Abstract
Hepatitis B virus (HBV) acute and chronic infections remain a major worldwide health problem. Towards developing an anti-HBV vaccine with single-dose scheme potential, we engineered infectious measles virus (MV) genomic cDNAs with a vaccine strain background and expression vector properties. Hepatitis B surface antigen (HBsAg) expression cassettes were inserted into this cDNA and three MVs expressing HBsAg at different levels generated. All vectored MVs, which secrete HBsAg as subviral particles, elicited humoral responses in MV-susceptible genetically modified mice. However, small differences in HBsAg expression elicited vastly different HBsAg antibody levels. The two vectors inducing the highest HBsAg antibody levels were inoculated into rhesus monkeys (Macaca mulatta). After challenge with a pathogenic MV strain (Davis87), control naive monkeys showed a classic measles rash and high viral loads. In contrast, all monkeys immunized with vaccine or a control nonvectored recombinant vaccine or HBsAg-expressing vectored MV remained healthy, with low or undetectable viral loads. After a single vaccine dose, only the vector expressing HBsAg at the highest levels elicited protective levels of HBsAg antibodies in two of four animals. These observations reveal an expression threshold for efficient induction of HBsAg humoral immune responses. This threshold is lower in mice than in macaques. Implications for the development of divalent vaccines based on live attenuated viruses are discussed.
Vaccines based on live viruses can be highly effective and easy to produce and deliver. Smallpox was eradicated with live vaccinia virus, and a live attenuated poliovirus vaccine has been at the core of the polio eradication campaign (17). Worldwide measles virus (MV) vaccination prevents an estimated 80 million cases and 4.5 million deaths annually (45) with minimal severe adverse effects, on average less than 10 in 1 million doses (34). With the currently recommended vaccination scheme, the first dose of vaccine given at 10 to 12 months of age confers long-lasting immunity to 95% of vaccinees (16). The second dose, given to 6-year-olds, raises the conversion rate to nearly 100%, eliminating primary vaccine failures (45). The two-dose strategy has been credited with elimination of indigenous measles in several countries (9), and the live attenuated MV vaccine is considered to be one of the safest and most cost-effective health tools available (28).
MV is a nonsegmented negative-strand RNA virus replicating in the cytoplasm. Vaccine safety and efficacy are sustained by lack of recombination, lack of a DNA replication phase, established vaccine production methods, and effective distribution networks (2, 50). A reverse genetic system (37) allows generation of recombinant MV expressing heterologous proteins, including those of other pathogens (48). For this, coding regions are inserted between duplicated MV-specific gene start and gene end motifs that direct transcription by the viral RNA-dependent RNA polymerase. These expression cassettes are named additional transcription units (ATUs). The genomic location of an ATU determines the amount of protein expressed due to the sequential attenuation of transcription at gene ends (6, 18).
Work towards developing recombinant MV with additional vaccine specificities has begun: genes from hepatitis B virus (HBV) (43), simian and human immunodeficiency viruses (24, 52), mumps virus (50), and West Nile virus (10) have been inserted into different positions in the MV genome and thus expressed at different levels. The immunogenicities of vectored MVs and, in one case, their vaccine efficacies have been characterized in rodent and primate animal models. An MV-based candidate vaccine protected interferon (IFN) receptor-deficient mice against West Nile virus challenge (10).
The widely used HBV vaccine is based on Saccharomyces cerevisiae-expressed small surface antigen (hepatitis B surface antigen [HBsAg]) and has a three-dose schedule (22). This vaccine provides enduring and protective immunity, but compliance with this regimen is often low, and a long-desired global immunization is not in sight. To facilitate HBV eradication, alternatives have been proposed, including a two-dose scheme (1, 4). Replicating HBsAg-expressing viral vectors have also been generated: vaccinia virus (27, 44)-, varicella-zoster virus (20, 42)-, adenovirus (25)-, and MV-based vectors (43) produce HBsAg to high levels and elicit protective anti-HBsAg antibodies in animal models.
Here, we report the production of an MV cDNA with a vaccine background and of vectored MVs based on this cDNA but expressing HBsAg at different levels. Three of these vectored MVs replicated to high titers, and two induced high levels of HBsAg antibodies in mice. These two viruses were inoculated into rhesus monkeys (Macaca mulatta). Both retained full vaccine competence, protecting the hosts against challenge with a wild-type MV, but only one vector induced protective levels of HBsAg antibodies.
MATERIALS AND METHODS
Cells and viruses.
Vero/hSLAM cells (31) and the helper 293-3-46 cell line (37) were maintained as monolayers in Dulbecco's modified Eagle's medium (Mediatech Inc., Herndon, VA) supplemented with 10% fetal calf serum, 1% penicillin-streptomycin (Mediatech), and 0.5 and 1.2 mg/ml G418 (Mediatech), respectively.
Recombinant MVs were generated as described by Radecke et al. (37). Briefly, the helper cell line 293-3-46 stably expressing MV-N, MV-P, and T7 polymerase was transfected by calcium phosphate precipitation using the ProFection kit (Promega, Madison, WI) with two plasmids, one containing the relevant MV genome and the other coding for the MV polymerase (pEMCLa). Three days after transfection, the helper cells were overlaid on Vero/hSLAM cells, and the appearance of infectious centers was monitored. Then single syncytia were picked and propagated on Vero/hSLAM cells. To prepare virus stocks, Vero/hSLAM cells were infected at a multiplicity of infection (MOI) of 0.03 and incubated at 32°C. Cells were scraped in Opti-MEM (Gibco) and particles released by two freeze-thaw cycles.
Virus growth characteristics were determined by infecting Vero/hSLAM cell monolayers at a MOI of 0.03 and incubating them at 37°C. Infected cells and supernatants were collected and lysed by one cycle of freeze and thaw 12, 24, 36, 48, 72, and 96 h postinfection to determine virus titers by 50% end point dilution (50% tissue culture infectious dose [TCID50]) on Vero/hSLAM cells using the Spearman-Kärber method (21).
Plasmid construction.
To correct three coding mutations in the polymerase (L) gene of the MV genome of pB(+)MVvac (11), site-directed mutagenesis was performed using the QuikChange system (Stratagene, La Jolla, CA). Mutagenesis (T331I, E429D, and N1805S) was executed in a cassette vector covering the L gene sequence, pSK-Lvac (our unpublished data). After mutagenesis the corrected L gene was reinserted into a genomic plasmid named pB(+)MVvac2. The complete coding identity of the plasmid sequence with those of both the Moraten and the Schwartz vaccine strains was confirmed (these two sequences are identical in spite of a nominally different origin, GenBank accession numbers AF266287 and AF266291) (32, 33).
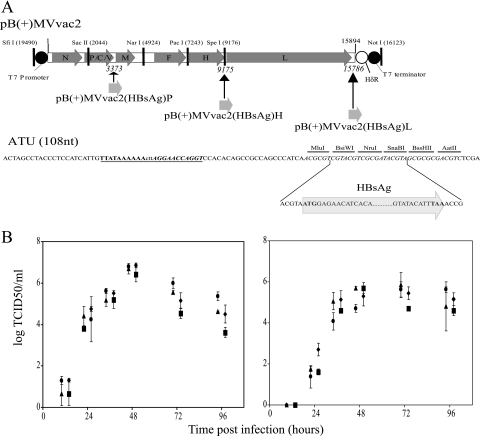
For construction of the vectored MV, a previously described ATU (37, 43) containing the N/P intergenic region (TTATAAAAAACTTAGGAACCAGGT; the three intergenic nucleotides are underlined) was amplified by PCR and transferred to pB(+)MVvac2-based vectors. The original ATU was amplified either from plasmid p(+)MPrGFPV or from plasmid p(+)MHrGFPV (50) for subcloning in position P or H, respectively. To facilitate future cloning of foreign genes and in compliance with the “rule of six”, six nonoverlapping restriction sites (36 nucleotides) were inserted as specified in Fig. 1A. These ATUs were then inserted in pB(+)MVvac2 in positions 3373, 9175, and 15786 of the MVvac2 genome (same numbers as in GenBank accession no. AF266287), giving rise to pB(+)MVvac2(ATU)P, pB(+)MVvac2(ATU)H, and pB(+)MVvac2(ATU)L, respectively.
FIG. 1.
Generation and characterization of vectored MV expressing HBsAg. (A, top) Map of pB(+)MVvac2, the plasmid containing an MV genome with vaccine-identical coding capacity and strategically located unique restriction sites. The coding regions of the MV genes are represented by arrowed dark gray boxes. The T7 promoter and terminator, as well as unique restriction sites, are indicated. HδR, hepatitis delta virus ribozyme. Names of the plasmids used to generate the vectored MVs that express HBsAg at different levels and the positions of the ATU inserted in these plasmids are shown below the pB(+)MVvac2 map. (A, bottom) Nucleotide sequence of the ATU. The conserved termination (boldface), intergenic (lowercase), and initiation (boldface italics) nucleotides are underlined. The restriction sites of the multicloning site are indicated. The sequences of the ends of the HBsAg coding region (light gray arrow) and the 9 nucleotides added upstream or downstream of this coding region to comply with the rule of six (38) are indicated below the ATU sequence. (B) Time course of cell-associated (left) and cell-free (right) virus production in Vero/hSLAM cells infected with four recombinant viruses: MVvac2 (triangles), MVvac2(HBsAg)P (dots), MVvac2(HBsAg)H (squares), and MVvac2(HBsAg)L (diamonds). Viral titers, indicated on the vertical axes, were measured 12, 24, 36, 48, 72, or 96 h postinfection. For clarity, symbols for viruses were moved slightly on the horizontal axes. Averages and standard deviations of three independent experiments are indicated.
The HBsAg coding sequence, obtained from p(+)MVHBs (43) as a MluI-BssHII restriction fragment, was then cloned into the corresponding sites of the ATUs of two pB(+)MVvac2(ATU) plasmids to obtain pB(+)MVvac2(HBsAg)P and pB(+)MVvac2(HBsAg)H. The plasmid pB(+)MVvac2(HBsAg)L was obtained after transferring an MluI-AatII restriction fragment from pB(+)MVvac2(HBsAg)P as the donor of the HBsAg coding sequence. The integrity of the new junctions was verified by sequencing.
HBsAg expression analysis.
For analysis of HBsAg expression Vero/hSLAM cells were seeded in a 35-mm-diameter six-well plate and infected with MVvac2 or its HBsAg-expressing derivates at a MOI of 0.5. Twenty hours after infection the cells were methionine and cysteine starved for 15 min and labeled with 100 μCi/ml of Tran 35S label (ICN Pharmaceuticals, Irvine, CA) for 4 h. The cells were lysed by incubation with radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) plus protease inhibitors (protease inhibitor cocktail set I; Calbiochem, Darmstadt, Germany). The antigenic material was immunoprecipitated with protein A-agarose (Pierce, Rockford, IL) using 2.5 μl of rabbit anti-MV H cytoplasmic tail polyclonal serum (5) or 2.5 μl of rabbit anti-HBsAg H651 polyclonal antibody (a kind gift from Heinz Schaller, University of Heidelberg).
For flow cytometry analysis Vero/hSLAM cells were infected at a MOI of 2. To avoid syncytium formation, 0.2 mM fusion inhibitor peptide Z-d-Phe-Phe-Gly-OH (40) was added after the inoculum was removed. After 24 h, cells were detached, fixed, permeabilized with 0.01% Triton X-100, and stained with 1:500 anti-MV N-fluorescein isothiocyanate (FITC) monoclonal antibody (Chemicon, Temecula, CA) or 1:100 anti-HBsAg mouse monoclonal antibody 3E7 (DakoCytomation, Carpinteria, CA). The reaction was developed by incubation with goat anti-mouse-FITC (Jackson Immunoresearch, West Grove, PA). Flow cytometry was performed using a BD-FACScalibur system (BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD Biosciences, San Jose, CA).
To quantitate HBsAg yields, 5 × 105 Vero/hSLAM cells were infected with the vectored MV at a MOI of 0.03 and supernatants were collected at 24, 48, and 72 h and precleared. HBsAg was detected by the HBsAg enzyme-linked immunosorbent assay (ELISA) Abazyme (Needham, MA) kit and quantified by a parallel line assay with a known standard (HBsAg ay, lot number 23090936; Chemicon, Temecula, CA).
HBsAg density determination.
Supernatants of 7 × 106 infected cells were first clarified by centrifugation at 8,000 rpm for 30 min in an SLA600TC rotor and then pelleted by ultracentrifugation at 35,000 rpm for 18 h in an SW41Ti rotor. The pellet was resuspended in 1 ml TNE buffer (1 mM Tris [pH 7.8], 100 mM NaCl, 10 mM EDTA), brought to 20% sucrose in TNE, and then layered on top of a 20, 30, 40, 50, and 60% (2 ml each) sucrose-TNE step gradient, followed by ultracentrifugation in the same rotor at 29,000 rpm for 16 h. To determine the particle density and contents, 1-ml fractions were taken from the top and weighed. An aliquot of each fraction was analyzed by ELISA, and a 25-μl aliquot was subjected to 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose for immunoblotting using a rabbit polyclonal anti-MV-N at 1:30,000 dilution and a horseradish peroxidase (HRP)-conjugated secondary antibody. The reaction was developed using a chemiluminescence kit (SuperSignal West Femto maximum; Pierce, Rockford, IL). To detect HBsAg, we used a monoclonal antibody (anti-HBsAg-HRP) supplied as a secondary antibody in the HBsAg ELISA Abazyme kit and developed the reaction with the ECL-plus detection system (Amersham Biosciences Corp., Piscataway, NJ).
Mouse inoculation.
All animal experimental procedures were performed according to a protocol previously approved by the Mayo Clinic Institutional Animal Care and Use Committee. The MV-susceptible mouse line Ifnarko-CD46Ge (29) was used as a host. Ten mice per group were inoculated by the intraperitoneal route with 5 × 104 TCID50 of either MVvac2 or its HBsAg-expressing derivatives. As a positive control a group of mice was inoculated with MVHBs, a previously reported HBsAg-expressing MV (43). Mice were bled at 14 and 28 days postinoculation, and serum was separated and stored at −20°C until use.
Inoculation of rhesus macaques.
MV-seronegative rhesus monkeys, housed at the California National Primate Research Center in accordance with the regulations of the Association for the Assessment and Accreditation of Laboratory Animal Care, were bled under ketamine sedation. Two control monkeys were vaccinated subcutaneously (s.c.) with 0.5 ml of live attenuated MV (Attenuvax; Merck and Co., NJ). The titer of a vaccine dose is declared as greater than or equal to 103 TCID50, but we repeatedly measured about 104 infectious units per dose. Therefore, 104 infectious units of the vectored MV was also used for s.c. vaccination.
The monkeys were challenged by conjunctival/intranasal inoculation of 105 infectious units of the Davis87 isolate of MV grown in Vero/hSLAM cells. The animals were monitored daily for anorexia, depression, coughing, diarrhea, and skin rash. They were bled on days 0, 4, 7, 14, and 28 postimmunization or postchallenge. Measles viremia was quantified by end point dilution coculture with Raji cells as described previously (36). Tissue culture infectious units were calculated by the method of Reed and Muench (39).
Characterization of the humoral immune response in mice and macaques.
MV neutralizing antibody titers were determined in a plaque reduction assay by incubating serum dilutions (mouse or macaque serum) with 50 PFU of MVvac(GFP)N (our unpublished data) expressing green fluorescence protein and were expressed as 90% plaque reduction fluorescence-forming units. Anti-HBsAg antibody titers were determined with a species-independent quantitative ELISA kit (Bioelisa anti-HBs; Biokit, Barcelona, Spain) and expressed as milli-international units per milliliter by comparison with World Health Organization standards supplied by the manufacturer.
Suppression of the antitetanus antibody response.
One week after vaccination with MVvac2 or MVvac2(HBsAg)H, monkeys were immunized with tetanus toxoid at 20 μl/kg body weight s.c. (unconcentrated tetanus toxoid; Colorado Serum Company, Denver, CO). Tetanus antibody titers were determined at 21 days postvaccination. Titers of serum antibody were measured by ELISA using purified tetanus toxoid (Accurate Chemical and Scientific Corp., Westbury, NY) and positive/negative control rhesus sera as described previously (36).
Cell-mediated immunity.
MV-specific T cells were counted using an gamma IFN (IFN-γ) enzyme-linked immunospot (ELISPOT) assay as previously described (36). Briefly, peripheral blood mononuclear cells (PBMC) were resuspended at 5 × 106 cells/ml in a 48-well flat-bottom plate in AIM V medium (Gibco/Invitrogen Corp., Grand Island, NY) supplemented with 10% fetal calf serum and stimulated overnight with live MV, Edmonston strain (American Type Culture Collection), at 103 TCID50/100 μl. Positive control stimulation was with 10 ng/ml phorbol 12-myristate 13-acetate and 1 μg/ml ionomycin (Sigma, St. Louis, MO). Following overnight incubation, the cells were transferred to a 96-well ELISPOT plate coated with antibody to rhesus IFN-γ (U-Cytech BV, Utrecht, The Netherlands) and developed as described by the manufacturer. Spot-forming cells (SFC) were counted under a dissecting microscope, and the numbers of spots in duplicate wells were averaged. A positive result was at least 10 spots per well and equal to or more than the mean plus 2 standard deviations of the medium control. The spot number in medium control wells was subtracted from the experimental spot count, and the number of SFC was adjusted to 106 PBMC.
RESULTS
Generation of vectored MVs expressing HBsAg.
We previously described an MV cDNA with vaccine-equivalent, but not vaccine-identical, coding capacity (11). Three coding differences between the polymerase (L) gene of this cDNA and those of vaccine strains Moraten and Schwartz (32, 33) exist. Although these three mutations concern nonconserved amino acids and although subsequent studies failed to reveal functional differences between viruses generated from the two genomic plasmids, it was considered appropriate to perform animal studies only with viruses having MV-identical coding capacity. Thus, the pB(+)MVvac2 backbone (Fig. 1A) was constructed as detailed in Materials and Methods. We refer to viruses derived from this backbone as having a vaccine strain background.
To express HBsAg at different levels, we then added to pB(+)MVvac2 an expression cassette (ATU). The ATU was inserted at three different positions: after the P gene (position 3373), after the H gene (position 9175), and after the L gene (position 15786). To direct HBsAg expression, the 681-nucleotide HBsAg (subtype ayw) coding region was then introduced in these plasmids to yield pB(+)MVvac2(HBsAg)P, pB(+)MVvac2(HBsAg)H, and pB(+)MVvac2(HBsAg)L (Fig. 1A). The corresponding recombinant MVs were then generated.
To assess the replication efficiency of these vectored MVs, growth kinetics were determined. As shown in Fig. 1B, the maximum titers reached by all three vectored viruses were equivalent to those of the parental strain, MVvac2. Peak titers of about 107 TCID50/ml were reached 48 h postinfection for the cell-associated virus and of 3 × 105 to 106 TCID50/ml 48 to 72 h postinfection for cell-free virus. Thus, the replication efficiency of these three HBsAg-vectored MVs was equivalent to that of the parental strain.
HBsAg expressed by vectored MVs is secreted in subviral particles.
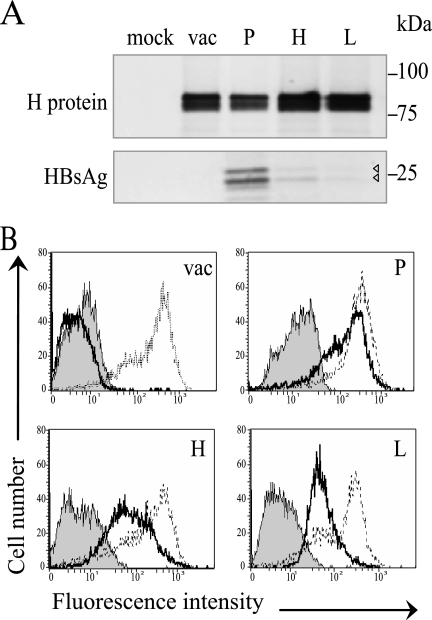
The HBsAg expressed from vectored MV was then characterized. Extracts of infected cells were assayed by immunoprecipitation using an anti-HBsAg rabbit polyclonal antiserum or an antiserum directed against the MV H glycoprotein. As shown in Fig. 2A, bottom, anti-HBsAg antibodies recognized the two differentially glycosylated HBsAg isoforms, with molecular masses of 26 and 24 kDa, respectively. As expected, MVvac2(HBsAg)P expressed HBsAg at the highest levels and MVvac2(HBsAg)L expressed HBsAg at the lowest (Fig. 2A, bottom, lanes P and L, respectively). H protein was expressed at similar levels by all four viruses and was detected as a doublet (Fig. 2A, top; the upper band corresponds to the mannose-rich endoplasmic reticulum form and the lower band to the post-Golgi form with processed oligosaccharides) (7).
FIG. 2.
HBsAg expression by vectored MV. (A) Immunoprecipitation of proteins produced by Vero/hSLAM cells infected with MVvac2 (vac), MVvac2(HBsAg)P (P), MVvac2(HBsAg)H (H), and MVvac2(HBsAg)L (L). Mock, mock-infected cells. Proteins were labeled with [35S]methionine 20 to 24 h postinfection and precipitated either with an H-specific serum (top) or an HBsAg-specific serum (bottom). The positions of molecular mass standards are indicated on the right. Arrowheads indicate two weak HBsAg signals. (B) Fluorescence-activated cell sorter analysis of HBsAg and MV N protein expression in infected cells. Thick lines, HBsAg expression; thin lines, N expression; shaded area, negative control without primary antibody.
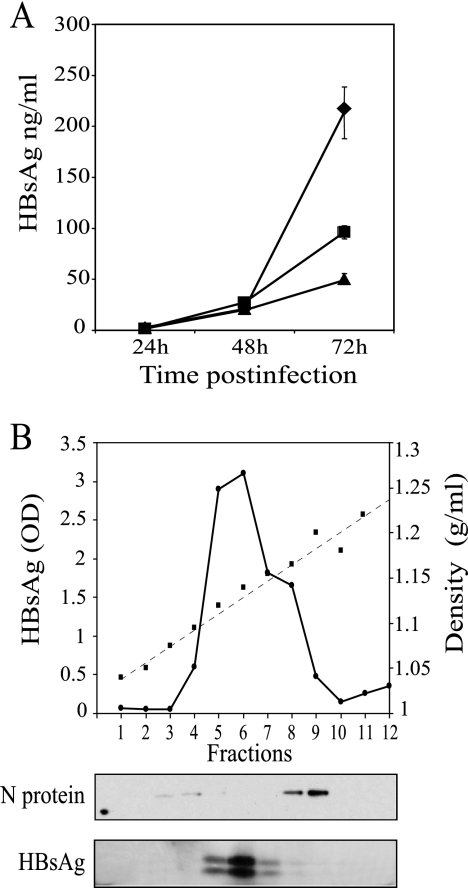
We also compared HBsAg expression levels by fluorescence-activated cell sorting using HBsAg monoclonal antibodies (Fig. 2B) and MV N protein antibodies (Fig. 2B). The intensity of HBsAg-specific fluorescence was highest for MVvac2(HBsAg)P-infected cells (mean = 286), intermediate for MVvac2(HBsAg)H-infected cells (mean = 153), and lowest for MVvac2(HBsAg)L-infected cells (mean = 74). The signal levels of the N expression signal and of the negative controls (secondary antibody alone) were similar among cells infected with MVvac2 and its derivates. In particular, the N protein expression means were 508, 405, and 267 for cells infected with vectors expressing HBsAg from the P, H, and L positions, respectively. To quantitate HBsAg secretion, we used an ELISA as illustrated in Fig. 3A. We found that cells infected with MVvac2(HBsAg)P secreted up to 210 ng/ml HBsAg, whereas cells infected with MV expressing HBsAg from the two downstream positions secreted two- and four-times-lower HBsAg concentrations, respectively.
FIG. 3.
Quantitation of HBsAg expression, and physical characteristics of released HBsAg. (A) Vero/hSLAM cells were infected with MVvac2(HBsAg)P (diamonds), MVvac2(HBsAg)H (squares), or MVvac2(HBsAg)L (triangles), and media were collected at the time points indicated and clarified. HBsAg was assayed by ELISA and quantified by comparison with a standard curve. Averages and standard deviations of a triplicate experiment are shown. (B, top) Materials released from Vero/hSLAM cells infected with MVvac2(HBsAg)P. Media collected 72 h after infection were clarified, and virus was pelleted, loaded on a 20 to 60% sucrose gradient, and centrifuged to equilibrium. One-milliliter fractions were collected from the top (left) to the bottom (right) and weighed. The density profile is shown by squares joined by a dashed line. The HBsAg concentration determined by ELISA is shown by dots joined by a continuous line. (Bottom) Aliquots of each fraction separated by 12.5% SDS-polyacrylamide gel electrophoresis, immunoblotted, and probed with the antibodies indicated on the left.
Most HBsAg in the serum of HBV-infected patients is in the form of spherical 22-nm particles devoid of nucleic acids, representing excess envelope material synthesized by HBV-infected cells (15). These subviral particles are also the principal constituent of the HBV vaccine. To assess whether HBsAg secreted by the vectored MV-infected cells associates in subviral particles, supernatants were clarified, concentrated, and subjected to equilibrium sedimentation through a discontinuous sucrose step gradient. A density profile of such a gradient is shown in Fig. 3B. HBsAg concentration (Fig. 3B) peaked at 1.12 to 1.15 g/ml, corresponding to the density of subviral particles.
Additionally, samples from the sucrose gradient were probed using HBsAg and MV N antibodies in immunoblots (Fig. 3B, bottom). The MV N protein was localized mainly in fractions 8 and 9, with a buoyant density of 1.16 to 1.18 mg/ml, the expected density of MV particles (13), and thus physically separated from HBsAg (Fig. 3B, lower panels). This result indicates that most HBsAg is not incorporated in MV virions and instead is secreted in subviral particles.
HBsAg-vectored MVs elicit a robust immune response in transgenic mice.
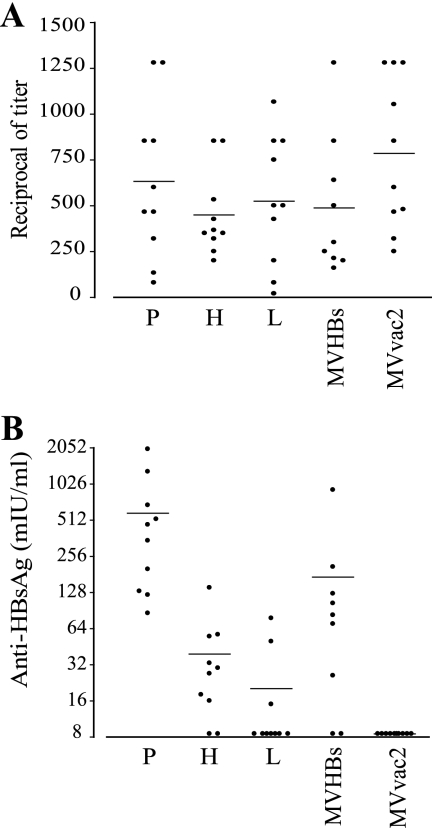
The efficiencies of vectored MVs in inducing humoral immune responses in Ifnarko-CD46Ge mice susceptible to MV infection were compared. These mice express the MV vaccine strain receptor human CD46 with human-like tissue specificity in an IFN type I receptor knockout background (29). Groups of 10 mice were inoculated intraperitoneally with the three new HBsAg-expressing recombinants. In addition, two groups of mice were inoculated with MVHBs as a reference (43) or with the parental strain, MVvac2, as a negative control. The anti-MV neutralization titer and the anti-HBsAg response were assessed 28 days postinoculation. As shown in Fig. 4A, all animals showed average MV neutralization titers in the 1:400 to 1:800 range.
FIG. 4.
Humoral immune response in genetically modified mice immunized with HBsAg-expressing MV. Groups of 10 animals were immunized with MVvac2(HBsAg)P (P), MVvac2(HBsAg)H (H), MVvac2(HBsAg)L (L), MVHBs, or MVvac2. Sera obtained 28 days postimmunization were assayed for MV neutralization (A; reciprocals of the titer) or for anti-HBsAg levels (B; values from quantitative ELISA). Each dot represents an animal; the mean of the group is indicated by a horizontal bar.
The anti-HBsAg response differed markedly between groups (Fig. 4B). The 10 mice immunized with MVvac2(HBsAg)P showed titers ranging from 80 to 2,000 mIU/ml, with an average of 590 mIU/ml. Eight of 10 mice inoculated with MVvac2(HBsAg)H had positive responses with titers up to 128 mIU/ml and an average of 39 mIU/ml. Only 3 of 10 mice immunized with MVvac2(HBsAg)L had an anti-HBsAg response with an average of 24 mIU/ml, around twice the protection level in humans (10 mIU/ml). The anti-HBsAg response observed in MVHBs-immunized mice was on average 140 mIU/ml, in line with a previous report (43). As expected none of the MVvac2-immunized mice had a positive anti-HBsAg response. Thus, HBsAg expression from position P induced an anti-HBsAg response 15 times higher than the one induced by the virus that expresses HBsAg from position H, which is remarkable because HBsAg expression levels differ only by a factor of about 2. Another twofold HBsAg expression level decrease in MVvac2(HBsAg)L also interfered strongly with HBsAg immunogenicity. To assess whether such effects occurred in another animal system, we inoculated macaques with the two viruses expressing HBsAg at the highest levels.
Vectored MV protects rhesus macaques from MV challenge.
To assess whether the vectored MV retained vaccine competence, groups of four rhesus macaques were immunized by the s.c. route with MVvac2 or its derivatives expressing HBsAg from position P or H. As controls, two animals were immunized with an equivalent dose of the vaccine Moraten strain. Two other monkeys were not vaccinated, for a total of 16 experimental animals that were all challenged 6 to 10 weeks after vaccination with 105 TCID50 of MV Davis87 by the conjuctival/intranasal route (26). To compare levels of protective efficacy and the induction of host responses, three quantitative parameters were measured: levels of viremia, anti-MV neutralization titers, and number of MV-specific IFN-γ-secreting T cells. These parameters were assayed before and after vaccination and challenge. In addition, rash appearance was scored.
Table 1 documents measles-specific rash appearance and levels of viremia. None of the animals developed a measles-specific skin rash at any time after vaccination. Viremia was assayed in PBMC samples collected 4, 7, and 14 days after immunization and challenge. Virus was isolated from two animals inoculated with MVvac2 and from one animal inoculated with MVvac2(HBsAg)P 7 days after inoculation. Low-level viremia, at ≤5 TCID50 per 106 PBMC, occurs after MV vaccination (47, 50). There was no rash or evidence of MV-induced suppression of the antibody response to tetanus immunization in all eight monkeys vaccinated with either MVvac2 or MVvac2(HBsAg)H in which the antitetanus antibody response was assayed (data not shown). In contrast, the two control naive animals presented a classic measles rash 7 days after challenge and levels of viremia of 103 to 104 TCID50 per 106 PBMC, characteristic of pathogenic MV infection (47, 51). One animal in three of the four vaccine groups had viremia, but at levels of 10 TCID50 or less per 106 PBMC.
TABLE 1.
Rash and viremia in immunized Rhesus monkeys
| Vaccine strain | Identification | Immunization
|
Challenge
|
||
|---|---|---|---|---|---|
| Rash | Viremiaa (TCID50/106 PBMC) | Rash | Viremiaa (TCID50/106 PBMC) | ||
| MVvac2 | 336 | No | 0 | No | 0 |
| 156 | No | 0 | No | 0 | |
| 157 | No | 100-100.75 | No | 100.75 | |
| 286 | No | 100-100.75 | No | 0 | |
| MVvac2(HBsAg)P | 547 | No | 0 | No | 0 |
| 676 | No | 100-100.75 | No | 0 | |
| 683 | No | 0 | No | 0 | |
| 782 | No | 0 | No | 0 | |
| MVvac2(HBsAg)H | 380 | No | 0 | No | 0 |
| 300 | No | 0 | No | 0 | |
| 360 | No | 0 | No | 0 | |
| 397 | No | 0 | No | 101 | |
| Moraten (Attenuvax) | 173 | No | 0 | No | 100.75 |
| 221 | No | 0 | No | 0 | |
| None (naive) | 183 | NAb | NA | Yes | 103.25 |
| 301 | NA | NA | Yes | 103.75 | |
On day 7 postimmunization or postchallenge.
NA, not applicable.
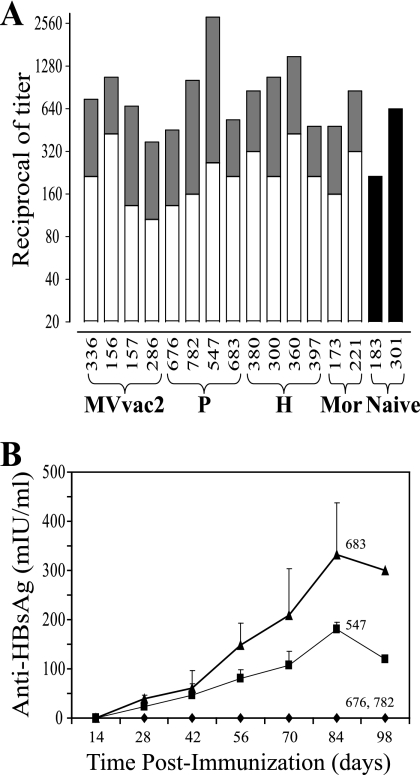
Figure 5A documents the MV neutralization titers 28 days after immunization or 28 days after challenge. Neutralization titers from 1:100 to 1:420 were monitored in response to vaccination. These titers increased by a factor of 3 to 10 in response to the challenge. The two naive monkeys developed neutralization titers of 1:200 and 1:640 28 days postchallenge, in line with previous reports (46, 47, 51).
FIG. 5.
Humoral immune response in rhesus monkeys immunized with HBsAg-expressing MV. (A) Monkeys were immunized with the vectored MV indicated at the bottom. P, MVvac2(HBsAg)P; H, MVvac2(HBsAg)H. Mor, monkeys immunized with a dose of Moraten vaccine. Naive, control animals that remained naive before challenge. Anti-MV neutralization titers measured 28 days postimmunization are indicated by the heights of the open bars, and the increase in titer measured 28 days postchallenge are indicated by the heights of the solid gray bars. Black bars, titers measured 28 days after challenge of naive monkeys. (B) Time course of the anti-HBsAg immune response in MVvac2(HBsAg)P-inoculated rhesus monkeys. The rhesus monkey identification number is shown above each curve. Averages and standard deviations of at least three independent determinations are shown.
To evaluate the cell-mediated immune (CMI) response, we isolated MV-specific IFN-γ-secreting T cells by ELISPOT at 0 and 30 days after vaccination and challenge (Table 2). Thirty days postvaccination, most of the animals immunized with recombinant MV presented a CMI response (group averages of 63 SFC/106 PBMC for MVvac2 and 44 and 55 SFC/106 PBMC for the P- and H-vectored HBsAg MV). This response was maintained until the day of challenge. Thirty days after challenge an increase in CMI response was documented in one animal per group. Overall, the CMI response observed in animals inoculated with vectored MV was equivalent to that from the Moraten (Attenuvax)-vaccinated animals and did not reveal differences between the groups. One monkey (no. 300) had detectable MV-specific T cells prior to vaccination at 7 SFC above the cutoff, which was unexpected.
TABLE 2.
MV-specific IFN-γ ELISPOT
| Vaccine strain | Identification | CMI responsea (SFC/106 PBMC) for:
|
|||
|---|---|---|---|---|---|
| Immunization on day:
|
Challenge on day:
|
||||
| 0 | 30 | 0 | 30 | ||
| MVvac2 | 336 | 0 | 16 | 0 | 29 |
| 156 | NDb | 85 | 108 | 111 | |
| 157 | 75 | 9 | ND | 83 | |
| 286 | 0 | 41 | ND | 21.5 | |
| MVvac2(HBsAg)P | 547 | 0 | 36 | 38 | 148 |
| 676 | 0 | 28 | 24 | 6 | |
| 683 | 7 | 74 | 28 | 67 | |
| 782 | 0 | 41 | 40 | 34 | |
| MVvac2(HBsAg)H | 380 | 0 | 18 | 60 | 58 |
| 300 | 27 | 57 | 54 | 150 | |
| 360 | 14 | 71 | 84 | 32 | |
| 397 | 0 | 38 | 70 | 45 | |
| Moraten (Attenuvax) | 173 | 5 | 136 | 139 | 161 |
| 221 | ND | 9 | 61 | 40 | |
| None (naive) | 183 | NAc | NA | 15 | 32 |
| 301 | NA | NA | 0 | 76 | |
Positive results (see Materials and Methods) are in boldface.
ND, not determined.
NA, not applicable.
HBsAg humoral response in macaques.
To assess the humoral immune response against HBsAg, antibody levels were measured by ELISA. Two of four monkeys immunized with MVvac2(HBsAg)P and no monkey vaccinated with MVvac2(HBsAg)H had an anti-HBsAg response. Figure 5B documents the time course of the HBsAg response in MVvac2(HBsAg)P-immunized monkeys: low levels 4 weeks postimmunization and a steady increase until week 12, reaching peak titers of 180 and 340 mIU/ml, or 18 to 34 times the reported protective level for humans. In summary, MVvac2 and its vectored derivatives elicited equivalent protection from pathogenic MV challenge as the standard vaccine. Only the vectored virus expressing HBsAg at the highest levels induced a strong immune response to HBsAg in two of four macaques.
DISCUSSION
We show here that vectored MVs expressing HBsAg are as effective as the live attenuated MV vaccine in protecting macaques from challenge with a pathogenic MV strain. Our study also revealed that only the MV expressing HBsAg at the highest levels elicited a strong humoral HBsAg response in some macaques. Similarly, in a rodent model HBsAg expression levels determined the strength of the humoral immune response but a lower expression level elicited a strong immune response. These observations have implications for the development of divalent vaccines based on MV and other live attenuated viruses.
Recombinant MVs with additional vaccine specificities have been generated and their immunogenicities tested in animal models (10, 24, 43, 50, 52). However, for none of these viruses was formal proof of retention of vaccine efficacy sought, as done here through challenge of vaccinated hosts with a macaque-adapted pathogenic MV strain. It appears likely that recombinant MVs with vaccine-identical coding sequences retain vaccine efficacy (8, 10, 24). However, in the original MV infectious cDNA (37) mutations interfering with the innate immunity control function of both V and P proteins exist (11, 30). Thus, recombinant viruses derived from it (43, 50, 52) may be overattenuated. We did not measure significant differences between the immunogenicity of the MVHBs vector (43) based on the original MV genomic cDNA and those of the new HBsAg-expressing viruses generated here. However, the IFN-defective mice we inoculated are not appropriate for investigating the effects of defects in viral proteins controlling innate immunity. On the other hand, another comparative analysis of the immunogenicities of MV derived from the original infectious cDNA and the Schwartz vaccine strain did suggest overattenuation (8).
The vast difference in the strength of the HBsAg-specific humoral immune response elicited from vectored MVs that express this protein at levels differing by a factor of 2 is remarkable. In macaques, only the vector expressing HBsAg at the highest level induced a detectable humoral response. In mice, this vector was strongly immunogenic; the vector expressing one-half as much HBsAg induced 10- to 20-times-lower levels of HBsAg antibodies, and the vector expressing about four times less HBsAg was weakly immunogenic in 3 of 10 animals. Thus, small HBsAg expression differences do have striking effects on the magnitude of humoral immune response induction in two different hosts.
The strength of the immune response may be strikingly concentration dependent also for antigens that, unlike HBsAg, do not form virus-like particles. A review of published studies on vectored MV indicates that most of the successful immunity induction strategies relied on P position expression, whereas the immune response to foreign proteins expressed from the ATU inserted at the H position was rarely documented (10, 24, 43, 50, 52). Other negative-strand RNA viruses with nonsegmented genomes have also been developed as vaccine vectors (2, 48). Several of the successful experimental vaccines are based on envelope protein exchange rather than on addition of the foreign envelope protein (3, 14, 19), but a bovine/human parainfluenza virus 3 expressing the human respiratory syncytial virus F glycoprotein in addition to the vector proteins has reached the clinical trial stage. This recombinant virus expresses the foreign antigen from upstream of N and therefore at the highest levels, another indication that high expression levels are important (41).
We did generate an MV expressing HBsAg from an ATU located upstream of N, but this virus reached titers of only 105 TCID50/ml (data not shown), which is 100 times lower than those of the other HBsAg-expressing viruses, suggesting interference with virus growth. We have expressed several other proteins from a location upstream of N; in this location, transcription initiates in the original position of N transcription initiation, conserving a hexameric phase supportive of high expression (23). Expression of most proteins at high levels did not result in interference effects, but two vectors had issues. First, a recombinant MV expressing the sodium-iodine symporter from upstream of N grew to low titers, whereas viruses expressing it from downstream of P or H grew to normal titers (12; D. Dingli and R. Cattaneo, unpublished observations). Second, a recombinant canine distemper virus expressing the green fluorescent protein from upstream of N was attenuated in ferrets, whereas viruses expressing it from downstream of P or H were fully virulent (49). Thus, high levels of expression of certain proteins, including HBsAg, can interfere with viral growth in cultivated cells or with spread in a natural host. On the other hand, we show here that strong HBsAg expression is necessary to elicit humoral immunity. Thus, for vaccination purposes proteins should be expressed at the highest levels compatible with efficient viral replication.
Expression of HBsAg from the L position was only 4 to 5 times lower than that from the P position, whereas in standard infections 10 to 20 times more P than L mRNAs are detected (6, 35). Thus, the ratio of HBsAg expression between the P and L positions was two to three times higher than expected. Several possibilities may account for this, including posttranscriptional effects or subtle differences in replication. Interestingly, initiation of transcription of the ATUs in the P and H positions was in the 0 hexameric phase, whereas initiation of transcription of the ATU in the L position is in the +5 hexameric phase.
It is possible that even the two macaques with negative HBsAg antibody counts after inoculation with MVvac2(HBsAg)P did develop HBV immunity. Chimpanzees inoculated with HBsAg-vectored vaccinia viruses or adenoviruses did not develop clinical hepatitis after HBV challenge, even when HBsAg antibodies had not been detected (25, 27). Moreover, a second dose of MVvac2(HBsAg)P may raise the HBsAg seroconversion rate. Alternatively, one dose of the protein-based HBV vaccine may be sufficient to elicit strong immunity in individuals primed with MVvac2(HBsAg)P that did not seroconvert. Finally, HBV immunity may be boosted through inoculation of other HBsAg-expressing vectors. For example, inoculation of children in need of variola virus or adenovirus immunization with HBsAg-vectored vaccinia viruses or adenoviruses may boost HBV immunity originally conferred by the MV-HBV vaccine. Thus, divalent or multivalent vaccines based on viruses from different families may be developed to offer appropriate vaccination solutions for the different necessities of populations or individuals.
Acknowledgments
We thank Heinz Schaller and Christa Kuhn for antibodies; Joseph Yao, Christoph Springfeld, and Chanakha Navaratnarajah for help and for reading the manuscript; Marie Frenzke and Sompong Vongpunsawad for excellent technical support; and Hannah Koble for secretarial assistance.
The National Institutes of Health (AI57761) and the Mayo Foundation supported this research. J.R.V. is a scholar of the National Researcher System, CONACyT, and IPN (Mexico).
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Boland, G., J. Beran, M. Lievens, J. Sasadeusz, P. Dentico, H. Nothdurft, J. N. Zuckerman, B. Genton, R. Steffen, L. Loutan, J. Van Hattum, and M. Stoffel. 2004. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 23:316-320. [DOI] [PubMed] [Google Scholar]
- 2.Bukreyev, A., M. H. Skiadopoulos, B. R. Murphy, and P. L. Collins. 2006. Nonsegmented negative-strand viruses as vaccine vectors. J. Virol. 80:10293-10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev, A., L. Yang, S. R. Zaki, W. J. Shieh, P. E. Rollin, B. R. Murphy, P. L. Collins, and A. Sanchez. 2006. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol. 80:2267-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassidy, W. M., B. Watson, V. A. Ioli, K. Williams, S. Bird, and D. J. West. 2001. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 107:626-631. [DOI] [PubMed] [Google Scholar]
- 5.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattaneo, R., G. Rebmann, A. Schmid, K. Baczko, V. ter Meulen, and M. A. Billeter. 1987. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 6:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo, R., and J. K. Rose. 1993. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J. Virol. 67:1493-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combredet, C., V. Labrousse, L. Mollet, C. Lorin, F. Delebecque, B. Hurtrel, H. McClure, M. B. Feinberg, M. Brahic, and F. Tangy. 2003. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 77:11546-11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutts, F. T., and L. E. Markowitz. 1994. Successes and failures in measles control. J. Infect. Dis. 170(Suppl. 1):S32-S41. [DOI] [PubMed] [Google Scholar]
- 10.Despres, P., C. Combredet, M. P. Frenkiel, C. Lorin, M. Brahic, and F. Tangy. 2005. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J. Infect. Dis. 191:207-214. [DOI] [PubMed] [Google Scholar]
- 11.Devaux, P., V. von Mesling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT 1 phosphorylation. Virology 360:72-83. [DOI] [PubMed] [Google Scholar]
- 12.Dingli, D., K. W. Peng, M. E. Harvey, P. R. Greipp, M. K. O'Connor, R. Cattaneo, J. C. Morris, and S. J. Russell. 2004. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 103:1641-1646. [DOI] [PubMed] [Google Scholar]
- 13.Galinski, M. S., and S. L. Wechsler. 1991. The molecular biology of the the paramyxovirus genus, p. 41-82. In D. W. Kingsbury (ed.), The paramyxoviruses. Plenum Press, New York, NY.
- 14.Geisbert, T. W., S. Jones, E. A. Fritz, A. C. Shurtleff, J. B. Geisbert, R. Liebscher, A. Grolla, U. Stroher, L. Fernando, K. M. Daddario, M. C. Guttieri, B. R. Mothe, T. Larsen, L. E. Hensley, P. B. Jahrling, and H. Feldmann. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerin, J. L., R. H. Purcell, M. D. Hoggan, P. V. Holland, and R. M. Chanock. 1969. Biophysical properties of Australia antigen. J. Virol. 4:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilleman, M. R. 2001. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651-665. [DOI] [PubMed] [Google Scholar]
- 17.Hinman, A. 1999. Eradication of vaccine-preventable diseases. Annu. Rev. Public Health 20:211-229. [DOI] [PubMed] [Google Scholar]
- 18.Iverson, L. E., and J. K. Rose. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477-484. [DOI] [PubMed] [Google Scholar]
- 19.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 20.Kamiyama, T., H. Sato, T. Takahara, S. Kageyama, and K. Shiraki. 2000. Novel immunogenicity of Oka varicella vaccine vector expressing hepatitis B surface antigen. J. Infect. Dis. 181:1158-1161. [DOI] [PubMed] [Google Scholar]
- 21.Karber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 22.Keating, G. M., and S. Noble. 2003. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 63:1021-1051. [DOI] [PubMed] [Google Scholar]
- 23.Kolakofsky, D., T. Pelet, D. Garcin, S. Hausmann, J. Curran, and L. Roux. 1998. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J. Virol. 72:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorin, C., L. Mollet, F. Delebecque, C. Combredet, B. Hurtrel, P. Charneau, M. Brahic, and F. Tangy. 2004. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 78:146-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubeck, M. D., A. R. Davis, M. Chengalvala, R. J. Natuk, J. E. Morin, K. Molnar-Kimber, B. B. Mason, B. M. Bhat, S. Mizutani, P. P. Hung, et al. 1989. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc. Natl. Acad. Sci. USA 86:6763-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McChesney, M. B., C. J. Miller, P. A. Rota, Y. D. Zhu, L. Antipa, N. W. Lerche, R. Ahmed, and W. J. Bellini. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 233:74-84. [DOI] [PubMed] [Google Scholar]
- 27.Moss, B., G. L. Smith, J. L. Gerin, and R. H. Purcell. 1984. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 311:67-69. [DOI] [PubMed] [Google Scholar]
- 28.Moszynski, P. 2007. Measles campaign's “historic victory” for global public health. Br. Med. J. 334:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mrkic, B., J. Pavlovic, T. Rulicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 85:2991-2999. [DOI] [PubMed] [Google Scholar]
- 31.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem. 2001. Analysis of the noncoding regions of measles virus strains in the Edmonston vaccine lineage. J. Virol. 75:921-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem. 2001. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J. Virol. 75:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pless, R. P., A. D. Bentsi-Enchill, and P. Duclos. 2003. Monitoring vaccine safety during measles mass immunization campaigns: clinical and programmatic issues. J. Infect. Dis. 187(Suppl. 1):S291-S298. [DOI] [PubMed] [Google Scholar]
- 35.Plumet, S., W. P. Duprex, and D. Gerlier. 2005. Dynamics of viral RNA synthesis during measles virus infection. J. Virol. 79:6900-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Premenko-Lanier, M., P. A. Rota, G. H. Rhodes, W. J. Bellini, and M. B. McChesney. 2004. Protection against challenge with measles virus (MV) in infant macaques by an MV DNA vaccine administered in the presence of neutralizing antibody. J. Infect. Dis. 189:2064-2071. [DOI] [PubMed] [Google Scholar]
- 37.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rager, M., S. Vongpunsawad, W. P. Duprex, and R. Cattaneo. 2002. Polyploid measles virus with hexameric genome length. EMBO J. 21:2364-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493-497. [Google Scholar]
- 40.Richardson, C. D., A. Scheid, and P. W. Choppin. 1980. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology 105:205-222. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, A. C., D. R. Wenzke, J. M. McAuliffe, M. St Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2002. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J. Virol. 76:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiraki, K., Y. Hayakawa, H. Mori, J. Namazue, A. Takamizawa, I. Yoshida, K. Yamanishi, and M. Takahashi. 1991. Development of immunogenic recombinant Oka varicella vaccine expressing hepatitis B virus surface antigen. J. Gen. Virol. 72:1393-1399. [DOI] [PubMed] [Google Scholar]
- 43.Singh, M., R. Cattaneo, and M. A. Billeter. 1999. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J. Virol. 73:4823-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, G. L., M. Mackett, and B. Moss. 1983. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 302:490-495. [DOI] [PubMed] [Google Scholar]
- 45.Strebel, P. M., M. J. Papania, and N. A. Halsey. 2004. Measles vaccine, p. 389-440. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 4th ed. Saunders-Elsevier, Philadelphia, PA.
- 46.van Binnendijk, R. S., M. C. Poelen, G. van Amerongen, P. de Vries, and A. D. Osterhaus. 1997. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 175:524-532. [DOI] [PubMed] [Google Scholar]
- 47.van Binnendijk, R. S., R. W. van der Heijden, G. van Amerongen, F. G. UytdeHaag, and A. D. Osterhaus. 1994. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J. Infect. Dis. 170:443-448. [DOI] [PubMed] [Google Scholar]
- 48.von Messling, V., and R. Cattaneo. 2004. Toward novel vaccines and therapies based on negative strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:281-312. [DOI] [PubMed] [Google Scholar]
- 49.von Messling, V., D. Milosevic, and R. Cattaneo. 2004. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 101:14216-14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Z., L. Hangartner, T. I. Cornu, L. R. Martin, A. Zuniga, M. A. Billeter, and H. Y. Naim. 2001. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine 19:2329-2336. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, Y. D., J. Heath, J. Collins, T. Greene, L. Antipa, P. Rota, W. Bellini, and M. McChesney. 1997. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology 233:85-92. [DOI] [PubMed] [Google Scholar]
- 52.Zuniga, A., Z. Wang, M. Liniger, L. Hangartner, M. Caballero, J. Pavlovic, P. Wild, J. F. Viret, R. Glueck, M. A. Billeter, and H. Y. Naim. 2007. Attenuated measles virus as a vaccine vector. Vaccine 25:2974-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]