Abstract
Prion diseases are fatal neurodegenerative disorders. Identification of possible therapeutic tools is important in the search for a potential treatment for these diseases. Congo red is an azo dye that has been used for many years to detect abnormal prion protein in the brains of diseased patients or animals. Congo red has little therapeutic potential for the treatment of these diseases due to toxicity and poor permeation of the blood-brain barrier. We have prepared two Congo red derivatives, designed without these liabilities, with potent activity in cellular models of prion disease. One of these compounds cured cells of the transmissible agent. The mechanism of action of these compounds is possibly multifactorial. The high affinity of Congo red derivatives, including compounds that are ineffective and are effective at the cure of prion disease, for abnormally folded prion protein suggests that the amyloidophylic property of these derivatives is not as critical to the mechanism of action as other effects. Congo red derivatives that are effective at the cure of prion disease increased the degradation of abnormal PrP by the proteasome. Therefore, the principal mechanism of action of the Congo red analogues was to prevent inhibition of proteasomal activity by PrPSc.
Prion diseases are neurodegenerative disorders otherwise known as transmissible spongiform encephalopathies (TSEs). There are inherited, transmitted, and sporadic forms of these diseases (54). The principal disease in humans is Creutzfeldt-Jakob disease (CJD), which is a sporadic form. These diseases are characterized by the accumulation of large amounts of protease-resistant aggregates of an altered isoform of the prion protein (54). The normal isoform of this protein (PrPc) is a copper binding glycoprotein expressed at the surface of a number of cell types but principally neurons (8, 58). This protein has a relatively short half-life of around an hour, but when converted to the protease-resistant isoform (PrPSc), it accumulates extracellularly and can remain there for much longer (16). The conversion process is unknown but is believed to involve a seeding mechanism in which small aggregates initially form over a long incubation period (15). These aggregates can then rapidly initiate the conversion of more protein, resulting in often high levels of PrPSc in the brains of individuals with the disease. It is largely assumed that the accumulation of these aggregates is responsible for the pathology of prion disease. A considerable amount of evidence suggests that PrPSc is directly toxic to neurons and initiates an apoptotic process, possibly also requiring the involvement of glia and oxidative stress (10). However, alternative processes may also play a role. An additional complication for the cell death mechanism is that cellular expression of PrPc is required (6, 7). Mice with the prion protein knocked out are resistant to infection with prion disease (10a).
Despite the considerable uncertainty about the mechanism of pathogenesis in prion diseases, there is a considerable demand for both diagnostic techniques and potential therapies. In some cases, there is no sure proof that a neurodegenerative condition is prion disease, such as CJD or BSE, without analysis of brain tissue for the presence of spongiform changes and PrPSc. Some evidence of neurological changes or the presence of CSF markers, such as 14-3-3 (30), can be helpful but are not specific. Tonsil biopsies in the case of variant CJD (28) or analyses of other lymphoid tissues in the case of scrapie (67) provide good evidence for the diagnosis of prion diseases. However, diagnosis usually can occur only when the disease has progressed to a point where the quality of life has deteriorated to such an extent that prevention of further neuronal death would have little benefit. The prion diseases all have long incubation periods, making diagnosis before clinical onset difficult (29). Nevertheless, there are currently considerable endeavors aimed at developing an effective test for the preclinical diagnosis of prion disease (43). Even if such a possibility is eventuated, without an effective treatment for these diseases, such a diagnosis remains a sure death sentence.
Treatment investigations target mostly the accumulation of PrPSc in the brain. The two most promising compounds, quinacrine and pentosan polysulphate, have largely been dismissed as ineffective in patients (27, 68). This fact implies that there is considerable pressure to thoroughly explore other possibilities. The standard techniques that are used for such investigations make use of either persistently infected cell lines or the mouse bioassay, which involves challenging mice with a mouse-passaged prion disease, such as scrapie. A number of compounds have shown antiprion activity in numerous studies using such assays (23, 41, 49, 62). Compounds shown to have antiprion activity include sulfated polysaccharides, which include pentosan polysulphate (21), Congo red and other azo dyes (12), amphotericin B and analogues (17), anthracyclines (63), phthalocyanines and porphyrins (13), phenanthridine derivatives (1), inorganic ions, branched polyamines, antagonists of the N-methyl-d-aspartate receptor, such as memantine (49), and acridine derivatives, such as quinacrine (19, 40, 47). Immunotherapeutic approaches are also being attempted for prion infection, with various levels of success (24, 50, 61). In addition, further screening methodologies have recently been reported to allow the screening of larger numbers of compounds in vitro (1, 3, 39).
There has been particular interest in Congo red derivatives for the treatment of prion diseases and other amyloidoses. In prion diseases, Congo red and some analogues and derivatives have been shown to be effective in a variety of models, including a cell-free polymerization assay (18, 35), cellular assays (48, 56, 57), surface plasmon resonance (33), and in vivo activity against prion disease in golden Syrian hamsters (2, 31, 52). Congo red itself has a number of shortfalls (18). It has a lack of specificity. In addition, it does not have significant permeability through the blood-brain barrier (37, 38), presumably due to its charged nature, primarily due to the presence of two sulfonate groups. Toxicity is also a problem; the diazo bonds in Congo red can be cleaved by enzymes present in the mammalian gut and intestines (5, 25). A side product of this cleavage is benzidine, a highly carcinogenic compound that is strongly associated with urinary bladder cancer (64). However, unlike many of the other compounds reported to have activity in various models of TSEs, Congo red is a small molecule which should be amenable to chemical synthesis and structure-activity relationship studies with the aim of producing a low-molecular-weight therapeutic agent. There has been considerable recent study to try to identify derivatives of Congo red which have antiprion activity but lack the shortfalls of the parent molecule (51, 56, 57, 59). In particular, two compounds with a nanomolar 50% effective concentration for clearing PrPSc from infected cells were identified (59). These compounds were considered worthy of further investigation.
In this report, we have studied the potential of two Congo red derivatives to effectively cure prion-infected cells and prevent prion protein aggregation. The mechanism of action was investigated. Although Congo red is active via its potential to interact with protein aggregates (11), our evidence suggests that the mechanism of action of these new compounds for the cure of prion disease is related to cellular effects independent of this protein interaction. It is, hence, probable that these compounds have a mechanism of action for the cure of prion disease that differs from that of Congo red.
MATERIALS AND METHODS
Experimental procedures.
Unless otherwise stated, reagents were purchased from Sigma-Aldrich.
Compound synthesis.
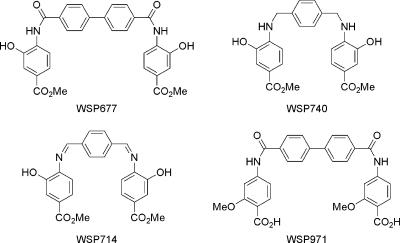
Compounds were prepared as described previously (59). The compounds are shown in Fig. 1. They include bis-methyl 4-({[4′-(aminocarbonyl)-1,1′-biphenyl-4-yl]carbonyl}amino)-3-hydroxybenzoate (WSP677), bis-4- ({[4′-(aminocarbonyl)-1, 1′-biphenyl-4-yl]carbonyl}-amino)-2-methoxybenzoic acid (WSP971), bis-methyl 4-{[4-(aminomethyl)benzyl]amino}-3-hydroxybenzoate (WSP74), and methyl 3-hydroxy-4-({(1Z)-[4-((Z)-{[2-hydroxy-4-(methoxycarbonyl) phenyl]imino}methyl)phenyl]methylene}-amino)benzoate (WSP714).
FIG. 1.
Chemical structures of the four Congo red derivatives studied are presented.
Cell culture.
SMB and SMB-PS cells were derived from the brain of a scrapie-infected mouse. The mouse had been infected with the Chandler strain of scrapie (14). The SMB cells persistently maintain the infection. The resulting cell line was cured of the infection by the use of pentosan sulfate (4) to generate an uninfected SMB-PS cell line that served as an appropriate control. The cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 5% newborn calf serum with 1% antibiotics (penicillin/streptomycin).
Plasmid constructs and transfection.
SMB cells were transfected for various experiments with a number of plasmid constructs. Two of these constructs expressed the whole mouse PrP open reading frame. The first of these was pCDNA3.1 (Invitrogen), with the PrP open reading frame cloned between the HindIII and EcoR1 sites of the multiple cloning site and green fluorescent protein (GFP)-PrP as previously described (26). The latter construct is based on pEGFP (Invitrogen), but the GFP gene was cloned between codons 37 and 38 of the PrP open reading frame.
For promoter studies, pd2-EGFP-1 (Clontech), a promoterless vector containing destabilized GFP with a 2-hour half-life, was used as a reporter construct. The mouse Prnp promoter, including exon 1, intron 1, and exon 2, was cloned into this construct, and the mouse Prnp promoter sequence (bases 1 to 8539) is available in the GenBank online service (accession number U29187). As a control, the cytomegalovirus (CMV) promoter was also cloned into the pd2-EGFP-1 vector.
All transfections were carried out using FuGENE 6 transfection reagent (Roche). Cells were plated at 50% confluence in six-well plates the day before the transfection. To perform the transfection, 100 μl of serum-free medium was pipetted into a microcentrifuge tube. Three microliters of the FuGENE 6 reagent was then added directly to this and mixed by gentle tapping. For each cell line to be created, 1 μg of DNA was added to the mixture, and the liquid was mixed as described above and incubated at room temperature for 15 min. After this time, the transfection mixture was added dropwise to the cells. The dish was swirled and returned to the incubator overnight. Cells were selected with 1 mg/ml Geneticin 24 h after transfection and then maintained in 0.5 mg/ml for routine culture.
Confocal microscopy.
The level of GFP expression in transfected cells was analyzed using an LSM 550 confocal microscope, and images were captured by using wavelengths for GFP (excitation, 488 nm; emission, 530 nm). GFP intensity was quantified using Scion imaging software (Scion Corporation).
Western blotting and PK resistance.
SMB cells were plated at 50% confluence per well in 24-well plates and left for 24 h to allow for attachment. Cells were treated with compounds for various lengths of time before being lysed in phosphate-buffered saline (PBS) containing 1% Triton X-100-1% Igepal CA-630 for 20 min at 37°C. Cell lysates were either placed on ice or treated with 80 μg/ml proteinase K (PK) for 1 h at 37°C. Proteins were concentrated from the total cell lysate by methanol precipitation, and the protein pellet was resuspended and denatured by boiling for 5 min in 8 M urea. Samples were electrophoresed on a 12% acrylamide gel and transferred electrophoretically to polyvinylidene difluoride membrane (Immobilon-P; Millipore). PrP was detected using the primary antibody DR1, as previously described (9), and a horseradish peroxidase-conjugated secondary antibody (Dako). Specific protein bands were visualized using ECL Plus chemiluminescent reagent (Amersham Pharmacia Biotech) followed by autoradiography. Autoradiographs were analyzed using Scion Image densitometric software.
Mouse bioassay of prion infectivity.
SMB cell extracts were prepared according to the method of Birkett et al. (4) for use in mouse bioassay experiments. Three sets of samples were prepared: untreated, Chandler-infected extracts (termed control); Chandler-infected and compound WSP740-treated samples (termed compound WSP740), and Chandler-infected and compound WSP677-treated samples (termed compound WSP677). Each of these preparations were used to intracerebrally (i.c.) inoculate a group of 15 CD-1 female mice at the age of 2 months, as previously described (60). Twenty-five microliters was used for each i.c. inoculation. Mice were assessed weekly for clinical signs of prion infection from 60 days postinoculation by observers blinded to the experimental status of the animals, who used a test of motor coordination for 3 consecutive weeks, following an established protocol (24, 60). The analysis of clinical symptoms consists of observing the activity level and competency of the mice on an apparatus containing a series of parallel bars (3 mm in diameter) placed 7 mm apart. The initial clinical findings are a reduction in activity and/or the ability of the mice to traverse the parallel bars. This clinical endpoint correlates to the pathological development of CNS scrapie infection (24, 60). Mice that never showed signs of prion infection were sacrificed at 400 days postinoculation to determine if any PrPSc was present in the spleen or brain. The brains and spleens from ketamine/xylazine-anesthetized, euthanized mice were removed. The right hemisphere was immersion fixed overnight in periodate-lysine-paraformaldehyde, whereas the left hemisphere was snap-frozen for Western blotting. The spleens were snap-frozen for Western blotting. The fixed brain hemispheres were subsequently transferred to a solution containing 20% glycerol and 2% dimethyl sulfoxide (DMSO) in 0.1 M sodium phosphate buffer and stored at 4°C until sectioned. Serial coronal sections (40 μm) were cut, and series of sections at 0.2-mm intervals were obtained for histological analysis. The diagnosis of prion disease was confirmed by staining brain sections with cresyl violet, immunostaining with a monoclonal anti-PrP antibody (6D11) (Signet Laboratories), and detecting PK-resistant PrP on Western blots as previously described (24). Ten percent brain homogenates were prepared in PBS. Twenty-microgram samples were incubated with 4 μg of PK at 37C for 1 h. The reaction was stopped with phenylmethylsulfonyl fluoride, and the samples were run on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Following transfer, Western blots were prepared using monoclonal anti-PrP 6D11 (50).
ITC.
The thermodynamics of Congo red-derivative binding to the prion were measured using an isothermal titration microcalorimeter (VP-ITC; MicroCal, Inc.). Prion protein samples were produced as previously described (9). Fresh monomeric PrP was prepared without binding metals. Aggregated PrP was generated from fresh PrP by interaction with manganese as previously described (9). This form of PrP has been previously shown to catalyze the seeded polymerization of monomeric PrP in a polymerization assay (59). Some of the Congo red derivatives, including WSP677 and WSP740, were previously shown to inhibit this polymerization reaction (59). Compounds WSP971 and WSP714 were not active in this assay. This result would suggest a difference in the abilities of these compounds to bind to PrP.
The samples were thoroughly degassed before each titration. The experiments were performed at 25°C, pH 7, with 20 μM PrP in the sample cell and 650 μM Congo red derivative in the titrant syringe. The sample in the cell was stirred at 300 rpm by the syringe, and 2 μl of titrant was delivered over 20 s with 180-s intervals between injections to allow complete equilibration. The data were collected automatically and subsequently analyzed by Origin software from MicroCal, Inc. Before the curve-fitting process, a background titration, consisting of the identical Congo red derivative solution in the syringe but only the buffer solution in the sample cell, was subtracted from each experimental titration to account for the heat of dilution. From the nonconstrained fitting to the plot of heat evolved/mol of derivative injected versus the molar ratio of Congo red derivative to PrP, the binding stoichiometry and the affinity constant were determined.
Redox assays.
Oxidative stress is associated with prion diseases. As Congo red derivatives could potentially have a curing effect via possible antioxidant activity, estimating the level of oxidative stress in scrapie-infected cells is important. Redox assays are a simple method of assessing the level of reactive oxygen species (ROS; mediators of oxidative stress) in cells.
Direct measurement of free radicals by microplate assay.
Direct measurement of ROS within cells was made using a microplate assay utilizing CM-H2DCFDA, which is a chemically reduced acetomethoxyl ester of 2′,7′-dichlorofluorescein (DCF). This compound is colorless until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. CM-H2DCFDA is cell permeable until it is oxidized to its fluorescent product inside the cell. Oxidation may be induced by hydrogen peroxide (with the assistance of endogenous metal ions), organic hydroperoxides, nitric oxide, and peroxynitrite.
F14 and F21 cells were plated in 96-well plates at 90 to 95% confluence and returned to the incubator overnight. Medium was removed from test wells, replaced by 50 μl of 5 μM probe in PBS, and incubated in the dark at 37°C for 20 min. The probe was removed from the cells and replaced by 100 μl of prewarmed OptiMEM. The test reagent was added to four wells per experiment, and the fluorescence intensity was measured using a microplate reader with excitation and emission wavelengths of 488 and 534 nm, respectively, at time zero, 30 min, and 1 h.
Direct measurement of free radicals by RedoxSensor red.
RedoxSensor red is a probe that passively enters live cells. Once inside the cell, the nonfluorescent probe is oxidized to a red fluorescent product, which then accumulates in the mitochondria. Alternatively, the nonfluorescent probe may be transported to lysozymes, where it is oxidized to the fluorescent product. The intensity of the fluorescent signal is determined by the redox potential of the cytosol, with increased free radicals in the cytosol increasing the degree of oxidation of the probe.
Cells were observed by confocal microscopy at the start of the assay and 5, 10, and 15 min after the addition of test reagent.
Proteasome activity assay.
The assay was performed as previously described (36). Ten micrograms of protein from cell lysates (SMB or SMB-PS) was added to 100 μl of reaction buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 1 mM dithiothreitol, 2 mM ATP, and a 100 μM concentration of either of the two substrate peptides. The peptide Suc-LLVL-AMC (Biomol) was used to assess chymotrypsin like activity and the peptide Ac-nLPnLD-AMC (Bachem) was used to measure caspase-like activity. Fluorescence was measured every minute for 30 min at room temperature using a Fluoroscan (ThermoElectron) plate reader (λex/λem = 360/440 nm). Nonspecific hydrolysis was assessed by preincubation with 50 μM epoxomicin and subtracted from each measurement. Values for treated and control SMB cells at the end of the 30 min were compared to those for SMB-PS cells as a percentage.
RESULTS
Previous studies with the Congo red derivatives identified two compounds that were able to abolish detectable levels of PrPSc in SMB cell cultures with particular potency (59). The chemical structure of these two compounds, referred to as WSP677 and WSP740, are shown in Fig. 1. (These are compounds 2a and 6b, respectively, in the original publication.) Compound WSP677 had a 50% effective dose of 25 to 50 nM for inhibition of PrPSc production in persistently scrapie-infected SMB cells, and WSP740 had a 50% effective dose of 75 nM. Furthermore, in a cell-free polymerization assay, WSP677 was able to reduce PrP aggregation compared to the control, while WSP740 actually caused an increase in levels of PrP aggregation. For further study of these two compounds, two others with structures similar to those of WSP677 and WSP740 but with no activity in terms of clearing PrPSc from cells or inhibiting prion protein polymerization were synthesized. These control compounds were WSP971 (homologue of WSP677) and WSP714 (homologue of WSP740) and are diagrammed in Fig. 1. WSP971 and WSP714 are compounds 2e and 5a, respectively, in the original publication. Neither WSP971 nor WSP714 showed an effect on levels of PrPSc in SMB cells at 10 μM, and both caused an increase in PrP aggregation levels in the cell-free polymerization assays (59).
Cure of infected cell cultures.
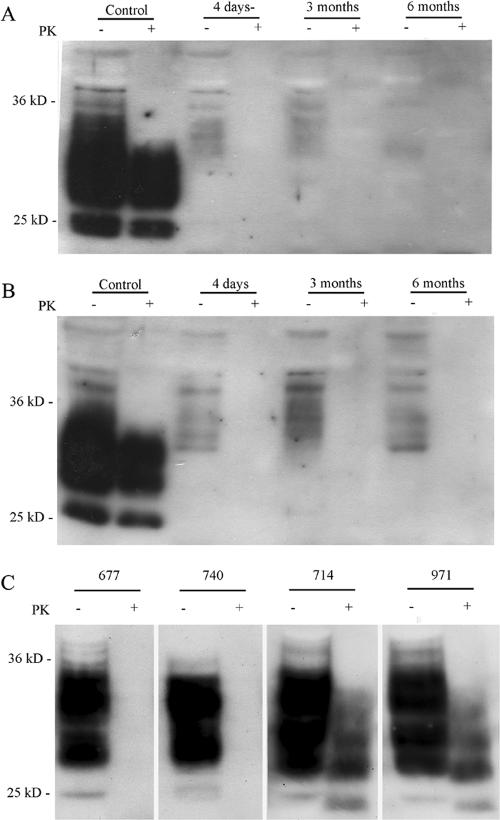
Although WSP740 and WSP677 were shown to clear PrPSc from SMB cells following a 4-day treatment at nanomolar concentrations, it was not clear if this treatment effectively cured the cells or whether the PrPSc level would return to normal. To determine if this treatment would effectively cure SMB cells, SMB cultures were treated with 1 μM of WSP677 or WSP740 for 4 days and then cultured in the absence of the drug for up to 6 months. Figure 2A and B shows the results of the treatment. Both WSP677 and WSP740 treatments clearly resulted in a long-lasting effect. Results of treatments with WSP914 and WSP971 are shown in comparison and indicate that these compounds had no effect on the level of PrPSc. In the control lanes, extracts treated with PK show bands for PrPSc only. However, for the control lanes not treated with PK, bands for both PrPSc and PrPc are present. For the treated cells, no bands are present when cell extracts are treated with PK, and only PrPc is present in the lanes for extracts not treated with PK.
FIG. 2.
Long-term cure of SMB cells. Scrapie-infected SMB cells were treated with Congo red derivatives for 4 days. After this time, the level of PK-resistant PrP was assessed by Western blotting. Parallel cultures similarly treated were maintained for extended periods of time, and the levels of PK-resistant PrP were reassessed periodically. Both WSP740 (A) and WSP677 (B) eliminated PK-resistant PrP from the cultures and effectively cured them, as there was no PK-resistant PrP present in these cultures after both 3 and 6 months of continued culture. (C) Although the Congo red derivative reduces the levels of PK-resistant PrP, there could still be low-level expression. Greatly increasing cellular levels of PrP could unmask residual levels of PrPSc. WSP740- and WSP677-cured cells were transfected with a plasmid construct (pCDNA3) to overexpress PrP in those cells. Infected cells were also transformed and treated with 10 μM of the control compounds WSP971 and WSP714 for 4 days. The cured cells showed no detectable PK-resistant protein, even when overexpressing PrP. The control cells overexpressing PrP but treated with WSP971 or WSP714 showed high levels of PK-resistant PrP.
It is possible that treatment with the compounds could reduce the levels of PrPSc to a low level, one that is below the limits of detection. Increasing the level of PrP in cells increases the amount of potential substrate for conversion to PrPSc. With higher levels of PrP, any residual PrPSc could then be detected. To test for this effect, SMB cells cured by WSP677 or WSP740 were transfected with a construct to overexpress PrP (Fig. 2C). Cells transfected with the pCDNA-PrP construct showed an 8.2 ± 1.2-fold increase in the level of total PrP expressed (n = 10). This overexpression resulted in a dramatic increase in PrP levels in the treated cells with no apparent increase in the levels of PK-resistant PrP. In contrast, cells treated with WSP714 and WSP971 showed much higher levels of PrPSc when transfected. The implication of these findings is that WSP677 and WSP740 are able to cure SMB cells of the persistent production of PrPSc.
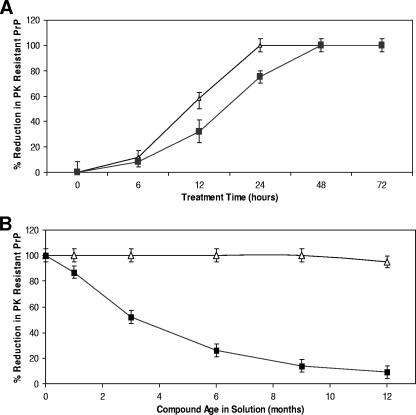
These studies were based on treatment for 4 days. Analysis of shorter treatment times demonstrated that WSP740 could cure cells with a single 24-h treatment at 1 μM, while WSP677 required 48 h (Fig. 3A). During the course of these studies, it was also shown that WSP740 was a more stable compound and retained its potency, while WSP677 lost potency. This resulted in a decreased potential to cure SMB cells at 1 μM for 4 days (Fig. 3B). The results indicate that WSP677 has a half-life in solution of approximately 3 months, while WSP740 remained stable for more than a year.
FIG. 3.
Congo red analogue effectiveness and stability. (A) Levels of effectiveness of WSP677 (squares) and WSP740 (triangles) at curing cells were compared. SMB cells were treated with 1 μM of either compound, and the level of PK-resistant PrP was assessed by Western blotting at time zero and at 6, 12, 24, 48, and 72 h after treatment. Both compounds effectively cured the cells by 48 h, while WSP740 cured the cells within 24 h. (B) During the experiments, it was noted that WSP677 was not as stable in solution as WSP740. Therefore, we compared the potentials of the two compounds to cure SMB cells when maintained as solutions over a period of a year (−20°C in DMSO). SMB cells were treated with 1 μM of WSP677 (triangles) or WSP740 (squares) for 4 days, and the level of PK-resistant PrP was measured by Western blotting and densitometric analysis. While the potential of WSP740 to cure cells remained unaffected by the time in solution, WSP677 rapidly lost its potential to cure. This result suggests that WSP677 is unstable is solution. Shown are the means and standard errors for four separate experiments.
Infectivity of cured cultures.
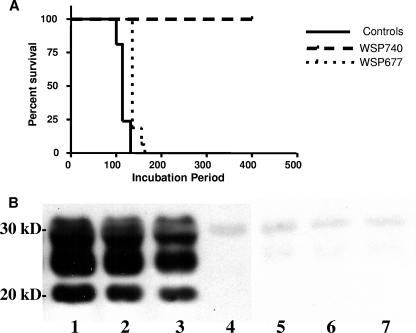
Levels of PrPSc expressed by cells do not necessarily correlate with the levels of the infective scrapie agent generated by the same cells. Verification that clearance of PrPSc correlates to loss of the infectious agent required additional experiments. In order to determine if the clearing of PrPSc from scrapie-infected SMB cell cultures equated to a loss of scrapie infectivity, extracts from SMB cells were prepared for testing by mouse bioassay for the presence of scrapie infectivity, as previously described (4). Extracts were prepared from control cells and WSP740- and WSP677-treated cells and were injected into mice. Figure 4A shows the Kaplan-Meier survival curves for the three different groups. Treatment with compound WSP740 led to a complete abrogation of infectivity, with all animals being clinically without symptoms of prion infection 400 days postinoculation. Western blot analysis of the brains and spleens of these animals showed an absence of PrPSc, indicating that these animals did not have subclinical infections (Fig. 4B). Treatment with compound WSP677 led to a significant prolongation of the incubation period (median survival of 135 days versus 114 days in the controls, P < 0.0001 using the log rank test [GraphPad Prism, version 4.0; GraphPad. Inc.]). As expected, the degree of spongiform changes in the brain and the levels of PrPSc did not differ significantly between controls and WSP677-treated mice at the time of sacrifice (data not shown).
FIG. 4.
Mouse bioassay of prion-cured cells. (A) Kaplan-Meier curve for the three different groups. By 132 days (median survival time, 114 days), all the control group animals were dead. By 163 days (median survival time, 135 days), all the compound WSP677 group were dead (P < 0.0001 versus the control by using the log rank test [GraphPad Prism, version 4; GraphPad, Inc.]). None of the animals in the compound WSP740-treated group were clinically sick at 400 days postinoculation, a result which demonstrated a complete abrogation of infectivity. (B) Western blot of brain homogenates treated with PK using anti-PrP monoclonal 6D11 antibody as a primary antibody. Lane 1, representative brain homogenate from an infected control mouse. Lanes 2 and 3, representative brain homogenates from mice i.c. inoculated with Chandler-infected and WSP677-treated cell extract. Lanes 4, 5, and 6, representative brain homogenates from mice i.c. inoculated with WSP740-treated cell extract. Lane 7, PK alone. The characteristic banding pattern of PrPSc is present in lanes 1 to 3, while no PrPSc is detected in lanes 4 to 6. The faint band at seen in lanes 4 to 7 is from the PK. The positions of molecular weight markers are shown on the left of the Western blot.
Binding of Congo red derivatives to PrP.
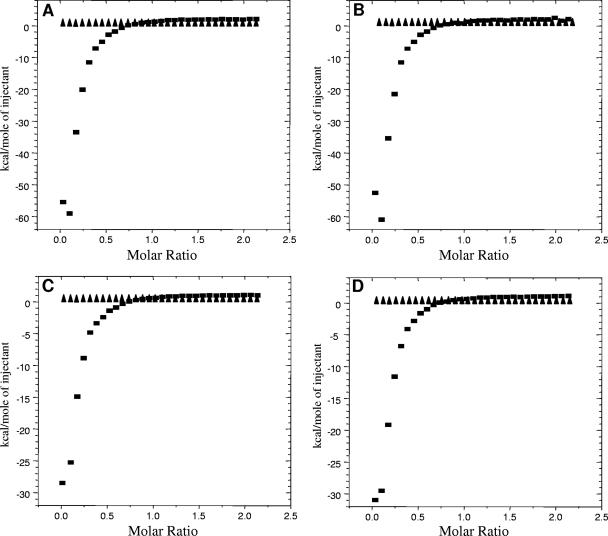
ITC has previously been used to demonstrate the interaction between Congo red and amyloidogenic proteins (34). If the differences in the potential of these new derivatives to cure SMB cells relates to the ability of the compounds to interact with PrP then there should be a difference in affinity for PrP between effective and ineffective compounds. Previously, we used a seeded polymerization assay to assess the potential of Congo red derivatives to inhibit PrP aggregation. The seeding material used was PrP refolded in the presence of manganese ions, which are known to alter the conformation of the protein (9, 59). WSP677 was able to inhibit seeded polymerization, a result which suggests that it is able to interact directly with either the substrate (soluble, monomeric PrP) or the seeding material (misfolded Mn-PrP). Conversely, WSP740 (and the controls, WSP714 and WSP971) were unable to inhibit seeded polymerization and, if anything, increased the amount of polymerization. ITC experiments were carried out to determine whether these compounds would bind to either form of PrP. ITC analysis of binding to fresh, monomeric recombinant mouse PrP showed that none of these compounds (WSP677, WSP971, WSP740, and WSP714), whether active or not, bind to this form of PrP (Fig. 5). Therefore, these compounds do not affect PrP polymerization by binding to the substrate. In contrast, all four Congo red derivatives showed a strong interaction with Mn-PrP (Fig. 5). Data fitting indicated that binding was one molecule of PrP to one molecule of compound. The affinity values were 2.6 × 109 M−1 for WSP677, 3.3 × 109 M−1 for WSP740, 6.5 × 106 M−1 for WSP714, and 2.1 × 105 M−1 for WSP971. Interestingly, the data indicate that the binding of WSP677 and WSP740 are about 103 times more potent than that of WSP714 and 104 times more potent than that of WSP971. Although the difference in affinity would suggest that WSP677 and WSP740 could be more effective at curing prion disease, it does not explain (i) why WSP677 reduces polymerization in the cell-free assay, while WSP740 increases polymerization, and (ii) why WSP971 and WSP714 are completely ineffective as antiprion agents. Therefore, the possibility of an alternative mechanism(s) of action was investigated.
FIG. 5.
ITC analysis of Congo red derivative affinity for PrP. ITC experiments were carried out to assess the affinity of the four compounds for fresh monomeric PrP (triangles) or aggregated PrP (squares). Aggregated, misfolded PrP was generated using interactions with manganese. The protein was placed into the ITC chamber with continuous stirring, and Congo red derivatives were titrated into the chamber and the specific heat was measured. All four compounds produced an isotherm that was consistent with high-affinity binding only for aggregated PrP. Plots show the enthalpy change for each titration event in terms of the molar ratio of injected compound to PrP in the chamber. Shown are WSP677 (A), WSP740 (B), WSP971 (C), and WSP714 (D).
Congo red derivatives and Redox status of cells.
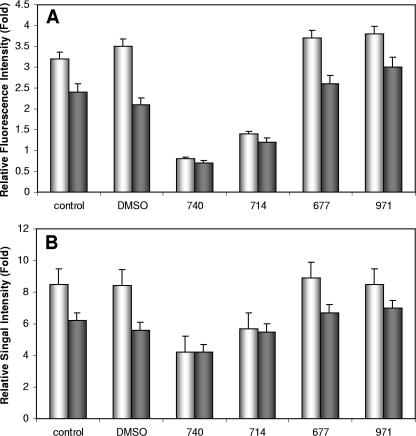
The redox status of a cell could favor the generation of PrPSc. In order to assess whether the redox status of cells is altered by Congo red derivatives, we used cellular redox assays to assess how infection alters the cellular redox status. The RedoxRed assay involved loading cells with a redox-sensitive compound that fluoresced red in accordance with the level of ROS in a cell. SMB and SMB-PS cells were loaded with the RedoxRed compound, and the intensity of the fluorescent signal was measured by quantitative confocal microscopy (Fig. 6A). The basal levels of fluorescence were higher in SMB cells, suggesting that prion infection causes an increase in ROS and, therefore, oxidative stress within cells. Cells loaded with the sensor dye were treated with the Congo red derivatives for 15 min, and the level of signal intensity was assessed (Fig. 6A). Neither WSP677 nor WSP971 had any effect on the redox status of the cells. However, WSP740 and WSP714 both reduced the level of fluorescent signal in SMB and in SMB-PS cells. The effect on the SMB cells was greater, abolishing the difference between the cells.
FIG. 6.
Antioxidant potential of Congo red derivatives. (A) SMB (white bars) and SMB-PS (gray bars) cells were loaded with the fluorescent dye RedoxRed. The relative change in fluorescence intensity for whole cells was measured with quantitative confocal microscopy. The change in fluorescence after 15 minutes was assessed. Increased fluorescence is indicative of the generation of ROS within the cells. Cells were treated either with DMSO or with one of the four Congo red derivatives. The level of fluorescence signal at time zero was measured, and the increase or decrease (n-fold) was measured and plotted. Shown are the means and standard errors for at least 100 cells from four separate experiments. (B) SMB (white bars) and SMB-PS (gray bars) cells were plated on 96-well plates. Cells were loaded with CM-H2DCFDA. The level of fluorescence in the cells was measured at time zero and after 1 h. Increased fluorescence is indicative of the generation of ROS within the cells. Cells were treated either with DMSO or with one of the four Congo red derivatives. The increase (n-fold) after 1 h above that measured at time zero was calculated and plotted. Shown are the means and standard errors for four experiments with six determinations each.
The assay described above was dependent on measurements taken directly from individual cells. A second assay, involving conversion of the radical-detecting dye CM-H2DCFDA into a fluorescent compound, was also used. This compound gives a direct measure of radical production in cells. Cells in microtiter plates were loaded with CM-H2DCFDA, and the change in the fluorescent emission at 534 nm was detected with a microtiter plate reader. This allowed studies of large cell populations. SMB and SMB-PS cells loaded with the CM-H2DCFDA were treated with the compounds for 1 h. The level of ROS in SMB cells was higher than that in SMB-PS cells. Treatment with 10 μM of WSP740 produced a significant decrease in the level of ROS detected and abolished differences between SMB and SMB-PS cells (Fig. 6B). WSP714 had a smaller effect, which was significantly different from control levels but also from the levels in cells treated with WSP740. In comparison, WSP677 and WSP971 had no significant effect on ROS levels when applied at 10 μM. These results suggest that some Congo red derivatives may have antioxidant properties. However, this finding does not provide an explanation as to why some Congo red derivatives have antiprion activities and others do not, as WSP677 was not active in this assay system.
Prion protein expression.
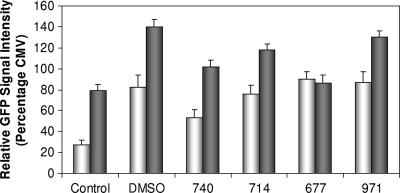
The level of expression of PrP is known to influence susceptibility to infection (22). Also, decreasing the expression of PrP has been shown to arrest prion disease progression (46). A promoter activity reporter system was used to study the activity of the murine Prnp gene in SMB cells. A large fragment of the gene that included the promoter, exons 1 and 2, and intron 1 was cloned into a reporter construct that drives the expression of destabilized GFP with a half-life of 2 h. The activity of the Prnp promoter was compared to that of the CMV promoter, a high-expression promoter that produces high levels of GFP in most cell types. The level of expression was determined by using quantitative confocal microscopy. The levels of CMV-driven GFP expression were equivalent in SMB and SMB-PS cells (data not shown). However, the level of Prnp-driven GFP expression in SMB cells was significantly lower than that in SMB-PS cells. This result implies that scrapie infection causes a significant decrease in the expression of PrP (Fig. 7). However, this system could not be used to assess the effect of the Congo red derivatives. Our Congo red derivatives had to be dissolved in DMSO, and the application of 0.2% DMSO to transfected SMB cells resulted in a fivefold increase in the level of GFP expression driven by Prnp. Some of the Congo red derivatives (WSP677 and WSP740) significantly (Student's t test, P < 0.05) reduced the level of reporter activity in SMB-PS cells compared to levels seen with DMSO treatment of SMB-PS cells. These effects were not seen for SMB cells except for those treated with compound WSP740. However, the net result was that reporter levels were not reduced to a level below those of the controls. In the presence of DMSO, Congo red derivatives at 10 μM showed no significant reduction in Prnp promoter activity compared to levels in untreated cells. Because DMSO increased the expression of PrP in all cases, the results suggest that the antiprion activity of Congo red derivatives is unlikely to be due to reduction of the level of expression of PrP.
FIG. 7.
Effects on PrP promoter activity. SMB (white bars) and SMB-PS (gray bars) cells were transfected with a construct containing the mouse Prnp promoter. The construct expressed destabilized GFP in the cells as a result of Prnp promoter activity. Therefore, GFP expression was indicative of the level factors stimulating PrP expression. DMSO and the Congo red derivatives were applied to the cultures, and the level of GFP expression was determined with confocal microscopy after 2 h. The level of GFP signal was compared to that generated by SMB and SMB-PS cells carrying a plasmid with GFP expression driven by the CMV promoter. The level of expression driven by the Prnp promoter was compared to that driven by the CMV promoter as a percentage. DMSO, the vector for all four Congo red derivatives, caused a great increase in the activity of the Prnp promoter, making it difficult to gauge any effect of the Congo red derivatives. However, WSP740 did reduce expression levels for both cell types to below the level for DMSO alone (Student's t test, P < 0.05). Shown are the means and standard errors for at least 100 cells per group.
Increased protein degradation.
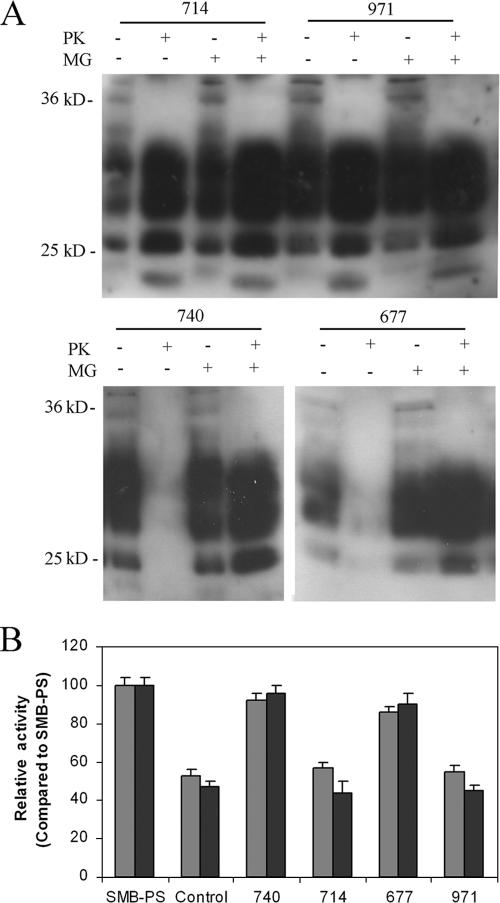
During protein synthesis, misfolded proteins detected in the endoplasmic reticulum are directed toward the proteasome for degradation. One possible reason Congo red derivatives could act as antiprion agents is that they stimulate the rate at which protein degradation occurs, either by the proteasome or a nonspecific pathway. Cells were treated with Congo red derivatives with and without the proteasome inhibitor MG132 at 50 μM. MG132 had no significant effect on the level of PK-resistant PrP generated by SMB cells or SMB cells treated with 10 μM WSP971 or WSP714 but completely blocked the antiprion effects of WSP677 and WSP740 (Fig. 8A). These results suggest that the antiprion effects of Congo red compounds could be due to increased proteasomal degradation of PrP. Although the degradation of total PrP may result in increased degradation of PrPc, the clearance of PrPSc implies that it is specifically PrPSc degraded.
FIG. 8.
Inhibition of proteasome activity. (A) SMB cells were treated for 4 days with the Congo red derivatives WSP677, WSP740, WSP714, and WSP971 with or without additional treatment with 50 μM MG132. Western blotting of protein extracts from the cells was carried out, and immunodetection identified specific PrP bands. Treatment with PK revealed that WSP677 and WSP740 decreased the expression of PK-resistant PrP, while WSP714 and WSP971 did not. The presence of MG132 blocked the effect of WSP677 and WSP740 on the expression of PK-resistant PrP but had no effect on the levels in cells treated with WSP714 or WSP971. (B) A fluorimetric assay was used to assess the activity of the proteasome in cell extracts from SMB cells. Activities analyzed were either chymotrypsin-like (gray bars) or caspase-like (black bars) activity. Activity of SMB cell extracts (either control or treated) was compared to that measured for SMB-PS cells. There was significantly lower proteasomal activity in SMB cells (control) than in SMB-PS cells. Four-day treatment with WSP677 and WSP740 blocked the inhibitory effect of scrapie infection, while treatment of SMB cells with WSP714 and WSP971 did not. Shown are means and standard errors for three experiments.
We directly measured the activity of the proteasome in extracts of SMB cells to determine whether infection and expression of PrPSc causes an inhibition of proteasome activity (Fig. 8B). Using an assay based on degradation of fluorogenic substrate peptides, we found that that the activity of the proteasome is inhibited in SMB cells relative to that in SMB-PS cells. This result suggests that the expression of PrPSc inhibited the activity of the proteasome. SMB cells treated for 4 days with 10 μM of WSP740 or WSP677 did not show this inhibition. This result suggests that the Congo red derivative blocks the inhibitory effect of PrPSc on the proteasome. In comparison, WSP714 and WSP971 did not block the inhibition. Taken together, these results suggest that Congo red derivatives that are effective at curing prion disease are able to cure cells by increasing the rate at which PrPSc is degraded. WSP740 or WSP677 did not alter the level of proteasomal activity in SMB-PS cells (data not shown).
Localization of PrP.
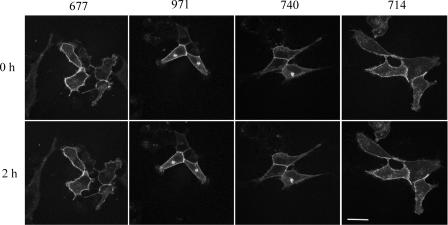
PrP is normally expressed at the cell surface. Increased degradation of PrP could be due to a change in localization of PrP. If PrP is internalized more rapidly in response to Congo red derivatives, such an internalization could explain the increased degradation of PrP. PrP has a short half-life at the cell surface (30 to 60 min) and is rapidly degraded after internalization. Therefore, the altered internalization must be assessed over a short time to distinguish this process from general increased cellular degradation or decreased synthesis of the protein. We used a GFP-tagged PrP system to look at the levels of PrP expression at the cell surface in SMB infected cells. Following treatment of the cells, the level of GFP signal was monitored for 2 h. This period of time was chosen because compounds that alter the rate of PrP internalization have been shown to be effective in this time range (26). None of the Congo red compounds tested had any effect on GFP-tagged PrP expression levels at the cell surface (Fig. 9). This suggests that Congo red derivatives do not have antiprion effects by altering the levels of PrP at the cell surface.
FIG. 9.
PrP turnover at the cell surface. SMB cells were transfected with a plasmid (pEGFP) expressing mouse PrP as a fusion with GFP. Previous studies have shown that PrP expressed with this tagged is transported to and from the surface in a way that is identical to that of untagged PrP (26). SMB cells expressing GFP-PrP were treated with 10 μM of the Congo red derivatives. The cells were photographed at time zero and at 2 h after treatment. There was no effect on the level of GFP-PrP at the cell surface.
DISCUSSION
The congophilic nature of amyloid proteins has been well documented for many years. The interaction of Congo red with PrPSc was a standard way of identifying prion rods in plaques before the use of immunohistochemistry (53). In recent years, the potential therapeutic properties of Congo red and its analogues have emerged (2, 12, 31, 52). In particular, in vitro studies have clearly demonstrated that some derivatives can abolish the continued production of PrPSc (18, 56, 59). We have verified in this study that the clearing of PrPSc from infected cells can equate to the cure of infected cells and the prevention of further scrapie agent replication. Despite these insights, the mechanism of action of these compounds remains poorly defined. Largely, previous studies have suggested that Congo red and its analogues and derivatives have their effect by interacting with the amyloid-forming proteins, such as prion fibrils. However, our studies clearly suggest that at least some Congo red derivatives can have a variety of other effects on cellular metabolism that might be more important or at least contribute to the process of curing prion disease. In particular, in this case, the derivatives that we have prepared show chemical structures that are significantly different from that of Congo red; hence, it might not be surprising that they show differences in their mode of action.
This study examined a series of possible factors that could contribute to the mechanism of curing prion disease. These factors included direct interaction with the protein (as shown by ITC), effects on the levels of expression, localization, and proteasome activity, and the redox status of the infected cells. Some of these factors clearly played little or no role in the ability of Congo red derivatives to diminish the level of PrPSc expressed by SMB cells. There were no changes in cell surface localization of PrP when treated with the compounds, and there was no evidence that the Congo red derivatives reduced the expression of PrP by reducing the activity of the promoter. Indeed, DMSO present when the derivatives were applied to the cells could result in the apparent increase in promoter activity observed. This result is in contrast to results from Western blots (Fig. 2), where there is a clear decrease in the total amount of protein present in the cells when treated with WSP677 or WSP740. This great decrease is possibly due to loss of the protease-resistant fraction. Additionally, as the proteasome inhibitor blocked the curing effects of WSP677 and WSP740, the loss of protein observed could be due to more rapid degradation of the protein because of increased proteasome activity.
The first and most obvious factor that could be playing a role in clearing of PK-resistant PrP is the ability of the compounds to bind to the protein. This activity is well known for Congo red, which binds to many amyloid-forming proteins. Our previous study (59) employed a polymerization assay in which aggregated PrP was generated by exposure to Mn. This form of PrP could catalyze polymerization of monomeric, nonaggregated PrP. WSP677 was active in this reaction (compound 2a in reference 59). The implication was that seed polymerization of PrP could be blocked by WSP677 binding either to the substrate or the seeding material. In contrast, WSP740 (compound 6b in reference 59) was not active in this assay.
We used ITC to determine the affinity of our compounds for PrP. Such an approach has been used previously on SMA, an immunoglobulin variable light chain domain (34). The authors showed that the amyloidogenic protein bound Congo red with a micromolar affinity. The affinity values we obtained were much higher. This binding was observed only for the Mn-aggregated form of PrP. Previous work has shown that treatment of PrP with Mn causes a change in the protein's conformation and the acquisition of PK resistance (9). As this form of PrP has some similarities to the PK-resistant form of PrP generated from infected cell cultures, it is quite possible that WSP677 and WSP740 interact with PrPSc directly. However, it is uncertain if this binding is responsible for the antiprion activity of these compounds, as WSP740 did not inhibit the seeded polymerization of PrP. Previous work has also suggested that Congo red interacts directly with PrPSc (11). It was suggested that PrPSc can convert PrPc to the abnormal isoform by partly unfolding, the implication being that Congo red could prevent the unfolding, thus preventing conversion.
Congo red and its analogues and derivatives could prevent polymerization by other mechanisms. Polarized light microscopy has been used to analyze the binding of Congo red to amyloid protein. The results indicated that Congo red molecules binding to fibrils align with respect to the long fiber axis (32). This kind of interaction could simply prevent monomeric nonmisfolded protein from interacting with the amyloid fiber or aggregates of the protein. As unfolding of the protein would be an energy-dependent reaction, the alternative model seems more likely.
Two of the compounds in this study, WSP714 and WSP971, were chosen because of their structural similarity to WSP677 and WSP740. However, neither compound was found to inhibit polymerization of PrP nor to cure infected cells and eliminate PrPSc formation (compounds 2e and 5a in reference 59). However, both compounds bound to Mn-PrP in the ITC experiments. The affinity was several orders of magnitude weaker than for WSP677 and WSP740 but implied that binding to PrPSc alone would not be sufficient to cure prion infection. It is possible that because of small structural differences in the compounds, the physical interaction between PrP and the compound was different, but it is also possible that the mechanism of curing prion disease is multifactorial.
An analysis of ROS generation in infected cells showed that the infected cells contain more ROS than noninfected cells. Treatment with some Congo red derivatives resulted in a clear reduction in the level of ROS, as determined by two different assay methods. The implication is that these compounds have antioxidant properties. This effect was limited to WSP740 and WSP714. It is possible to envisage oxidation of WSP740 and WSP714 to some kind of quinone-type structure. In WSP677, this type of oxidation of the compound is much less likely, as the amino group is tied up as an amide, and for WSP971, this type of oxidation is not possible. As this effect was not limited to the compounds with the capacity to cure prion disease, it cannot be concluded that it is necessary for the cure of prion disease per se. However, as WSP740 has the capacity to cure prion disease, it is possible that this oxidation does play a role in the mechanism of action of WSP740 but not WSP677. This implies that Congo red derivatives could have their effect via differing mechanisms. Other compounds with antiprion activities have been shown to have antioxidant effects. These include uric acid (44) and quinicrine (66). Such results point to the potential of antioxidants in their own right as therapeutic agents for prion diseases. There is currently no evidence that antioxidants can cure prion disease but there is evidence that they can slow disease progression (20).
There is stronger evidence that WSP677 and WSP740 alter the rate at which PrPSc is degraded. The proteasome inhibitor MG132 inhibited the curing effect of WSP677 and WSP740 but did not alter the levels of PK-resistant PrP in cells treated with WSP971 or WSP714. The implication of this result is that WSP677 and WSP740 alter the rate at which PrPSc is recognized as a misfolded protein and degraded by the intrinsic cellular machinery for clearing misfolded proteins. There is no evidence that WSP677 or WSP740 directly stimulate proteasome activity or alter cellular transport of PrPSc, but the result demonstrates that proteasome activity is a requirement for the cure of prion disease. It could be postulated that the increased activity of the proteasome in WSP677- and WSP740-treated cells is a downstream result of the curing but is not the cause of the curing. However, as inhibition of the proteasome with MG132 prevents PrPSc clearance, this is highly unlikely.
A recent study has shown conclusively that prion infection of cells inhibits the activity of the 26S proteasome in those cells (42). Our results confirm these findings, as the proteasome in SMB cells also appears to have less activity. Therefore, the ability of Congo red derivatives to block this inhibition is possibly central to their mechanism of cure of prion-infected cells, as this ability allows the proteasome to increase the rate at which PrPSc is degraded and cleared from the cell.
This finding has important implications for any potential therapeutic strategy for prion diseases. If proteasome activity is important for the cure of prion disease, then new strategies could be developed for altering proteasome activity or increasing the access of the proteasome to PrPSc. There is some evidence that even small changes to PrP cause a change in the ability of the proteasome to degrade PrP (65). The use of proteasome inhibitors in previous PrP studies resulted in the accumulation of PrP in the cytoplasm and apparent cytotoxic effects. The cytotoxic effects were suggested to be related to the increase in cytoplasmic PrP (45). Although it has been shown previously that misfolded PrP can be exported into the cytoplasm for proteasomal degradation (69), the potential cytotoxicity of this protein has been disputed (55). Nevertheless, the hypothesis that inhibition of the proteasome could trigger the production or accumulation of PrPSc has recently been proposed (29a). Our evidence clearly demonstrates that proteasome inhibitors prevent clearance of PrPSc under certain circumstances, but there is currently no evidence that increasing proteasome activity is beneficial. One possible hypothesis for the mode of action of our compounds is that their complexation to PrPSc increases the rate of degradation by the proteasome.
The potential of WSP677 and WSP740 to cure cells of the potential to generate PrPSc is based on the detectible levels of PrPSc in the cells. Cells overexpressing PrP were used to confirm this. In addition, the persistence of the cured state over many months also supported the idea that cells were cured of prion infection. A mouse bioassay was used to assess whether this equated to curing of the cells in terms of the transmissible agent, which is thought to be independent of the level of PrPSc expression by the cells. The results clearly demonstrated that cells treated with WSP740 were cured of the transmissible agent. However, the cells “cured” by WSP677 were not free of the transmissible agent, as the mice injected with the WSP677-treated cells did develop disease, but with a longer incubation period. As the results show, WSP677 is a less-stable chemical agent and loses its cell-curing potential over time. Therefore, further study could identify conditions where WSP677 would also cure the cells of both carrying the transmissible agent of prion disease and generating PrPSc. However, WSP740 is simply a better compound in terms of true cure of prion disease. This was also shown by the fact that WSP740 could cure cells within 24 h, while WSP677 could not. These studies emphasize that the fact that the absence of PrPSc does not equate to an absence of the transmissible agent of the disease. Although it has recently been suggested that cell-based bioassays could replace the mouse bioassay for studies of infectivity, this suggestion may be premature, and the mouse bioassay remains the sole effective assay for the presence of the transmissible agent of prion disease.
In summary, we have shown that two Congo red derivatives (termed WSP677 and WSP740) are able to cause a persistent clearing of PK-resistant protein from scrapie-infected cells. We also showed that WSP740 could eliminate the agent of disease transmission. WSP677 showed an incomplete effect in this regard, but this could have been due to an incomplete cure of the cells used for the experiment because of the lower stability of WSP677 in solution. The mechanism of action of the compounds potentially involves the binding of PrP aggregates and increased activity of the proteasome in breaking them down. Antioxidant activity of some of the compounds could also play a role but would be secondary to other factors. The results highlight the potential of Congo red derivatives for the treatment of prion diseases. The results here also provide mechanistic insights for the development of other novel therapeutic agents by highlighting cellular mechanisms that can be altered to accelerate the destruction of prions by cells. The use of cell-based studies such as these has provided an efficient means of thoroughly testing the activity of antiprion agents. Such tests in animals are not feasible until cell-based studies are completed. We anticipate that the present work will facilitate further studies to determine if WSP740 is also an effective antiprion agent in vivo.
Acknowledgments
This research was supported by a grant from the Medical Research Council of the United Kingdom and by NIH grant NS047433.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Bach, S., N. Talarek, T. Andrieu, J. M. Vierfond, Y. Mettey, H. Galons, D. Dormont, L. Meijer, C. Cullin, and M. Blondel. 2003. Isolation of drugs active against mammalian prions using a yeast-based screening assay. Nat. Biotechnol. 21:1075-1081. [DOI] [PubMed] [Google Scholar]
- 2.Beringue, V., K. T. Adjou, F. Lamoury, T. Maignien, J.-P. Deslys, R. Race, and D. Dormont. 2000. Opposite effects of dextran sulfate 500, the polyene antibiotic MS-8209, and Congo red on accumulation of the protease-resistant isoform of PrP in the spleens of mice inoculated intraperitoneally with the scrapie agent. J. Virol. 74:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertsch, U., K. F. Winklhofer, T. Hirschberger, J. Bieschke, P. Weber, F. U. Hartl, P. Tavan, J. Tatzelt, H. A. Kretzschmar, and A. Giese. 2005. Systematic identification of antiprion drugs by high-throughput screening based on scanning for intensely fluorescent targets. J. Virol. 79:7785-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkett, C. R., R. M. Hennion, D. A. Bembridge, M. C. Clarke, A. Chree, M. E. Bruce, and C. J. Bostock. 2001. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture EMBO J. 20:3351-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos, R. P., J. P. Koopman, J. L. G. Theuws, and P. T. Henderson. 1987. The essential role of the intestinal flora in the toxification of orally-administered benzidine-based dyes—internal exposure of rats to benzidine after intestinal azo reduction. Mutat. Res. 181:327. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. R., J. Herms, and H. A. Kretzschmar. 1994. Mouse cortical cells lacking cellular PrP survive in culture with a neurotoxic PrP fragment. Neuroreport 5:2057-2060. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. R., B. Schmidt, and H. A. Kretzschmar. 1996. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345-347. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. R., K. Qin, J. W. Herms, A. Madlung, J. Manson, R. Strome, P. E. Fraser, T. Kruck, A. von Bohlen, W. Schulz-Schaeffer, A. Giese, D. Westaway, and H. A. Kretzschmar. 1997. The cellular prion protein binds copper in vivo. Nature 390:684-687. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. R., F. Hafiz, L. L. Glasssmith, B.-S. Wong, I. M. Jones, C. Clive, and S. J. Haswell. 2000. Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J. 19:1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, D. R. 2002. Mayhem of the multiple mechanisms: modelling neurodegeneration in prion disease. J. Neurochem. 82:209-215. [DOI] [PubMed] [Google Scholar]
- 10a.Büeler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 11.Caspi, S., M. Halimi, A. Yanai, S. B. Sasson, A. Taraboulos, and R. Gabizon. 1998. The anti-prion activity of Congo red. Putative mechanism. J. Biol. Chem. 273:3484-3489. [DOI] [PubMed] [Google Scholar]
- 12.Caughey, B., D. Ernst, and R. E. Race. 1993. Congo red inhibition of scrapie agent replication. J. Virol. 67:6270-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, M. C., and D. A. Haig. 1970. Evidence for the multiplication of scrapie agent in cell culture. Nature 225:100-101. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, F. E., and S. B. Prusiner. 1998. Pathologic conformations of prion proteins. Annu. Rev. Biochem. 67:793-819. [DOI] [PubMed] [Google Scholar]
- 16.DeArmond, S. J., M. P. McKinley, R. A. Barry, M. B. Braunfeld, J. R. McColloch, and S. B. Prusiner. 1985. Identification of prion amyloid filaments in scrapie-infected brain. Cell 41:221-235. [DOI] [PubMed] [Google Scholar]
- 17.Demaimay, R., K. T. Adjou, V. Beringue, S. Demart, C. I. Lasmezas, J. P. Deslys, M. Seman, and D. Dormont. 1997. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J. Virol. 71:9685-9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demaimay, R., J. Harper, H. Gordon, D. Weaver, B. Chesebro, and B. Caughey. 1998. Structural aspects of Congo red as an inhibitor of protease-resistant prion protein formation. J. Neurochem. 71:2534-2541. [DOI] [PubMed] [Google Scholar]
- 19.Doh-Ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drisko, J. A. 2002. The use of antioxidants in transmissible spongiform encephalopathies: a case report. J. Am. Coll. Nutr. 21:22-25. [DOI] [PubMed] [Google Scholar]
- 21.Farquhar, C., A. Dickinson, and M. Bruce. 1999. Prophylactic potential of pentosan polysulphate in transmissible spongiform encephalopathies. Lancet 353:117. [DOI] [PubMed] [Google Scholar]
- 22.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, I. H., and H. Rudyk. 1999. Inhibitors of protease resistant prion formation. Int. Antivir. News 7:78-82. [Google Scholar]
- 24.Goni, F., E. Knudsen, F. Schreiber, H. Scholtzova, J. Pankiewicz, R. Carp, H. C. Meeker, D. R. Brown, J. A. Chabalgoity, E. M. Sigurdsson, and T. Wisniewski. 2005. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience 133:413-421. [DOI] [PubMed] [Google Scholar]
- 25.Gray, L. E., and J. S. Ostby. 1993. The effects of prenatal administration of azo dyes on testicular development in the mouse—a structure activity profile of dyes derived from benzidine, dimethylbenzidine, or dimethoxybenzidine. Fundam. Appl. Toxicol. 20:177-183. [DOI] [PubMed] [Google Scholar]
- 26.Haigh, C. L., K. Edwards, and D. R. Brown. 2005. Copper binding is the governing determinant of prion protein turnover. Mol. Cell Neurosci. 30:186-196. [DOI] [PubMed] [Google Scholar]
- 27.Haik, S., J. P. Brandel, D. Salomon, V. Sazdovitch, N. Delasnerie-Laupretre, J. L. Laplanche, B. A. Faucheux, C. Soubrie, E. Boher, C. Belorgey, J. J. Hauw, and A. Alperovitch. 2004. Compassionate use of quinacrine in Creutzfeldt-Jakob disease fails to show significant effects. Neurology 63:2413-2415. [DOI] [PubMed] [Google Scholar]
- 28.Hill, A. F., R. J. Butterworth, S. Joiner, G. Jackson, M. N. Rossor, D. J. Thomas, A. Frosh, N. Tolley, J. E. Bell, M. Spencer, A. King, S. Al-Sarraj, J. W. Ironside, P. L. Lantos, and J. Collinge. 1999. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353:183-189. [DOI] [PubMed] [Google Scholar]
- 29.Hill, A. F., and J. Collinge. 2003. Subclinical prion infection in humans and animals. Br. Med. Bull. 66:161-170. [DOI] [PubMed] [Google Scholar]
- 29a.Hooper, N. M. 2003. Could inhibition of the proteasome cause mad cow disease? Trends Biotechnol. 21:144-145. [DOI] [PubMed] [Google Scholar]
- 30.Hsich, G., K. Kenney, C. J. Gibbs, K. H. Lee, and M. G. Harrington. 1996. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N. Engl. J. Med. 335:924-930. [DOI] [PubMed] [Google Scholar]
- 31.Ingrosso, L., A. Ladogana, and M. Pocchiari. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin, L. W., K. A. Claborn, M. Kurimoto, M. A. Geday, I. Maezawa, F. Sohraby, M. Estrada, W. Kaminksy, and B. Kahr. 2003. Imaging linear birefringence and dichroism in cerebral amyloid pathologies. Proc. Natl. Acad. Sci. USA 100:15294-15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawatake, S., Y. Nishimura, S. Sakaguchi, T. Iwaki, and K. Doh-Ura. 2006. Surface plasmon resonance analysis for the screening of anti-prion compounds. Biol. Pharm. Bull. 29:927-932. [DOI] [PubMed] [Google Scholar]
- 34.Kim, Y. S., T. W. Randolph, M. C. Manning, F. J. Stevens, and J. F. Carpenter. 2003. Congo red populates partially unfolded states of an amyloidogenic protein to enhance aggregation and amyloid fibril formation. J. Biol. Chem. 278:10842-10850. [DOI] [PubMed] [Google Scholar]
- 35.Kirby, L., C. R. Birkett, H. Rudyk, I. H. Gilbert, and J. Hope. 2003. In vitro cell-free conversion of bacterial recombinant PrP to Prpres as a model for conversion. J. Gen. Virol. 84:1013-1020. [DOI] [PubMed] [Google Scholar]
- 36.Kisselev, A. F., and A. L. Goldberg. 2005. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 298:364-378. [DOI] [PubMed] [Google Scholar]
- 37.Klunk, W. E., M. L. Debnath, and J. W. Pettegrew. 1994. Development of small-molecule probes for the beta-amyloid protein of Alzheimers disease. Neurobiol. Aging 15:691-698. [DOI] [PubMed] [Google Scholar]
- 38.Klunk, W. E., M. L. Debnath, A. M. C. Koros, and J. W. Pettegrew. 1998. Chrysamine-G, a lipophilic analogue of Congo red, inhibits A beta-induced toxicity in PC12 cells. Life Sci. 63:1807-1814. [DOI] [PubMed] [Google Scholar]
- 39.Kocisko, D. A., G. S. Baron, R. Rubenstein, J. Chen, S. Kuizon, and B. Caughey. 2003. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J. Virol. 77:10288-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korth, C., B. C. H. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koster, T., K. Singh, M. Zimmermann, and E. Gruys. 2003. Emerging therapeutic agents for transmissible spongiform encephalopathies: a review. J. Vet. Pharmacol. Ther. 26:315-326. [DOI] [PubMed] [Google Scholar]
- 42.Kristiansen, M., P. Deriziotis, D. E. Dimcheff, G. S. Jackson, H. Ovaa, H. Naumann, A. R. Clarke, F. W. van Leeuwen, V. Menendez-Benito, N. P. Dantuma, J. L. Portis, J. Collinge, and S. J. Tabrizi. 2007. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol. Cell 26:175-188. [DOI] [PubMed] [Google Scholar]
- 43.Lehto, M. T., H. E. Peery, and N. R. Cashman. 2006. Current and future molecular diagnostics for prion diseases. Expert Rev. Mol. Diagn. 6:597-611. [DOI] [PubMed] [Google Scholar]
- 44.Lekishvili, T., J. Sassoon, A. R. Thompsett, A. Green, J. W. Ironside, and D. R. Brown. 2004. BSE and vCJD cause disturbance to uric acid levels. Exp. Neurol. 190:233-244. [DOI] [PubMed] [Google Scholar]
- 45.Ma, J., and S. Lindquist. 2002. Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science 298:1785-1788. [DOI] [PubMed] [Google Scholar]
- 46.Mallucci, G., A. Dickinson, J. Linehan, P. C. Klohn, S. Brandner, and J. Collinge. 2003. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302:871-874. [DOI] [PubMed] [Google Scholar]
- 47.May, B. C., A. T. Fafarman, S. B. Hong, M. Rogers, L. W. Deady, S. B. Prusiner, and F. E. Cohen. 2003. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc. Natl. Acad. Sci. USA 100:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milhavet, O., A. Mange, D. Casanova, and S. Lehmann. 2000. Effect of Congo red on wild-type and mutated prion proteins in cultured cells. J. Neurochem. 74:222-230. [DOI] [PubMed] [Google Scholar]
- 49.Müller, W. E. G., J. L. Laplanche, H. Ushijima, and H. C. Schroder. 2000. Novel approaches in diagnosis and therapy of Creutzfeldt-Jakob disease. Mech. Ageing Dev. 116:193-218. [DOI] [PubMed] [Google Scholar]
- 50.Pankiewicz, J., F. Prelli, M. S. Sy, R. J. Kascsak, R. B. Kascsak, D. S. Spinner, R. I. Carp, H. C. Meeker, M. Sadowski, and T. Wisniewski. 2006. Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur. J. Neurosci. 23:2635-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poli, G., W. Ponti, G. Carcassola, F. Ceciliani, L. Colombo, P. Dall'Ara, M. Gervasoni, M. L. Giannino, P. A. Martino, C. Pollera, S. Villa, and M. Salmona. 2003. In vitro evaluation of the anti-prionic activity of newly synthesized Congo red derivatives. Arzneimittelforschung 53:875-888. [DOI] [PubMed] [Google Scholar]
- 52.Poli, G., P. A. Martino, S. Villa, G. Carcassola, M. L. Giannino, P. Dall'Ara, C. Pollera, S. Iussich, V. M. Tranquillo, S. Bareggi, P. Mantegazza, and W. Ponti. 2004. Evaluation of anti-prion activity of Congo red and its derivatives in experimentally infected hamsters. Arzneimittelforschung 54:406-415. [DOI] [PubMed] [Google Scholar]
- 53.Prusiner, S. B., M. P. McKinley, K. A. Bowman, D. C. Bolton, P. E. Bendheim, D. F. Groth, and G. G. Glenner. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358. [DOI] [PubMed] [Google Scholar]
- 54.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roucou, X., Q. Guo, Y. Zhang, C. G. Goodyer, and A. C. LeBlanc. 2003. Cytosolic prion protein is not toxic and protects against Bax-mediated cell death in human primary neurons. J. Biol. Chem. 278:40877-40881. [DOI] [PubMed] [Google Scholar]
- 56.Rudyk, H., S. Vasiljevic, R. M. Hennion, C. R. Birkett, J. Hope, and I. H. Gilbert. 2000. Screening Congo red and its analogues for their ability to prevent the formation of PrP-res in scrapie-infected cells. J. Gen. Virol. 81:1155-1164. [DOI] [PubMed] [Google Scholar]
- 57.Rudyk, H., M. H. Knaggs, S. Vasiljevic, J. Hope, C. Birkett, and I. H. Gilbert. 2003. Synthesis and evaluation of analogues of Congo red as potential compounds against transmissible spongiform encephalopathies. Eur. J. Med. Chem. 38:567-579. [DOI] [PubMed] [Google Scholar]
- 58.Sales, N., K. Rodolfo, R. Hassig, B. Faucheux, L. Di Giamberardino, and K. L. Moya. 1998. Cellular prion protein localization in rodent and primate brain. Eur. J. Neurosci. 10:2464-2471. [DOI] [PubMed] [Google Scholar]
- 59.Sellarajah, S., T. Lekishvili, C. Bowring, A. R. Thompsett, H. Rudyk, C. R. Birkett, D. R., Brown, and I. H. Gilbert. 2004. Synthesis of analogues of Congo red and evaluation of their anti-prion activity. J. Med. Chem. 47:5515-5534. [DOI] [PubMed] [Google Scholar]
- 60.Sigurdsson, E. M., D. R. Brown, M. Daniels, R. J. Kascsak, R. Kascsak, R. Carp, H. C. Meeker, B. Frangione, and T. Wisniewski. 2002. Immunization delays the onset of prion disease in mice. Am. J. Pathol. 161:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigurdsson, E. M., and T. Wisniewski. 2005. Promising developments in prion immunotherapy. Expert Rev. Vaccines 4:607-610. [DOI] [PubMed] [Google Scholar]
- 62.Supattapone, S., K. Nishina, and J. R. Rees. 2002. Pharmacological approaches to prion research. Biochem. Pharmacol. 63:1383-1388. [DOI] [PubMed] [Google Scholar]
- 63.Tagliavini, F., R. A. McArthur, B. Canciani, G. Giaccone, M. Porro, M. Bugiani, P. M. J. Lievens, O. Bugiani, E. Peri, P. Dall'Ara, M. Rocchi, G. Poli, G. Forloni, T. Bandiera, M. Varasi, A. Suarato, P. Cassutti, M. A. Cervini, J. Lansen, M. Salmona, and C. Post. 1997. Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science 276:1119-1122. [DOI] [PubMed] [Google Scholar]
- 64.Talaska, G. 2003. Aromatic amines and human urinary bladder cancer: exposure sources and epidemiology. J. Environ. Sci. Health C 21:29-43. [DOI] [PubMed] [Google Scholar]
- 65.Tenzer, S., L. Stoltze, B. Schonfisch, J. Dengjel, M. Muller, S. Stevanovic, H. G. Rammensee, and H. Schild. 2004. Quantitative analysis of prion-protein degradation by constitutive and immuno-20S proteasomes indicates differences correlated with disease susceptibility. J. Immunol. 172:1083-1091. [DOI] [PubMed] [Google Scholar]
- 66.Turnbull, S., B. J. Tabner, D. R. Brown, and D. Allsop. 2003. Quinacrine acts as an antioxidant and reduces the toxicity of the prion peptide PrP106-126. Neuroreport 14:1743-1745. [DOI] [PubMed] [Google Scholar]
- 67.van Keulen, L. J., B. E. Schreuder, R. H. Meloen, G. Mooij-Harkes, M. E. Vroman, and J. P. Langeveld. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J. Clin. Microbiol. 34:1228-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whittle, I. R., R. S. Knight, and R. G. Will. 2006. Unsuccessful intraventricular pentosan polysulphate treatment of variant Creutzfeldt-Jakob disease. Acta Neurochir. 148:WSP677-WSP679. [DOI] [PubMed] [Google Scholar]
- 69.Yedidia, Y., L. Horonchik, S. Tzaban, A. Yanai, and A. Taraboulos. 2001. Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J. 20:5383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]