Abstract
The molecular mechanisms underlying the directional neuron-to-epithelial cell transport of herpesvirus particles during infection are poorly understood. To study the role of the viral glycoprotein D (gD) in the directional spread of herpes simplex virus (HSV) and pseudorabies virus (PRV) infection, a culture system consisting of sympathetic neurons or epithelial cells in different compartments was employed. We discovered that PRV infection could spread efficiently from neurons to cells and back to neurons in the absence of gD, the viral ligand required for entry of extracellular particles. Unexpectedly, PRV infection can also spread transneuronally via axo-axonal contacts. We show that this form of interaxonal spread between neurons is gD independent and is not mediated by extracellular virions. We also found that unlike PRV gD, HSV-1 gD is required for neuron-to-cell spread of infection. Neither of the host cell gD receptors (HVEM and nectin-1) is required in target primary fibroblasts for neuron-to-cell spread of HSV-1 or PRV infection.
Alphaherpesviruses are pantropic, capable of infecting a variety of tissues and cell types. They are also able to establish latent infections in the peripheral nervous systems (PNSs) of their natural hosts. When latent PNS infections are reactivated, the infection spreads back to the peripheral tissues innervated by the PNS neurons. Occasionally, the infection spreads into the central nervous system, with serious consequences (21, 51). A fascinating, yet poorly understood aspect of alphaherpesvirus infection is the control of directional spread in and out of the PNS. In particular, the ability of the virus to spread directionally from infected peripheral neurons is crucial for the continued survival of the virus and the host since reinfection of the surface epithelium often leads to minor skin lesions, such as cold sores, while directional spread into the brain often results in fatal encephalitis.
Another well-known, but poorly understood phenomenon during neuroinvasion is the capacity of most tested alphaherpesviruses to spread between synaptically connected neurons (circuit-specific transneuronal spread). Indeed, in vivo infections of various animal models have consistently recapitulated spread within specified neuronal circuits (3, 7, 9, 19). Recent work demonstrates that particularly for attenuated strains of pseudorabies virus (PRV), viral infection can predict neural circuitry that had not been established previously (14, 22, 39-41, 57, 58). However, the molecular mechanisms that guide this precise spread of alphaherpesvirus infection between neurons cannot be studied readily in animal models and, hence, remain poorly understood. To investigate the molecular basis of directional spread, in vitro systems that can successfully recapitulate directional spread of in vivo infections must be developed.
Recently, we and others have begun to study the directional spread of alphaherpesvirus infection by culturing neurons in modified Campenot chambers (6). We first reported the use of a trichamber system (10) as a simple and tractable method to study neuron-to-cell transmission of infection in vitro. By physically isolating the cell bodies and distal axons of sympathetic neurons cultured from rat superior cervical ganglia in different compartments, we were able to study infection initiated from cell bodies and examine the spread of infection from neurons to epithelial cells during anterograde spread of infection. Alternatively, the chambers could be used to study the entry of virus at growth cones and the subsequent axon-mediated infection of the cell bodies.
In this report, we alter the location of neurons and target cells in the trichamber system in order to allow directional spread of infection from neurons to epithelial cells and then to naive, uninfected neurons in an opposing chamber. This plating method allowed us to study alphaherpesvirus spread from infected epithelial cells to neurons without having to introduce exogenous extracellular viral particles in the medium. We utilized herpes simplex virus type 1 (HSV-1) and PRV strains that do not express glycoprotein D (gD), the viral ligand required for receptor binding during initial entry of extracellular particles, to study contact-mediated, cell-to-neuron spread of infection (4, 25, 31, 32, 44, 47, 53). In the process of studying this direct cell-to-neuron spread, we discovered that PRV is capable of gD-independent, interaxonal spread between neurons. To our knowledge, this type of spread has not been reported during alphaherpesvirus infections. In addition, we used the trichamber system to examine the role of gD and two gD receptors, HVEM and nectin-1, in PRV and HSV-1 infection.
MATERIALS AND METHODS
Cells and virus strains.
Porcine kidney epithelial cells (PK15) and monkey kidney epithelial cells (Vero C1008) were purchased from the American Type Culture collection (CCL-33 and CRL-1586, respectively). Mouse embryo fibroblasts were cultured from gestation day 13 embryos of Tnfrsf14−/− (HVEM-deficient) mice (62) on the C57BL/6 background (F20 cells), C57BL/6 mice (F19 cells), Pvrl1−/− (nectin-1-deficient) mice (30) of mixed genotype (F13 cells), and wild-type littermate controls for the Pvrl1−/− mice (F12 cells). All nonneuronal cells were cultured in Dulbecco's modified Eagle medium supplemented in 10% fetal calf serum and 1% penicillin-streptomycin. PRV stocks used in this report include PRV Becker (Be), a virulent isolate (50), and PRV Bartha (Ba), an attenuated vaccine strain (37). Both PRV 151 (Be-GFP) and PRV 152 (Ba-GFP), which encode green fluorescent protein (GFP) and are expressed under the cytomegalovirus promoter in the gG locus, have been described previously (16). PRV GS442 (a gD-null mutant in which the GFP gene replaces the gD open reading frame) was kindly provided by G. Smith (Northwestern University). This mutant also has been described previously (10). The PRV strains that express the red fluorescence proteins (RFPs), PRV 614 (Ba-mRFP) and PRV 616 (Be-mRFP), are isogenic strains of PRV 152 and PRV 151, respectively, and were obtained from B. Banfield (University of Colorado) (5). All PRV stocks were propagated in PK15 cells except for complemented PRV GS442, which was expanded in a gD-expressing cell line (47).
Viral stocks of the PRV gD-null mutant, PRV GS442, that were not complemented with gD were prepared as follows: confluent dishes of PK15 cells were transfected with the bacterial artificial chromosome infectious clone pGS442. After several days, many infectious centers were visualized on the lawn of cells. These cells were then trypsinized and replated on a fresh monolayer of PK15 cells to expand the infection. The trypsinization and replating process was repeated three times until a complete cytopathic effect was observed for the entire monolayer of PK15 cells. Finally, both the PRV GS442-infected cells and the medium were harvested and used as viral stocks. The titer for the noncomplemented gD-null virus was determined on PK15 cells after treatment with polyethylene glycol (fusion assay) (55).
For HSV-1 strains, we used the wild-type (F) strain as well as the gD-null mutant that expresses enhanced GFP (EGFP) (vRR1097; R. Roller, University of Iowa) (52). The wild-type HSV-1 (F) strain was propagated in Vero C1008 cells, while the vRR1097 stocks were expanded in HSV gD complementing cells (VD-60; D. Johnson, Oregon Health and Science University).
Antibodies and dyes.
We used antibodies that detected HSV VP16 and HSV gC (H. Friedman, University of Pennsylvania). Secondary Alexa fluorophores and the Hoechst nuclear dye were purchased from Molecular Probes.
Neuronal cultures.
Detailed protocols for dissecting and culturing neurons have been published previously (11). Briefly, sympathetic neurons from the superior cervical ganglia (SCG) were dissected from embryonic day 15.5 to embryonic day 16.5 pregnant Sprague-Dawley rats (Hilltop Labs, Inc., Scottdale, PA) and incubated in 250 μg/ml of trypsin (Worthington Biochemicals) for 10 min. Trypsin inhibitor (1 mg/ml; Sigma Aldrich) was added to neutralize the trypsin for 3 min and then removed and replaced with neuron culture medium (described below). Prior to plating, the ganglia were triturated using a fire-polished Pasteur pipette and then plated in the S, N, or both compartments of the Teflon ring, depending on the experiment. The Teflon ring was placed within a 35-mm plastic tissue culture dish coated with 500 μg/ml of poly-dl-ornithine (Sigma Aldrich) diluted in borate buffer and 10 μg/ml of natural mouse laminin (Invitrogen). The neuron culture medium is serum free and consists of Dulbecco's modified Eagle medium (Invitrogen) and Ham's F12 (Invitrogen) in a 1:1 ratio. The serum-free medium was further supplemented with 10 mg/ml of bovine serum albumin (Sigma Aldrich), 4.6 mg/ml glucose (J. T. Baker), 100 μg/ml of holotransferrin (Sigma Aldrich), 16 μg/ml of putrescine (Sigma Aldrich), 10 μg/ml of insulin (Sigma Aldrich), 2 mM of l-glutamine (Invitrogen), 50 μg/ml or U of penicillin-streptomycin (Invitrogen), 30 nM of selenium (Sigma Aldrich), 20 nM of progesterone (Sigma Aldrich), and 100 ng/ml of nerve growth factor 2.5S (Invitrogen). Two days after plating, the neuronal cultures were treated with 1 μM of antimitotic drug cytosine β-d-arabinofuranoside (AraC; Sigma Aldrich) to eliminate any nonneuronal cells. The neuron culture medium was replaced every 3 to 4 days, and cultures were maintained in a humidified, CO2-regulated, 37°C incubator. All experimental protocols related to animal use were approved by the Institutional Animal Care and Use Committee of the Princeton University Research Board under protocol number 1452-AR2 and are in accordance with the regulations of the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (public law 99-198).
Trichamber culture system.
Protocols for assembling the trichamber system have been described previously (10). Briefly, all Teflon rings were purchased from Tyler Research (Alberta, Canada) and protocols were modified from previously published reports for Campenot chambers (6, 10). Tools and reagents, including the Teflon rings and the silicone grease-loaded syringe (Dow Corning), were sterilized by autoclaving prior to assembly. To facilitate both the penetration of axons across the Teflon barriers and the processing for immunofluorescence, the chambers were assembled on the surface of the flexible thermoplastic fluoropolymer film known as Aclar (EM Sciences). Next, the Aclar was etched with a pin rake, creating a series of 16 evenly spaced grooves. The Aclar was then placed inside 35-mm tissue culture dishes, first coated with 500 μg/ml of poly-dl-ornithine (Sigma Aldrich), followed by 10 μg/ml of natural mouse laminin (Invitrogen), and then washed and dried. We used a silicone grease-loaded syringe attached to an 18-gauge truncated hypodermic needle to apply a thin, continuous strip of silicone grease over the entire bottom surface of the Teflon ring. Next, a 50-μl drop of neuron medium containing 1% methocel (serum free) was placed in the center of each tissue culture dish covering the etched grooves. This step prevents the seal from being entirely devoid of moisture, which is needed for axon penetration. Finally, the silicone grease-coated ring was gently seated on the tissue culture dish or the surface of the Aclar such that the etched grooves spanned all three compartments, forming a watertight seal between compartments. Neuron medium was then placed in all three compartments immediately after the chamber was assembled. Once the SCG neurons were dissected and dissociated, approximately one half of a single ganglion was plated into the S, N, or both compartments, depending on experimental parameters (Fig. 1). Neuron cultures were then maintained according to the protocols for culturing neurons reported above.
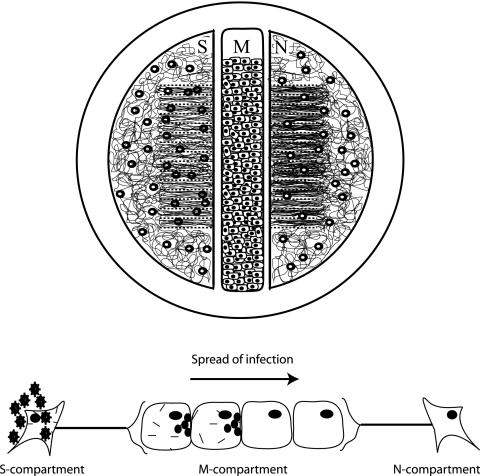
FIG. 1.
Setup of the trichamber system for the assay of neuron-cell-neuron spread of infection. SCG neurons were dissected and plated in both the S and the N compartments. After the appropriate time (4 to 7 days), PK15 cells were plated in the M compartment. Twenty-four hours after plating, methocel was added to the M compartment and infectious virions were added to the S compartment to initiate infection of the neurons. The infection then spreads from the neurons in the S compartment to the PK15 cells in the M compartment. Since the neurons from the N compartment also extend neurites into the M compartment, the infected PK15 cells will transmit the infection to the neurons in the N compartment.
Assaying neuron-to-cell spread of infection.
Neurons were cultured for approximately 2 weeks in the trichamber system with frequent medium changes. After 2 weeks, axon penetration into the M and N compartments was assessed visually and only cultures with comparable axon densities were used for experiments. After axons penetrated the N compartment, nonneuronal epithelial cells or fibroblasts permissive for either PRV or HSV infections were plated in the N compartment. For PK15 cells, the neuron medium in the N compartment was supplemented with 1% fetal bovine serum and allowed to attach and expand for 24 h prior to any experiment. However, for mouse embryonic fibroblasts, the neuron medium in the N compartment was supplemented with 10% fetal bovine serum and the cells were allowed to attach and expand for 48 h after plating to increase the viability of the cells.
Once the target cells in the N compartment were plated, neuron medium containing 1% methocel was placed in the M compartment. After 30 min, the neuronal cell bodies in the S compartment were infected with virus diluted in neuron medium (approximately 105 PFU) sufficient to essentially infect all cells. After 1 h, the viral inoculum was removed and replaced with neuron medium. The chambers were incubated in a humidified 37°C incubator until the time when the production of infectious virus in the S and N compartments was determined. Unless otherwise stated, both intracellular and extracellular virions in the S and N compartments were carefully harvested by scraping the bottom of the dish with the pointed end of a gel-loading tip. The cells and medium were then pooled and freeze-thawed, and titers were determined for either PK15 cells or Vero C1008 cells, depending on whether it was a PRV or HSV infection.
Studying axon-mediated infection of neuron cell bodies.
Neurons were grown and cultured as described above except that epithelial cells were not plated in the N compartment. Neuron medium including 1% methocel was added to the M compartment and allowed to incubate for 30 min prior to infection. A viral inoculum with a high multiplicity of infection (MOI) was added to the N compartment and incubated for 1 h to allow virus entry. After 1 h of incubation, the viral inoculum was removed and replaced with neuron medium. At the appropriate time after infection, both intra- and extracellular virions were harvested from the S compartment and titers were determined for PK15 cells.
Assaying neuron-cell-neuron spread of PRV infection.
The trichamber system was assembled as described above. However, SCG neurons were plated in both the S and the N compartments. After 4 to 7 days postplating, epithelial cells were usually plated in the M compartment. In some experiments where interaxonal spread of infection was studied, epithelial cells were omitted from the M compartment. After 24 h postplating, neuron medium made with 1% methocel was added to the M compartment and allowed to incubate for 30 min prior to infection. A viral inoculum with a high MOI was then added to S, N, or both compartments, depending on experimental parameters. The viral inoculum was incubated for 1 h before being replaced with neuron medium. At the appropriate time after infection, the total contents of the compartments were harvested and titers were determined for PK15 cells.
Indirect immunofluorescence and epifluorescence microscopy.
Immunofluorescence protocols have been described previously (11). After the infection of neurons in the S compartment, all three compartments were washed once with phosphate-buffered saline (PBS) and fixed with 3.2% paraformaldehyde for 10 min at room temperature. The fixative was removed, and the cells were washed two to three times with PBS, depending on the integrity of the neuron cultures. After the wash, the Teflon ring was gently separated from the Aclar and the remaining silicone grease was carefully removed so as not to dislodge the neurons. The Aclar was trimmed to facilitate processing of the samples for immunofluorescence imaging.
The Aclar sample was then incubated in PBS containing 3% bovine serum albumin and 0.5% saponin for 10 min before the addition of primary antibodies for 1 h. After the incubation, the primary antibodies were removed and the sample was washed three times with PBS containing 3% bovine serum albumin and 0.5% saponin. The process was repeated with secondary antibodies before the sample on Aclar was mounted on a glass slide by using Aqua poly/mount (Polysciences). A coverslip was then placed on top of the sample, and the Aqua poly/mount was allowed to dry for 24 h prior to imaging.
For direct live fluorescence imaging of green and red fluorescence proteins, the entire trichamber system in the tissue culture dish was placed on the stage of an inverted epifluorescence microscope (Nikon Eclipse TE300) and imaged directly using the appropriate excitation and emission filters. For the live imaging experiments, neurons in the chambers were often imaged prior to being harvested for titers to be determined. Since the excitation beam path has to penetrate the plastic tissue culture dishes before reaching the sample, the resolution and intensity of the images collected are often lower than those of confocal microscopy. While the fine structures of axons are difficult to discern, the state of infection of individual cells can be determined easily.
RESULTS
Studying neuron-cell-neuron spread of PRV infection in the trichamber system.
We prepared a compartmented system of cultured neurons and epithelial cells that mimicked the basic parameters of spread of alphaherpesvirus infection in and out of the peripheral nervous system. The major components of the trichamber system have been described previously (10). As shown in Fig. 1, the basic setup to study neuron-cell-neuron spread of infection is similar to that used to study neuron-to-cell spread of infection. In the neuron-to-cell spread assay, dissociated neurons are plated in only the S compartment and are allowed to mature and extend axons into the M compartment and then into the N compartment. However, in the neuron-cell-neuron assay, dissociated neurons are plated in both the S and the N compartments so that their axons enter the M compartment from either side. Once the neurons mature for 4 to 7 days and extend their neurites into the M compartment, epithelial cells (PK15, a transformed swine kidney cell line) are plated in the M compartment and allowed to attach to the surface of the dish for at least 24 h. The neuron medium in the M compartment is then replaced with 1% methocel prior to infection. This method and timing of plating allow the neuron cell bodies plated in the side compartments to extend their axons and contact the epithelial cells plated in the M compartment. Infection is initiated from neuron cell bodies in the S compartment and spreads via axons to the epithelial cells in the M compartment and then enters axons from neuronal cell bodies in the N compartment. The infection of neuron cell bodies in the N compartment completes the neuron-cell-neuron circuit.
PRV gD is not required for neuron-cell-neuron spread of infection.
Using the initial setup described in the legend for Fig. 1, we wanted to determine whether the spread of PRV infection occurs from neurons to target cells and back to neurons. We also wanted to examine whether the viral ligand gD is required for this type of spread since PRV gD is not required for cell-to-cell spread of infection. We infected neurons in the S compartment with either PRV 151 (wild-type PRV expressing GFP) or PRV GS442 (gD-null PRV expressing GFP and grown on a gD-complementing cell line) at a high MOI. Note that PRV 151 has the GFP gene inserted in the gG locus and is transcribed from the human cytomegalovirus promoter. PRV GS442 has the GFP gene replacing the gD gene and is transcribed from the gD promoter. As a result, GFP expression in PRV 151-infected cells is earlier and stronger than GFP expression from PRV GS442-infected cells.
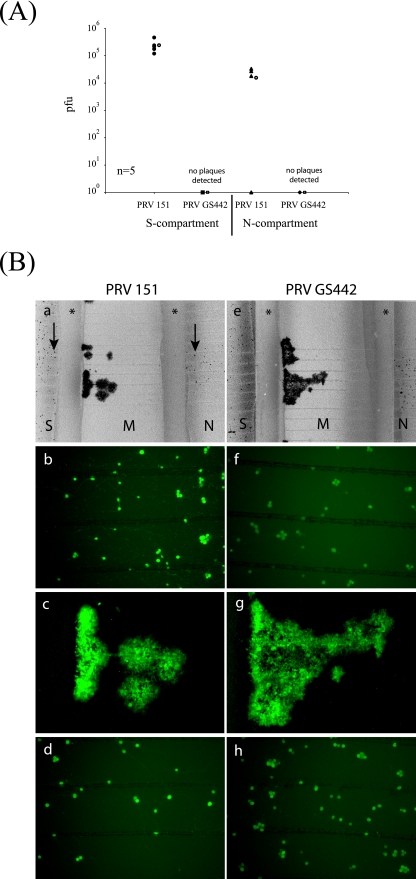
Five duplicate chambers were used for each set of infections. After 48 h, the total contents of the S and N compartments were harvested and titers were determined for PK15 cells. As shown in Fig. 2A, we detected approximately 105 PFU in the S and N compartments of neurons infected with PRV 151. We could also detect GFP expression by 24 h postinfection. PRV 151-infected neuron cell bodies in both S and N compartments were infected and expressing GFP (Fig. 2B, panel a). By comparing GFP-positive cells with the total neuronal cells, we noted that all the neuronal cell bodies in the S compartment (Fig. 2B, panel b), but not in the N compartment, were infected (Fig. 2B, panel d).
FIG. 2.
PRV can spread from neuron to cell to neuron, and gD is not required for this spread of infection. (A) SCG neurons were cultured in both S and N compartments. After 5 days, PK15 cells were plated in the M compartment and 24 h later, the neurons in the S compartment were infected with PRV 151 or PRV GS442 grown in gD-complementing cell lines at a high MOI. At 48 h postinfection, the total contents of S and N compartments were harvested and titers were determined for PK15 cells. Five chambers were used in each type of infection. The open circle beside each data set represents the average value for that particular set of data. The standard deviations are ±1.2 × 106 for the S compartment and ±1.5 × 105 for the N compartment. (B) Chambers were prepared as described above, and neurons were infected with PRV 151 (a to d) or PRV GS442 (e to h) for 48 h before being imaged live with an epifluorescence microscope. Both PRV 151 and GS442 express EGFP in infected cells. Wide-field images of each infection were obtained using a 2× objective (a and e). The corresponding higher magnifications from the S (b and f), M (c and g), and N compartments (d and h) were also obtained for each wide-field image. Asterisks indicate the central Teflon barrier, while arrows indicate infected neuron cell bodies (a and e).
Unlike PRV 151 infections, no infectious particles were detected in any of the compartments infected with PRV GS442 (Fig. 2A). As predicted, the complemented gD-null virus can infect once but the resulting gD-null progeny from the infection are noninfectious. We also observed GFP expression in neuronal cell bodies in both the S and the N compartments. This result is similar to that observed for a PRV 151 infection, but the expression and GFP fluorescence were weaker in a GS442 infection (Fig. 2B, panel h). In addition, epithelial cells in the M compartment became infected (Fig. 2B, panel g). Apparently, gD is not required for the transmission of infection from epithelial cells to neurons (Fig. 2B, panel e).
PRV infection spreads from neuron to neuron via adjacent axons in a gD-independent manner and in the absence of epithelial cells.
The previous experiment established that gD is not required for neuron-cell-neuron spread of infection. Next, we sought to detect in more direct fashion the apparent spread of infection from axon to an uninfected axon. Based on our initial experiments described above, we hypothesized that in the absence of epithelial cells in the M compartment, no spread should occur. Dissociated neurons were cultured in both the S and the N compartments. After 6 days, we visually confirmed that axons from both side compartments had successfully penetrated the silicone grease barrier and reached the midsection of the M compartment. We then added methocel in the M compartment prior to infecting neurons in the S compartment with either PRV 151 or PRV GS442. At 24 h postinfection, the total contents of the S and N compartments were harvested and titers were determined for PK15 cells.
Remarkably, we recovered approximately 104 PFU of PRV 151 from the neurons in the N compartment (Fig. 3A). By GFP expression, neurons in both the S and the N compartments were infected with either PRV 151 (Fig. 3B, panels a through d) or PRV GS442 (Fig. 3B, panels e through h). The number of neurons infected with PRV GS442 in the N compartment was lower than the number found with PRV 151 infection. This experiment supports the notion that infection can spread directly between adjacent axons that are presumably in close proximity. This form of interaxonal spread is gD independent.
FIG. 3.
PRV can spread interaxonally in a gD-independent manner from neuron to neuron in the absence of target cells in the M compartment. (A) SCG neurons were plated in both S and N compartments. At 6 days after plating, neurons in the S compartment were infected with PRV 151 and PRV GS442 at a high MOI. After 24 h, the total contents of the S and N compartments were harvested and titers were determined for PK15 cells. Five chambers were used for each set of infections. The open circle beside each data set represents the average value for that particular set of data. The standard deviations are ±1.5 × 105 for the S compartment and ±3.9 × 103 for the N compartment. (B) Chambers were set up and infected with PRV as described above. At 24 h postinfection, the EGFP expressed in cells infected with PRV 151 (a to d) or PRV GS442 (e to h) was imaged live using an epifluorescence microscope (b, d, f, and h).
Neurons in the side compartments do not extend axons beyond the M compartment.
The unexpected spread of infection between neurons via adjacent axons in the absence of epithelial cells in the M compartment (Fig. 3) could be explained by the penetration of axons through the M compartment into the opposing compartment. For example, neurons cultured in the S compartment might extend axons into the N compartment and vice versa. While this occurrence is unlikely given the short duration that neurons are grown in culture prior to infection (5 to 8 days), it was important to eliminate the possibility that the spread of infection is due to the infection of cell bodies by rare axons that completely penetrate the M compartment.
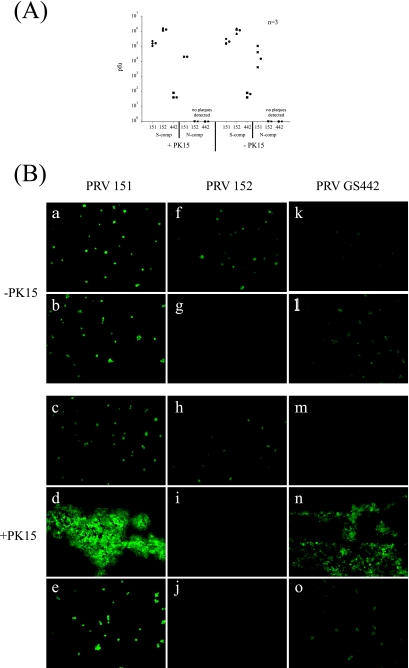
We took advantage of a directional spread mutant, PRV Bartha. PRV Bartha can spread only from postsynaptic to presynaptic neurons; it cannot spread from presynaptic to postsynaptic neurons. This phenotype results because virion structural proteins cannot be targeted to axons in PRV Bartha-infected cells; hence, the spread of infection via axons is impossible. We infected neurons in the S compartment with PRV 152, a GFP reporter derivative of PRV Bartha. We reasoned that if neurons cultured in the N compartment extended axons to the S compartment, then PRV 152 will infect these particular neurons in the N compartment only if they project axons into the S compartment.
We assembled the chambers and cultured neurons in both the S and the N compartments for 6 days as previously described. One day prior to infection, we plated epithelial cells in the M compartment of half the chambers used in the experiment. We then infected the neurons in the S compartment with PRV 151, PRV 152, and GS442 at a high MOI. At 24 h postinfection, epifluorescence images of the compartments were obtained and the contents of both the S and the N compartments were harvested and titers were determined for PK15 cells. Our results indicate that regardless of whether epithelial cells were plated in the M compartment, PRV 152 was never detected in the N compartment either by the titer assay (Fig. 4A) or by the visualization of GFP expression of infected neurons (Fig. 4B, panels g and j).
FIG. 4.
Neurons cultured up to 6 days extend neurites into the M but not into the N compartment (comp). (A) SCG neurons were cultured in both S and N compartments. After 6 days, we plated PK15 cells in the M compartment of half the chambers used in the experiment. Twenty-four hours after we plated the PK15 cells, we infected the neurons in S compartments with PRV 151, PRV 152, or GS442. At 24 h postinfection, the total contents of the S and N compartments were harvested and titers were determined for PK15 cells. Three chambers were used in each type of infection. The circle beside each data set represents the average value for that set of data. The standard deviations for the S compartment are ±5.8 × 104 for cells with PK151 (+PK151), ±2.4 × 104 for +PK152, ±4 × 101 for +PK442, ±8.8 × 104 for cells without PK151 (−PK151), ±4.2 × 105 for −PK152, and ±2.3 × 101 for −PK442; those for the N compartment are 0 for +PK151 and ±5.1 × 104 for −PK151. (B) SCG neurons were cultured as described above, with (c to e, h to j, and m to o) or without (a, b, f, g, k, and l) PK15 cells in the M compartment. Neurons in the S compartment were then infected with PRV 151 (a through e), PRV 152 (f through j), or PRV GS442 (k through o). The expression of EGFP in infected cells was visualized live with an epifluorescence microscope. Images were obtained from the S compartment (a, f, k, c, h, and m), M compartment (d, i, and n), and N compartment (b, g, l, e, j, and o). −, absence of; +, presence of.
We also performed experiments with neurons plated at 5, 6, and 8 days. In all the experiments, PRV 152 never infected neurons in the N compartment, indicating that at least up to 8 days postplating, axons extending from the N compartment do not grow rapidly enough to penetrate into the S compartment (data not shown). We also repeated the above experiment with PRV 614 and PRV 616, derivatives of PRV Bartha that express RFPs (Table 1), and obtained similar results (data not shown).
TABLE 1.
Virus strains used in this study
| PRV strain | Description |
|---|---|
| Becker | PRV wild-type strain |
| Bartha | Attenuated vaccine strain |
| 151 | Be-GFP; green Becker |
| 152 | Ba-GFP; green Bartha |
| GS442a | PRV gD-null virus expressing GFP |
| 614 | Ba-mRFP; red Bartha |
| 616 | Be-mRFP; red Becker |
| HSV (F) | HSV wild-type strain |
| RR1097b | HSV gD-null virus expressing GFP |
GS442 was usually grown in a gD-complementing cell line unless otherwise stated.
RR1097 was grown in VD-60, a gD-complementing cell line.
Axons in the M compartment must be in close proximity for spread of PRV infection.
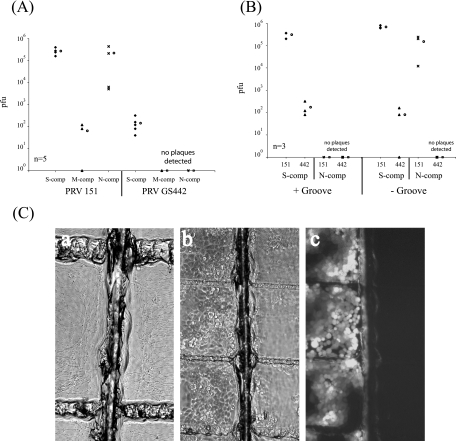
Previously, we reported that few, if any, extracellular virions were released into the media from axons in the N compartment (10). However, we cannot rule out the possibility that a few extracellular virions were released and subsequently entered an adjacent, uninfected growth cone. We wanted to determine whether the axon-axon spread of infection we are postulating requires close contact of infected and uninfected axons. To test this hypothesis, we prevented close contact of the axons in the M compartment that extended from either of the side compartments. We did this by using an insect pin to etch a narrow groove in the middle of the M compartment parallel with the central Teflon barriers but perpendicular to the series of 16 evenly spaced grooves. As a result, when neurons are cultured in both the S and the N compartments, the axons will penetrate through the silicone grease barriers into the M compartment but the perpendicular groove will stop further axon growth. Since the groove is small, the axons still will be in close proximity but will not be in close contact with each other (Fig. 5C, panel a).
FIG. 5.
Interaxonal spread of infection requires close contact of axons in the M compartment (comp). (A) SCG neurons were plated in both S and N compartments. For all the chambers, a midline groove parallel to the central Teflon barriers was etched onto the tissue culture dish prior to plating the neurons. At 7 days after plating, neurons in the S compartment were infected with PRV 151 and PRV GS442 at a high MOI. After 24 h, the total contents of the S, M, and N compartments were harvested and titers were determined for PK15 cells. Five chambers were used for each set of infections. The open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±1.5 × 105 for strain 151 and ±1.1 × 102 for strain 442, that for the M compartment is ±6.0 × 101, and that for the N compartment is ±2.2 × 105. (B) SCG neurons were plated in both S and N compartments. For half the chambers, a midline groove parallel to the central Teflon barriers was etched onto the tissue culture dish prior to plating the neurons. At 10 days after plating, we used an insect pin to sever the axons that penetrate the midline groove by gently dragging the pin over the midline groove 10 times. The S compartment was then infected with PRV 151 or PRV GS442 at a high MOI. After 48 h, the total contents of the S and N compartments were harvested and titers were determined for PK15 cells. Three chambers were used for each set of infections. The open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±9.2 × 104 (+Groove 151), ±2.0 × 102 (+Groove 442), ±1.1 × 105 (−Groove 151), and ±7.9 × 101 (−Groove 442), and that for the N compartment is ±1.2 × 105 (−Groove 151). (C) Chambers were set up as described for panel A, and a transmitted light image of the M compartment near the midline groove was taken (a). Chambers were also set up using conditions described for panel B, and in addition, PK15 cells were plated in the M compartment. No cells attached on the midline groove. The S compartment was subsequently infected with PRV 151, and the expression of EGFP in infected PK15 cells in the M compartment was visualized using epifluorescence microscopy (b and c).
We prepared the chambers as described above and etched the perpendicular groove in the M compartment prior to plating dissociated neurons. We then infected the neurons in the S compartment after 7 days postplating with either PRV 151 or GS442 at a high MOI. At 48 h, we harvested, and titers were determined for, the total contents of the S, M, and N compartments. Unexpectedly, we detected virus in the N compartment even in the presence of the groove (Fig. 5A). Upon closer examination of the midline groove, we noticed that a small number of axons managed to migrate over the groove, allowing the infection to spread from neurons in the S to N compartments. In these studies, it is noteworthy that we recovered a small amount of infectious material in the M compartment.
To rectify this problem, we again assembled chambers as described above, but only half of the chambers were etched with the midline groove in the M compartment. At 10 days postplating, we gently dragged the insect pin 10 times over the original midline groove to ensure that all remaining axonal connections were severed. Neurons in the S compartment were then infected at a high MOI with PRV 151 or GS442. After 48 h, the total contents of the compartments were harvested and titers were determined for PK15 cells. By severing the axons prior to infection, the presence of the midline groove completely blocked transmission of infection to cell bodies in the N compartment (Fig. 5B). No plaques were detected in the N compartment during either PRV 151 or GS442 infection. The absence of infected neurons in the N compartment was also verified by the lack of GFP expression in infected neurons (data not shown). In contrast, neurons grown in chambers without the midline groove were readily infected with either PRV 151 or GS442 as determined by the presence of infectious virions (Fig. 5B) or by GFP expression in infected neurons in the N compartment (data not shown).
Next, we demonstrated that if extracellular virions were produced, they would not diffuse easily in methocel-containing medium. We prepared chambers containing the midline groove similar to those described for Fig. 5B and plated PK15 epithelial cells in the M compartment (Fig. 5C, panel b). While PK15 epithelial cells readily attached to the surface of the dish in the M compartment, no cells were able to attach on the midline groove. We then infected the neurons in the S compartment with PRV 151 and observed infection in the epithelial cells. In each of the chambers tested, methocel was present in the M compartment. In all cases, the infection was unable to spread beyond the midline groove and only the epithelial cells closest to the S compartment were infected and expressed GFP (Fig. 5C, panel c). This result confirms that (i) methocel-containing medium is an excellent retardant of virion diffusion, (ii) no axons can grow across the midline groove after our treatment, and (iii) interaxonal spread of infection between neurons requires adjacent axons to either be in close proximity or be in contact with each other.
PRV gD is required for virion entry at growth cones as well as in axon-axon-mediated infection.
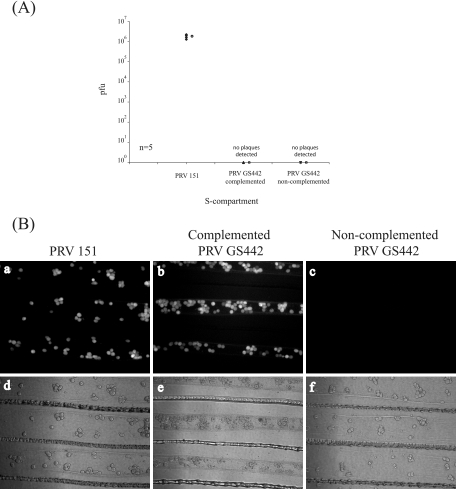
gD is the viral ligand responsible for the engagement of receptors required for the attachment and entry of alphaherpesviruses at the plasma membrane. However, the role of gD in virion entry at growth cones or axon terminals has not been well studied. To study this question, we cultured neurons in the S compartment for 2 weeks and then added viral inoculum from noncomplemented PRV GS442 in the axons that penetrated into the N compartment. We then measured and observed the spread of infection to the cell bodies in the S compartment (axon-mediated infection).
To prepare gD-null virions, we transfected pGS442 bacterial artificial chromosome DNA into noncomplementing PK15 cells and isolated the noninfectious virions. Using the polyethylene glycol fusion assay described previously by Spear and colleagues (55), we calculated the titer of these gD-null virions to be approximately 106 PFU/ml. Based on that titer, we added an equivalent high MOI viral inoculum of PRV 151, PRV GS442, or the noncomplemented PRV GS442 virions to axons in the N compartment. At 48 h postinfection, the contents of the S compartment were harvested and titers were determined for PK15 cells. Only PRV 151-infected neurons produced infectious particles (106 PFU) (Fig. 6A). As expected, no plaques were detected after infection with either PRV GS442-complemented or noncomplemented infected neurons. GFP expression was easily detected for both PRV 151- and complemented PRV GS442-infected neurons, but it was absent for the noncomplemented PRV GS442 (Fig. 6B). We conclude that gD is required for the entry of extracellular virions at growth cones and terminals. In addition, we conclude that gD is not required for the spread of infection from neuron to neuron via adjacent axons in the M compartment as shown in Fig. 3.
FIG. 6.
PRV gD is required for entry at growth cones and subsequent axon-mediated infection of neuron cell bodies. (A) SCG neurons were plated in the S compartment and cultured for 2 weeks to allow neurites to penetrate the N compartment. High-MOI viral inocula of PRV 151, PRV GS442 propagated in cells expressing gD (GS442 complemented), and PRV GS442 propagated in noncomplemented PK15 cells (GS442 noncomplemented) were then added to the exposed neurites in the N compartment. At 48 h postinfection, the total contents of the S compartment were harvested and titers were determined for PK15 cells. Five chambers were used for each set of infections, and the open circle beside each data set represents the average value for that particular set of data. The standard deviation for the S compartment is ±3.9 × 105. (B) Chambers were set up as described above and images of the infected neurons in the S compartment were obtained, live, via epifluorescence microscopy prior to harvesting the cells for titers to be determined. Both PRV 151 (a and d) and PRV GS442 (b, c, e, and f) express EGFP in infected cells.
PRV spreads efficiently in a gD-independent manner from neurons to nonneuronal cells lacking either HVEM or nectin-1.
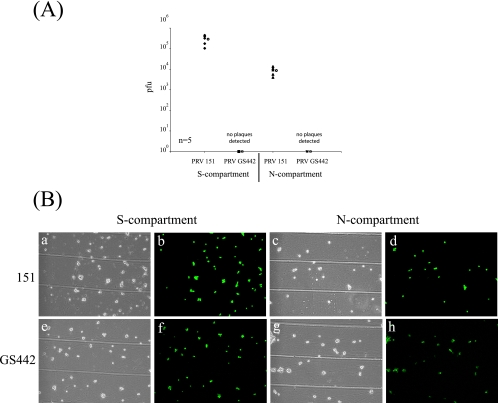
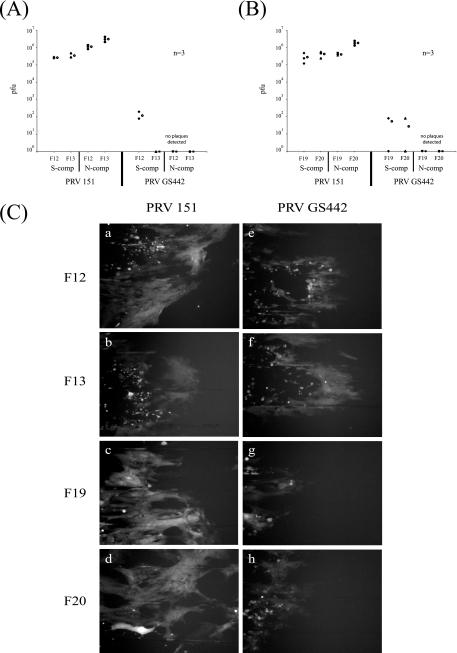
Both HVEM and nectin-1 have been well established as cellular receptors that can mediate entry of HSV (60). However, for PRV, only nectin-1 or other members of the nectin family have been demonstrated as entry receptors (24). To test whether nectin-1 or HVEM is required for the spread of infection from neurons to cells, we used primary mouse embryonic fibroblasts lacking either nectin-1 (F13) or HVEM (F20) as target nonneuronal cells (30, 63). As controls, we used fibroblasts from the corresponding wild-type mice for either nectin-1 knockout mice (F12) or HVEM knockout mice (F19). Neurons were cultured in the S compartment for 2 weeks to allow maximum axonal penetration into the N compartment. The mouse embryonic fibroblasts (F12, F13, F19, and F20) were then plated in the N compartment for 48 h.
We infected the neurons in the S compartment with either PRV 151 or GS442 at a high MOI for 48 h before the total contents of both the S and the N compartments were harvested and titers were determined for PK15 cells. The results show that both wild-type PRV and GS442 spread efficiently from neurons to the fibroblasts in the absence of nectin-1 (Fig. 7A) or HVEM (Fig. 7B). While GS442 can spread to fibroblasts in the N compartment, the rate of spread is lower than that with wild-type infection, as evident by the slower appearance of GFP expression and lower viral titers (data not shown). Furthermore, viral infection, as assayed by GFP fluorescence, is harder to detect in the plated fibroblasts. We observed that unlike epithelial cells, infected fibroblasts rapidly detach from the surface of the dish, making it harder to detect GFP fluorescence of infected cells. Thus, we conclude that anterograde spread from neuron to nonneuronal cells does not require gD and also does not require nectin-1 or HVEM.
FIG. 7.
PRV spreads efficiently in a gD-independent manner from neurons to target cells lacking either HVEM or nectin-1. (A) SCG neurons were plated in the S compartment (comp) for 2 weeks to allow neurite maturation. Mouse embryonic fibroblasts that do not express nectin-1 (F13) and cells derived from the wild-type littermate control for the nectin-1 knockout mouse (F12) were plated in the N compartment. After 48 h, the neurons in the S compartment were infected with PRV 151 and PRV GS442 at a high MOI and the infection was allowed to proceed for another 48 h before the total contents of the S and N compartments were harvested and titers were determined for PK15 cells. Three chambers were used for each set of infections and the open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±2.0 × 104 for 151-F12, ±1.2 × 105 for 151-F13, and ±6.9 × 101 for GS442-F12. The standard deviations for the N compartment are ±2.7 × 105 for 151-F12 and ±1.0 × 106 for 151-F13. (B) The same experiment as that described for panel A, except the target cells plated in the N compartment were mouse embryonic fibroblasts that do not express HVEM (F20) and the corresponding wild-type littermate control for the HVEM knockout mouse (F19). Three chambers were used for each set of infections, and the open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±1.8 × 105 for F-F19, ±1.7 × 105 for F-F20, ±4.6 × 101 for 1097-F19, and ±4.6 × 101 for 1097-F20; those for the N compartment are ±6.9 × 104 for F-F19 and ±5.6 × 105 for F-F20. (C) Chambers were set up as described for panels A and B, and fibroblasts in the N compartment infected with PRV 151 (a to d) or PRV GS442 (e to h) expressing EGFP were imaged live with an epifluorescence microscope prior to harvesting the cells for the determination of titers.
Wild-type HSV-1 can spread from neurons to fibroblasts with single gene knockouts of either nectin-1 or HVEM.
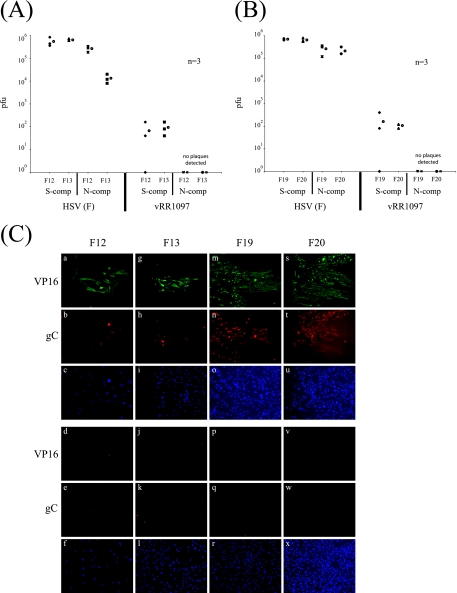
These experiments determined whether the lack of cellular receptor nectin-1 or HVEM in the fibroblasts prevents wild-type HSV-1 from spreading across the neuron-cell junction. We assembled the chamber and cultured neurons exactly as described for the previous experiment for PRV infections. Next, we infected neurons in the S compartment with wild-type HSV (F). At 48 h postinfection, total cell lysates in both the S and the N compartments were harvested and titers were determined for Vero cells. HSV-1 (F) can spread efficiently from neurons to fibroblasts lacking HVEM (F20) as well as to cells derived from wild-type control mice (F19). We conclude that the lack of HVEM in the fibroblasts does not compromise the ability of wild-type HSV-1 to spread efficiently from SCG neurons to nonneuronal cells (Fig. 8B). However, we noted that HSV (F) produces lower titers in the N compartment when the epithelial cells do not express nectin-1 (F13) (Fig. 8A). This finding may indicate that nectin-1 is required for the efficient spread of HSV (F) across the neuron-cell junction or that nectin-1 is required for the efficient spread among fibroblasts in the N compartment. We also immunostained infected fibroblasts with antibodies against VP16 or gC. All the cells used in this experiment, F12, F13, F19, and F20, could be infected with HSV (F) by these measurements (Fig. 8C).
FIG. 8.
HSV gD is required for neuron-to-cell spread of infection, and the absence of nectin-1 but not HVEM in the target cells reduces the efficiency of spread. (A) SCG neurons were plated in the S compartment (comp) for 2 weeks to allow neurite maturation. Mouse embryonic fibroblasts that do not express nectin-1 (F13) and cells derived from the wild-type littermate control for the nectin-1 knockout mouse (F12) were plated in the N compartment. After 48 h, the neurons in the S compartment were infected at a high MOI with HSV-1 (F) strain or vRR1097 (gD-null virus propagated in a gD-expressing cell line) and the infection was allowed to proceed for another 48 h before the total contents of the S and N compartments were harvested and titers were determined for Vero C1008 cells. Three chambers were used for each set of infections, and the open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±2.6 × 105 for F-F12, ±6.9 × 104 for F-F13, ±8.3 × 101 for 1097-F12, and ±9.3 × 101 for 1097-F13; those for the N compartment are ±7.6 × 104 for F-F12 and ±6.1 × 103 for F-F13. (B) The same experiment as described for panel A, except the target cells plated in the N compartment were mouse embryonic fibroblasts that do not express HVEM (F20) and the corresponding wild-type littermate control for the HVEM knockout mouse (F19). Three chambers were used for each set of infections, and the open circle beside each data set represents the average value for that particular set of data. The standard deviations for the S compartment are ±6.1 × 104 for F-F19, ±1.3 × 105 for F-F20, ±2.1 × 102 for 1097-F19, and ±2.3 × 101 for 1097-F20; those for the N compartment are ±1.3 × 105 for F-F19 and ±9.2 × 104 for F-F20. (C) Chambers were set up as described above, and neurons in the S compartment were infected with either HSV-1 (F) (a to c, g to i, m to o, and s to u) or vRR1097 (d to f, j to l, p to r, and v to x). At 48 h postinfection, the chambers were disassembled and cells were fixed and immunostained with VP16 (a, d, g, j, m, p, s, and v), gC (b, e, h, k, n, q, t, and w), or Hoechst nuclear dye (c, f, i, l, o, r, u, and x). Images shown in this figure are fibroblasts F12 (a to f), F13 (g to l), F19 (m to r), and F20 (s to x) plated in the N compartment.
HSV gD is absolutely required for spread from neurons to epithelial cells.
Unlike PRV gD, HSV-1 gD is essential for transneuronal spread of infection in the rat retina (18). For both PRV and HSV-1, gD is required for infection by extracellular particles as well (26, 36). We determined whether gD is required for HSV to spread from neurons to target cells in the chamber system. We assembled the chambers as described for the previous experiment, represented by Fig. 8A and B, and infected neurons in the S compartment with HSV gD null (vRR1097) grown in a complementing cell line at a high MOI. The gD open reading frame of vRR1097 has been replaced with the GFP gene. As expected, vRR1097-infected neurons produced no detectable infectious particles in either the S or the N compartment. The small number of plaques detected in the N compartment represents the residual complemented gD plus virus inoculum (Fig. 8A and B).
Importantly, all the neurons in the S compartment were infected by complemented vRR1097 as evident by the expression and autofluorescence of GFP (data not shown). In the N compartment, all target cells, regardless of genotype, infected with the wild-type HSV (F) strain were stained with antibodies against VP16 (Fig. 8C, panels a, g, m, and s) and gC (panels b, h, n, and t). In all cases, VP16 and gC were expressed in all infected cells, indicating that the wild-type HSV (F) strain can infect cells that do not express nectin-1 or HVEM. In contrast, vRR1097 did not spread from infected neurons to any of the plated fibroblasts in the N compartment. This conclusion comes from the lack of staining with either VP16 (Fig. 8C, panels d, j, p, and v) or gC (panels e, k, q, and w) antibodies. We conclude that, unlike the case for PRV, the HSV gD protein is absolutely required for neuron-to-cell spread and that gD-null mutants cannot spread across the neuron-cell junction. However, the absence of either HVEM or nectin-1 in the target fibroblast did not prevent neuron-to-cell spread.
DISCUSSION
In this report, we initially set out to demonstrate the flexibility of the trichamber system by developing a method to study directional spread of infection between epithelial cells and PNS neurons. However, we quickly discovered that plating epithelial cells in the M compartment was unnecessary in this study since PRV is able to spread directly between axons in a gD-independent manner. Hence, we were unable to study the direct spread of infection from neurons to cells and vice versa. Whether all alphaherpesviruses are able to spread directly between axons remains to be tested. However, for viruses that are not capable of spreading interaxonally, the trichamber system remains a feasible method to study neuron-cell-neuron spread of infection.
In our experiments, we repeatedly demonstrated that gD is not required for neuron-to-cell spread. This result is not entirely surprising given the numerous reports in the literature that have demonstrated that PRV gD is not required for cell-to-cell and transneuronal spread of infection both in cell culture and in animal models (10, 25, 45, 47, 48). However, our observations that the virus is able to spread directly between axons was unexpected since we initially postulated that the PK15 epithelial cell monolayer in the M compartment was required as a bridge or conduit for the infection to spread between the neuronal cultures from either side compartment. We also discovered that this form of axon-axon spread is gD independent and requires that the adjacent axons growing from opposing compartments be in close proximity since a small groove in the M compartment blocks any form of spread.
Rather than axon-axon spread, an alternative explanation is that axons from cell bodies in one side compartment have completely penetrated the M compartment and entered the other side compartment. This possibility seems unlikely for two reasons. First, based on previous observations, we noted that up to 7 days after plating, axon growth cones do not grow fast enough to penetrate from S to N compartment and vice versa. Second, we used a directional spread PRV mutant strain to prove that rare axons from opposite compartment neurons were not present in our cultures. PRV Bartha, which is defective for anterograde, but not retrograde spread of infection, was used as a probe for retrograde entry into axons and infection of distant cell bodies (10). If any exposed growth cones or varicosities were present in the S compartment where the PRV Bartha infection was initiated, then neurons in the N compartment would be infected. We did not observe any infection of cell bodies in the N compartment by PRV Bartha. We also performed independent infections with other Bartha strains with different reporters and showed essentially the same result (data not shown).
How can PRV infection spread transneuronally across adjacent axons in a gD-independent manner in the absence of cocultured epithelial cells? One possibility is that free particles mediate this axon-axon infection, but gD is not required for the entry of extracellular particles in distal axons. While we previously reported that no detectable extracellular particles were released in the medium of distal infected axons, it is possible that released particles remain associated with the axolemma. The prediction is that gD-null virions will readily enter and infect a naïve distal axon. We prepared stocks of gD-null viral mutants in noncomplementing PK15 cells by transfection of gD-null mutant DNA. Using a high MOI infection of the gD-null virions, we infected the distal axons in the N compartment. We saw no signs of infection and we concluded that PRV gD is absolutely required for the entry of virions at distal axons. Furthermore, the spread of infection between adjacent axons most likely does not occur via extracellular particle infection.
How then does PRV spread between adjacent axons in a gD-independent manner? We propose that this interaxonal spread is mediated at specific axo-axonal contacts between neurons, perhaps through interactions at the varicosities and growth cones from adjacent neurons. Indeed, cultured sympathetic neurons form not only axo-axonal synapses but also axo-dendritic and axo-glial synapses (2). These synapses are functional and are likely to have in vivo significance since sympathetic neuron innervation regulates and maintains normal function and phenotypes of parasympathetic neurons (59). In support of this idea, recent publications described below demonstrate that egressing capsids can exit at specific sites along distal axon shafts, such as axon terminals, varicosities, and growth cones. We previously showed in the trichamber system that clusters of infected epithelial cells could be found at discrete sites along distal axons that enter the N compartment (data not shown) (10). Some in vivo data support the idea that PRV exits at distinct sites along axon shafts. Tomishima and Enquist demonstrated that in a rodent visual system of PRV infection, infection can spread not only from the axon terminals in the optic tectum but also at specific sites in the optic nerve where glial cells make contact along the axon shafts during anterograde spread of infection (62). More recently, De Regge et al. showed that PRV glycoprotein D interacts with sensory trigeminal ganglion neurons and triggers the formation of varicosities that serve as axonal exit sites for egressing virus (17). Saksena et al. also reported that HSV-1 accumulates, envelopes, and exits near growth cones and varicosities in mid-distal regions of the axon (54).
While these papers present compelling evidence that alphaherpesviruses can egress and exit from specific axonal structures, the nature of transneuronal spread and the composition of the viral particle that mediates this spread remain contested. Part of this long-standing controversy surrounds the location where the viral capsid obtains its final envelope in neurons. Data accumulated from work with PRV and HSV suggest two possibilities. First, the capsid could acquire the membrane in the neuronal cell body and rely on the neuronal secretory and axonal targeting pathway to transport the mature virus particle to distal axonal structures (1, 8, 10, 13, 15, 20, 27, 28, 33, 35, 38, 54, 64). Alternatively, the unenveloped viral capsid with inner tegument proteins could engage the axonal transport machinery and travel down the length of the axon before acquiring the final envelope and outer tegument proteins at or near distal axonal structures, such as varicosities and growth cones or terminals (29, 34, 42, 43, 46, 49). Currently, we know little about the process of release of virions at or near a synapse in vivo. It also is unclear how infection can spread transneuronally or from neuron to cell or vice versa in a gD-independent manner. The implication has been that an extracellular particle is not involved, but direct proof for this suggestion has not been easy to obtain. It is plausible that closely apposed pre- and postsynaptic membranes could fuse in some unknown limited fashion and mediate the spread of infection, but this mechanism begs the question of what type of particle is transferred between the axons. Alternatively, if a mature viral particle is released in the synaptic cleft, it is possible that the virus particle may be trapped in the cleft and fusion with the postsynaptic membrane may occur in a gD- and receptor-independent manner simply because the membranes are in such close opposition. This controversy can be resolved once we can visualize and track particle movement across the synapse via a combination of fluorescence live-cell microscopy and correlative electron microscopy.
Our experiments with fibroblasts lacking nectin-1 or HVEM indicated that PRV can enter and spread efficiently in fibroblasts lacking either of those two proteins. It has been shown that nectin-1 interacts with PRV gD and serves as the cellular entry receptor (12, 23). However, it is likely that other cellular receptors besides nectin-1 will function as entry receptors for PRV. Indeed, PRV gD-null mutants that are capable of infecting and spreading in cell cultures have been isolated under a selective environment (32, 56). This indicates that non-gD-mediated cellular entry receptors exist and can be used by PRV to enter cells even in the absence of nectin-1 or HVEM. The same reasoning can be applied to HSV-1 infection of cultured fibroblasts lacking either HVEM or nectin-1. In the absence of HVEM, HSV-1 will use nectin-1 as the primary entry receptor and vice versa (60). The fact that lower titers were observed in nectin-1 knockout fibroblasts may suggest that nectin-1 is a more efficient entry receptor than HVEM is. To summarize, a single gene knockout of either HVEM or nectin-1 in fibroblasts does not prevent entry and subsequent spread of either HSV-1 or PRV. However, a double knockout of HVEM and nectin-1 genes in mutant mice made those mice nonsusceptible to HSV-1 infection in the vaginal epithelia (61).
Our results from the trichamber system agree with data collected from studies of PRV and HSV-1 spread of infection in animal nervous system models. Indeed, a PRV gD-null mutant cannot infect neurons at growth cones but once it manages to enter neurons, subsequent transneuronal spread between neurons or between neurons and cells is gD independent (10, 25, 45, 47, 48). In contrast, gD is required for HSV to successfully infect cells via extracellular particles as well as subsequent cell-to-cell spread of infection (18).
Acknowledgments
We acknowledge T. del Rio and B. Feierbach for valuable advice on the preparation of the manuscript. We also thank L. Pomeranz for preparing and generously sharing her valuable GS442 viral stock grown in a gD-complementing cell line.
This work was supported primarily by the National Institute of Neurological Disorders and Stroke (NIH-NINDS grant R01 33506).
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Antinone, S. E., and G. A. Smith. 2006. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 80:11235-11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archakova, L. I., I. A. Bulygin, and N. I. Netukova. 1982. The ultrastructural organization of sympathetic ganglia of the cat. J. Auton. Nerv. Syst. 6:83-93. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones, G., and J. P. Card. 2000. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J. Neurosci. Methods 103:51-61. [DOI] [PubMed] [Google Scholar]
- 4.Babic, N., T. C. Mettenleiter, A. Flamand, and G. Ugolini. 1993. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J. Virol. 67:4421-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banfield, B. W., J. D. Kaufman, J. A. Randall, and G. E. Pickard. 2003. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J. Virol. 77:10106-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campenot, R. B. 1977. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA 74:4516-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card, J. P. 2001. Pseudorabies virus neuroinvasiveness: a window into the functional organization of the brain. Adv. Virus Res. 56:39-71. [DOI] [PubMed] [Google Scholar]
- 8.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card, J. P., L. Rinaman, J. S. Schwaber, R. R. Miselis, M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1990. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J. Neurosci. 10:1974-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 12.Connolly, S. A., J. J. Whitbeck, A. H. Rux, C. Krummenacher, S. van Drunen Littel-van den Hurk, G. H. Cohen, and R. J. Eisenberg. 2001. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC(nectin-1) with different affinities. Virology 280:7-18. [DOI] [PubMed] [Google Scholar]
- 13.Cook, M. L., and J. G. Stevens. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7:272-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuccurazzu, B., F. Deriu, E. Tolu, B. J. Yates, and I. Billig. 2007. A monosynaptic pathway links the vestibular nuclei and masseter muscle motoneurons in rats. Exp. Brain Res. 176:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demmin, G. L., A. C. Clase, J. A. Randall, L. W. Enquist, and B. W. Banfield. 2001. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J. Virol. 75:10856-10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Regge, N., H. J. Nauwynck, K. Geenen, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, and H. W. Favoreel. 2006. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enquist, L. W., and J. P. Card. 2003. Recent advances in the use of neurotropic viruses for circuit analysis. Curr. Opin. Neurobiol. 13:603-606. [DOI] [PubMed] [Google Scholar]
- 20.Feierbach, B., M. E. Bisher, J. Goodhouse, and L. W. Enquist. 2007. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J. Virol. 81:6846-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields, B. N., D. M. Knipe, and P. M. Howley. 1996. Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 22.Geerling, J. C., T. C. Mettenleiter, and A. D. Loewy. 2003. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 122:541-550. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty, R. J., C. R. Jogger, and P. G. Spear. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268:147-158. [DOI] [PubMed] [Google Scholar]
- 24.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 25.Hanssens, F. P., H. J. Nauwynck, and T. C. Mettenlieter. 1995. Role of glycoprotein gD in the adhesion of pseudorabies virus infected cells and subsequent cell-associated virus spread. Arch. Virol. 140:1855-1862. [DOI] [PubMed] [Google Scholar]
- 26.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill, T. J., and H. J. Field. 1973. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. J. Gen. Virol. 21:123-133. [DOI] [PubMed] [Google Scholar]
- 28.Hill, T. J., H. J. Field, and A. P. Roome. 1972. Intra-axonal location of herpes simplex virus particles. J. Gen. Virol. 15:233-235. [DOI] [PubMed] [Google Scholar]
- 29.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki, M., K. Irie, H. Ishizaki, M. Tanaka-Okamoto, K. Morimoto, E. Inoue, T. Ohtsuka, J. Miyoshi, and Y. Takai. 2005. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 132:1525-1537. [DOI] [PubMed] [Google Scholar]
- 31.Karger, A., and T. C. Mettenleiter. 1993. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology 194:654-664. [DOI] [PubMed] [Google Scholar]
- 32.Karger, A., J. Schmidt, and T. C. Mettenleiter. 1998. Infectivity of a pseudorabies virus mutant lacking attachment glycoproteins C and D. J. Virol. 72:7341-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristensson, K., B. Ghetti, and H. M. Wisniewski. 1974. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 69:189-201. [DOI] [PubMed] [Google Scholar]
- 34.LaVail, J. H., A. N. Tauscher, J. W. Hicks, O. Harrabi, G. T. Melroe, and D. M. Knipe. 2005. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J. Virol. 79:11142-11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaVail, J. H., K. S. Topp, P. A. Giblin, and J. A. Garner. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 36.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomniczi, B., M. L. Blankenship, and T. Ben-Porat. 1984. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J. Virol. 49:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lycke, E., K. Kristensson, B. Svennerholm, A. Vahlne, and R. Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 65:55-64. [DOI] [PubMed] [Google Scholar]
- 39.Mack, S. O., M. Wu, P. Kc, and M. A. Haxhiu. 2007. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J. Appl. Physiol. 102:189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marson, L., and A. Z. Murphy. 2006. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291:R419-R428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metts, B. A., G. D. Kaufman, and A. A. Perachio. 2006. Polysynaptic inputs to vestibular efferent neurons as revealed by viral transneuronal tracing. Exp. Brain Res. 172:261-274. [DOI] [PubMed] [Google Scholar]
- 42.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulder, W., J. Pol, T. Kimman, G. Kok, J. Priem, and B. Peeters. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J. Virol. 70:2191-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulder, W. A., J. Priem, K. L. Glazenburg, F. Wagenaar, E. Gruys, A. L. Gielkens, J. M. Pol, and T. G. Kimman. 1994. Virulence and pathogenesis of non-virulent and virulent strains of pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus. J. Gen. Virol. 75:117-124. [DOI] [PubMed] [Google Scholar]
- 46.Ohara, P. T., A. N. Tauscher, and J. H. LaVail. 2001. Two paths for dissemination of herpes simplex virus from infected trigeminal ganglion to the murine cornea. Brain Res. 899:260-263. [DOI] [PubMed] [Google Scholar]
- 47.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peeters, B., J. Pol, A. Gielkens, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platt, K. B., C. J. Mare, and P. N. Hinz. 1979. Differentiation of vaccine strains and field isolates of pseudorabies (Aujeszky's disease) virus: thermal sensitivity and rabbit virulence markers. Arch. Virol. 60:13-23. [DOI] [PubMed] [Google Scholar]
- 51.Pomeranz, L. E., A. E. Reynolds, and C. J. Hengartner. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69:462-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch, D. A., N. Rodriguez, and R. J. Roller. 2000. Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread. J. Virol. 74:11437-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 80:3592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarmiento, M., M. Haffey, and P. G. Spear. 1979. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J. Virol. 29:1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt, J., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J. Virol. 71:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shekhtman, E., J. C. Geerling, and A. D. Loewy. 2007. Aldosterone-sensitive neurons of the nucleus of the solitary tract: multisynaptic pathway to the nucleus accumbens. J. Comp. Neurol. 501:274-289. [DOI] [PubMed] [Google Scholar]
- 58.Sík, A., A. Cote, and Z. Boldogkoi. 2006. Selective spread of neurotropic herpesviruses in the rat hippocampus. J. Comp. Neurol. 496:229-243. [DOI] [PubMed] [Google Scholar]
- 59.Smith, P. G., J. D. Warn, J. J. Steinle, D. Krizsan-Agbas, and W. Hasan. 2002. Modulation of parasympathetic neuron phenotype and function by sympathetic innervation. Auton. Neurosci. 96:33-42. [DOI] [PubMed] [Google Scholar]
- 60.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 61.Taylor, J. M., E. Lin, N. Susmarski, M. Yoon, A. Zago, C. F. Ware, K. Pfeffer, J. Miyoshi, Y. Takai, and P. G. Spear. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease as assessed by intravaginal challenge of mutant mice. Cell Host Microbe 2:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomishima, M. J., and L. W. Enquist. 2002. In vivo egress of an alphaherpesvirus from axons. J. Virol. 76:8310-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, Y., S. K. Subudhi, R. A. Anders, J. Lo, Y. Sun, S. Blink, Y. Wang, J. Wang, X. Liu, K. Mink, D. Degrandi, K. Pfeffer, and Y. X. Fu. 2005. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J. Clin. Investig. 115:711-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto, T., S. Otani, and H. Shiraki. 1973. Ultrastructure of herpes simplex virus infection of the nervous system of mice. Acta Neuropathol. 26:285-299. [DOI] [PubMed] [Google Scholar]