Abstract
N-methyl-d-aspartate (NMDA) glutamate receptor-mediated increases in intracellular calcium are thought to play a critical role in synaptic plasticity. The mechanisms by which changes in cytoplasmic calcium transmit the glutamate signal to the nucleus, which is ultimately important for long-lasting neuronal responses, are poorly understood. We show that NMDA receptor stimulation leads to activation of p21ras (Ras) through generation of nitric oxide (NO) via neuronal NO synthase. The competitive NO synthase inhibitor, l-nitroarginine methyl ester, prevents Ras activation elicited by NMDA and this effect is competitively reversed by the NO synthase substrate, l-arginine. NMDA receptor stimulation fails to activate Ras in neuronal cultures from mice lacking neuronal NO synthase. NMDA-induced Ras activation occurs through a cGMP-independent pathway as 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ), a potent and selective inhibitor of guanylyl cyclase, has no effect on NMDA receptor-induced activation of Ras, and the cell-permeable cGMP analog, 8Br-cGMP, does not activate Ras. Furthermore, NO directly activates immunoprecipitated Ras from neurons. NMDA also elicits tyrosine phosphorylation of extracellular signal-regulated kinases, a downstream effector pathway of Ras, through a NO/non-cGMP dependent mechanism, thus supporting the physiologic relevance of endogenous NO regulation of Ras. These results suggest that Ras is a physiologic target of endogenously produced NO and indicates a signaling pathway for NMDA receptor activation that may be important for long-lasting neuronal responses.
Neuronal survival, differentiation, and plasticity involve signal transduction cascades that occur in large part through activation of p21ras (Ras) (1–4). Ras is highly expressed in the developing and adult nervous systems and plays an important classical role in mediating growth factor responses (1–4). Recent studies suggest that increases in intracellular calcium also can activate Ras (5–10). Activation of Ras by increases in cytoplasmic calcium levels may play critical roles in mediating and/or modulating activity-induced changes such as neuronal differentiation, synaptic strength, and neuronal survival, in part, through activation of extracellular signal-regulated kinases (Erks) (5–12). The molecular mechanisms by which changes in intracellular calcium levels in neurons activate Ras is not known. However, recent studies in tumor cell lines suggest the existence of multiple pathways that could be important in generating calcium-mediated activation of Ras, including Src, Ras-GRF, PYK2, and epidermal growth factor receptor (5–10). Despite the identification of these calcium-dependent pathways to Ras, none of these pathways have been directly demonstrated to mediate calcium-dependent Ras activation in neurons (5–10).
Nitric oxide (NO) is an important messenger molecule with many diverse actions in the nervous, vascular, and immune systems (13–15). NO is produced in a calcium/calmodulin-dependent fashion from l-arginine (l-Arg) by the enzyme NO synthase (NOS) (16, 17). A family of related NOS proteins are the products of different genes and include neuronal NOS (nNOS-type 1), macrophage or immunologic NOS (iNOS-type 2), and endothelial NOS (eNOS-type 3) (16, 17). nNOS is localized to discreet neuronal populations (18–20) and is transiently expressed during neuronal development (21, 22) and in response to neuronal injury (18, 23, 24). Despite the involvement of NO in glutamate neurotoxicity (18, 25, 26), there is some suggestion that NO may be involved in neuronal survival, differentiation, and plasticity (20, 27–31). Preliminary studies showed that exogenously applied NO activates Ras in tumor cell lines through redox-sensitive mechanisms (32, 33). Because NO generation in neurons is calcium dependent and NO may play a role in activity-dependent changes in neuronal function, we wondered whether endogenous NO could activate Ras and its downstream effector, the Erk pathway, in response to N-methyl-d-aspartate (NMDA) glutamate receptor activation. We now report that endogenous NO is a major mediator of calcium-dependent activation of Ras through NMDA receptor stimulation in neurons.
MATERIALS AND METHODS
Cell Culture.
Primary cortical cultures were prepared from gestational day 15–16 fetal rats or mice in a procedure modified from that described previously (34). Briefly, the cortex from fetal mice was dissected, and the cells were dissociated by trituration in modified Eagle’s medium (MEM), 20% horse serum, 25 mM glucose, and 2 mM l-glutamine after a 30-min digestion in 0.027% trypsin/saline solution. Cortex from fetal rats was dissociated by trituration in MEM, 10% fetal bovine serum, 10% horse serum, and 2 mM l-glutamine after a 30-min digestion in 0.027% trypsin/saline solution. The cells were plated on 15-mm multiwell plates coated with polyornithine. Four days after plating, the cells were treated with 5-fluoro-2-deoxyuridine for 3 days to inhibit proliferation of non-neuronal cells. Mice cultures then were maintained in MEM, 10% horse serum, 25 mM glucose, and 2 mM l-glutamine in an 8% CO2 humidified 37°C incubator. Rat cultures then were maintained in MEM, 5% horse serum, and 2 mM l-glutamine in an 8% CO2 humidified 37°C incubator. The growth medium was refreshed twice per week, and the neurons were allowed to mature for 14 days in culture when nNOS is expressed at mature levels (34).
In Situ Ras Activation Assay.
Ras activity assessments were performed as described (11). Briefly, primary cultures of cortical neurons were metabolically labeled for 4 hr at 37°C in a solution containing 10 mM Hepes (pH 7.4), 114 mM NaCl, 26 mM NaHCO3, 5.3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM glucose, 1 mM glycine, 0.5 mM sodium pyruvate, plus 1 mCi/ml [32P]H3PO4 and 1 μM tetrodotoxin. After metabolic labeling, cells were pretreated with 500 μM l-nitroarginine methyl ester (l-NAME) or 500 μM l-NAME plus 5 mM l-Arg for 10 min before stimulation with 50 nM-500 μM NMDA or 0.1 mM α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid or 0.1 mM kainate for 5 min. Cells were lysed 0.5 min later in 0.1 ml/well of lysis buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM MgCl2/1% Triton X-100) containing 2 μg of anti-Ras mAb Y13–259 (Oncogene Science). Extracts were drawn 12 times through a 0.22 gauge needle, mixed with 1:10 vol of PBS/1% BSA/10% charcoal slurry, and rocked at 4°C for 45 min. After centrifugation for 10 min at 14,000 rpm, the supernatants were mixed with 20 μl of protein A Sepharose precoupled to 2 μg of goat anti-rat secondary antibody. Samples were rocked at 4°C for 45 min, and washed once with lysis buffer and once with PBS. Pellets were resuspended in 12 μl of 1 M KH2PO4 (pH 3.4) and incubated at 85°C for 5 min. Samples were centrifuged, and the supernatants were transferred to new tubes and stored at −20°C overnight. The next day samples were spotted onto polyethyleneimine-cellulose TLC plates (EM Science), and guanine nucleotides were eluted and separated in 1 M KH2PO4 (pH 3.4) for 3 hr.
In Vitro Ras Activation Assay.
Ras assays were performed as described previously for in vitro Ras activity (35). Briefly, rat cortical neurons were harvested and lysed with 1 ml of lysis buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM MgCl2/1% Triton X-100) containing 1 μg of anti-Ras mAb Y13–259 (Oncogene Science). Samples were incubated for 30 min at 4°C with 10 μl of protein A-Sepharose precoupled to 1 μg goat anti-rat secondary antibody and then washed three times with lysis buffer. [35S]GTPγS binding assay was performed with immunoprecipitated Ras, and data were presented as percent of the control [(cpm of [35S]GTPγS bound to immunoprecipitated Ras) − (cpm of [35S]GTPγS bound to goat anti-rat secondary antibody-coupled protein A-Sepharose)].
Tyrosine Phosphorylation of Erk.
Cultures were pretreated with 1 μM of tetrodotoxin for 30 min, and glutamate receptor agonists were applied for 5 min. At various time points after stimulation, cell extracts were lysed in boiling SDS sample buffer (160 mM Tris⋅HCl, pH 6.8/4% SDS/30% glycerol/5% β-mercaptoethanol/0.02% bromophenol blue). Lysates were separated by electrophoresis on 10% SDS-polyacrylamide gels and electrotransferred to nitrocellulose membranes. Membranes were incubated for 1 hr at room temperature in blocking solution [5% (wt/vol) nonfat dry milk in TTBS buffer (20 mM Tris⋅HCl, pH 7.6/137 mM NaCl/0.05% Tween-20)] and for 1 hr in phospho-specific anti-Erk antibody and control anti-Erk antibody (New England Biolabs) (1:1,000 dilution) in TTBS. After four washes with TTBS, membranes were incubated for 20 min with donkey anti-rabbit IgG-horseradish peroxidase (Amersham) (1:5,000 dilution) in TTBS. After four washes with TTBS, membranes were subjected to ECL (Amersham) detection.
RESULTS
NMDA Receptor Stimulation Activates Ras.
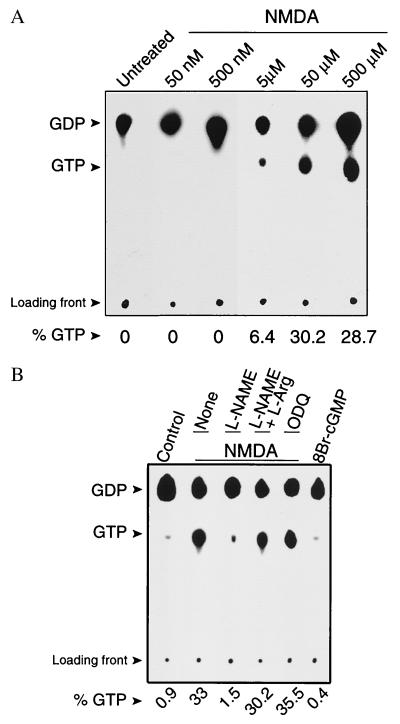
To directly investigate whether NO activates endogenous Ras in response to NMDA receptor stimulation in intact cortical neurons we used an in situ GTP loading assay in which Ras activation is monitored by the exchange of bound GDP for GTP (11) (Fig. 1). NMDA receptor stimulation potently increases the proportion of GTP bound to Ras, reflecting Ras activation. The activation of Ras by NMDA is dose-dependent with 50 μM NMDA eliciting maximal activation (Fig. 1A). This activation is blocked by the competitive NOS inhibitor, l-NAME (Fig. 1B). To confirm the specificity of the blockade of NMDA-induced Ras activation by l-NAME we examined the effects of adding excess substrate, l-Arg. l-Arg completely reverses the blockade of NMDA-induced Ras activation by l-NAME (Fig. 1B). Furthermore, the inactive d-isomer of l-NAME, d-NAME has no effect on NMDA-induced Ras activation (data not shown). Because a majority of NO’s physiologic actions are thought to be mediated through increases in cGMP through activation of soluble guanylyl cyclase (GC), we evaluated the effects of the potent and selective inhibitor of GC, 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ) (36), on NMDA-stimulated activation of Ras as well as the effects of the cell-permeable cGMP analog, 8-Br-cGMP, on Ras activation (Fig. 1B). ODQ fails to inhibit NMDA-stimulated Ras activation and 8Br-cGMP fails to activate Ras, thus ruling out a possible secondary activation of Ras through NO-activating GC (Fig. 1B). α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and kainate in the presence of 5 μM of MK801 to prevent secondary activation of NMDA receptors failed to activate Ras (data not shown).
Figure 1.
(A) In situ Ras activation assay on primary rat cortical neuronal cultures shows that NMDA induces Ras activation in a dose-dependent manner. Activated Ras is indicated by the detection of GTP on TLC plates. Cells were exposed to control saline solution (untreated) or treated with increasing concentrations of NMDA (50 nM to 500 μM) for 5 min. (B) NMDA induces Ras activation through an NO-dependent, cGMP-independent mechanism. Cells were exposed to control saline solution (Control), or treated with 50 μM NMDA for 5 min with no previous pretreatment (None), or pretreated for 10 min with 500 μM l-NAME, 500 μM l-NAME plus 5 mM l-Arg, or 10 μM ODQ before exposure to 50 μM NMDA for 5 min. Cells also were treated with 5 mM 8Br-cGMP for 5 min. Cells were lysed and collected for in situ Ras activation assay 0.5 min after each of the treatments. The NOS inhibitor, l-NAME, blocks NMDA-induced activation of Ras, and this blockade is reversed by the NOS substrate, l-Arg. Inhibition of GC by ODQ has no effect on NMDA-induced activation of Ras, and 8Br-cGMP does not activate Ras. Shown is a representative experiment. These results have been replicated three times with similar results.
To confirm the potential involvement and to determine the source of NO in NMDA-stimulated Ras activation we evaluated the ability of NMDA to activate Ras in neuronal cultures from mice lacking the gene for nNOS (nNOS−/−) (37) (Fig. 2). NMDA (50 μM) fails to activate Ras in cortical cultures from nNOS−/− mice. NMDA (500 μM) also did not activate Ras in nNOS−/− cortical cultures (data not shown). Brain-derived nerve growth factor (BDNF) (100 ng/ml) activates Ras in nNOS−/− cortical cultures, thus indicating that Ras is still functional in nNOS−/− cultures (Fig. 2A). NMDA (50 μm) and BDNF (100 ng/ml) potently activate Ras in wild-type cortical cultures (Fig. 2B).
Figure 2.
(A) In situ Ras activation assay on primary cortical neuronal cultures from nNOS−/− mice shows that NMDA fails to activate Ras, and that Ras can be activated in these cultures by BDNF. Cultures from nNOS−/− mice or wild-type mice were left untreated or exposed to 50 μM NMDA for 5 min, or to recombinant 100 ng/ml BDNF (Intergen, Purchase, NY) for 10 min. (B) NMDA induces Ras activation in neuronal cultures from wild-type littermates of the nNOS−/− mice. Cells were collected for in situ Ras activation assay 0.5 min after each treatment. Shown is a representative experiment. These results have been replicated twice with similar results.
NO Directly Activates Ras in Cortical Neurons.
To ascertain whether NO itself elicits Ras activation in cortical neurons we assessed Ras activity via a GTPγS binding assay (Fig. 3) (35). Ras protein immunoprecipitated from primary cortical neuronal culture extracts is potently activated by the NO releasors sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine (SNAP) in a dose-dependent manner. At higher concentrations SNAP modestly decreases Ras activity. SNP and SNAP depleted of NO by incubating SNP and SNAP in buffer for 24 hr under light fails to activate Ras (data not shown), thus excluding potential nonspecific effects of the parent compounds.
Figure 3.
NO directly activates Ras. Ras immunopurified from cultured cortical neurons was assayed in vitro for [35S]GTPγS binding in the presence of various concentrations of SNP (•) or SNAP (○). These experiments have been replicated three times with similar results.
NO Mediates Tyrosine Phosphorylation of Erk.
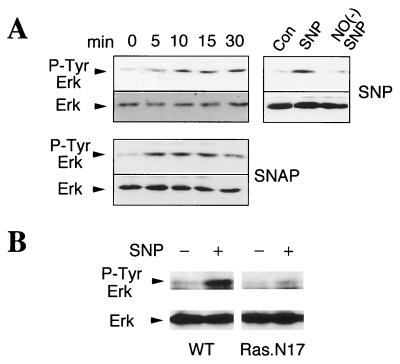
Tyrosine phosphorylation of Erk is thought to be mediated through a Ras-dependent pathway (1–3). To ascertain whether NO itself elicits tyrosine phosphorylation of Erk in cortical neurons we administered the NO donors, SNP and SNAP (Fig. 4A). A brief (5 min) pulse of SNP and SNAP (500 μM each) leads to tyrosine phosphorylation of p42 Erk with different time course kinetics presumably caused by the different NO release rate of each compound. At 30 min there is a modest decrease in Erk phosphorylation elicited by SNAP. SNP incubated overnight in buffer to deplete SNP of NO does not cause tyrosine phosphorylation of p42 Erk (Fig. 4A). To directly determine whether NO activates Ras, which then elicits tyrosine phosphorylation of Erk in neuronal cells, we monitored tyrosine phosphorylation of Erk in wild-type pheochromocytoma cells (PC12) and a mutant PC12 cell line containing a dominant negative mutant of Ras (Ras.N17) (38). In wild-type PC12 cells SNP markedly stimulates tyrosine phosphorylation of p42 Erk. In striking contrast, SNP barely stimulates tyrosine phosphorylation of p42 Erk in Ras.N17 cells (Fig. 4B). Thus, NO-induced phosphorylation of Erk requires functional Ras.
Figure 4.
Activation of Erk by NO occurs through Ras activation. (A) Time course of Erk tyrosine phosphorylation of cortical cultures treated with SNP or SNAP (500 μM each, 5 min); NO(−)SNP was obtained by incubation of 50 mM SNP at room temperature under light for at least 24 hr. (B) Wild-type (WT) PC12 cells or PC12 cells containing dominant negative mutant Ras (Ras.N17) were treated with SNP (500 μM, 5 min) and analyzed for Erk tyrosine phosphorylation after 5 min. These experiments have been replicated three times with similar results, and each individual blot was stripped and probed with control anti-Erk antibody.
NO Mediates NMDA Receptor-Induced Erk Activation.
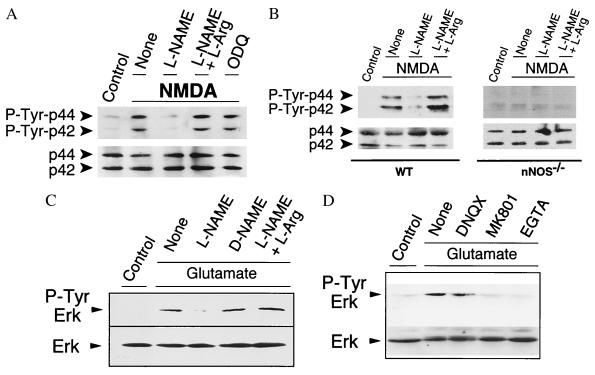
To further evaluate the significance of NMDA receptor-induced activation of Ras through NO and its potential physiologic relevance we explored the potential role of NO in mediating NMDA receptor-stimulated tyrosine phosphorylation of Erk. The NOS inhibitor, l-NAME, dramatically reduces NMDA-mediated tyrosine phosphorylation of p42 and p44 Erk (Fig. 5A). To confirm the specificity of the blockade of NMDA-induced p42 and p44 Erk phosphorylation by l-NAME we examined the effects of adding excess substrate, l-Arg. l-Arg completely reverses the reduction in tyrosine phosphorylation by l-NAME (Fig. 5A). ODQ (10 μM) fails to inhibit NMDA-stimulated phosphorylation of Erk, thus ruling out secondary activation of Erk through increases in cGMP by NO activating GC. Reduced hemoglobin (500 μM), which complexes and inactivates NO, blocks Erk activation by NMDA (data not shown). Thus, NO diffuses from nNOS neurons and acts as an intercellular messenger to activate Erk in adjacent neurons. Confirming the role of nNOS in NMDA-induced tyrosine phosphorylation of Erk is our observation that NMDA fails to elicit tyrosine phosphorylation of Erk in cortical cultures from nNOS−/− mice, whereas NMDA potently stimulates tyrosine phosphorylation of p42 and p44 Erk in wild-type cultures (Fig. 5B). Similar findings as noted above for NMDA were observed with glutamate-mediated tyrosine phosphorylation of p42 Erk (Fig. 5C).
Figure 5.
(A) Western blot analysis of tyrosine-phosphorylated Erks in primary cortical neurons shows that NMDA induces Erk phosphorylation through a NO-dependent, cGMP-independent mechanism. Treatment of rat cortical cultures with 50 μM of NMDA for 5 min induces a dramatic increase of the phosphorylated forms of p44 and p42 Erks 10 min after treatment. Pretreatment of cells with 500 μM l-NAME for 10 min before exposure to NMDA blocks NMDA-induced activation of Erks, and this blockade is reversed by preincubation of cells with l-NAME (500 μM) in the presence of l-Arg (5 mM). Inhibition of GC with 10 μM of ODQ for 10 min before and during exposure to NMDA (50 μM) does not prevent NMDA-induced phosphorylation of Erks. (B) NMDA induces Erk phosphorylation in cortical neurons from wild-type (WT) mice, but not in nNOS−/− mice. Cortical cultures pretreated with l-NAME (500 μM) or l-NAME (500 μM) plus l-Arg (5 mM) for 10 min and treated with 50 μM NMDA for 5 min were harvested after 10 min and analyzed for Erk tyrosine phosphorylation. (C) Glutamate-stimulated Erk phosphorylation is mediated by NO. Cortical cultures pretreated with l-NAME (500 μM), d-NAME (500 μM), or l-NAME (500 μM) plus l-Arg (5 mM) for 10 min and treated with 500 μM of glutamate for 5 min were harvested after 10 min and analyzed for Erk tyrosine phosphorylation. (D) Cortical cultures pretreated with 6,7-dinitroquinoxaline-2,3-dione (DNQX; 50 μM), MK801 (5 μM), or EGTA (3 μM) for 5 min and treated with 500 μM of glutamate for 5 min were harvested after 10 min and analyzed for Erk tyrosine phosphorylation. These experiments have been replicated three times with similar results.
We also confirmed the work of previous investigations (39, 40) and found that glutamate caused tyrosine phosphorylation of p42 Erk, which was markedly attenuated by the NMDA receptor antagonist, MK801 (Fig. 5D.) Chelating extracellular calcium with EGTA completely blocks tyrosine phosphorylation of p42 Erk, which confirms the calcium dependence of Erk activation (Fig. 5D). The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, DNQX, partially blocks glutamate-induced tyrosine phosphorylation of p42 Erk (Fig. 5D).
DISCUSSION
The major finding of this report is our demonstration that stimulation of the NMDA receptor directly activates Ras through endogenously formed NO. NMDA-induced Ras activation is a NO-dependent process as indicated by the blockade of this event by the NOS inhibitor, l-NAME, and the reversal by excess substrate, l-Arg. Furthermore, NMDA-induced Ras activation occurs through activation of nNOS as NMDA fails to induce Ras activation in neuronal cultures from nNOS−/− mice that have a functional Ras as indicated by our observations that BDNF activates Ras in nNOS−/− neuronal cultures. The blockade of Ras activation is unlikely to occur through NMDA-receptor blockade or through changes in cytoplasmic calcium levels as the inhibitors used in this study do not influence NMDA-receptor calcium currents (41–44) and NMDA-induced calcium currents in nNOS−/− neuronal cultures are equivalent to wild-type NMDA induce calcium currents (43, 44). Furthermore, NMDA stimulated NO activation of Ras is not mediated through increases in cGMP because the potent and selective GC inhibitor, ODQ, and the cell permeable cGMP analog, 8Br-cGMP, have no effects on Ras activation.
Previous studies in tumor cell lines suggest the existence of multiple pathways that could be important in generating calcium-mediated activation of Ras, including Src, Ras-GRF, PYK2 and epidermal growth factor receptor (5–10). Despite the identification of these calcium-dependent pathways to Ras, none of them have been directly demonstrated to mediate calcium-dependent Ras activation in neurons (5, 39) nor has NMDA receptor stimulation been shown to directly activate Ras (5, 39). Our results demonstrate that NMDA can activate Ras, and that NO is a key mediator in neurons for activation of Ras by NMDA receptor stimulation. Our observations of potent activation of Ras after NMDA receptor stimulation may be caused by the high levels of nNOS, equivalent to adult brain, that are present in our culture system (34). NMDA-induced activation of Ras probably occurs through a direct redox-sensitive modulation of Ras by NO. Consistent with this notion are our observations that exogenous NO directly activates immunopurified Ras from cortical neurons in an in vitro Ras activation assay. Previous investigations indicate that exogenous NO can activate Ras in tumor cell lines (32, 33), and that Ras has a critical cysteine at Cys-118 (45), which fits with the consensus sequence for a NO-sensitive redox modulation site (46). Site-directed mutagenesis of Cys-118 to Ser-118 eliminates NO ability to activate Ras, but this mutant Ras retains the ability to be stimulated by growth factors (47). Thus, Ras appears to be a direct target of endogenously formed NO that may subserve important redox signaling and physiologic functions in the nervous system as well as in non-neuronal tissue.
We also confirm and extend previous observations that tyrosine phosphorylation of Erk occurs through NMDA receptor activation (39). NMDA-induced tyrosine phosphorylation of Erk is known to occur through changes in cytoplasmic calcium, but the mechanism by which changes in intracellular calcium induce tyrosine phosphorylation of Erk is poorly understood (5, 39). Our findings indicate that NMDA-induced tyrosine phosphorylation of Erk is mediated, in part, through endogenous NO as indicated by the blockade of this event by the NOS inhibitor, l-NAME, and the reversal by excess substrate, l-Arg, and by the failure of NMDA to induce tyrosine phosphorylation of Erk in nNOS−/− neuronal cultures. NMDA-induced tyrosine phosphorylation of Erk is not mediated through increases in cGMP as ODQ has no effects. Furthermore, exogenous NO causes tyrosine phosphorylation of Erk in cortical neurons as indicated by the ability of NO donors to stimulate tyrosine phosphorylation of Erk, consistent with previous observations in which Erk is tyrosine-phosphorylated by NO donors in tumor cell lines (47). NMDA-induced tyrosine phosphorylation of Erk appears to occur through direct activation of Ras by NO. Consistent with this notion are our observations that NO causes guanine nucleotide exchange on immunopurified Ras from cortical cultures, NO fails to stimulate tyrosine phosphorylation of Erk in PC12 cells containing a dominant negative mutant of Ras and NMDA receptor-mediated increases in NO directly activate Ras.
NO previously has been implicated in short-term changes in neuronal plasticity (20, 31, 43, 48–51). For instance, NO may be involved in certain forms of long-term potentiation in the hippocampus (20, 31, 43, 48–51). Most of NO’s physiologic effects in the nervous system are attributable to activation of GC and increases in intracellular cGMP levels (18–20), or through interactions with the superoxide anion to mediate neurotoxicity (25, 52). Our results indicate that not only may NO be involved in short-term changes in synaptic plasticity, but that it may be involved with long-term changes, which might involve alterations in gene transcription through activation of Ras and subsequent tyrosine phosphorylation of Erk. These processes may occur through redox-sensitive modulation of Ras and suggest that Ras is a potential endogenous NO-redox sensitive effector molecule mediating the intercellular actions of NO in the central nervous system.
Acknowledgments
We thank Ann Schmidt for secretarial assistance, Galina Mukhin and Brian Hoffman for providing and caring for the neuronal cultures used in this study, and G. M. Cooper for the gift of Ras N17 PC12 cells. M.G.-Z. is supported by a postdoctoral research award from the Boehringer Ingelheim Fonds (Stuttgart, Germany). V.L.D. is supported by U.S. Public Health Service Grant NS 33142, the American Heart Association, the Muscular Dystrophy Association, and the Amyotrophic Lateral Sclerosis Association. T.M.D. is an Established Investigator of the American Heart Association and is supported by U.S. Public Health Service Grants NS 37090 and NS 33277 and the Paul Beeson Faculty Scholar Award in Aging Research. Under an agreement between the Johns Hopkins University and Guilford Pharmaceuticals, T.M.D. and V.L.D. are entitled to a share of sales royalty received by the university from Guilford. T.M.D. and the university also own Guilford stock, and the university stock is subject to certain restrictions under university policy. The terms of this arrangement are being managed by the university in accordance with its conflict of interest policies.
ABBREVIATIONS
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- l-Arg
l-arginine
- l-NAME
l-nitroarginine methyl ester
- GC
guanylyl cyclase
- NMDA
N-methyl-d-aspartate
- Erk
extracellular signal-regulated kinase
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
- BDNF
brain-derived nerve growth factor
- ODQ
1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one
References
- 1.Barbacid M. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 2.Lowy D R, Willumsen B M. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 3.Macara I G, Lounsbury K M, Richards S A, McKiernan C, Bar-Sagi D. FASEB J. 1996;10:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 4.Schlessinger J, Ullrich A. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 5.Finkbeiner S, Greenberg M E. Neuron. 1996;16:233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh A, Greenberg M E. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 7.Rusanescu G, Qi H, Thomas S M, Brugge J S, Halegoua S. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 8.Farnsworth C L, Freshney N W, Rosen L B, Ghosh A, Greenberg M E, Feig L A. Nature (London) 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 9.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 10.Rosen L B, Greenberg M E. Proc Natl Acad Sci USA. 1996;93:1113–1118. doi: 10.1073/pnas.93.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen L B, Ginty D D, Weber M J, Greenberg M E. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 12.Miranti C K, Ginty D D, Huang G, Chatila T, Greenberg M E. Mol Cell Biol. 1995;15:3672–3684. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs A. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro L J. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C, Xie Q-W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 16.Bredt D S, Snyder S H. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 17.Marletta M A. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 18.Dawson T M, Snyder S H. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent S R, Hope B T. Trends Neurosci. 1992;15:108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- 20.Garthwaite J, Boulton C L. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- 21.Bredt D S, Snyder S H. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 22.Roskams A J, Bredt D S, Dawson T M, Ronnett G V. Neuron. 1994;13:289–299. doi: 10.1016/0896-6273(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 23.Dawson T M, Gonzalez-Zulueta M, Kusel J, Dawson V L. The Neuroscientist. 1998;4:96–112. [Google Scholar]
- 24.Yun H-Y, Dawson V L, Dawson T M. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 25.Samdani A, Dawson T M, Dawson V L. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 26.Iadecola C. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 27.Farinelli S E, Park D S, Greene L A. J Neurosci. 1996;16:2325–2334. doi: 10.1523/JNEUROSCI.16-07-02325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peunova N, Enikolopov G. Nature (London) 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- 29.Kalb R G, Agostini J. Neuroscience. 1993;57:1–8. doi: 10.1016/0306-4522(93)90107-q. [DOI] [PubMed] [Google Scholar]
- 30.Aoki C, Fenstenmaker S, Lubin M, Go C-G. Brain Res. 1993;620:97–113. doi: 10.1016/0006-8993(93)90275-r. [DOI] [PubMed] [Google Scholar]
- 31.Schuman E M, Madison D V. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 32.Lander H M, Ogiste J S, Pearce S F A, Levi R, Novogrodsky A. J Biol Chem. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 33.Lander H M, Milbank A J, Tauras J M, Hajjar D P, Hempstead B L, Schwartz G D, Kraemer R T, Mirza U A, Chait B T, Burk S C, Quilliam L A. Nature (London) 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 34.Samdani A, Newcamp C, Resink A, Facchinetti F, Hoffman B E, Dawson V L, Dawson T M. J Neurosci. 1997;17:4633–4641. doi: 10.1523/JNEUROSCI.17-12-04633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lander H M, Sehajpal P K, Novogrodsky A. J Immunol. 1993;151:7182–7187. [PubMed] [Google Scholar]
- 36.Southam E, Charles S L, Garthwaite J. Br J Pharmacol. 1996;119:527–532. doi: 10.1111/j.1476-5381.1996.tb15703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 38.Szeberenyi J, Cai H, Cooper G M. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bading H, Greenberg M E. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- 40.Fiore R S, Murphy T H, Sanghera J S, Pelech S L, Baraban J M. J Neurochem. 1993;61:1626–1633. doi: 10.1111/j.1471-4159.1993.tb09796.x. [DOI] [PubMed] [Google Scholar]
- 41.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson V L, Dawson T M, Bartley D A, Uhl G R, Snyder S H. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Dell T J, Huang P L, Dawson T M, Dinerman J L, Snyder S H, Kandel E R, Fishman M C. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- 44.Linden D J, Dawson T M, Dawson V L. J Neurosci. 1995;15:5098–5105. doi: 10.1523/JNEUROSCI.15-07-05098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lander H M, Hajjar D P, Hempstead B L, Mirza U A, Chait B T, Campbell S, Quilliam L A. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 46.Stamler J S, Toone E J, Lipton S A, Sucher N J. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 47.Mott H R, Carpenter J W, Campbell S L. Biochemistry. 1997;36:3640–3644. doi: 10.1021/bi962790o. [DOI] [PubMed] [Google Scholar]
- 48.Zorumski C F, Izumi Y. Biochem Pharmacol. 1993;46:777–785. doi: 10.1016/0006-2952(93)90484-e. [DOI] [PubMed] [Google Scholar]
- 49.Arancio O, Kiebler M, Lee C J, Lev-Ram V, Tsien R Y, Kandel E R, Hawkins R D. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- 50.Son H, Hawkins R D, Martin K, Kiebler M, Huang P L, Fishman M C, Kandel E R. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 51.Kantor D B, Lanzrein M, Stary S J, Sandoval G M, Smith B, Sullivan B M, Davidson N, Schuman E M. Science. 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- 52.Beckman J S, Koppenol W H. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]