Abstract
Treatment of chronic hepatitis B virus (HBV) infection could combine potent antiviral drugs and therapeutic vaccines to overcome immunological tolerance and induce the recovery phenotype to protect against disease progression. Conventional vaccination of woodchucks chronically infected with the woodchuck hepatitis virus (WHV) elicited differential T-cell response profiles depending on whether or not carriers were treated with the potent antiviral drug clevudine (CLV), which significantly reduces viral and antigen loads. The differential T-cell responses defined both CLV-dependent and CLV-independent epitopes of the pre-S and S regions of the WHV envelope protein. Only combined treatment involving CLV and conventional vaccine therapeutically restored the T-cell response profile of chronic WHV carrier woodchucks to that seen in prophylactic vaccination and in recovery from acute WHV infection. The results have implications for mechanisms of immunological tolerance operating in chronic HBV infection and suggest that such combined chemoimmunotherapy may be useful for treatment of humans with chronic HBV infection.
Chronic hepatitis B virus (HBV) infection is often associated with immunological tolerance to the virus characterized by hyporesponsive T helper (Th) cells, reduced numbers of cytolytic T lymphocytes, diminished Th1-type cytokine responses, and undetectable virus-neutralizing antibodies to viral envelope proteins (3, 8, 9, 16, 20, 24, 27, 42, 46). When HBV-specific cellular immune responses sometimes become detectable in HBV carriers, they are often suboptimal and contribute more to disease progression than to viral clearance and recovery (8, 16, 27, 46). Immunological tolerance in chronic HBV infection (2, 4, 6, 7, 37, 38) may arise theoretically from central or peripheral tolerance mechanisms (or both). Central tolerance could involve negative selection of antigen-specific T cells by thymic deletion in the presence of antigen (6, 7, 37, 38). Peripheral tolerance may follow after positive selection of antigen-specific T cells and result from clonal anergy, immunological exhaustion, or altered regulation between Th1 and Th2 cells (1, 43). The development and/or maintenance of central and peripheral tolerance in chronic HBV infection may be a consequence of the high viral and antigen loads often observed in chronic carriers. The development of immunotherapeutics able to circumvent T-cell tolerance, alone or in combination with antiviral therapeutics, represents a further critical step toward the successful treatment of chronic HBV infection.
Vaccines for HBV will ultimately interdict transmission of the virus and eradicate HBV-related diseases. However, there are currently more than 350 million chronic carriers of HBV worldwide who are at risk of developing chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) (47). Treatment options for chronic HBV infection are limited presently. Pegylated alpha interferon brings about sustained antiviral responses in approximately one-third of patients (25) but is associated with frequent side effects. Nucleoside and nucleotide analogs, such as lamivudine, entecavir, and adefovir dipivoxil, produce antiviral effects with minimal toxicity, but there is the risk of a relapse if treatment is discontinued and the emergence of drug-resistant variants with continued treatment (25). Accordingly, chronic HBV carriers could benefit immensely from more-effective therapies.
One new strategy has been modeled with woodchucks (Marmota monax) chronically infected with the woodchuck hepatitis virus (WHV) (12, 21, 29, 44). It involves combination chemoimmunotherapy using potent experimental antiviral drugs and vaccine immunizations. Clevudine (CLV) significantly reduces serum concentrations of both WHV DNA and WHV surface antigen (WHsAg) in woodchucks and also the levels of WHV covalently closed circular DNA and WHV RNA in liver (40). Chronic WHV carrier woodchucks treated with CLV for 32 weeks often have sustained antiviral effects lasting well beyond the cessation of drug treatment (22, 33, 35). When followed by a conventional vaccine (WHsAg-alum), CLV-treated WHV carriers developed detectable antibody responses to WHsAg (anti-WHs) and significant T-cell responsiveness to WHsAg, and the onset of end-stage chronic active hepatitis and HCC was delayed significantly compared to results for placebo controls (22, 33, 35, 44). T-cell responses in the group receiving combination therapy also were broadened to recognize additional WHV antigens (22, 33, 35). WHV carriers treated with vaccine alone had limited immune responses by comparison, with no reduction in serum viral and antigen loads (22, 33, 35).
We now extended these model studies (22, 33, 35) to include mapping of the T-cell responses to the pre-S and S regions of WHsAg (i.e., the WHV envelope protein). T-cell responses to the pre-S1, pre-S2, and S regions of WHsAg were often diminished and were clearly restricted among WHV carriers administered vaccine alone (i.e., in a high viral and antigen load setting). In contrast, vaccination in a setting of reduced viral and antigen loads induced by prior treatment with CLV restored the carrier T-cell response profile to WHsAg to more closely resemble that observed in vaccinated WHV-negative woodchucks and in adult woodchucks during recovery from acute WHV infection.
MATERIALS AND METHODS
Experimental animals.
Woodchucks were born to WHV-negative females and reared in environmentally controlled laboratory animal facilities at Cornell University. All experiments involving woodchucks were performed according to protocols approved by the Cornell University Institutional Animal Care and Use Committee. Ten adult woodchucks, approximately 1 year of age, all seronegative for markers of WHV infection, were used to demonstrate vaccine safety (n = 3) or immunogenicity (n = 7). Seven additional adult, WHV-negative woodchucks, approximately 1 year of age, were inoculated intravenously with 1 × 107 woodchuck infectious doses of a standardized WHV inoculum (WHV7P1) (11) to characterize T-cell responses to WHsAg during recovery from acute WHV infection for qualitative comparison with the present results; a more complete description of this study will be presented elsewhere. For present purposes, recovery was based on the loss of detectable WHV DNA and WHsAg in serum and liver and on the detection of serum antibodies to WHV core antigen (anti-WHc) and serum anti-WHs following inoculation. Thirty-two adult, chronic WHV carrier woodchucks, 1 to 2 years of age, were used to determine the effect of antiviral drug treatment and therapeutic vaccination on chronic WHV infection as described previously (22, 33, 35). The woodchucks were infected experimentally as neonates at 3 days of age by subcutaneous inoculation with 5 × 106 woodchuck infectious doses of WHV7P1. Persistence of WHV infection was based on the constitutive detection of WHV DNA and WHsAg in serum from 3 months of age. On entry into the study, woodchucks had minimal chronic hepatitis based on histology and serum enzyme profiles. All were considered to be free of HCC based on hepatic ultrasound examinations and on the γ-glutamyltranspeptidase activity of serum.
Drug and vaccine.
The antiviral drug CLV (L-FMAU) (1-[2-fluoro-5-methyl-β-l-arabinofuranosyl]-uracil) was provided by Triangle Pharmaceuticals, Inc. (Research Triangle Park, NC). The subunit vaccine consisted of 22-nm WHsAg particles purified by zonal ultracentrifugation from serum of WHV7P1-infected WHV carriers (17), inactivated with formalin, and adsorbed onto alum. The WHsAg in the vaccine was homologous to the infecting WHV and to serum WHsAg circulating in the WHV carriers used in this study. The purified WHsAg within the vaccine was not pretreated with enzymes that remove pre-S sequences. Prior to alum adsorption, the purified and formalin-inactivated WHsAg was tested for safety in three WHV-negative woodchucks by using a single dose of 50 μg of aqueous WHsAg administered intravenously in order to assure inactivation of any residual infectious WHV. The vaccine was uniformly immunogenic when administered intramuscularly to seven WHV-negative woodchucks as four 50-μg doses at 0, 4, 8, and 16 weeks (i.e., the same dosing regimen and interval used with the present chronic WHV carriers). In a separate study, this same WHsAg vaccine also was uniformly immunogenic when administered to four WHV-negative woodchucks as three 10-μg doses (data not shown).
Influence of antiviral drug treatment on T-cell responses to therapeutic vaccination.
The design of the therapeutic study has been described elsewhere in detail. Briefly, 16 WHV carriers received CLV (CLV+ carriers; 10 mg/kg of body weight/day, one dose orally for 32 weeks), and 16 received a saline placebo (CLV− carriers). CLV and the placebo were discontinued at week 32. Eight CLV+ carriers and 8 CLV− carriers were then administered 50 μg of the WHsAg vaccine by intramuscular injection at weeks 32, 36, 40, and 48 (CLV+V+ and CLV−V+ carriers). Seven CLV+ carriers and 7 CLV− carriers received saline as an injection control at these time points (CLV+V− and CLV−V− carriers). T-cell responses were measured by an in vitro proliferation assay using peripheral blood mononuclear cells (PBMCs) isolated from whole blood as described previously (33, 35). Most woodchucks in each group (63 to 86%) were monitored at 2- to 4-week intervals, with the remaining woodchucks tested at intervals of 8 to 12 weeks. As a further control for T-cell responses induced by the WHsAg vaccine, the responses of the seven WHV-negative woodchucks in the immunogenicity study (see above) were analyzed at 2- to 4-week intervals following vaccination In addition, another seven WHV-infected woodchucks were monitored at weekly intervals during the acute and recovery phases of infection; these responses served as additional control for T-cell responses induced by the virus. Four unvaccinated, WHV-negative woodchucks were monitored at appropriate intervals to serve as a negative control for T-cell responses induced by vaccine or virus.
Assays of viral and antibody markers.
Serum WHV DNA was measured by dot blot Southern hybridization, either directly or following PCR-based amplification, as described previously (22, 33, 35). WHsAg, anti-WHs, and antibodies against WHV core antigen in serum were measured by enzyme-linked immunosorbent assay as described previously (22, 33, 35).
Polyclonal activators, viral antigens, and synthetic peptides.
In vitro stimulators were used at concentrations previously determined as optimal for cultures of woodchuck PBMCs (28). Concanavalin A (8 μg/ml; Sigma, St. Louis, MO), lipopolysaccharide (0.5 μg/ml; Sigma), and human recombinant interleukin 2 (100 IU/ml; Cetus, Emeryville, CA) were used as positive control stimulators. Viral antigen stimulators consisted of native 22-nm WHsAg (0.5, 2, and 5 μg/ml, i.e., the same purified antigen used to prepare the vaccine) and a panel of synthetic WHsAg peptides (WHs peptides) (8 to 23 amino acids long; 10 μg/ml; Sigma-Genosys, The Woodlands, TX) which included nearly the entire open reading frame (pre-S1/pre-S2/S regions) of the viral envelope protein. The S prefix designations for synthetic peptides indicate “surface” (see Table 1). Culture medium, bovine serum albumin (0.5 and 2 μg/ml; Sigma), and a WHV-unrelated synthetic peptide (10 μg/ml; Sigma-Genosys) were used as stimulators to control for non-WHs-specific (negative) T-cell responses.
TABLE 1.
Synthetic peptides used for detection of WHs-specific T-cell responses and responder rates and sample frequencies with positive T-cell responses following WHsAg vaccination of WHV-negative woodchucksa
| Peptide or antigen | Region | Protein specificity (aa coordinates)
|
Cumulative rate or frequency
|
|||
|---|---|---|---|---|---|---|
| Large (1c) | Middle (150c, 1d) | Small (210c, 61d, 1e) | CGRR (%) | CSRF (%) | ||
| S1 | Pre-S1 | 1-15 | 71 | 16 | ||
| S2 | Pre-S1 | 16-36 | 0 | 0 | ||
| S3 | Pre-S1 | 37-59 | 0 | 0 | ||
| S4 | Pre-S1 | 60-80 | 0 | 0 | ||
| S5 | Pre-S1 | 81-100 | 14 | 3 | ||
| S6 | Pre-S1 | 101-120 | 0 | 0 | ||
| S7 | Pre-S1 | 121-140 | 57 | 11 | ||
| S7/8 | Pre-S1 | 131-150 | 1 | ND | ND | |
| S8 | Pre-S1/S2 | 140-160 | 1-11 | 57 | 19 | |
| S9 | Pre-S2 | 161-180 | 12-31 | 0 | 0 | |
| S10 | Pre-S2 | 181-202 | 32-53 | 0 | 0 | |
| S11 | Pre-S2/S | 203-225 | 54-76 | 1-16 | 57 | 9 |
| S12 | S | 226-234/235-242 | 77-85/86-93 | 17-25/26-33 | 0 | 0 |
| S12/13 | S | 226-245 | 77-96 | 17-36 | ND | ND |
| S13 | S | 243-260 | 94-111 | 34-51 | 29 | 6 |
| S14 | S | 261-280 | 112-131 | 52-71 | 42 | 6 |
| S15 | S | 281-290/291-300 | 132-141/142-151 | 72-81/82-91 | 14 | 3 |
| S16 | S | 301-320 | 152-171 | 92-111 | 14 | 4 |
| S17 | S | 321-340 | 172-191 | 112-131 | 0 | 0 |
| S18 | S | 341-360 | 192-211 | 132-151 | 100 | 30 |
| S19 | S | 361-370/371-380 | 212-221/222-231 | 152-161/162-171 | 14 | 8 |
| S20 | S | 381-400 | 232-251 | 172-191 | 0 | 0 |
| S21 | S | 411-431 | 262-282 | 202-222 | 29 | 22 |
| WHsAgb | Native | 86 | 23 | |||
Peptides S12, S15, and S19 could not be synthesized as full-length 15- to 20mers and therefore were synthesized as two nonoverlapping 8- to 10mers. CGRR (= no. of woodchucks with positive T-cell responses/total no. of woodchucks × 100) and CSRF (= no. of samples with positive T-cell responses/total no. of samples × 100) were calculated for the postvaccination period, defined as week 2 up to week 28. The T-cell response was positive if the SI was ≥3.1. For CGRR, seven woodchucks were studied. For CSRF, the denominator sample observation for each stimulator used for calculating percentages was 64. aa, amino acid; ND, not done.
Composition of WHsAg was as follows: 1 to 2% large protein, 18 to 19% middle protein, and 80% small protein.
Coordinate of the first amino acid within the large (pre-S1) structural region of the WHV envelope protein.
Coordinate of the first amino acid within the middle (pre-S2) structural region of the WHV envelope protein.
Coordinate of the first amino acid within the small (S) structural region of the WHV envelope protein.
T-cell assay and parameters of response.
The in vitro proliferation assay using woodchuck PBMCs is comparable to those performed in human studies (14, 20), except that dividing cells were labeled with [2-3H]adenine (28). Counts per minute of triplicate PBMC cultures were averaged and expressed as a stimulation index (SI) by dividing the average sample cpm in the presence of the stimulator by that observed in the absence of stimulator (seven replicates). A SI value of ≥3.1 was considered to represent a positive, specific T-cell response (28). This positive cutoff is conservative in relationship to the dynamic range of observed positive stimulations by viral antigens and peptides (i.e., the maximal antigen-specific SIs ranged from 7 to 12). The 3.1 cutoff level represents 25 to 45% of the maximal range of observed positive stimulations induced by antigens or peptides. At the 3.1 SI cutoff, sample cpm always were greater than 2 standard deviations above the mean cpm of unstimulated control cultures from the same woodchuck and were typically more than 2 standard deviations above the cpm for antigen- or peptide-stimulated cultures from WHV-negative control woodchucks. Antibody markers that define T-cell subsets in woodchucks are not available currently; however, woodchuck PBMCs stimulated with WHV core antigen and stained with cross-reacting anti-CD3 antibody indicate conclusively that the proliferating cell subpopulation in such cultures is indeed composed of CD3+ T cells (32). Furthermore, activation of woodchuck PBMCs by WHV antigens and peptides results in the activation and proliferation of antigen-specific T cells that express interleukin 2 and can be expanded further in the presence of exogenous interleukin 2 (31, 32).
For data analysis, the group responder rate at a given time point was defined as a percentage as the number of woodchucks with positive T-cell responses divided by the total number of woodchucks × 100; these data were converted as indicated to a cumulative group responder rate (CGRR) for a given study interval. The cumulative sample response frequency (CSRF) was defined as a percentage as the number of samples with positive T-cell responses divided by the total number of samples × 100 during a given study interval. This parameter was used as an indicator of sustained T-cell responses.
Statistical analysis.
CGRRs and CSRFs were compared by Fisher's test for proportions (two-tailed). P values of <0.05 were considered statistically significant.
RESULTS
Vaccination of WHV-negative woodchucks elicits antibody and T-cell responses to WHsAg.
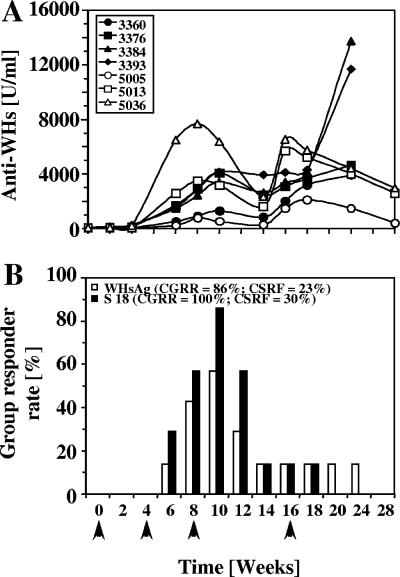
WHsAg-alum vaccine administered to seven WHV-negative woodchucks elicited high-titer and sustained anti-WHs responses (>2,000 U/ml) (Fig. 1A). Indicative of the sustained responses, 95% of all serum samples measured were positive for anti-WHs (>101 U/ml) during the postvaccination period between week 2 and 28. Four of the seven woodchucks (57%) actually became low-level positive for anti-WHs (150 to 300 U/ml) after the first immunization; subsequent immunizations resulted in the more robust responses. Vaccinated woodchucks had variable in vitro T-cell responder rates to native WHsAg at each time point upon stimulation of PBMC. Rates ranged from 0% detectability after one immunization to 14% (one of seven) after the second immunization and up to 57% (four of seven) shortly after the third immunization, with lower rates thereafter (Fig. 1B). The kinetic correlated initially with the onset of anti-WHs responses through week 10, at which time most woodchucks with elevated anti-WHs titers also demonstrated T-cell responses to WHsAg (Fig. 1A and B). Although anti-WHs titers were boosted in all woodchucks following the fourth immunization, T-cell responses were less detectable.
FIG. 1.
Humoral and cellular immune responses of WHV-negative woodchucks following WHsAg vaccination. (A) Serum anti-WHs response. (B) Rates of responders with positive T-cell responses to WHsAg and WHs peptide S18. Arrows indicate the four immunizations with 50-μg doses of a WHsAg vaccine at weeks 0, 4, 8, and 16. CGRRs ([woodchucks with positive T-cell responses/total number of woodchucks] × 100) and CSRFs ([number of samples with positive T-cell responses/total number of samples] × 100) were calculated for the postvaccination period, defined as being from week 2 up to week 28. The T-cell response was positive if the SI was ≥3.1. For CGRRs, seven woodchucks were studied. For CSRFs, the denominator sample observation for both stimulators used for calculating percentages was an n value of 64.
Overall, six of seven vaccinated woodchucks had positive T-cell responses to native WHsAg at least once, corresponding to a CGRR of 86% (i.e., 86% of woodchucks responded positively at least once (SI ≥ 3.1) (Fig. 1B; Table 1). The CSRF to WHsAg was 23% (i.e., 23% of the samples tested during the interval indicated positive T-cell responses). Use of synthetic WHs peptide S18 detected WHs-specific T-cell responses for the one remaining woodchuck at additional time points (Fig. 1B). Accordingly, all seven woodchucks had T-cell responses to S18 during the postvaccination period (CGRR = 100%) and with a generally higher CSRF (30%) than for native WHsAg (Fig. 1B; Table 1).
WHs peptides S1, S7, S8, S11, S13, S14, and S21 detected T-cell responses in vaccinated woodchucks at CGRRs ranging between 29% and 71% of woodchucks, with CSRFs ranging between 6% and 22% (Table 1). T-cell responses were negligible for other WHs peptides (S5, S15, S16, and S19) and undetectable for others (S2, S3, S4, S9, S10, S12, S17, and S20) (Table 1). Thus, vaccine-induced T-cell responses of WHV-negative woodchucks were highly selective for the different structural regions of WHsAg (i.e., pre-S and S regions) and also for individual T-cell epitopes within those regions.
Vaccination of chronic WHV carrier woodchucks in high versus low viral and antigen load settings elicits differential T-cell response profiles.
For subsequent comparisons of T-cell responses to WHs peptides in various settings, we point out that administration of CLV to WHV carriers for 32 weeks resulted in rapid and sustained reductions in both serum WHV DNA (>6 to 8 logs) and serum WHsAg (>50- to 500-fold) (22, 33, 35). These were sustained in most woodchucks for several months beyond the cessation of drug treatment, providing opportunity to compare immune responses to those with subsequent vaccination in WHV carriers that had low viral and antigen loads (CLV+V+) and in WHV carriers that had typical high viral and antigen loads at the start of vaccination (CLV−V+). In CLV−V+ carriers, low-level anti-WHs responses (130 to 540 U/ml maximum) became detectable in response to the vaccine, along with modest T-cell responses to native WHsAg, but there was no significant reduction in serum viremia and antigenemia (22, 33, 35). CLV+V+ carriers also developed low-level anti-WHs responses following vaccination with slightly higher titers (110 to 1,000 U/ml maximum) but in contrast had enhanced T-cell responses to WHsAg and improved endogenous collateral T-cell immunity to other WHV antigens, including WHV core antigen (22, 33, 35).
CLV-dependent T-cell responses to pre-S- and S-region epitopes of WHsAg in chronic WHV carrier woodchucks following vaccination.
T-cell responses of WHV carriers to WHsAg were determined further with two specifically designed WHs peptides for the pre-S and S regions of WHsAg (WHs peptides S7/8 and S12/13, respectively). In the drug-treated WHV carriers (CLV+ group), treatment with CLV for 32 weeks did not unmask detectable T-cell responses to the entire WHsAg or to these two WHs peptides (Table 2), despite rapid and marked reductions in serum WHV DNA and WHsAg. Although T-cell responses to viral antigens other than WHsAg were observed sporadically and at a low frequency during the drug treatment period between weeks 4 and 32 (22, 33, 35), overall these T-cell responses for CLV+ carriers were not different from those observed for placebo-treated WHV carriers (CLV− group). During the vaccination period between weeks 36 and 60, T-cell responses to WHsAg and the two WHs peptides were detected most frequently among WHV carriers receiving combined therapy with CLV and vaccine (CLV+V+) (Table 2). No T-cell responses were observed in the double placebo control group (CLV−V−) because WHV carriers with high viral and antigen loads are typically tolerant to WHsAg and other viral antigens (32, 33, 35). Consistent with this idea, administration of vaccine alone to carrier woodchucks not treated with CLV (CLV−V+) did not elicit T-cell responses to these two peptides, although responses to native WHsAg were observed (Table 2). Administration of CLV alone (CLV+V−) reduced the viral and antigen loads significantly as indicated previously (22, 33, 35), and a few responses in this group to native WHsAg (Table 2) resulted mainly just after drug withdrawal and were probably in response to a slight rebound in serum viremia and antigenemia observed at the time (33, 35). However, drug alone and this slight rebound were ineffective at the time in promoting significant endogenous T-cell responses to the two peptides (Table 2). Thus, the vaccine-induced T-cell responses in CLV+V+ WHV carriers to these selected peptide epitopes within the pre-S and S structural regions of WHsAg were more dependent on treatment with CLV.
TABLE 2.
Combination of CLV treatment and therapeutic vaccination of chronic WHV carrier woodchucks overcomes tolerance and stimulates T-cell responses to pre-S and S regions of WHsAga
| Treatment group | CSRF
|
|||||
|---|---|---|---|---|---|---|
| Wk 4-32
|
Wk 36-60
|
|||||
| WHsAg | Peptide S7/8 | Peptide S12/13 | WHsAg | Peptide S7/8 | Peptide S12/13 | |
| CLV− | 0 | 0 | 0 | |||
| CLV+ | 1 | 0 | 1 | |||
| CLV−V− | 0 | 0 | 0 | |||
| CLV−V+ | 40 | 0 | 0 | |||
| CLV+V− | 28 | 0 | 4 | |||
| CLV+V+ | 71 | 19 | 38 | |||
CLV−, drug placebo group; CLV+, CLV group; CLV−V−, drug placebo/vaccine placebo group; CLV+V−, CLV/vaccine placebo group; CLV−V+, drug placebo/vaccine group; CLV+V+, CLV/vaccine group. CSRFs were calculated for the drug treatment period between weeks 4 and 32 and for the postvaccination period between weeks 36 and 60 following cessation of treatment with drug or saline placebo at week 32. For CSRF, the denominator sample observations for the three stimulators used for calculating percentages are as follows: CLV−, n = 98; CLV+, n = 103 (drug treatment period); CLV−V−, n = 46; CLV+V−, n = 47; CLV−V+, n = 45; CLV+V+, n = 58 (postvaccination period). Statistical comparisons by Fisher's test (two-tailed; only P values for significant comparisons during the postvaccination period are presented): CLV+V+ versus CLV−V−, S7/8, <0.002; S12/13, <0.00001; CLV+V+ versus CLV+V−, S7/8, <0.001; S12/13, <0.00001; CLV+V+ versus CLV−V+, S7/8, <0.003; S12/13, <0.00001.
CLV-dependent and CLV-independent T-cell responses to pre-S- and S-region epitopes of WHsAg in chronic WHV carrier woodchucks following vaccination.
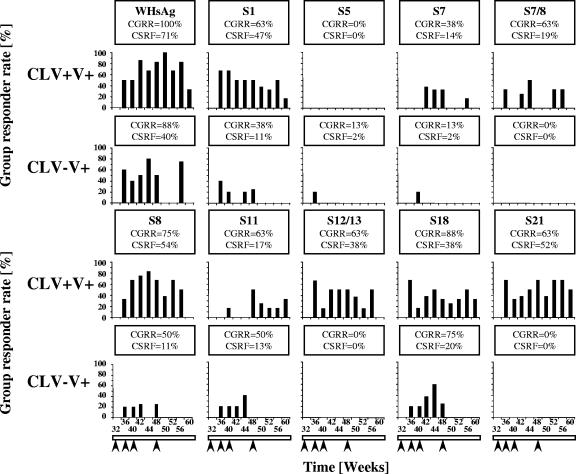
Neither double placebo control woodchucks (CLV−V−) nor woodchucks receiving CLV alone (CLV+V−) became highly or consistently responsive to native WHsAg during the vaccination period (Table 2) (22, 33, 35). Therefore, WHs-specific T-cell responses were compared and mapped only for the vaccinated groups in this study and using only selected WHs peptides that represented important epitopes recognized in other settings (S1, S5, S7, S7/8, S8, S11, S12/13, S14, S18, and S21). Each of these peptides, except S5 and S14, detected significant T-cell responses in CLV+V+ carriers during the vaccination period, with CGRRs between 38 and 88% of woodchucks, and CSRFs between 14 and 54% of samples (Fig. 2). These responses correlated also with robust T-cell responses to native WHsAg (Fig. 2, upper left panel). The T-cell responses were detected as early as week 36 of the study (4 weeks after the first vaccination at week 32), with occasional response variation among individual woodchucks through the course of vaccination and follow-up until week 60.
FIG. 2.
T-cell responses of chronic WHV carrier woodchucks during WHsAg vaccination following treatment with CLV or placebo. Responder rates for positive T-cell responses to WHsAg and selected WHs peptides at each time point, by treatment group and postvaccination period, are shown. Arrows indicate the four immunizations with 50-μg doses of a WHsAg vaccine at weeks 32, 36, 40, and 48. The postvaccination period was defined as being between weeks 36 and 60 following cessation of treatment with drug or saline placebo at week 32. CLV+V+, CLV/vaccine group; CLV−V+, drug placebo/vaccine group. CGRRs and CSRFs are given within each graph. For CGRRs, the number of WHV carriers was eight for both groups. For CSRFs, the denominator sample observations for all stimulators used for calculating percentages were as follows: for the CLV+V+ group, n = 58; for the CLV−V+ group, n = 45. The T-cell response was positive if the SI was ≥3.1. Statistical comparisons by Fisher's test (two-tailed; only P values for significant comparisons are presented) are as follows: for CGRR, CLV+V+ versus CLV−V+, peptide S7/8, <0.03; peptide S12/13, <0.03; peptide S21, <0.03; for CSFR, CLV+V+ versus CLV−V+, WHsAg, <0.003; peptide S1, <0.0001; peptide S7, <0.05; peptide S7/8, <0.003; peptide S8, <0.00001; peptide S12/13, <0.00001; peptide S18, <0.04; peptide S21, <0.00001.
In contrast, T-cell responses in CLV−V+ carriers were diminished to native WHsAg and were diminished or absent across the entire panel of peptides tested (Fig. 2). In addition to the lack of T-cell response to WHs peptides S7/8 and S12/13 (Fig. 2 and as mentioned earlier in Table 2), there was a further complete lack of response to an additional WHs peptide of the S region, S21, thus indicating another CLV-dependent epitope of WHsAg. CLV−V+ carriers did have occasional T-cell responses to some of the WHs peptides (S1, S5, S7, S8, and S11), with S18 being recognized most often. This indicated that responses to certain WHsAg-specific T-cell epitopes were CLV independent. As with native WHsAg, such peptide-specific T-cell responses developed in CLV−V+ carriers mainly in association with the first three doses of vaccine and not with the fourth dose administered at week 48 (Fig. 2). Aside from the results shown in Table 2, we did not determine which if any of the remaining CLV-dependent and CLV-independent epitopes may have contributed to the nominal T-cell responses in CLV+V− carriers following drug withdrawal.
Overall, the diminished T-cell responses in CLV−V+ carriers to native WHsAg were due to the decreased scope and intensity of the response to the peptide panel tested. The CLV−V+ carriers failed to respond to the CLV-dependent epitopes identified, and they had less-frequent immediate and long-term responses to those CLV-independent epitopes that were recognized. In the CLV−V+ carriers, although the recognition of WHs peptides early in the course of vaccination was considered CLV independent, these woodchucks appeared to become refractory in the recognition of such peptides upon further vaccination.
Chemoimmunotherapy of chronic WHV carrier woodchucks with CLV and vaccine restores T-cell responses to resemble those following prophylactic vaccination and recovery from acute WHV infection.
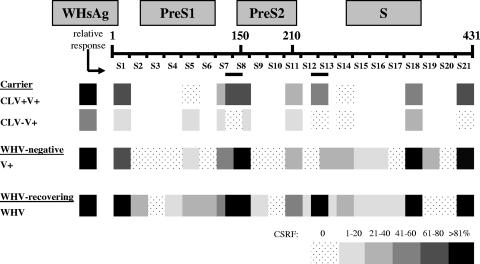
A summary comparison of WHs-specific T-cell responses was developed from different woodchuck studies using increasingly shaded blocks to represent more-intense T-cell responses to WHsAg and WHs peptides. The different studies included the treated WHV carriers and the WHV-negative vaccine recipients discussed here, along with a separate group of adult woodchucks undergoing recovery from acute WHV infection (see Methods). The observed CSRFs for WHs peptides were expressed relative to those for native WHsAg in each respective study, i.e., 23% for vaccinated, WHV-negative woodchucks and 41% for WHV-infected adult woodchucks that recovered; and for the therapeutic study, the data for both vaccinated groups were normalized to the 71% CSRF for WHsAg for the CLV+V+ group.
In CLV+V+ carriers, T-cell responses were observed frequently to the pre-S1-region peptides S1 and S7 and also to peptide S8, which is a hybrid pre-S1/2 segment contiguous only in the large surface protein. The pre-S1 peptide S7/8 was likewise well recognized by CLV+V+ carriers. Robust T-cell responses to these regions of WHsAg were observed similarly in blood from vaccinated, WHV-naive woodchucks and also from the group of WHV-infected adult woodchucks that recovered from WHV infection. The significantly weaker responses (or lack of responses) to these regions by CLV−V+ carriers are evident (Fig. 3). T-cell responses to peptide S5 were more evident among recovering woodchucks and less so in the therapeutic setting (and were not significantly different between carrier groups). Overall, T-cell responses for CLV+V+ carriers were restored more fully to those observed during vaccination of naive woodchucks and in recovery of infected woodchucks. The restored responses were clearly predicated on the use of CLV in combination with vaccine. The CLV-dependent T-cell response to peptide S7/8 in CLV+V+ carriers was well recognized also with recovering woodchucks, so the importance of this therapeutically induced T-cell response to recovery is indeed noteworthy.
FIG. 3.
Comparison of WHs-specific T-cell responses of chronic WHV carrier woodchucks during WHsAg vaccination following treatment with CLV or placebo, of WHV-negative woodchucks during WHsAg vaccination, and of woodchucks recovering from acute WHV infection. CSRFs to WHsAg and WHs peptides were converted to relative values within ranges shown for each experimental group. The T-cell response was positive if the SI was ≥3.1. CLV+V+, CLV/vaccine group; CLV−V+, drug placebo/vaccine group (16 WHV carriers [n = 8 in each group]); the postvaccination period was defined between weeks 36 and 60 following cessation of treatment with drug or saline placebo at week 32. V+, vaccine group (seven vaccinated WHV-negative woodchucks; the postvaccination period was defined to be from week 2 up to week 28. WHV, recovering group (seven WHV-infected woodchucks; the observation period for recovery was 12 weeks following experimental WHV infection in week 0). Denominator sample observations for all stimulators used for calculating the observed CSRFs were as follows: for CLV+V+, n = 58; for CLV-V+, n = 45; for V+, n = 64; for WHV, n = 84. Relative CSRFs were calculated by setting the observed CSRF to WHsAg at 100% for each experimental group. For comparison of CLV−V+ versus CLV+V+ carriers, the observed CSRF was normalized to the response to WHsAg observed in serum from CLV+V+ carriers. Increasingly shaded blocks represent an increasing relative CSFR range for each stimulator, as indicated. The relative positions of the WHs peptides in the pre-S1, pre-S2, and S regions of the viral envelope protein are displayed. S1, N terminus of WHsAg; S21, C terminus of WHsAg.
Based on the panel of WHs peptides used in WHV carriers, it was not possible to say more about specific T-cell responses to the pre-S2 region of the large and middle envelope proteins, but S-region responses differed considerably. Peptide S18 induced frequent T-cell responses in serum from all of the vaccinated, WHV-negative woodchucks and in all woodchucks recovering from acute WHV infection. This peptide was considered a potent, CLV-independent stimulator for both of the vaccinated WHV carrier groups (at least earlier on for the carriers receiving only vaccine). A similar case can be made for S11, a pre-S2/S hybrid peptide. T-cell responses of CLV+V+ carriers to peptides S12/13 and S21 were clearly CLV dependent and were similar to those observed for vaccinated, WHV-negative woodchucks and for recovering woodchucks.
Woodchucks recovering from acute WHV infection had frequent T-cell responses to peptides S2, S5, S6, S9, S12, S13, S14, S15, and S16. Among these, CLV+V+ carriers did not respond to S-region peptide S14, although it was recognized moderately by vaccinated, WHV-negative woodchucks. In fact, T-cell responses to peptide S14 were consistently absent in all 16 CLV+V+ and CLV−V+ carriers. They were evident at CGRRs of 43% for naive vaccine recipients (P < 0.05) and of 57% for recovering woodchucks (P < 0.02). Thus, although T-cell tolerance had been overcome to a significant extent by combination therapy in the WHV carriers, a portion of the S region remained tolerant. However, the overall T-cell response profiles associated with vaccine treatment in high versus low viral and antigen load settings demonstrated significant differential recognition of the pre-S1 and S regions of the envelope protein. Moreover, the therapeutically restored T-cell responses in WHV carriers given combination therapy are also important in prophylactic vaccination and in recovery from acute WHV infection.
Correlation of immune response parameters with therapeutic outcome.
The above results greatly extend those from prior studies by defining envelope peptide-specific T-cell responses and their correlation with therapeutic outcome in terms of disease progression (22, 33, 35) (Table 3). The reduction of viral and antigen loads in serum and liver by CLV and subsequently improved envelope-specific and core-specific collateral T-cell responses to vaccine were associated with an improved therapeutic outcome in terms of reduced or delayed chronic hepatitis, liver injury, and onset of HCC (Table 3) (22). We show here that the improved T-cell immunity correlate was due to broader and more-sustained T-cell responses to WHsAg that involved both CLV-independent and CLV-dependent epitopes (Table 3). Moreover, those improved response correlates closely mimicked those observed for naive vaccine recipients and for recovering woodchucks that are essentially protected against infection and/or disease progression. It is noteworthy that evidence for WHV nucleic acids may persist long term even in serologically recovered woodchucks (10, 30, 36). This may even be associated occasionally with a low lifetime risk of HCC (23) and also with reactivation of virus replication during experimental immunosuppression (30). Overall, the present combination treatment of chronic WHV infection very closely approached the virologic and host profiles, and the lower risk of disease progression, that are observed for woodchucks that recover early on from acute WHV infection. Thus, the viral and host responses of chronic WHV carrier woodchucks treated with both CLV and vaccine are restored to those resembling recovery with partial or complete protection against disease outcome (Table 3) (22, 33, 35).
TABLE 3.
Correlation of humoral and cellular immune responses with therapeutic outcome for chronic WHV carrier woodchucks following CLV treatment and/or vaccinationa
| Marker | Presence of marker in treatment groupf
|
|||
|---|---|---|---|---|
| CLV−V− | CLV−V+ | CLV+V− | CLV+V+ | |
| WHV replication | ||||
| Serum WHV DNA | +++ | +++ | +/− | +/− |
| Serum WHsAg | +++ | +++ | +/− | +/− |
| Hepatic WHV antigens | +++ | +++ | +/− | +/− |
| Hepatic WHV DNA RI | +++ | +++ | +/− | +/− |
| Hepatic WHV RNA | +++ | +++ | +/− | +/− |
| Hepatic WHV cccDNAb (implied) | +++ | +++ | +/− | +/− |
| Liver disease progression | ||||
| Chronic hepatitis | +++ | +++ | + | +/− |
| Liver injury | +++ | +++ | + | − |
| HCC (onset) | +++ | +++ | + | +/− |
| Immunological correlates | ||||
| Anti-WHs antibodyc,d | − | + | − | + |
| T-cell responses to WHsAgc,d | − | +/− | +/− | +++ |
| T-cell responses to pre-S and S peptides (2 CLV-dependent prototypes) | − | − | − | +++ |
| T-cell responses to pre-S and S peptides (extended panel) | ND | + | ND | +++ |
| Collateral T-cell responses to WHcAgc | − | − | +/− | +++ |
| Collateral T-cell responses to C100-119 peptidec | − | − | +/− | +++ |
| Collateral T-cell responses to WHV e and x antigensc | − | − | +/− | +++ |
| Recovery phenotypee | − | +/− | +/− | +++ |
An overall relative assessment of the indicated markers of WHV replication, liver disease progression, and immunological correlates for the different therapies is presented. The table was adapted from reference 22. Data from the present study are boldfaced. WHV DNA RI, WHV DNA replicative intermediates.
Covalently closed circular DNA; see reference 40; reductions in WHV covalently closed circular DNA are deduced from reductions in WHV DNA replicated intermediates and WHV RNA from reference 22.
WHV core antigen; see reference 33.
See reference 35.
The recovery phenotype was defined as a virological profile with loss of WHV replication, RNA expression, and protein expression in liver, reduction in serum WHV DNA and WHsAg, and an immunological profile with anti-WHs and T-cell responses to WHV core antigen, WHsAg, and other WHV antigens and T-cell recognition of many WHsAg epitopes, all leading to reduced and/or delayed disease progression.
+++, maximum relative presence/occurrence of the individual marker; −, essentially undetectable level; +/−, absence of marker in some woodchucks in an experimental group.
DISCUSSION
Rational treatment of chronic HBV infection ultimately may combine antiviral drugs and immunotherapy. This study of chronic WHV carrier woodchucks advances our understanding of T-cell responses to therapeutic vaccination following treatment with a potent antiviral drug. It also provides insight into basic cellular immunological mechanisms associated with chronicity in hepadnavirus infection versus vaccine protection and resolution of acute infection. Results using synthetic peptides provide strong evidence for underlying differences in T-cell response profiles of WHV carriers in two therapeutic settings. Here, only the combination of a potent antiviral drug and vaccine could therapeutically restore the T-cell response to WHsAg to that seen typically with prophylactic vaccination and recovery from acute infection.
Serum anti-WHs titers for vaccinated WHV-negative woodchucks reached levels that are considered protective against experimental challenge with WHV (18) and that are similar to those for woodchucks recovering from acute WHV infection (48). This implies that the associated T-cell response profiles would be adequate for protection against WHV infection as well. Indeed, vaccination of WHV-negative woodchucks elicited multiple T-cell responses to peptides specific for the pre-S1 and S regions of the viral envelope protein. The correlation between detectability of anti-WHs and associated T-cell responses in serum from vaccinated WHV-naive woodchucks in part may reflect differences in the assay sensitivities, but it may also reflect low antigenicity of particulate WHsAg used as a T-cell stimulator. Synthetic peptides can be of additional value, not only for mapping T-cell responses to WHsAg but potentially also for enhanced detection of such responses, because they are used at a concentration of 10 μg/ml, which represents a substantial molar excess compared to the 22-nm native WHsAg particles used at concentrations of 0.5 to 5 μg/ml of protein.
The vaccine-induced T-cell responses to WHsAg in serum from woodchucks were essentially polyclonal, a result which is analogous to those for human vaccine recipients receiving HBsAg vaccines (5, 13, 15, 19). The WHs-specific T-cell responses observed for outbred, WHV-negative woodchucks were directed against several fairly dominant sites (epitopes), each of which probably plays a role in vaccine protection, and correlate with recovery from WHV infection. This indicates an importance of these epitopes not only in vaccine protection from WHV infection but also in controlling WHV replication during infection. T-cell responses to WHsAg in CLV+V+ carriers involved frequent and broad recognition of WHs peptides that sometimes exceeded those of the vaccinated, WHV-negative woodchucks. This observation may relate to an additional low-level endogenous stimulation by WHsAg once antigenic tolerance was mitigated by CLV (but still requiring vaccine in combination). Successful T-cell responses to certain WHs peptides in the pre-S and S regions were clearly dependent on achieving reduced levels of viremia and antigenemia by the prior treatment with CLV, with the resulting profile comparable to that for vaccinated, WHV-negative woodchucks and for recovering woodchucks. Hence, the CLV+V+ carriers demonstrated nearly the same phenotypic profile as that seen in natural recovery from acute WHV infection, and this was associated with significant delays in disease progression (22) (Table 3). Thirty-eight percent of the WHV carriers given the combination therapy remained free of HCC through weeks 80 to 104 of the study, long after the placebo controls had succumbed to HCC (22).
WHs-specific T-cell responses were not detected in WHV carriers during the actual treatment with CLV. This is consistent with other studies where no improvements in T-cell responses to HBV core antigen were observed during treatment of HBV carriers with lamivudine alone or in combination with alpha interferon (26, 41). In those studies, T-cell responses became detectable only after discontinuation of drug treatment during viral recrudescence. Slight increases in viral replication associated with drug withdrawal in our study appear to have acted as an endogenous stimulus even in the absence of vaccine, but the responses there were not nearly as robust or sustained as when vaccine was also administered. In another study (4), HBV carriers treated with lamivudine developed strong and sustained T-cell responses to HBV core antigen, HBV e antigen, and several HBc peptides shortly after initiation of treatment. Possible explanations for variability in the unmasking of T-cell responses during antiviral treatment may relate to the following: (i) the use of drugs with different potencies (lamivudine versus CLV), (ii) the host age at the time of infection and onset mechanisms of chronicity (adult-acquired versus neonatally acquired chronicity), and (iii) any underlying liver disease at the start of treatment. The WHV carriers in the present study were experimentally infected with WHV as neonates and were therefore immunotolerant to WHsAg (33-35). In addition, they were preselected for minimal liver disease on entry in the drug treatment period. Accordingly, there was little or no probability that concurrent reduction in viral and antigen loads by CLV would unmask T-cell responses to WHsAg and other viral antigens. Further consistent with this explanation was the inability of CLV treatment alone to result in concurrent anti-WHs antibody induction, which is T cell dependent.
Although T-cell responses to WHsAg and WHs peptides differed between CLV−V+ and CLV+V+ carriers, their respective low-level anti-WHs responses, detected by enzyme-linked immunosorbent assay, were similar (22, 33, 35). The observed seroconversion to anti-WHs was mainly a consequence of WHsAg vaccination and less due to CLV treatment and its discontinuation. The maximum mean titers of antibody following therapeutic vaccination of the WHV carriers were approximately 20-fold lower than those for typical naive vaccine recipients. The class of antibodies (i.e., immunoglobulin M or immunoglobulin G) elicited and their fine epitope specificity and potential immune complex composition are unknown. The similarity in anti-WHs responses detected in the vaccinated WHV carriers in high or low viral load settings could be explained by various mechanisms that will require further study. For example, low anti-WHs titers in serum from vaccinated carriers may reflect the presence of excess WHsAg in serum, and the resulting degree of exchange of complexed antibody with antigen on the solid-phase assay supports this. Some circulating WHsAg was still detectable at very low levels in serum from most of the treated WHV carriers by the end of drug treatment and thereafter. Alternatively, the similarity in the anti-WHs titer, with differences in T-cell responses, could reflect different biases in the Th1-type versus Th2-type immune responses in the different treatments. For example, combination treatment (i.e., CLV treatment and WHsAg vaccination), together with endogenous stimulations (i.e., slight increases in viral replication following discontinuation of drug), may preferentially stimulate Th1-type responses more than Th2-type responses, thus resulting in proportionately less anti-WHs than that seen with vaccine given alone in the high-viral-load setting. As with the present dissection of T-cell responses, additional tests of anti-WHs in immune complexes and reactivity with synthetic peptide, may uncover differences in fine specificity of the humoral immune responses between CLV+V− and CLV+V+ carriers.
The S-region sequences of the WHV envelope protein are shared by the overlapping small, middle, and large surface proteins and therefore comprise the vast majority of the surface antigen load to which an untreated WHV carrier is tolerant. Cellular immunologic unresponsiveness to the S region in certain mouse model systems can be circumvented by the inclusion of pre-S2-region sequences in the immunogen (vaccine) (39). Pre-S1- and pre-S2-region sequences were present in the WHsAg particles used as a vaccine in this study. Pre-S1-region sequences present in the WHsAg vaccine may function similarly to pre-S2-region sequences in circumventing T-cell unresponsiveness to S-region sequences. Certain CLV-independent pre-S1-region sequences were recognized occasionally by CLV−V+ carriers (e.g., peptide S1), but recognition of other pre-S1 peptides, some of which are located only in the large surface protein (i.e., peptides S7 and S7/8), was dependent on the prior reduction in viral and antigen loads by CLV. This suggests a model in which the expansion of T-cell responses in vaccinated WHV carriers to include additional S-region sequences also may depend on responses to critical pre-S1-region sequences present only in the large surface protein that require prior reduction of viral and antigen loads for recognition.
Our results for CLV-dependent versus CLV-independent T-cell epitopes have implications for possible mechanisms of central and/or peripheral immunological tolerance that may operate in chronic HBV infection. Clearly, some T-cell responses to WHsAg can be elicited upon vaccination even in the high viral and antigen load setting (i.e., CLV-independent epitopes), but others are not (CLV-dependent epitopes). In one possible mechanism involving peripheral tolerance, T-cell clonal anergy may result at the postthymic stage due to high levels of antigenemia following experimental neonatal WHV infection that are associated with the chronic outcome instead of recovery. Once viral and antigen loads are reduced sufficiently by drug treatment (or modulated by any subsequent drug withdrawal), the anergized T cells then could be poised to have their reactivities restored and therefore become available for stimulation by exogenous antigen during therapeutic vaccination (as indicated by the present results). Our observations could also be explained similarly by antigen-dependent thymic depletion operating during central tolerance. In this case, however, a more extended time of reduced viral and antigen load may be required (as seen with CLV) in order to allow such clones to be replenished in sufficient numbers to gain responsiveness to vaccine.
On a related note, T-cell responses to peptide S14 were observed for some of the WHV-negative, vaccinated woodchucks and also for the majority of woodchucks recovering from WHV infection, but they were not observed for any of the treated WHV carriers. This could suggest that some anergized T cells may not regain their potential for reactivity with a reduction of viremia and antigenemia. On the other hand, such a loss of T-cell specificities in established carriers also may be a result of more-extensive clonal depletion during progression to chronicity. Due to the dynamic functional nature of T-cell ontogeny, an irreversible deletion of virus-specific T-cell clones for S14 is probably not a major factor in the onset or maintenance of chronic WHV infection, but a model for extensive T-cell depletion and lack of restoration may represent one possible alternative. However, improved therapeutic outcomes were possible even among CLV+V+ carriers that did not respond to this peptide. Accordingly, responses to other regions of WHsAg (e.g., CLV-dependent epitopes) may be sufficient for a positive therapeutic outcome.
Based on our present results with chemoimmunotherapeutic modeling in chronic WHV infection, we conclude that combination therapy with potent antiviral drugs and conventional vaccine overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. In combination with potent antiviral drugs that reduce both viral and surface antigen loads, conventional vaccination was able to therapeutically restore the T-cell response profile to that observed during vaccine prophylaxis and recovery from acute WHV infection. Such experimental therapy should be useful for the treatment of humans with chronic HBV infection.
Acknowledgments
This work was supported by contract N01-AI-05399 (College of Veterinary Medicine, Cornell University) and contract N01-AI-95390 (Division of Molecular Virology and Immunology, Georgetown University, School of Medicine) from the National Institute of Allergy and Infectious Diseases (NIAID).
We gratefully acknowledge the expert assistance of Betty Baldwin, Lou Ann Graham, Richard Moore, and Chris Bellezza (Cornell University) and of Karen Gay, Christine Ferrar, and Frances Wells (Georgetown University). We gratefully acknowledge the generous supply of CLV by Triangle Pharmaceuticals, Inc., for this study.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Abbas, A. K., J. Lohr, B. Knoechel, and V. Nagabhushanam. 2004. T cell tolerance and autoimmunity. Autoimmun. Rev. 3:471-475. [DOI] [PubMed] [Google Scholar]
- 2.Bertoletti, A., M. M. D'Elios, C. Boni, M. De Carli, A. L. Zignego, M. Durazzo, G. Missale, A. Penna, F. Fiaccadori, G. Del Prete, and C. Ferrari. 1997. Different cytokine profiles of intrahepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology 112:193-199. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti, A., and C. Ferrari. 2003. Kinetics of the immune response during HBV and HCV infection. Hepatology 38:4-13. [DOI] [PubMed] [Google Scholar]
- 4.Boni, C., A. Bertoletti, A. Penna, A. Cavalli, M. Pilli, S. Urbani, P. Scognamiglio, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 1998. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J. Clin. Investig. 102:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celis, E., D. Ou, and L. Otvos, Jr. 1988. Recognition of hepatitis B surface antigen by human T lymphocytes. Proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J. Immunol. 140:1808-1815. [PubMed] [Google Scholar]
- 6.Chen, M., M. Sallberg, J. Hughes, J. Jones, L. G. Guidotti, F. V. Chisari, J. N. Billaud, and D. R. Milich. 2005. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 79:3016-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, M. T., J. N. Billaud, M. Sallberg, L. G. Guidotti, F. V. Chisari, J. Jones, J. Hughes, and D. R. Milich. 2004. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 101:14913-14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari, F. V. 2000. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 156:1117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 17:261-281. [DOI] [PubMed] [Google Scholar]
- 10.Coffin, C. S., and T. I. Michalak. 1999. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J. Clin. Investig. 104:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31:190-200. [DOI] [PubMed] [Google Scholar]
- 12.Cote, P. J., I. Toshkov, I. Nakamura, S. Menne, B. Korba, B. Tennant, and J. Gerin. 2002. Chronicity as an outcome of experimental neonatal woodchuck hepatitis virus infection results from a deficient type 1 immune response to acute infection, p. 280-285. In H. S. Margolis, M. J. Alter, T. J. Liang, and J. L. Dienstag (ed.), Viral hepatitis and liver diseases: proceedings of the 10th International Symposium on Viral Hepatitis and Liver Disease. International Medical Press Ltd., Atlanta, GA.
- 13.Couillin, I., S. Pol, M. Mancini, F. Driss, C. Brechot, P. Tiollais, and M. L. Michel. 1999. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis. 180:15-26. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari, C., A. Bertoletti, A. Penna, A. Cavalli, A. Valli, G. Missale, M. Pilli, P. Fowler, T. Giuberti, F. V. Chisari, et al. 1991. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J. Clin. Investig. 88:214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari, C., A. Cavalli, A. Penna, A. Valli, A. Bertoletti, G. Pedretti, M. Pilli, P. Vitali, T. M. Neri, T. Giuberti, et al. 1992. Fine specificity of the human T-cell response to the hepatitis B virus preS1 antigen. Gastroenterology 103:255-263. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 17.Gerin, J. L., R. M. Faust, and P. V. Holland. 1975. Biophysical characterization of the adr subtype of hepatitis B antigen and preparation of anti-r sera in rabbits J. Immunol. 115:100-105. [PubMed] [Google Scholar]
- 18.Gerin, J. L., B. C. Tennant, H. Popper, F. J. Tyeryar, and R. H. Purcell. 1986. The woodchuck model of hepadnavirus infection and disease, p. 383-386. In F. Brown, R. Chanock, and R. Lerner (ed.), Vaccines 86: new approaches to immunization. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 19.Honorati, M. C., P. Dolzani, E. Mariani, A. Piacentini, G. Lisignoli, C. Ferrari, and A. Facchini. 1997. Epitope specificity of Th0/Th2 CD4+ T-lymphocyte clones induced by vaccination with rHBsAg vaccine. Gastroenterology 112:2017-2027. [DOI] [PubMed] [Google Scholar]
- 20.Jung, M. C., H. M. Diepolder, U. Spengler, E. A. Wierenga, R. Zachoval, R. M. Hoffmann, D. Eichenlaub, G. Frosner, H. Will, and G. R. Pape. 1995. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J. Virol. 69:3358-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korba, B. E., P. Cote, W. Hornbuckle, B. C. Tennant, and J. L. Gerin. 2000. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 31:1165-1175. [DOI] [PubMed] [Google Scholar]
- 22.Korba, B. E., P. J. Cote, S. Menne, I. Toshkov, B. H. Baldwin, F. V. Wells, B. C. Tennant, and J. L. Gerin. 2004. Clevudine therapy with vaccine inhibits progression of chronic hepatitis and delays onset of hepatocellular carcinoma in chronic woodchuck hepatitis virus infection. Antivir. Ther. 9:937-952. [PubMed] [Google Scholar]
- 23.Korba, B. E., F. V. Wells, B. Baldwin, P. J. Cote, B. C. Tennant, H. Popper, and J. L. Gerin. 1989. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology 9:461-470. [DOI] [PubMed] [Google Scholar]
- 24.Lohr, H. F., W. Weber, J. Schlaak, B. Goergen, K. H. Meyer zum Buschenfelde, and G. Gerken. 1995. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology 22:61-68. [DOI] [PubMed] [Google Scholar]
- 25.Marcellin, P., T. Asselah, and N. Boyer. 2005. Treatment of chronic hepatitis B. J. Viral Hepat. 12:333-345. [DOI] [PubMed] [Google Scholar]
- 26.Marinos, G., N. V. Naoumov, and R. Williams. 1996. Impact of complete inhibition of viral replication on the cellular immune response in chronic hepatitis B virus infection. Hepatology 24:991-995. [DOI] [PubMed] [Google Scholar]
- 27.Marinos, G., F. Torre, S. Chokshi, M. Hussain, B. E. Clarke, D. J. Rowlands, A. L. Eddleston, N. V. Naoumov, and R. Williams. 1995. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology 22:1040-1049. [DOI] [PubMed] [Google Scholar]
- 28.Menne, S., and P. J. Cote. 2004. Measurement of cell-mediated immune response in woodchucks. Methods Mol. Med. 96:27-36. [DOI] [PubMed] [Google Scholar]
- 29.Menne, S., and P. J. Cote. 2007. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 13:104-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menne, S., P. J. Cote, S. D. Butler, I. A. Toshkov, J. L. Gerin, and B. C. Tennant. 2007. Immunosuppression reactivates viral replication long after resolution of woodchuck hepatitis virus infection. Hepatology 45:614-622. [DOI] [PubMed] [Google Scholar]
- 31.Menne, S., J. Maschke, M. Lu, H. Grosse-Wilde, and M. Roggendorf. 1998. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge J. Virol. 72:6083-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menne, S., J. Maschke, T. K. Tolle, M. Lu, and M. Roggendorf. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 71:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menne, S., C. A. Roneker, B. E. Korba, J. L. Gerin, B. C. Tennant, and P. J. Cote. 2002. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J. Virol. 76:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menne, S., C. A. Roneker, M. Roggendorf, J. L. Gerin, P. J. Cote, and B. C. Tennant. 2002. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J. Virol. 76:1769-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menne, S., C. A. Roneker, B. C. Tennant, B. E. Korba, J. L. Gerin, and P. J. Cote. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237-250. [DOI] [PubMed] [Google Scholar]
- 36.Michalak, T. I., I. U. Pardoe, C. S. Coffin, N. D. Churchill, D. S. Freake, P. Smith, and C. L. Trelegan. 1999. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 29:928-938. [DOI] [PubMed] [Google Scholar]
- 37.Milich, D. R., M. K. Chen, J. L. Hughes, and J. E. Jones. 1998. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J. Immunol. 160:2013-2021. [PubMed] [Google Scholar]
- 38.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLachlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 87:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milich, D. R., M. K. McNamara, A. McLachlan, G. B. Thornton, and F. V. Chisari. 1985. Distinct H-2-linked regulation of T-cell responses to the pre-S and S regions of the same hepatitis B surface antigen polypeptide allows circumvention of nonresponsiveness to the S region. Proc. Natl. Acad. Sci. USA 82:8168-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33:254-266. [DOI] [PubMed] [Google Scholar]
- 41.Pontesilli, O., A. Van Nunen, D. Van Riel, S. Bruijns, B. Niesters, F. Uytdehaag, R. A. De Man, S. W. Schalm, and A. D. Osterhaus. 2000. Immune responses during lamivudine/interferon-combination therapy of chronic HBV infection. Antivir. Ther. 5(Suppl. 1):B18. [Google Scholar]
- 42.Rehermann, B. 2000. Intrahepatic T cells in hepatitis B: viral control versus liver cell injury. J. Exp. Med. 191:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romagnani, S. 2006. Immunological tolerance and autoimmunity. Intern. Emerg. Med. 1:187-196. [DOI] [PubMed] [Google Scholar]
- 44.Tennant, B. C., I. A. Toshkov, S. F. Peek, J. R. Jacob, S. Menne, W. E. Hornbuckle, R. D. Schinazi, B. E. Korba, P. J. Cote, and J. L. Gerin. 2004. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127(Suppl. 1):S283-S293. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.Tsai, S. L., P. J. Chen, M. Y. Lai, P. M. Yang, J. L. Sung, J. H. Huang, L. H. Hwang, T. H. Chang, and D. S. Chen. 1992. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J. Clin. Investig. 89:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vryheid, R. E., M. A. Kane, N. Muller, G. C. Schatz, and S. Bezabehd. 2000. Infant and adolescent hepatitis B immunization up to 1999: a global overview. Vaccine 19:1026-1037. [DOI] [PubMed] [Google Scholar]
- 48.Wong, D. C., J. W. Shih, R. H. Purcell, J. L. Gerin, and W. T. London. 1982. Natural and experimental infection of woodchucks with woodchuck hepatitis virus, as measured by new, specific assays for woodchuck surface antigen and antibody. J. Clin. Microbiol. 15:484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]