfections were normalized with the protein lysate concentration (indicated by the symbol “[ ]”) determined by Bradford assay (Bio-Rad) (A) or by cotransfecting the phRL plasmid without any promoter driving the Renilla gene (B). The bars indicate the means ± the standard deviations of three independent transfections made in duplicate.
Transfection of MeWo cells with ΔI, ΔII, and ΔIII promoter constructs with or without IE62 showed that IE62-mediated activation of the gE promoter was dramatically reduced by the ΔIII mutation, whereas the ΔI and ΔII deletions did not cause any significant decrease (Fig. 2B). These results suggest that only the cis-elements removed by the ΔIII promoter deletion, which eliminates the two Sp1 sites, are essential for IE62-mediated activation of the gE promoter in vitro.
Role of Sp1 in IE62 transactivation of the gE promoter in vitro.
Based on these observations, we investigated whether IE62 transactivation of the gE promoter is mediated by Sp1, as demonstrated for the VZV gI promoter (37). For this purpose, three luciferase reporter constructs were produced, including the SP1A and SP1B constructs, in which each Sp1 site was mutated individually by two base pair substitutions, and the SP1AB construct, in which both Sp1 sites were mutated (Fig. 3A). An EMSA confirmed that the Sp1 binding site mutations abolished the binding of the transcriptional factor to both sites (Fig. 3B).
FIG. 3.
Effect of Sp1 mutations on transactivation activity of the gE promoter by IE62. (A) Schematic representation of the four probes used in EMSA. wt, wild-type sequence; SP1A, mutation of the A site; SP1B, mutation of the B site; SP1AB, mutation of the A and B sites. (B) EMSA results. Sp1 binds specifically to the gE promoter, as unlabeled wild-type probe competes for binding. Mutation of the Sp1 binding sites abolished complex formation. (C) Luciferase assay in melanoma cells at 24 h after transfection with the Sp1 mutant constructs and pCMV-IE62 vector. Transfections were normalized by cotransfecting the phRL plasmid without any promoter driving the Renilla gene. The results shown were obtained in three independent transfections made in duplicate. The standard deviation is indicated.
When MeWo cells were transfected with the SP1A, SP1B, and SP1AB constructs and the pCMV-IE62 plasmid, IE62-mediated promoter activity was reduced by about 65-fold by SP1A and about 20-fold by SP1B. The luciferase activity measured in the reporter assay with the SP1B promoter construct was higher than that with the SP1A construct. These results correlated with those obtained by EMSA in which the probe containing the SP1A mutation showed less affinity than the SP1B probe for binding to Sp1. These data suggested that the Sp1-A site has higher affinity for the Sp1 protein and that mutation of this site in the gE promoter has a greater impact on the activity of the promoter in vitro. Finally, the decrease in promoter activity was even more dramatic when both Sp1 sites were modified (170-fold decrease) (Fig. 3C). The decrease in responsiveness of the SP1AB promoter to IE62 was comparable to the change caused by the ΔIII deletion, suggesting that the Sp1 binding sites are the cis elements removed in the ΔIII deletion that blocked IE62-mediated activation.
Sp1 is essential for gE promoter activation during VZV infection and replication.
MeWo cells transfected with mutated gE promoter constructs and infected with rOka showed that the ΔI and ΔII mutant promoters were transactivated to levels comparable to the intact gE promoter, whereas the ΔIII deletion caused a significant reduction (40-fold decrease) (Fig. 4A). Mutation of the Sp1 sites decreased luciferase expression, which was greatest (53-fold decrease) when both sites were mutated (Fig. 4A). The decrease of luciferase expression associated with the SP1AB mutation was comparable to that observed with the ΔIII promoter construct, indicating that the two Sp1 binding sites are essential for gE promoter expression in the context of VZV infection.
FIG. 4.
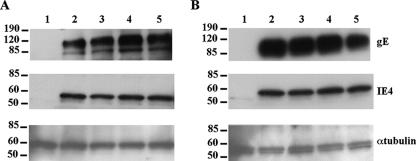
Effect of Sp1 mutations during VZV infection and replication. (A) Melanoma cells were transfected with the wild-type gE promoter construct, or the constructs with the deletion ΔI, ΔII, ΔIII, or Sp1 mutant promoter element. rOka-infected melanoma cells were added to transfected cells (ratio 1:8) at the time of medium addition. Cells were harvested 24 h after transfection/infection, and the luciferase assay was performed. phRL plasmid without any promoter was used for normalization. The error bars indicate the standard deviations. (B) Plaque morphology in the rOka-SP1B virus. Melanoma cells infected with the rOka or rOka-SP1B viruses were analyzed 4 days postinfection by immunohystochemistry with polyclonal anti-VZV human immune serum (32). (C) Expression of gE in the rOka-SP1B virus. Melanoma cells infected with rOka-SP1B virus were analyzed by Western blotting with mouse anti-gE monoclonal antibody 3B3 (top panel), anti-rabbit polyclonal antibody to IE4 (central panel), and monoclonal antibody anti-α-tubulin (bottom panel). Lane 1, cell lysate from uninfected melanoma cells; lane 2, cell lysate from rOka-infected melanoma cells; lane 3, cell lysate from rOka-SP1B-infected melanoma cells. The molecular markers are indicated on the left in kilodaltons.
To demonstrate the essential role of Sp1 in gE promoter activation during VZV replication, we introduced the mutation of the two Sp1 binding sites (SP1AB) into the viral genome in the ORF67-ORF68 intergenic region of the cosmid pSpe23. The mutated cosmid, pSpe23-SP1AB, was transfected along with the cosmids pPme2, pSpe14, and pFsp73; intact pSpe23 was transfected along with the other cosmids as a control. Mutation of the two Sp1 binding sites was incompatible with VZV replication, as indicated by a failure to recover recombinant virus from transfections of two independently derived pSpe23-SP1AB clones (data not shown). Recombinant virus was recovered when a wild-type copy of ORF68 and its flanking regions were inserted at the unique AvrII restriction site in the pSpe23 cosmid (4, 30; data not shown). These results indicate that Sp1 has a major role in the gE promoter activation and that its contribution to the transactivation of the gE promoter is essential for replication.
Our data from the luciferase assay and EMSA indicated that the SP1A mutation had a stronger effect on the gE promoter expression than the SP1B. To further investigate the contribution of each of these two sites on gE promoter expression, we introduced the A and B mutations individually into the VZV genome. Melanoma cells were transfected with two independently derived pSpe23-SP1A or pSpe23-SP1B clones along with the cosmids pPme2, pSpe14, and pFsp73. Recombinant virus was recovered from transfection with the pSpe23-SP1B clones but not from the pSpe23-SP1A clones; rOka control virus was recovered in each transfection as expected. Interestingly, replication of the recombinant virus carrying the SP1B mutation was impaired. The mutant virus was recovered with a delay of 4 to 5 days compared to the rOka control, and it showed a small-plaque phenotype (Fig. 4B). Reduced levels of gE expression were detected in the SP1B mutant virus (Fig. 4C, top panel) when analyzed at a similar or even higher level of infection compared to the rOka control, as indicated by the levels of expression of IE4 (Fig. 4C, central panel). These results indicate that while the Sp1-A site is essential for gE promoter expression, this site is not sufficient since mutation of the Sp1-B site strongly affected the promoter expression and VZV replication.
Effect of stepwise deletions on gE promoter regulation in melanoma and T cells in vitro.
Bioinformatic analysis of the gE promoter region by MatInspector (5, 42) revealed potential consensus sites for other transcriptional factors (Fig. 5). To determine whether cellular transcriptional factors other than Sp1 might be involved in regulating the gE promoter, the ΔI and ΔII mutations that removed the putative binding sites for several cellular proteins (Fig. 5B) from the gE 5′-untranslated region (5′UTR) were introduced into the P-Oka genome, using the pSpe23 cosmid. We also introduced the ΔIII mutation, which removes the Sp1 sites and was therefore predicted to be lethal based on the results with SP1AB mutagenesis. Mutations of the gI-gE intergenic region affect the 3′UTR of gI. Therefore, to create these VZV mutants, it was necessary to make the ΔI, ΔΙΙ, and ΔIII deletions from intergenic region of the ΔgI-N pSpe23 cosmid (25) and avoid the consequences of the gI truncation by inserting the intact gI sequence and its flanking regions into the AvrII site in the pSpe23 cosmid, as previously described (25). As a control for these constructs with gE promoter mutations, a mutant cosmid was made that had an intact gI-gE intergenic region, the gI-N-terminal truncation, and insertion of gI at the AvrII site.
FIG. 5.
Bioinformatic analysis of the ORF67-ORF68 intergenic region. (A) ORF67-ORF68 intergenic region containing the gE promoter. Numbers indicate the nucleotide position; the filled and the open lollipops indicate the ΔI and ΔII boundaries, respectively. The Sp1 sites are underlined. (B) Summary of the bioinformatics analysis performed with Matinspector. The “core similarity” is the level of similarity between the “core sequence” of the matrix used by the program and the sequence; the value 1.000 represents the maximum of similarity.
Melanoma cells were transfected with the mutated pSpe23 cosmids and the other three cosmids—pFsp73, pPme2, and pSpe14. Recombinant virus was recovered with the mutated pSpe23 that had the intact gI-gE intergenic region and gI at the AvrII site and was designated rOka-gEpro. The ΔI and ΔII changes also yielded viruses, designated rOka-gEproΔI and rOka-gEproΔII, but transfection experiments with the cosmid carrying the ΔIII deletion did not, as expected. The growth kinetics of the recombinant viruses with the gE promoter mutations did not differ from rOka over 6 days in melanoma cells (data not shown). The levels of gE expression were monitored in melanoma cells infected with each recombinant virus at 24 and 72 h postinfection by Western blotting (Fig. 6A and B). No differences were observed at a comparable level of infection, as indicated by the expression of IE4, at either time point. These results indicated that the ΔI and ΔII gE promoter deletions were compatible with viral replication in vitro; in addition, they did not alter the growth kinetics or gE expression compared to rOka-gEpro or rOka generated from cosmids with no mutations.
FIG. 6.
Analysis of gE expression in infected melanoma cells. Melanoma cells were inoculated with the gE promoter mutant viruses and analyzed by Western blotting with mouse anti-gE monoclonal antibody 3B3 (top panel), anti-rabbit polyclonal antibody to IE4 (central panel), and monoclonal antibody anti-α-tubulin (bottom panel). Cell lysates from uninfected melanoma cells (lane 1) or melanoma cells inoculated with rOka (lane 2), rOka-gEpro (lane 3), rOka-gEproΔI (lane 4), or rOka-gEproΔII (lane 5) were analyzed at 24 h (A) and 72 h (B) postinfection. The molecular markers are indicated on the left in kilodaltons.
To analyze whether the regions deleted in the gE promoter mutants were important for the regulation of the gE promoter in T cells, primary human tonsil T cells were infected with rOka or rOka-gEpro, rOka-gEproΔI, and rOka-gEproΔΙΙ by coculturing them with infected fibroblasts. Infection was analyzed by flow cytometry after staining the cells with the T-cell marker, CD3, and with the polyclonal anti-VZV human immune serum to detect VZV-infected cells. No significant differences were observed in the level of infection of the T cells by the promoter mutants compared to the rOka or rOka-gEpro controls (Fig. 7A). The minor reduction of T-cell infection with rOka-gEproΔΙ was consistent with a lower titer of this virus in the fibroblast monolayer compared to the titers in fibroblasts infected with rOka or rOka-gEpro (Fig. 7B).
FIG. 7.
Analysis of the gE promoter mutants replication in human tonsil T cells. Primary human tonsil T cells were infected by coculture with HELF-infected monolayers; the cells were stained for CD3 and VZV proteins and analyzed by flow cytometry 48 h postinfection. (A) Tonsil T cells were analyzed by flow cytometry; the bars represent the percentage of T cells (CD3 positive) VZV-positive for each viruses compared to the uninfected control. The error bar represents the standard deviation. (B) Titer of the HELF-infected monolayers. The virus titer of each monolayer used to infect T cells was determined by infectious focus assay onto melanoma cells. Each column represents the mean titer; the bars represent the standard deviation. The asterisk indicates significance (P < 0.05).
Effect of gE promoter mutations on VZV replication in human skin xenografts in vivo.
We analyzed the effect of the gE promoter deletions in human skin xenografts implanted in SCID mice. The skin xenografts were inoculated with fibroblasts infected with rOka, rOka-gEpro, rOka-gEproΔΙ, or rOka-gEproΔΙΙ viruses; the inoculum titers were determined on melanoma cells at the time of infection. The replication of the gE promoter deletion mutants did not differ from rOka or rOka-gEpro at day 10, whereas a minimal reduction in the rOka-gEproΔΙΙ growth kinetics (P = 0.048) compared to rOka-gEpro was observed at day 21 (Fig. 8). At day 21, both of the gE promoter mutants showed a significant decrease in growth kinetics compared to the rOka control but not to rOka-gEpro (Fig. 8). When this experiment was repeated and both data sets were analyzed together, both rOka-gEpro and rOka-gEproΔII had lower titers compared to rOka control in skin xenografts tested at day 10, and rOka-gEpro, rOka-gEproΔI, and rOka-gEproΔII titers were lower than the rOka control at day 21 (data not shown). These observations indicate that the ectopic expression of gI reduced the virulence of VZV in skin compared to rOka but that the ΔI and ΔII deletions of putative binding sites for cellular factors in the gE promoter did not contribute to alter virulence. Of interest, the initial (day 10) titers were higher in skin xenografts infected with rOka-gEproΔΙ compared to rOka-gEpro (P = 0.0035) (data not shown). Sequencing of samples recovered after 10 and 21 days showed persistence of the expected mutations (data not shown).
FIG. 8.
Effect of the gE promoter deletions on VZV replication in skin xenografts. Skin xenografts were inoculated with HELF infected with rOka, rOka-gEpro, rOka-gEproΔI, or rOka-gEproΔII. The inoculum titers were 2.2 × 105 PFU/ml for rOka, 1.5 × 105 PFU/ml for rOka-gEpro, 2.5 × 105 PFU/ml for rOka-gEproΔI, and 2.5 × 105 PFU/ml for rOka-gEproΔII. The infected xenografts were collected at days 10 and 21. Six xenografts were inoculated with each virus; the titers of samples from which infectious virus was not recovered were considered equal to 1 PFU/implant. Each bar represents the mean titer, and the error bar indicates the standard deviation. The asterisks indicate significance (P < 0.05). *, Significant difference between rOka and the gE promoter mutants; **, significant difference between rOka-gEpro and rOka-gEproΔII.
DISCUSSION
VZV gE is a multifunctional protein, involved in cell-cell fusion and cell-to-cell spread of viral particles and secondary envelopment of the cytoplasmic virions in the trans-Golgi network (9, 26, 27, 48). VZV gE has unique characteristics compared to gE in the other alphaherpesviruses. We have shown that, in contrast to these other viruses, VZV gE is required for VZV replication (25, 30) and that VZV gE has a nonconserved N-terminal region of the ectodomain that is essential for VZV replication (4). Importantly, specific VZV gE functional motifs are necessary for T-cell and skin tropisms involved in VZV pathogenesis (4, 31).
Given the importance of gE for VZV replication and pathogenesis, we investigated in detail the role of VZV transactivators and cellular transcriptional factors in the regulation of the VZV gE promoter in vitro and in vivo. We found that the major VZV transactivator, IE62 (38-40), is the viral regulatory protein with the most potent effect on the gE promoter compared to ORF61, IE4, and IE63. Interestingly, we found that IE63 alone had no activating or repressor effects on the gE promoter but that IE63 enhanced the IE62-mediated transactivation of this promoter. IE63 has been shown to physically interact with IE62 and the cellular RNA polymerase II and to enhance the IE62 transactivation of the VZV gI promoter in a similar way (24).
Further analysis showed that deletions of the region containing the two Sp1 binding sites in the gE promoter eliminated IE62-mediated transactivation in transient-expression assays and expression from the gE promoter reporter construct in VZV-infected cells. Sp1 is a ubiquitous cellular transcriptional factor that binds GC-rich sequences and interacts with the TATA-binding protein and other components of the transcriptional machinery (22). Sp1 binding sites have been identified in several VZV promoters (47), including the gI and the gE 5′UTR (14, 44, 46). In addition to the effects of deleting the gE promoter region containing the two Sp1 binding sites, targeted mutations of these two sites dramatically affected IE62-mediated transactivation. Like IE63, Sp1 has been shown to physically interact with IE62, and Sp1 recruits the viral transactivator to the gI promoter (37). Interestingly, the VZV gI promoter contains a unique Sp1 binding site, which is noncanonical (14), whereas the gE promoter contains two Sp1 binding sites: a typical consensus site, which we have designated Sp1-B (11), and an atypical consensus sequence, designated Sp1-A, identical to the one identified in the gI promoter (14, 46, 47). Our luciferase assays and EMSA experiments indicate that the atypical consensus site, Sp1-A, has a higher binding affinity for Sp1 that correlated with a more dramatic effect of its mutation on IE62-mediated transactivation of the gE promoter compared to the mutation of the canonical binding site. Interestingly, when each of the Sp1 binding sites was mutated individually in the VZV genome, altering the atypical Sp1 site (Sp1-A) was lethal, whereas mutation of the canonical site (Sp1-B) was compatible with virus recovery but significantly impaired VZV replication and plaque formation. These results indicate that the Sp1-A site is essential for VZV replication but not sufficient, whereas the Sp1-B site although not essential is strictly required for optimal gE promoter expression and VZV replication.
The atypical consensus sequence has been predicted to be more common in promoter regions in the VZV genome (47). Further investigations of its usage in VZV gene transcription would be of interest, since this sequence does not appear to be the major Sp1 site used in mammalian cells. The presence of a high-affinity atypical Sp1 consensus site in VZV promoter regions might be a mechanism to effectively divert Sp1 to the VZV promoters and allow efficient transcription of the viral proteins. The requirement for two Sp1 binding sites in the gE promoter, an atypical consensus site and a canonical consensus site with different characteristics, could be a mechanism to ensure expression of an essential protein for VZV replication.
Simian varicella virus is closely related to VZV. Although the simian varicella virus gE promoter does not show many similarities in the region upstream and downstream of the transcriptional start site (12), it also contains a potential Sp1 consensus site. The gE promoter in pseudorabies virus contains several potential Sp1 binding sites and is transactivated by the only pseudorabies virus IE protein, IE180 (15), the herpes simplex virus ICP4 homolog, which is the counterpart of VZV IE62 (6). Sp1 regulation of the gE promoter may be a conserved characteristic of alphaherpesviruses.
The critical importance of cooperation between the cellular factor Sp1 and VZV IE proteins for VZV gE expression was demonstrated in experiments showing that viral replication from cosmids was blocked by targeted mutations of the two Sp1 sites. This observation was confirmed by failure to recover virus from cosmids with the ΔIII deletion where these sites are located. Since gE expression is essential, these results indicate that the Sp1 sites are necessary for IE62-mediated gE promoter transactivation in the context of viral genome as well as in transient-expression systems. As noted above, gI promoter regulation by IE62 also requires Sp1 (37), but mutation of the Sp1 site in this promoter was not lethal for VZV replication in vitro; nevertheless, mutating the Sp1 site in the gI promoter significantly impaired VZV virulence in skin and T-cell xenografts in SCIDhu model (17).
Cellular transcriptional factors other than Sp1 have also been implicated in the regulation of VZV gene transcription, and consensus sites for cellular factors were present in the gE promoter by bioinformatics analysis. The USF protein physically interacts with IE62 (43) and cooperates with this viral factor in the regulation of the ORF28/ORF29 bidirectional promoter (28, 53) and the ORF4 and the ORF10 promoters (7, 29). In addition, a USF binding site is present in the gI promoter, in the AUS region, where the Sp1 atypical consensus site and the binding site for the cellular factor AP-1 were also identified (14). These cellular transcriptional factors were found to differentially influence VZV virulence in skin and in T-cell xenografts in vivo (17). Therefore, in addition to targeted analysis of the Sp1 sites, we examined the effects of deletions in the gE promoter on the gE expression by using reporter constructs and cosmid mutagenesis. Despite eliminating predicted consensus binding motifs for 13 and 6 cellular factors with the ΔI and ΔII mutations, respectively, no major differences were observed in VZV replication in melanoma cells or primary human tonsil T-cell in vitro or in human skin xenografts in vivo. A minor increase in VZV replication during the initial stage of skin infection was detected when the ΔI region was deleted, which could result from abolishing consensus sites for a transcriptional repressor. For instance, a potential consensus site for the transcriptional repressor E4BP4 is present in the ΔI region. This protein, first identified for the ability to bind and repress viral promoters, belongs to the PAR family of basic leucine zipper (bZIP) factors and, in contrast to the other members of this family, it acts as a transcriptional repressor (10). Mutagenesis of the E4BP4 binding site in the gE promoter sequence and analysis of the binding of this protein to the consensus site would be necessary to demonstrate a role for this factor as a repressor of gE expression.
Some of the potential consensus sites identified in the gE promoter by bioinformatics analysis, such as Brn-5, are specific for transcriptional factors expressed in the nervous system (41). Other members of this family, Oct-1 and Brn-3, regulate viral genes in herpes simplex virus (21, 52). Interestingly, isoforms of the cellular Oct-2 transcriptional factor that are specifically expressed in neuronal cells appear to repress the activation of the VZV IE62 promoter (36). It is possible that members of the POU family regulate the expression of gE in the dorsal root ganglia; it would be interesting to investigate the significance of the potential Brn-5 binding site for VZV replication in vivo in human dorsal root ganglia in the SCIDhu model (55).
In conclusion, we defined the cellular transcriptional factor Sp1 as the major cell protein involved in gE promoter regulation and showed that binding of this protein to the gE promoter is essential for IE62-mediated transactivation and therefore for VZV replication, since gE is a required protein. Cellular factors other than Sp1 may be important for a fine-tuning of the gE promoter regulation in a cell-specific manner. However, VZV infection of human T cells in vitro and skin xenografts in vivo does not require consensus sites for at least 19 such factors in the gE promoter elements. Based on its effects on both gI and gE expression, Sp1 plays a pivotal role in the capacity of VZV to infect the human host.
Acknowledgments
This study was supported by grants AI20459 and AI053846 from the National Institute of Allergy and Infectious Diseases and grant CA49605 (A.M.A.) from the National Cancer Institute.
We thank Reija Matheson for the preparation of the primary human tonsil cells.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Angel, P., and M. Karin. 1991. The role of Jun, Fos, and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 2.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2768. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 3.Baiker, A., C. Bagowski, H. Ito, M. Sommer, L. Zerboni, K. Fabel, J. Hay, W. Ruyechan, and A. M. Arvin. 2004. The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 78:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berarducci, B., M. Ikoma, S. Stamatis, M. Sommer, C. Grose, and A. M. Arvin. 2006. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J. Virol. 80:9481-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartharius, K., K. Frech, K. Grote, B. Klocke, M. Haltmeier, A. Klingenhoff, M. Frisch, M. Bayerlein, and T. Werner. 2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933-2942. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. Y., M. L. Wong, H. W. Lin, and T. J. Chang. 2002. Cloning and regulation of the promoter of pseudorabies virus (TNL strain) glycoprotein E gene. Virus Genes 24:235-241. [DOI] [PubMed] [Google Scholar]
- 7.Che, X., B. Berarducci, M. Sommer, W. T. Ruyechan, and A. M. Arvin. 2007. The ubiquitous cellular transcriptional factor USF targets the varicella-zoster virus open reading frame 10 promoter and determines virulence in human skin xenografts in SCIDhu mice in vivo. J. Virol. 81:3229-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 9.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 10.Cowell, I. G. 2002. E4BP4/NFIL3, a PAR-related bZIP factor with many roles. Bioessays 24:1023-1029. [DOI] [PubMed] [Google Scholar]
- 11.Dynan, W. S., and R. Tjian. 1983. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell 35:79-87. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher, T. M., III, and W. L. Gray. 1994. Transcriptional analysis of two simian varicella virus glycoprotein genes which are homologous to varicella-zoster virus gpI (gE) and gpIV (gI). Virology 205:352-359. [DOI] [PubMed] [Google Scholar]
- 13.Grose, C., D. M. Perrotta, P. A. Brunell, and G. C. Smith. 1979. Cell-free varicella-zoster virus in cultured human melanoma cells. J. Gen. Virol. 43:15-27. [DOI] [PubMed] [Google Scholar]
- 14.He, H., D. Boucaud, J. Hay, and W. T. Ruyechan. 2001. cis and trans elements regulating expression of the varicella zoster virus gI gene. Arch. Virol. Suppl. :57-70. [DOI] [PubMed]
- 15.Ihara, S., L. Feldman, S. Watanabe, and T. Ben-Porat. 1983. Characterization of the immediate-early functions of pseudorabies virus. Virology 131:437-454. [DOI] [PubMed] [Google Scholar]
- 16.Inchauspe, G., S. Nagpal, and J. M. Ostrove. 1989. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology 173:700-709. [DOI] [PubMed] [Google Scholar]
- 17.Ito, H., M. H. Sommer, L. Zerboni, H. He, D. Boucaud, J. Hay, W. Ruyechan, and A. M. Arvin. 2003. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mice in vivo. J. Virol. 77:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinchington, P. R., J. P. Vergnes, P. Defechereux, J. Piette, and S. E. Turse. 1994. Transcriptional mapping of the varicella-zoster virus regulatory genes encoding open reading frames 4 and 63. J. Virol. 68:3570-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku, C. C., J. A. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaBoissiere, S., S. Walker, and P. O'Hare. 1997. Concerted activity of host cell factor subregions in promoting stable VP16 complex assembly and preventing interference by the acidic activation domain. Mol. Cell. Biol. 17:7108-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lania, L., B. Majello, and P. De Luca. 1997. Transcriptional regulation by the Sp family proteins. Int. J. Biochem. Cell Biol. 29:1313-1323. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., M. A. Ali, and J. I. Cohen. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch, J. M., T. K. Kenyon, C. Grose, J. Hay, and W. T. Ruyechan. 2002. Physical and functional interaction between the varicella zoster virus IE63 and IE62 proteins. Virology 302:71-82. [DOI] [PubMed] [Google Scholar]
- 25.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maresova, L., T. J. Pasieka, E. Homan, E. Gerday, and C. Grose. 2005. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J. Virol. 79:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier, J. L., and S. E. Straus. 1995. Interactions between varicella-zoster virus IE62 and cellular transcription factor USF in the coordinate activation of genes 28 and 29. Neurology 45:S30-S32. [DOI] [PubMed] [Google Scholar]
- 29.Michael, E. J., K. M. Kuck, and P. R. Kinchington. 1998. Anatomy of the varicella-zoster virus open-reading frame 4 promoter. J. Infect. Dis. 178(Suppl. 1):S27-S33. [DOI] [PubMed] [Google Scholar]
- 30.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella-zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 31.Moffat, J., C. Mo, J. J. Cheng, M. Sommer, L. Zerboni, S. Stamatis, and A. M. Arvin. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 78:12406-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou, Y., and W. L. Gray. 2006. Simian varicella virus gene 28 and 29 promoters share a common upstream stimulatory factor-binding site and are induced by IE62 transactivation. J. Gen. Virol. 87:1501-1508. [DOI] [PubMed] [Google Scholar]
- 36.Patel, Y., G. Gough, R. S. Coffin, S. Thomas, J. I. Cohen, and D. S. Latchman. 1998. Cell type specific repression of the varicella-zoster virus immediate-early gene 62 promoter by the cellular Oct-2 transcription factor. Biochim. Biophys. Acta 1397:268-274. [DOI] [PubMed] [Google Scholar]
- 37.Peng, H., H. He, J. Hay, and W. T. Ruyechan. 2003. Interaction between the varicella zoster virus IE62 major transactivator and cellular transcription factor Sp1. J. Biol. Chem. 278:38068-38075. [DOI] [PubMed] [Google Scholar]
- 38.Perera, L. P., J. D. Mosca, W. T. Ruyechan, and J. Hay. 1992. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J. Virol. 66:5298-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and J. Hay. 1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol. 67:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perera, L. P., J. D. Mosca, M. Sadeghi-Zadeh, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein, IE62, can positively regulate its cognate promoter. Virology 191:346-354. [DOI] [PubMed] [Google Scholar]
- 41.Phillips, K., and B. Luisi. 2000. The virtuoso of versatility: POU proteins that flex to fit. J. Mol. Biol. 302:1023-1039. [DOI] [PubMed] [Google Scholar]
- 42.Quandt, K., K. Frech, H. Karas, E. Wingender, and T. Werner. 1995. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahaus, M., N. Desloges, M. Yang, W. T. Ruyechan, and M. H. Wolff. 2003. Transcription factor USF, expressed during the entire phase of varicella-zoster virus infection, interacts physically with the major viral transactivator IE62 and plays a significant role in virus replication. J. Gen. Virol. 84:2957-2967. [DOI] [PubMed] [Google Scholar]
- 44.Rahaus, M., and M. H. Wolff. 1999. Influence of different cellular transcription factors on the regulation of varicella-zoster virus glycoproteins E (gE) and I (gI) UTR's activity. Virus Res. 62:77-88. [DOI] [PubMed] [Google Scholar]
- 45.Rahaus, M., and M. H. Wolff. 2003. Reciprocal effects of varicella-zoster virus (VZV) and AP1: activation of Jun, Fos and ATF-2 after VZV infection and their importance for the regulation of viral genes. Virus Res. 92:9-21. [DOI] [PubMed] [Google Scholar]
- 46.Rahaus, M., and M. H. Wolff. 2000. Transcription factor Sp1 is involved in the regulation of varicella-zoster virus glycoprotein E. Virus Res. 69:69-81. [DOI] [PubMed] [Google Scholar]
- 47.Ruyechan, W. T., H. Peng, M. Yang, and J. Hay. 2003. Cellular factors and IE62 activation of VZV promoters. J. Med. Virol. 70(Suppl. 1):S90-S94. [DOI] [PubMed] [Google Scholar]
- 48.Santos, R. A., C. C. Hatfield, N. L. Cole, J. A. Padilla, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306-317. [DOI] [PubMed] [Google Scholar]
- 49.Sawadogo, M. 1988. Multiple forms of the human gene-specific transcription factor USF. II. DNA binding properties and transcriptional activity of the purified HeLa USF. J. Biol. Chem. 263:11994-12001. [PubMed] [Google Scholar]
- 50.Sommer, M. H., E. Zagha, O. K. Serrano, C. C. Ku, L. Zerboni, A. Baiker, R. Santos, M. Spengler, J. Lynch, C. Grose, W. Ruyechan, J. Hay, and A. M. Arvin. 2001. Mutational analysis of the repeated open reading frames, ORFs 63 and 70 and ORFs 64 and 69, of varicella-zoster virus. J. Virol. 75:8224-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suske, G. 1999. The Sp-family of transcription factors. Gene 238:291-300. [DOI] [PubMed] [Google Scholar]
- 52.Turner, E. E., J. M. Rhee, and L. T. Feldman. 1997. The POU-domain factor Brn-3.0 recognizes characteristic sites in the herpes simplex virus genome. Nucleic Acids Res. 25:2589-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, M., J. Hay, and W. T. Ruyechan. 2004. The DNA element controlling expression of the varicella-zoster virus open reading frame 28 and 29 genes consists of two divergent unidirectional promoters which have a common USF site. J. Virol. 78:10939-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yee, S. P., and P. W. Rigby. 1993. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 7:1277-1289. [DOI] [PubMed] [Google Scholar]
- 55.Zerboni, L., C. C. Ku, C. D. Jones, J. L. Zehnder, and A. M. Arvin. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. USA 102:6490-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]