Abstract
Mice carrying a wild-type Mx1 gene (Mx1+/+) differ from standard laboratory mice (Mx1−/−) in being highly resistant to infection with common laboratory strains of influenza A virus. We report that Mx1 also protects mice against the pandemic human 1918 influenza virus and a highly lethal human H5N1 strain from Vietnam. Resistance to H5N1 of Mx1+/+ but not Mx1−/− mice was enhanced if the animals were treated with a single dose of exogenous alpha interferon before infection. Thus, the interferon-induced resistance factor Mx1 represents a key component of the murine innate immune system that mediates protection against epidemic and pandemic influenza viruses.
Mice are frequently used as an animal model to study the pathogenesis of influenza A viruses (FLUAV). However, standard laboratory mice do not possess a complete antiviral defense system, as they carry defective alleles of the Mx1 gene (15). The Mx1 gene is under tight transcriptional control of alpha/beta interferon (IFN-α/β) and codes for a nuclear 72-kDa protein which represents a decisive antiviral factor that controls FLUAV infections in mice (7, 16). Mx1 inhibits primary transcription of the FLUAV genome by a poorly defined mechanism (11). In humans, IFN-regulated Mx genes are also present (1). MxA, the product of the human MX1 gene, inhibits a step of the FLUAV replication cycle which follows primary transcription (11). In spite of these differences between the Mx systems of humans and mice, Mx1+/+ mice which carry functional Mx1 alleles may better mimic the innate immune system of humans than Mx1−/− mice.
Mouse-adapted laboratory strains of influenza virus are usually well controlled by the action of the Mx1 proteins in mice (6). However, we recently identified an exceptionally virulent FLUAV strain that kills Mx1+/+ mice, presumably because it replicates at a higher efficiency and overruns the host innate immune system (4). Efficient replication is also a hallmark of the pandemic 1918 FLUAV strain (10, 17) and of current highly pathogenic avian influenza H5N1 viruses that have resulted in sporadic human infections with a high fatality rate (8, 13). The question therefore arose as to whether these naturally aggressive viruses, which are highly pathogenic for standard laboratory mice, would also be highly virulent in mice with an intact Mx1 resistance gene.
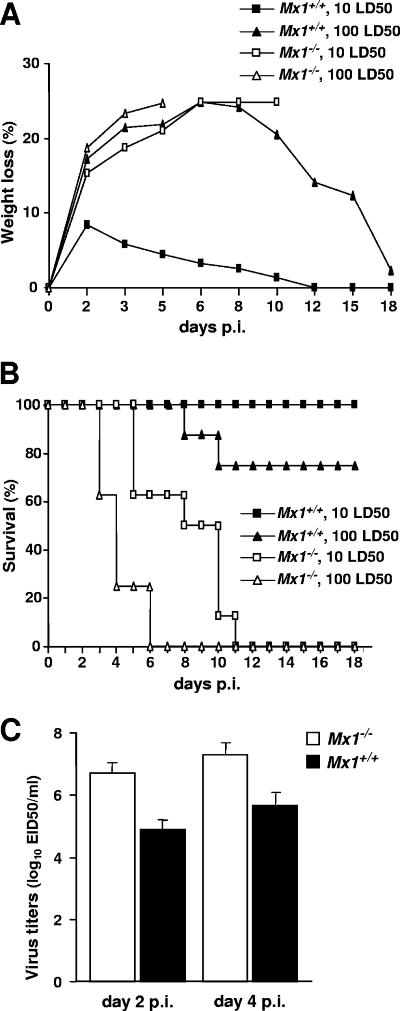
We first determined whether the 1918 pandemic FLUAV strain that caused high mortality in standard laboratory mice is highly virulent for Mx1+/+ mice. Groups of eight standard BALB/c mice carrying a defective allele of the Mx1 resistance gene (15) or congenic BALB.A2G-Mx1 mice carrying the functional Mx1 allele (14) were anesthetized by intraperitoneal injection of 0.2 ml of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Aldrich Chemical Co., Milwaukee, WI) before they were infected intranasally with either 10 or 100 50% mouse lethal doses (LD50) for BALB/c mice of the reconstructed 1918 virus (17). As expected, under these conditions, all Mx1−/− BALB/c mice quickly lost weight (Fig. 1A), became severely ill, and succumbed to infection (Fig. 1B). By contrast, weight loss of the Mx1+/+ BALB.A2G-Mx1 mice infected with 10 LD50 of the 1918 virus for BALB/c mice was minimal (Fig. 1A) and all animals survived (Fig. 1B). Six of the eight BALB.A2G-Mx1 mice infected with 100 LD50 of the 1918 virus for BALB/c mice survived, indicating a difference in the LD50s between Mx1-competent and Mx1-incompetent mice of at least 100-fold. The enhanced resistance of the Mx1+/+ mice also was apparent when virus titers in the lungs of the infected animals were compared. Whole lung tissues from individual mice were removed aseptically and homogenized in 1 ml of sterile phosphate-buffered saline (PBS), and tissue debris was removed by low-speed centrifugation. Virus titers in the supernatants were then determined in embryonated chicken eggs by performing serial 10-fold dilutions in PBS. The average virus titers in the lungs of Mx1−/− mice infected with the low dose of the 1918 virus were at least 50-fold higher than those in Mx1+/+ mice at both days 2 and 4 postinfection (Fig. 1C).
FIG. 1.
Enhanced resistance of Mx1+/+ mice to the 1918 pandemic influenza virus. Standard BALB/c (Mx1−/−) mice (open symbols) and congenic BALB.A2G-Mx1 (Mx1+/+) mice (closed symbols) were challenged with either 10 LD50 for BALB/c mice (squares) or 100 LD50 (triangles) of the pandemic 1918 strain of influenza A virus by the intranasal route. Weight loss (A) and survival (B) of the animals (eight per group) were monitored for 18 days following infection. (C) At days 2 and 4 postinfection (p.i.), four additional Mx1+/+ and Mx1−/− animals infected with the low virus dose were euthanized, and virus titers in the whole lungs were determined. Fifty percent egg infectious dose (EID50) titers were determined by serial titration of clarified homogenates in eggs (9).
We next determined whether the Mx1+/+ mice would also exhibit enhanced resistance to highly pathogenic H5N1 FLUAV. In a first experiment, groups of Mx1+/+ and Mx1−/− mice were challenged with 10 LD50 for BALB/c mice of A/Vietnam/1203/04 (VN1203), a virus isolated in 2004 from a fatal human case in Vietnam (9). Weight loss in the Mx1+/+ mice was moderate and all animals survived, whereas all Mx1−/− control mice developed severe disease and succumbed to infection between days 4 and 7 postinfection (Fig. 2A). Virus titers in the lungs of Mx1+/+ mice were approximately 50-fold lower than in Mx1−/− mice at both day 2 and day 4 postinfection (Fig. 2B). When a dose of 100 LD50 for BALB/c mice of VN1203 was used for the challenge, the weight loss in the Mx1+/+ BALB.A2G-Mx1 mice was more severe and six of eight animals died (data not shown). In a second experiment in which the animals were infected with 1,000 LD50 for BALB/c mice of VN1203, the survival rate of the BALB.A2G-Mx1 mice was 50% (compared to saline-treated controls) (Fig. 3A). Under these conditions, the virus titers in the lungs of the BALB.A2G-Mx1 mice on day 3 postinfection were about 50 times lower than in the Mx1−/− BALB/c control mice (Fig. 3B), indicating that the Mx1 resistance gene confers similar degrees of protection against both highly lethal H5N1 virus and the 1918 pandemic virus.
FIG. 2.
Enhanced resistance of Mx1+/+ mice to highly lethal human H5N1 influenza virus. Standard BALB/c (Mx1−/−) mice (open symbols) and congenic BALB.A2G-Mx1 (Mx1+/+) mice (closed symbols) were challenged with 10 LD50 for BALB/c mice of VN1203. (A) Survival of the animals (eight per group) was monitored for 18 days following infection. (B) At days 2 and 4 postinfection (p.i.), four additional Mx1+/+ and four Mx1−/− animals were euthanized and virus titers in the lung were determined.
FIG. 3.
Resistance of Mx1+/+ mice to highly lethal human H5N1 virus can be enhanced by intranasal treatment with exogenous IFN. Eight hours prior to infection with 1,000 LD50 for BALB/c mice of strain VN1203, BALB/c (Mx1−/−) mice (open symbols) and congenic BALB.A2G-Mx1 (Mx1+/+) mice (closed symbols) were treated with PBS (triangles) or 10,000 units of recombinant human IFN-αB/D (squares) by the intranasal route. (A) Survival of the animals (eight per group) was monitored for 18 days following infection. (B) At day 3 postinfection (p.i.), three additional Mx1+/+ mice and four Mx1−/− animals of each treatment group were killed and virus titers in the lungs, brains, and spleens were determined. The dotted line indicates the detection limit (101.2 50% egg infectious dose/ml) in this assay.
VN1203 and other highly virulent strains of H5N1 cause a systemic infection in standard inbred strains of laboratory mice (13). In our experiments, we also detected infectious H5N1 virus in the brains and spleens of Mx1−/− BALB/c mice at 3 days postinfection (Fig. 3B). In contrast, virus titers in the same organs of Mx1+/+ BALB.A2G-Mx1 mice were below the limit of detection, demonstrating that the Mx1 resistance factor also limits the systemic spread of FLUAV in infected mice and suggesting that this could also be the case in humans, where high viral titers are observed only in the respiratory tract of infected individuals (2).
Since human infections with currently circulating H5N1 viruses are frequently fatal (12) and since only few treatment options are available for the victims, we used the mouse model to evaluate the protective potential of recombinant IFN-α. Groups of mice were treated by the intranasal route with either PBS or 10,000 units of hybrid human IFN-αB/D, which is highly active in mouse cells (3). Eight hours later, the animals were infected with 1,000 LD50 for BALB/c mice of VN1203. None of the eight IFN-treated Mx1+/+ mice became severely ill (Fig. 3A), and the weight loss in these animals was less than 10% (data not shown). At day 3 postinfection, virus titers in the lungs of IFN-treated Mx1+/+ mice were about 1,000 times lower than those in PBS-treated Mx1+/+ control mice (Fig. 3B), demonstrating that IFN conferred very efficient protection. Interestingly, the IFN treatment was completely ineffective in Mx1−/− animals. The IFN-treated BALB/c mice died almost as quickly as the PBS-treated control mice (Fig. 3A), and the virus titers in the lungs, brains, and spleens were comparably high in both IFN-treated and untreated Mx1−/− mice (Fig. 3B). IFN treatment of Mx1−/− mice was similarly ineffective when the challenge virus dose was reduced to 100 LD50 for BALB/c mice (data not shown). These results are in good agreement with an earlier report in which a mouse-adapted FLUAV strain with high virulence for Mx1+/+ mice was used for IFN treatment studies (4).
Our study shows that the IFN-induced resistance factor Mx1 is a key component of the innate immune system of mice that mediates protection against both zoonotic and human pandemic strains of influenza viruses of high virulence. We demonstrated that the lethal doses of the pandemic 1918 virus and of a highly virulent H5N1 strain were at least 2 orders of magnitude different between Mx1+/+ and Mx1−/− mice. A similar degree of protection by Mx1 was previously reported for several mouse-adapted FLUAV strains of human and avian origins (5, 6). Thus, neither the 1918 virus nor the currently circulating H5N1 viruses have acquired Mx resistance or developed efficient strategies to prevent Mx gene induction in response to infection. Using a mouse-adapted model virus, we recently demonstrated that a specific mouse-adapted strain of influenza virus displayed a simple strategy for evading the Mx system without acquiring insensitivity to the Mx1 resistance factor (4). In this case, escape from Mx1-mediated virus control was based on the high speed of virus replication, which subverts the timely induction of the dormant IFN-regulated innate immune system of the infected host. It should be noted that our present work cannot exclude the possibility that the high virulence for humans of the pandemic 1918 virus and certain highly pathogenic H5N1 strains is based on a similar evasion strategy which, however, is not apparent in mice since the 1918 and H5N1 viruses employed here were not adapted to the mouse. Therefore, these viruses might not have revealed their maximal growth potential in our system.
A final interesting aspect of our work is that prophylactic treatment with recombinant human IFN-α increased the resistance to the H5N1 virus. It will be important to determine in future experiments whether IFN treatment shortly after virus infection is also beneficial. Furthermore, it is of interest to know whether exogenous IFN can also prevent disease in the ferret model, which is believed to represent a better animal model for human influenza virus infections. If IFN holds its promise as an antiviral against influenza in these animal models, it might be considered as an emergency drug for treatment of pandemic influenza in times when neuraminidase inhibitors and other antiviral agents are expected to be in short supply.
Acknowledgments
This work was partially supported by a grant to P.S. and G. K. from the Deutsche Forschungsgemeinschaft (DFG) and by NIH grant P01 AI058113 to A.G.-S.
We thank Heinz Hochkeppel (Novartis) for providing recombinant human IFN-αB/D.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agencies.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Aebi, M., J. Fah, N. Hurt, C. E. Samuel, D. Thomis, L. Bazzigher, J. Pavlovic, O. Haller, and P. Staeheli. 1989. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 9:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong, M. D., V. C. Bach, T. Q. Phan, M. H. Vo, T. T. Tran, B. H. Nguyen, M. Beld, T. P. Le, H. K. Truong, V. V. Nguyen, T. H. Tran, Q. H. Do, and J. Farrar. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352:686-691. [DOI] [PubMed] [Google Scholar]
- 3.Gangemi, J. D., J. Lazdins, F. M. Dietrich, A. Matter, B. Poncioni, and H. K. Hochkeppel. 1989. Antiviral activity of a novel recombinant human interferon-alpha B/D hybrid. J. Interferon Res. 9:227-237. [DOI] [PubMed] [Google Scholar]
- 4.Grimm, D., P. Staeheli, M. Hufbauer, I. Koerner, L. Martinez-Sobrido, A. Solorzano, A. García-Sastre, O. Haller, and G. Kochs. 2007. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc. Natl. Acad. Sci. USA 104:6806-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller, O. 1981. Inborn resistance of mice to orthomyxoviruses. Curr. Top. Microbiol. Immunol. 92:25-52. [DOI] [PubMed] [Google Scholar]
- 6.Haller, O., H. Arnheiter, I. Gresser, and J. Lindenmann. 1979. Genetically determined, interferon-dependent resistance to influenza virus in mice. J. Exp. Med. 149:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Tech. Off. Int. Epizoot. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 8.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3:591-600. [DOI] [PubMed] [Google Scholar]
- 9.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L. M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. Nguyen, J. H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palese, P. 2004. Influenza: old and new threats. Nat. Med. 10:S82-87. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovic, J., O. Haller, and P. Staeheli. 1992. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 66:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris, J. S., M. D. de Jong, and Y. Guan. 2007. Avian influenza virus (H5N1): a threat to human health. Clin. Microbiol. Rev. 20:243-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 14.Staeheli, P., P. Dreiding, O. Haller, and J. Lindenmann. 1985. Polyclonal and monoclonal antibodies to the interferon-inducible protein Mx of influenza virus-resistant mice. J. Biol. Chem. 260:1821-1825. [PubMed] [Google Scholar]
- 15.Staeheli, P., R. Grob, E. Meier, J. G. Sutcliffe, and O. Haller. 1988. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 8:4518-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staeheli, P., O. Haller, W. Boll, J. Lindenmann, and C. Weissmann. 1986. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44:147-158. [DOI] [PubMed] [Google Scholar]
- 17.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. García-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]