Abstract
Induction of autophagy has been shown to be beneficial for the replication of poliovirus, a phenomenon that might also apply for other picornaviruses. We demonstrate that de novo synthesis of human rhinovirus type 2 (HRV2), an HRV of the minor receptor group, is unaffected by tamoxifen, rapamycin, and 3-methyladenine (3-MA), drugs either stimulating (tamoxifen and rapamycin) or inhibiting (3-MA) autophagic processes. Furthermore, LC3-positive vesicles (i.e., autophagosomes) are not induced upon infection. Therefore, multiplication of this particular picornavirus is not dependent on autophagy.
Human rhinoviruses (HRVs) are responsible for roughly 50% of respiratory tract infections. As picornaviruses, they are nonenveloped, with a positive-sense RNA genome (12). Of the 99 HRV serotypes, 12 (the minor group) use members of the low-density lipoprotein receptor family and 87 (the major group) use intercellular adhesion molecule 1 for cell entry (13). The prototype minor-group virus HRV2 is internalized and routed to late endosomes, where RNA uncoating occurs (2, 9). Subsequently, viral protein synthesis is initiated and followed by RNA replication. Finally, infective viruses are assembled and released from the host cell. Replication has been most extensively studied for the closely related poliovirus, and Schlegel and coworkers found double-membraned vesicles in infected cells. These compartments could be immunoisolated with an antibody against viral protein 2C and contained markers of the secretory pathway and lysosomal membrane protein 1 (LAMP-1), indicative of an autophagic origin (11). Furthermore, induction of autophagy by tamoxifen and rapamycin increased poliovirus yield (6). Based on immunofluorescence colocalization of green fluorescent protein (GFP)-microtubule-associated protein light chain kinase 3 (LC3) (an autophagosome marker) and LAMP-1 as well as staining with monodansylcadaverine (MDC), autophagosomes were also observed after infection with HRV2 and the major-group virus HRV14. Focusing on HRV2, we therefore investigated whether replication of this rhinovirus is also affected by modulators of autophagy.
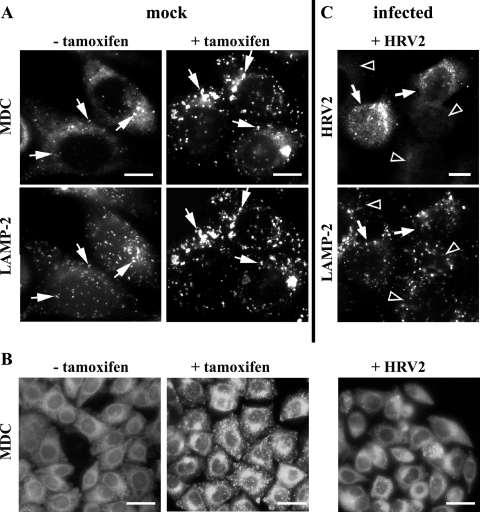
Autophagosomes are induced by treatment with tamoxifen in cells expressing the estrogen receptor. In accordance with Bursch et al. (3), 1 μM tamoxifen was sufficient to induce autophagy in all HeLa-H1 cells after 48 h. MDC and LAMP-2 staining was used to reveal autophagic vacuoles (3, 5). Following incubation with tamoxifen, MDC (10 μM in dimethyl sulfoxide-ethanol) was added for 1 h prior to fixation with 4% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS) for 15 min at room temperature (3). The cells were viewed under a Zeiss Axioscop2 microscope. Subsequently, cells were permeabilized with 0.02% Triton X-100 in PBS for 10 min, quenched with 50 mM NH4Cl in PBS, and incubated with monoclonal anti-LAMP-2 antibody (1:200; H4B4; BD Pharmingen) followed by Alexa 488-labeled goat anti-mouse immunoglobulin G (1:1,000; Invitrogen). The samples were washed and mounted in glycerol-PBS (9:1), and the areas previously photographed for MDC staining were relocated for digital imaging. As depicted in Fig. 1A, tamoxifen treatment induced large, MDC-positive compartments that also contained LAMP-2. Then, the effects of viral infection on MDC (Fig. 1B) and LAMP-2 (Fig. 1C) staining were studied. Cells were challenged for 1 h with HRV2 (300 50% tissue culture infective doses [TCID50]/cell; 34°C), washed, and further incubated. MDC was added at 5.5 h postinfection (p.i.), and the cells were fixed and immediately photographed as described above. For comparison, a tamoxifen control was included (Fig. 1B) and showed the expected increase of vesicular MDC staining (compare to Fig. 1A). No such increase was seen in the infected cells (Fig. 1B, right). Next, cells infected as described above were fixed and permeabilized at 6.5 h p.i. and stained for viral protein with rabbit anti-HRV2 antiserum (1:100) followed by Alexa 568-conjugated goat anti-rabbit immunoglobulin G (1:1,000; Invitrogen) (Fig. 1C, upper) and for LAMP-2 as described for Fig. 1A (Fig. 1C, lower). No increase in the intensity or number of LAMP-2-positive compartments was seen in cells replicating virus compared to that in cells not expressing viral proteins (Fig. 1C).
FIG. 1.
Visualization of autophagosomes by MDC and LAMP-2. (A) Cells were incubated with (+) or without (−) 1 μM tamoxifen for 48 h and stained with MDC and anti-LAMP-2 antibody. A few LAMP-2-positive vesicles (arrows) were also stained with MDC in the absence of tamoxifen. However, large MDC- and LAMP-2-positive compartments are induced in all cells by the drug (arrows). (B) Control cells with or without tamoxifen and cells infected with HRV2 at 300 TCID50/cell were stained with MDC. Infected cells lacked the typical tamoxifen-induced MDC staining (right). (C) Infected cells were stained with anti-HRV2 and anti-LAMP-2 antibodies followed by Alexa 568 and Alexa 488-conjugated secondary antibodies. No alteration in LAMP-2-positive compartments was observed in cells actively replicating virus (arrows). Arrowheads, noninfected cells. Bars, 10 μm (A, C) and 30 μm (B).
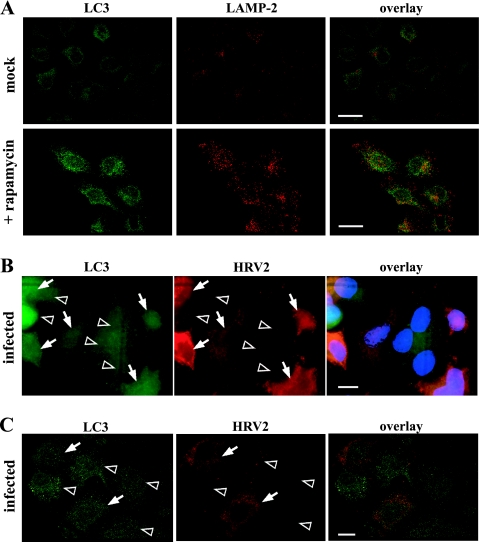
To further verify the lack of induction of autophagic vacuoles in HRV2-replicating cells, endogenous LC3 was monitored (4, 10). As a control, autophagic vesicles were induced by rapamycin (100 nM, 13 h) (7), and after permeabilization with 0.1% saponin, LC3 was detected with rabbit anti-LC3 antibody PD015 (MBL; 1:300) and LAMP-2 with mouse anti-LAMP-2 antibodies. As expected, rapamycin treatment resulted in increased and costaining of the two markers (Fig. 2A). Upon infection (Fig. 2B), virus-producing cells were revealed with monoclonal anti-HRV2 antibody (8F5; 1:500) (2). No increased LC3-staining was seen in HRV2-replicating cells compared to that in noninfected cells (Fig. 2B). It is of note that LC3 expression levels differed to similar extents in infected and noninfected cells. Furthermore, no colocalization between HRV2 replication sites and LC3 was observed (Fig. 2C).
FIG. 2.
HRV2 infection does not induce LC3-positive vesicles. (A) Mock- or rapamycin-treated cells (100 nM, 13 h) (A) and HRV2-infected cells (1-h challenge with 300 TCID50/cell) (B, C) were stained at 7.5 h p.i. for LC3 and LAMP-2 or for LC3 and HRV2. Arrows, HRV2-positive cells; arrowheads, noninfected cells. Bars, 50 μm (A) and 10 μm (B, C). Panels A and C are confocal images. Compared to that in rapamycin-treated cells (A), LC3 in infected cells is not increased (B) and does not colocalize with HRV2 (C).
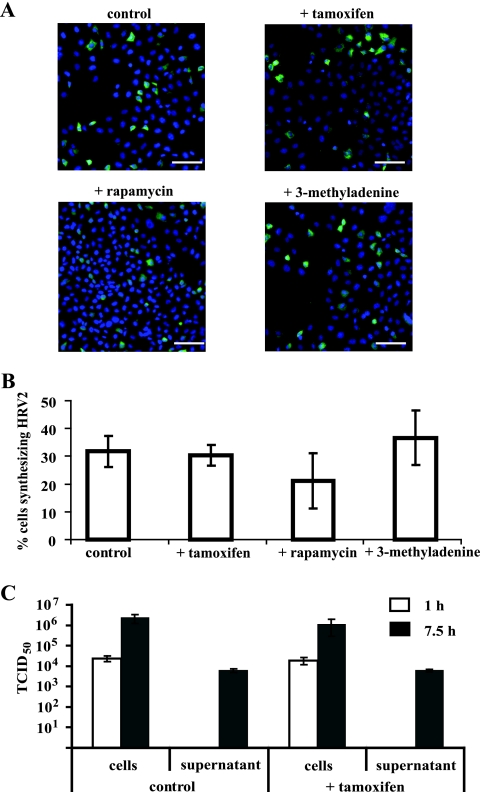
Finally, we analyzed whether the autophagy inducers tamoxifen and rapamycin or the inhibitor 3-methyladenine (3-MA) (8) affects viral yield. Cells were pretreated with tamoxifen for 48 h, with rapamycin for 13 h, or with 5 mM 3-MA for 3 h, and virus was internalized for 1 h at 34°C. After further incubation in culture medium for 6.5 h in the presence of the respective drug, viral proteins and nuclei were revealed by fluorescence microscopy (Fig. 3A) as described above. HRV2-positive cells were quantified using TissueQuest software (TissueGnostics Gmbh, Vienna) by analyzing 10,000 cells/treatment (Fig. 3B). About 32% of the cells were producing virus, with no significant difference upon drug treatment. This is in line with the lack of influence of tamoxifen on viral titer (Fig. 3C). The amounts of virus taken up during 1 h and of de novo-synthesized, cell-associated virus at 7.5 h p.i. were virtually identical in the absence and presence of the drug. In addition, no influence of tamoxifen on virus release into the supernatant was observed. Taken together, our data demonstrate that autophagic vesicles are not involved in replication or release of HRV2 in HeLa cells.
FIG. 3.
Modulation of autophagy does not affect HRV2 synthesis. Cells were incubated with (+) or without 1 μM tamoxifen for 48 h, with 100 nM rapamycin for 13 h, or with 5 mM 3-MA for 3 h. (A) HRV2 (300 TCID50/cell) was internalized for 1 h, and cells were further incubated for 6.5 h in the presence of the respective drug. Virus was visualized with monoclonal antibody 8F5 and nuclei with Hoechst dye. Bars, 50 μm. (B) Cells replicating virus were quantified with respect to the total number of cells per group (∼10,000) by using TissueQuest software. Data are means ± standard deviations for 15 images. (C) Cells were pretreated with tamoxifen and infected as described for panel A but with 100 TCID50/cell, and the amount of infectious virus cell associated or released into the supernatant was determined at 1 and 7.5 h p.i. Data are means ± standard deviations for four parallel samples.
Autophagy generates sites that may facilitate virus replication (14). For poliovirus, Jackson and colleagues demonstrated that modulation of autophagy affected virus yield as well as release from the cell (6). Moreover, they observed colocalization of poliovirus 3A with GFP-LC3 in infected cells. For HRV2, they took colocalization of GFP-LC3 and LAMP-1 only to indicate autophagosome formation following infection. Using a different experimental approach, we demonstrate that (i) endogenous LC3 and LAMP-2 are unchanged in cells actively replicating HRV2 and (ii) these markers do not colocalize with viral proteins, but on the other hand, (iii) infection is affected neither by induction of autophagy by tamoxifen or by rapamycin acting via different signaling pathways nor by inhibition of autophagy by 3-MA, and finally, (iv) viral yield and virus release into the medium are not increased by tamoxifen treatment. These results are in line with previous data demonstrating that the phosphatidylinositol 3-kinase and autophagy inhibitor wortmannin (8) does not modify viral yield (1). Thus, at least this particular rhinovirus serotype does not induce the synthesis of LAMP-2- and LC3-positive compartments, nor does modification of autophagy result in increased viral synthesis.
Acknowledgments
This work was supported by Austrian Science Fund P17590 to R.F. and Austrian Academy of Sciences DOC-FORTE to U.B.
We thank Irene Goesler for TCID50 determination and Francesca Demarchi for providing the detailed protocol for LC3 staining.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Brabec, M., D. Blaas, and R. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348:741-749. [DOI] [PubMed] [Google Scholar]
- 2.Brabec, M., D. Schober, E. Wagner, N. Bayer, R. F. Murphy, D. Blaas, and R. Fuchs. 2005. Opening of size-selective pores in endosomes during human rhinovirus serotype 2 in vivo uncoating monitored by single-organelle flow analysis. J. Virol. 79:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bursch, W., K. Hochegger, L. Torok, B. Marian, A. Ellinger, and R. S. Hermann. 2000. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J. Cell Sci. 113:1189-1198. [DOI] [PubMed] [Google Scholar]
- 4.Demarchi, F., C. Bertoli, T. Copetti, I. Tanida, C. Brancolini, E. L. Eskelinen, and C. Schneider. 2006. Calpain is required for macroautophagy in mammalian cells. J. Cell Biol. 175:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Polo, R. A., P. Boya, A. L. Pauleau, A. Jalil, N. Larochette, S. Souquere, E. L. Eskelinen, G. Pierron, P. Saftig, and G. Kroemer. 2005. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J. Cell Sci. 118:3091-3102. [DOI] [PubMed] [Google Scholar]
- 6.Jackson, W. T., T. H. Giddings, Jr., M. P. Taylor, S. Mulinyawe, M. Rabinovitch, R. R. Kopito, and K. Kirkegaard. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai, A., S. Takano, N. Nakamura, and S. Ohkuma. 2006. Quantitative monitoring of autophagic degradation. Biochem. Biophys. Res. Commun. 351:71-77. [DOI] [PubMed] [Google Scholar]
- 8.Meley, D., C. Bauvy, J. H. Houben-Weerts, P. F. Dubbelhuis, M. T. Helmond, P. Codogno, and A. J. Meijer. 2006. AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 281:34870-34879. [DOI] [PubMed] [Google Scholar]
- 9.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar, S., R. A. Floto, Z. Berger, S. Imarisio, A. Cordenier, M. Pasco, L. J. Cook, and D. C. Rubinsztein. 2005. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlegel, A., T. H. Giddings, M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semler, B. L., and E. Wimmer. 2002. Molecular biology of picornaviruses. ASM Press, Washington, DC.
- 13.Vlasak, M., M. Roivainen, M. Reithmayer, I. Goesler, P. Laine, L. Snyers, T. Hovi, and D. Blaas. 2005. The minor receptor group of human rhinovirus (HRV) includes HRV23 and HRV25, but the presence of a lysine in the VP1 HI loop is not sufficient for receptor binding. J. Virol. 79:7389-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wileman, T. 2006. Aggresomes and autophagy generate sites for virus replication. Science 312:875-878. [DOI] [PubMed] [Google Scholar]