Abstract
West Nile virus (WNV) infection causes neurological disease at all levels of the neural axis, accompanied by neuroinflammation and neuronal loss, although the underlying mechanisms remain uncertain. Given the substantial activation of neuroinflammatory pathways observed in WNV infection, we hypothesized that WNV-mediated neuroinflammation and cell death occurred through WNV infection of both glia and neurons, which was driven in part by WNV capsid protein expression. Analysis of autopsied neural tissues from humans with WNV encephalomyelitis (WNVE) revealed WNV infection of both neurons and glia. Upregulation of proinflammatory genes, CXCL10, interleukin-1β, and indolamine-2′,3′-deoxygenase with concurrent suppression of the protective astrocyte-specific endoplasmic reticulum stress sensor gene, OASIS (for old astrocyte specifically induced substance), was evident in WNVE patients compared to non-WNVE controls. These findings were supported by increased ex vivo expression of these proinflammatory genes in glia infected by WNV-NY99. WNV infection caused endoplasmic reticulum stress gene induction and apoptosis in neurons but did not affect glial viability. WNV-infected astrocytic cells secreted cytotoxic factors, which caused neuronal apoptosis. The expression of the WNV-NY99 capsid protein in neurons and glia by a Sindbis virus-derived vector (SINrep5-WNVc) caused neuronal death and the release of neurotoxic factors by infected astrocytes, coupled with proinflammatory gene induction and suppression of OASIS. Striatal implantation of SINrep5-WNVC induced neuroinflammation in rats, together with the induction of CXCL10 and diminished OASIS expression, compared to controls. Moreover, magnetic resonance neuroimaging showed edema and tissue injury in the vicinity of the SINrep5-WNVc implantation site compared to controls, which was complemented by neurobehavioral abnormalities in the SINrep5-WNVc-implanted animals. These studies underscore the important interactions between the WNV capsid protein and neuroinflammation in the pathogenesis of WNV-induced neurological disorders.
In North America, widespread West Nile virus (WNV) infection was first recognized in 1999 during an outbreak of viral encephalitis in New York City (32). Infection by WNV causes a spectrum of neurological disorders and ensuing death in a subset of infected individuals (24, 26). WNV belongs to the flavivirus family (reviewed in references 11, 14, and 34), which are enveloped viruses with a genome consisting of one 10- to 11-kb single-stranded RNA molecule of positive-strand mRNA polarity. WNV genomic RNA contains one large open reading frame, which is translated into a single polypeptide and cleaved by viral and cellular proteases into three structural proteins and several nonstructural (NS) proteins (11, 34). The WNV structural proteins include the capsid (C) protein, the small transmembrane protein (M and its precursor preM), and the surface or envelope glycoprotein (E), which are all involved in pathogenesis. It is clear that infection of the central nervous system (CNS) by WNV results in induction of neuroinflammation and neuronal loss (26). It is also well documented that neurons are the prime target for WNV infection, with ensuing neuronal apoptosis (47, 66). To what extent glial cells are infected and contribute to WNV-related pathogenesis is unclear. Infection of astrocytes by WNV has been reported to alter major histocompatibility complex class I expression in vitro (29), but other pathogenic consequences are largely unknown (15).
There is a broad appreciation for the contributions of both structural and nonstructural viral proteins to various aspects of WNV pathogenesis (4, 5, 36, 41, 65, 66). Studies of neurovirulent WNV strains and other neurovirulent flaviviruses suggest that the WNV envelope (E) and capsid (C) proteins play important roles in neuropathogenesis (3, 5, 33, 43, 44). Direct evidence for neuropathogenic contributions exists only for the WNV capsid protein since it affects the survival of neuronal cells by inducing apoptosis (66). Given the latter properties of WNV capsid protein and the marked neuroinflammatory changes caused by WNV, we hypothesized that the WNV capsid protein might also participate in the generation of neuroinflammation with ensuing neurotoxic effects. Thus, the objective of the present study was to determine the mechanism(s) by which non-neuronal cells, infected or activated by WNV, contribute to neuropathogenesis. Given the putative role of the capsid protein in pathogenesis, we also examined its effects by using both ex vivo and in vivo models. Our results indicate that neural cells, including astrocytic and monocytoid cells, are infected by WNV in the human CNS and that infection of these cell types results in the induction of neuroinflammation, as well as the release of neurotoxic molecules. Moreover, dysregulation of the endoplasmic reticulum (ER) stress genes is an important component of this neuroinflammatory host response. Ex vivo data, together with results obtained using a rat in vivo model, suggest that the WNV capsid protein might exert a key role in this respect by downmodulating the astrocyte-specific protective ER stress response gene OASIS (for old astrocyte specifically induced substance) (25, 31).
MATERIALS AND METHODS
Viruses.
Stocks of WNV strain New York 99 (WNV-NY99) (obtained from the Alberta Provincial Laboratory, Edmonton, Alberta, Canada) were grown and plaqued on Vero cells according to previously described protocols (16). In addition, titers of viral stocks were determined on BHK-21 cells by an immunofluorescence assay using a polyclonal rabbit antibody (3G3) directed against a purified recombinantly expressed WNV capsid protein and counting the immunopositive cells 24 h after infection.
Production of EGFP and WNVC virus stocks.
cDNA was obtained from RNA isolated from WNV-NY99 virus stocks and used as a template to amplify the entire WNV capsid (C) region by PCR using high-fidelity platinum Taq polymerase (Invitrogen, Burlington, Ontario, Canada) and oligonucleotide primers (Table 1), which contained the XbaI restriction site and a stop codon introduced at the 3′ end of the capsid coding sequence. The resulting fragment was gel purified, digested with XbaI, and inserted into the XbaI restriction site of the polylinker of the pSINrep5 expression vector (pSINrep5-WNVC). Correct insertion of the fragment was confirmed via sequencing. All restriction and other enzymes were obtained from New England Biolabs (Mississauga, Ontario, Canada) or Invitrogen and used according to their specifications. The SINrep5 vector system, the construction of the SINrep5 vector expressing the enhanced green fluorescent protein (EGFP), and the production of recombinant viruses have been described previously (10, 63). Briefly, capped in vitro runoff RNA transcripts were generated with the SP6 mMESSAGE mMACHINE kit (Ambion, Inc., Austin, TX) using the linearized pSINrep5-EGFP, pSINrep5-WNVC, and the helper virus construct pDH-BB as templates. DH-BB and the EGFP or WNVC vector RNA transcripts were transfected into BHK-21 cells in phosphate-buffered saline (PBS) by electroporation according to the protocol of Bredenbeek et al. (10). Media containing the recombinant SINrep5 viruses were harvested 20 to 24 h after transfection. The titers of the recombinant SINrep5 stocks were determined on BHK-21 cells. For the EGFP virus, the titer was determined by counting the number of EGFP-positive cells using fluorescence microscopy at 24 h postinfection. To determine the titer of the pSINrep5-WNVC virus, cells were subjected to immunofluorescence analyses with a polyclonal rabbit antibody directed against WNV capsid protein (3G3), and the immunopositive cells were counted. On average, 106 to 107 infectious virus particles/ml for the EGFP and 106 particles/ml for the WNVC virus stocks were obtained.
TABLE 1.
Primers used for PCR
| Targeta | Sequence (5′→3′)
|
Tm (°C) | Concn (μM) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| CXCL10 | CCTTCCTGTATGTGTTTGGA | CCTGCTTCAAATATTTCCCT | 55 | 1.25 |
| CCL2 | GCGAGCTATAGAAGAATCACC | ATAAAACAGGGTGTCTGGGG | 60 | 2.5 |
| IL-1β | CCAAAGAAGAAGATGGAAAAGCG | GGTGCTGATGTACCAGTTGGG | 58-59 | 2.5 |
| TNF-α | ACCTCATCTACTCCCAGGTCC | CTCTTGATGGCAGAGAGGAGG | 55 | 2.5 |
| IDO | GGCAAACTGGAAGAAAAAAGG | ATTTCCACCAATAGAGAGAC | 50 | 2.5 |
| GAPDH | AGCCTTCTCCATGGTGGTGAAGAC | CGGAGTCAACGGATTTGGTCC | 53-60 | 2.5 |
| OASIS | CAACGCACCCCACTCACAGACACC | GGAGCAGCAAAGCCCGCACTAACT | 54 | 2.5 |
| BiP | TCATCGGACGCACTTGGAA | CAACCACCTTGAATGGCAAGA | 54 | 2.5 |
| PERK | AAGTAGATGACTGCAATTACGCTATCAA | TTTAACTTCCCGCATTACCTTCTC | 56 | 2.5 |
| GADD153 | AACCAGCAGAGGTCACAAGC | AGCCGTTCATTCTCTTCAGC | 56 | 2.5 |
| iNOS | ACTTTGATCAGAAGCTGTCCC | CAAAGGCTGTGAGTCCTGCAC | 60 | 2.5 |
| WNVC | CTAGTCTAGACAGTGCGAGCTGTTTCTTAGC | CTAGTCTAGATTATGCCTCCTACGCTGGCGATCAGGCC | 50 | 2.5 |
CXCL10 (IP-10); CCL2 (MCP-1); TNF-α, tumor necrosis factor alpha; BiP, immunoglobulin binding protein (GRP78); PERK, RNA-dependent protein kinase-like ER kinase; GADD153, growth arrest and DNA-damage-inducible protein 153. All other targets are as defined in the text.
Human brain tissue samples.
CNS tissues were collected with consent at autopsy from WNV-seropositive individuals (n = 2) with encephalomyelitis (12) or other inflammatory neurological diseases (HIV/AIDS, n = 3; multiple sclerosis [MS], n = 3) and stored at −80°C from which RNA was extracted, as described below (8, 61). In addition, 10% formalin-fixed and paraffin-embedded 10-μm CNS tissue sections were used for immunohistochemical studies.
Cell culture and treatments.
Primary human fetal astrocytes and monocyte-derived macrophage cultures were prepared as previously described (27, 46). In addition, astrocytic (U373), monocytoid (U-937), and neuronal (LAN-2) cell lines were used (8, 27, 46). Human cholinergic neuronal LAN-2 cells were differentiated with dibutyryl cyclic AMP before use (46). To study the effects of OASIS expression on astrocytic cells, 5 μg of the construct OASIS-FLAG (a gift from K. Imaizumi, Miyazaki University, Miyazaki, Japan) was transfected into astrocytes by using Transfectin lipid reagent (5 μl) (Bio-Rad). In addition, astrocytes were treated with sodium nitroprusside (100 nM; Axxora Life Sciences, Inc.) to study the effects of nitric oxide (NO) on these cells.
Analysis of ex vivo cytotoxicity.
Cells were infected for 24 and 48 h with WNV-NY99, SINrep5-EGFP, or -WNVC virus using a multiplicity of infection (MOI) ranging from 0.01 to 1. As controls (mock), equal volumes of conditioned medium obtained from mock-transfected BHK-21 cells were incubated with the cells. The cytotoxicity was tested in triplicate in two separate experiments for each virus. After 24 and 48 h the percentage of cell death was assessed by trypan blue exclusion and compared to the SINrep5-EGFP- and mock-infected controls. For the DNA laddering experiments chromosomal DNA was isolated from 106 WNV- or mock-infected LAN-2 cells 48 h after infection by using previously described protocols (17), separated on 1.5% agarose gels, and visualized by using ethidium bromide.
Cell viability and activation of caspase-3 by WNV infection or the WNVC was assessed by in-cell quantitative immunocytochemistry using the Odyssey infrared-imaging system (LI-COR Biosciences, Lincoln, NE) as previously described (46). Briefly, neuronal (LAN-2) cells and human fetal astrocytes in 96-well plates were infected at various MOIs with WNV or SINrep5-WNVC, using cells mock infected or infected with SINrep5-EGFP as controls. Cells treated with 0.5 μM staurosporin were used as positive controls. Cells were fixed with 4% paraformaldehyde at 48 h postinfection, washed, permeabilized with PBS-0.1% Triton X-100, and blocked with Odyssey blocking buffer. Subsequently, cells were labeled using a primary polyclonal rabbit anti-cleaved caspase-3 (1/100 dilution; Cell Signaling), a mouse monoclonal anti-βIII tubulin (1/100 dilution; Santa Cruz Biotechnology), or an anti-WNV capsid polyclonal (3G3) antibody and a goat anti-rabbit IR Dye800 secondary antibody (1/800 dilution; Rockland Immunochemicals). Immunoreactivity was measured and quantified by using the Odyssey system. For fluorescence labeling, the same antibodies were used followed by the appropriate Alexa 488-conjugated or Cy3-conjugated secondary antibodies (Molecular Probes). For the indirect cytotoxicity experiments, U373 cells were infected at various MOIs with WNV or SINrep5-WNVC, using SINrep5-EGFP and cells incubated with media from mock-transfected BHK cells as controls. After 4 h the cells were washed three times with AIM-V media to remove residual virus and incubated with fresh AIM-V for another 20 h. This conditioned medium was subsequently harvested, diluted 1:2 with fresh AIM-V medium, and applied to differentiated LAN-2 cells in 96-well plates for 24 and 48 h, and cell death and activation of caspase-3 were assessed as described above.
In vivo neurovirulence of WNV capsid protein.
Three-week-old male Sprague-Dawley rats were implanted in the right striatum (0.2 mm posterior, 3.5 mm lateral, 6 mm deep relative to the bregma) with 5 μl of the EGFP (n = 6) or WNV capsid protein (n = 6) expressing Sindbis vectors (0.5 × 106 infectious units/ml) injected over a 10-min time period, using conditioned media of mock-transfected cells as a control (n = 6). Neurobehavioral testing was performed at days 3, 7, and 14 postimplantation. The behavioral tests included the ladder rung test and the cylinder test (7), and the total neurological deficit score (NDS) was obtained by summing the scores from all tests (48). In addition, the implanted animals were subjected to magnetic resonance imaging (MRI) examinations on a Bruker MSL-X Biospec 7T/21 MR system using a volume coil 2 cm in diameter. At days 3, 7, and 14 postimplantation of virus, quantitative T2 images were obtained for all animals per group for evaluation of the edema. Animals were euthanized after completion of the MR study and neurobehavioral testing on day 14 by pentobarbital overdose together with PBS perfusion. The brains were removed; the brain anterior to the lesion site was snap-frozen in liquid nitrogen for subsequent isolation of RNA, and the posterior brain was fixed in 4% paraformaldehyde and used for immunohistochemical analysis (49).
Immunofluorescence and immunocytochemical analyses of cultured cells and tissue sections.
Cells mock infected or infected with WNV-NY99 virus and SINrep5-EGFP or -WNVC viruses were fixed in 4% paraformaldehyde for 20 min and washed with PBS and subjected to immunofluorescence analysis with an antibody raised in rabbits using purified recombinantly expressed capsid protein of WNV (3G3) and the astrocyte marker glial fibrillary acidic protein (GFAP; Dako, Glostrup, Denmark), using the corresponding Cy3- or Alexa488-conjugated secondary antibodies (Molecular Probes, Eugene, OR), and analyzed by using standard fluorescence microscopy (Axioskop 2; Carl Zeiss Canada, Ltd., Toronto, Ontario, Canada).
Human and rat CNS tissues were embedded in paraffin (51), and 10-μm tissue sections were cut by using a microtome (Leica, Richmond Hill, Ontario, Canada). Tissue sections were deparaffinized, rehydrated, and subjected to immunohistochemical analysis with antibodies directed against GFAP, the activated microglial marker ionized calcium binding adaptor molecule 1 (Iba-1; Wacko, Inc., Tokyo, Japan), the rat-specific macrophage marker ED-1 (Chemicon, Temecula, CA), neurofilament 200 kDa (NF200) (Chemicon), CD45 (Chemicon), and the 3G3 antibody for detecting the WNV capsid protein, followed by the appropriate horseradish peroxidase- or alkaline phosphatase-conjugated secondary antibodies (Sigma, St. Louis, MO) according to previously described procedures (51). All tissue sections were analyzed with a standard bright-field microscope, and immunopositivity including double-labeled cells was counted in serial sections (Axioskop 2).
Real-time reverse transcription-PCR (RT-PCR) analysis.
RNA was extracted by using TRIzol reagent (Invitrogen) from rat brains, cortical autopsy brain tissue, and spinal cords of patients who died from WNV encephalitis (WNVE), using brains of healthy individuals, HIV-infected patients, and MS patients as controls. Similarly, cellular RNA was isolated from cells 24 h postinfection with the WNV-NY99, WNVC, or EGFP viruses at an MOI ranging from 1 to 0.01 using mock-infected cells as a control. The RNA was used for cDNA synthesis and subsequent real-time quantitative PCR analysis using the iCycler IQ system (Bio-Rad, Mississauga, Ontario, Canada) as described previously (64). The primers used for real-time PCR analysis are listed in Table 1. mRNA levels were normalized against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and are expressed as the fold increase over RNA levels in mock-infected cells or appropriate control tissues.
To determine the relative levels of infection, U-937, U373, and LAN-2 cells were infected for 2 h with WNV at MOIs ranging from 1 to 0.05. Cells were washed three times with PBS to remove residual virus, and fresh media were added. RNA for real-time RT-PCR analysis was isolated from the different cells 24 h after infection by using TRIzol reagent. Viral RNA levels were normalized against GAPDH and are expressed as relative fold change over viral RNA levels in the cells infected with the lowest MOI.
All real-time PCR quantifications were performed in duplicate and repeated with different preparations of cDNA. For all experiments real-time PCR quantifications were performed on cDNAs from two or more independent experiments.
Statistical analyses.
All statistical tests, including parametric and nonparametric analyses, were performed by using Graphpad InStat version 3.01. (GraphPad Software, San Diego, CA), and P values of <0.05 were considered significant.
RESULTS
Neuroinflammatory gene expression in human WNVE cases.
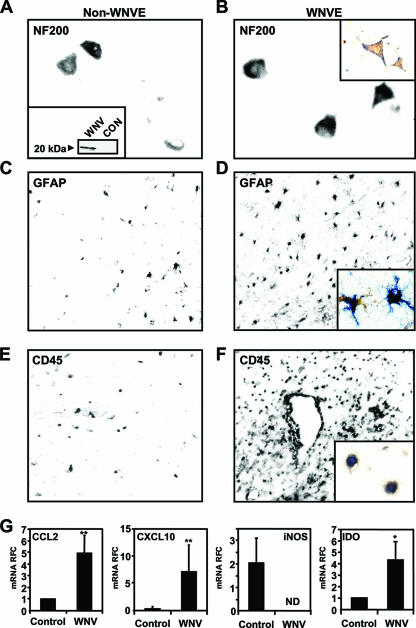
Although WNV infection is widely recognized as a cause of encephalomyelitis, the extent to which the virus infects different neural cell types and causes neuroinflammation in humans remains uncertain. WNV is assumed to infect primarily neuronal cells (24), although there are reports suggesting that glial cells in the CNS might be infected by WNV (53, 55). To define WNV's in vivo cell tropism and neuroimmune pathology, brain and spinal cord sections from two human WNVE cases were examined for WNV immunoreactivity using a polyclonal antiserum, which recognized the WNV capsid protein at a molecular mass of approximately 20 kDa (Fig. 1A, inset). In non-WNVE (Fig. 1A) and WNVE (Fig. 1B) brain sections displaying NF200 immunoreactive neurons (Fig. 1A), neurons showed colocalization with WNV capsid protein immunoreactivity in WNVC cases (Fig. 1B, inset), which resembled previous reports (53, 55). Colocalization of WNVE immunoreactivity was evident in 8.2% of NF200-positive cells. Moreover, astrocytosis was present in WNVE sections (Fig. 1D) compared to non-WNVE (Fig. 1C), together with colocalization of GFAP with WNV capsid immunoreactivity (Fig. 1D, inset). Among GFAP-immunopositive cells, 3.3% were WNV capsid immunopositive. Similarly, there were numerous CD45-immunopositive ramified cells detected in the parenchyma of WNVE sections (Fig. 1F) resembling microglia and macrophages compared to non-WNVE sections (Fig. 1E), but WNV capsid protein immunoreactivity was colocalized in a small subset, 1.9% of CD45-positive cells in both spinal cord and brain (Fig. 1F, inset). Although there was a trend toward greater WNV capsid expression in neurons compared to astrocytes and leukocytes, it did not achieve statistical significance (Kruskal-Wallis, P > 0.05). Colocalization between WNV capsid and cell type-specific markers (NF200, GFAP, and CD45) was not evident in non-WNVE sections.
FIG. 1.
Infection of neural cells by WNV and neuroinflammation in CNS of human WNVE cases. WNVC immunoreactivity (brown) colocalized with NF200 immunoreactivity (blue), indicating infection of neurons by WNV (B) compared to the non-WNVE CNS (control) sections (A). The specificity of the WNV capsid antibody was confirmed by Western blotting of lysates of WNV-infected and uninfected LAN-2 cells (A, inset). Increased GFAP immunoreactivity, indicative of neuroinflammation, was observed in the WNVE CNS (D) compared to non-WNVE CNS sections (C). In addition, colocalization (inset) of WNVC immunoreactivity (brown) was observed with the astrocyte marker GFAP (blue), indicating a low level of infection of cells of astrocytic lineage by WNV (D, inset). CD45 immunoreactivity was also increased in WNVE sections, and double labeling (inset) for CD45 (blue) and WNV capsid protein (brown) (F, inset) indicated these leukocytoid cells were also infected by WNV relative to non-WNVE controls (E). (G) Real-time RT-PCR analyses using RNA isolated from the CNS of the WNVE cases revealed upregulation of CCL2, CXCL10, and IDO mRNA expression (mean relative fold change [RFC]) in WNV-infected tissue, but iNOS was suppressed compared to non-WNVE control tissues. *. P < 0.05; **, P < 0.01 (Dunn's multiple-comparison test).
Using cDNA derived from RNA of brain and spinal cord in a nested RT-PCR protocol targeted to the capsid region (WNVC) of the WNV genome, we detected the presence of viral genome in both the brain and the spinal cord of each WNVE case but not in the non-WNVE tissues (data not shown), confirming the presence of virus in the CNS tissues of the WNVE cases. Given the glial activation present in the WNVE CNS sections (Fig. 1D and F), we examined the neuroinflammatory gene expression profile using the same cDNA in real-time RT-PCR analyses, which revealed an increase in RNA expression for CCL2, CXCL10, and indolamine-2′,3′-deoxygenase (IDO) (P < 0.01) (Fig. 1D) compared to CNS tissues from healthy individuals (non-WNVE). This pattern of upregulation of RNA expression of these neuroinflammatory genes was similar to the pattern observed in tissues of other neuroinflammatory conditions (i.e., HIV/AIDS and MS [data not shown]). However, inducible nitric oxide synthase (iNOS) transcripts were not detected in WNVE-derived samples. In addition, interleukin-1β (IL-1β) RNA expression was elevated depending on the individual WNVE CNS tissues but, due to the limited amount of samples available, a consistent upregulation was not observed (data not shown). These results indicated that in addition to CNS neurons, WNV also infected neuroimmune effector cells (astrocytic and monocytoid cells), which coincided with the induction of genes commonly associated with neuroinflammation.
Differential neuroinflammatory gene induction by WNV in astrocytic and monocytoid cells.
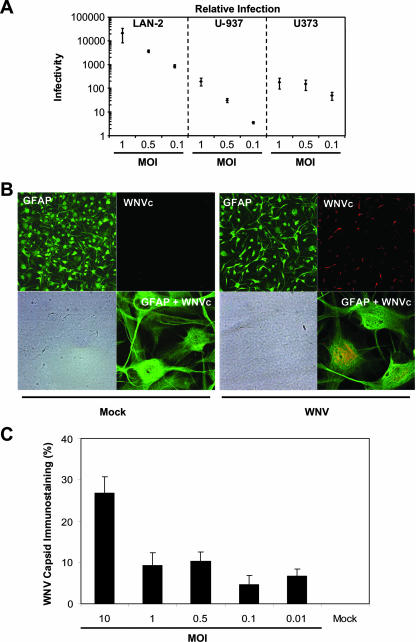
Given the present in vivo evidence for WNV infection of different CNS cell types, we compared the relative infectivity of WNV in several neural cell types at a range of input titers (Fig. 2A). Human neuronal (LAN-2) cells were 10- to 100-fold more permissive to WNV infection than human monocytoid (U-937) and astrocytic (U373) cells at matched input titers (MOI = 0.1, 0.5, and 1.0) (Fig. 2A). Glial cell infection or exposure to different viruses often results in innate immune activation and contributes to neuroinflammation (9, 50). Indeed, these findings were confirmed by comparing virus titers in supernatants from WNV-infected BHK-21 and U373 cells infected at the same input titer (MOI = 1.0), respectively, which revealed that the mean log10 titers (with the standard deviation) from BHK-21 supernatants were 8.08 ± 0.076 PFU/ml, while the titers in U373-derived supernatants were 5.97 ± 0.059. In astrocytic (U373) cultures infected with WNV (MOI = 1.0), WNV capsid-immunopositive cells were frequently observed, a finding similar to the observations with WNV-infected human fetal astrocytes without evidence of obvious cytopathic effect (Fig. 2B, right side). Moreover, there was clear evidence of colocalization of GFAP and WNV capsid immunoreactivity (Fig. 2B, right side, GFAP+WNVc inset). To corroborate further the real-time RT-PCR data, we assessed the percent WNV capsid immunoreactivity by quantitative immunocytochemistry in human fetal astrocytes infected with WNV at various MOIs (Fig. 2C), disclosing increased infection at higher input titers. The restricted infection of these glial cells by WNV corresponded to the low number of WNV capsid-immunopositive non-neuronal cells observed in the immunohistochemical analyses described above (Fig. 1).
FIG. 2.
Susceptibility of astrocytic and monocytoid cells to WNV infection and the induction of neuroinflammatory gene expression. (A) Semiquantitative real-time RT-PCR analyses indicating that ex vivo human astrocytic (U373) and monocytoid (U-937) cells were infected at an ∼100-fold-lower level compared to neuronal cells (LAN-2). (B) Similarly, WNV capsid immunopositive cells were detected in WNV-infected human fetal astrocyte cultures (WNV, right side) but not in mock-infected cultures (Mock, left side). GPAP immunoreactivity is displayed in the upper left quadrant for both mock- and WNV-infected cultures, while the corresponding bright-field image is shown in the left lower quadrant. Double immunolabeled cultures are present in the right lower quadrants, but only the WNV-infected human astrocytes exhibit both GFAP (green) and WNV capsid (red) immunoreactivity. (C) Quantitative immunocytochemical analysis of WNV capsid protein immunopositive cells revealed an MOI-dependent infection; WNV capsid immunoreactivity was expressed as the percentage of immunopositive cells. Original magnification: GFAP, WNV, and bright-field, ×200; GFAP+WNV (overlay), ×630.
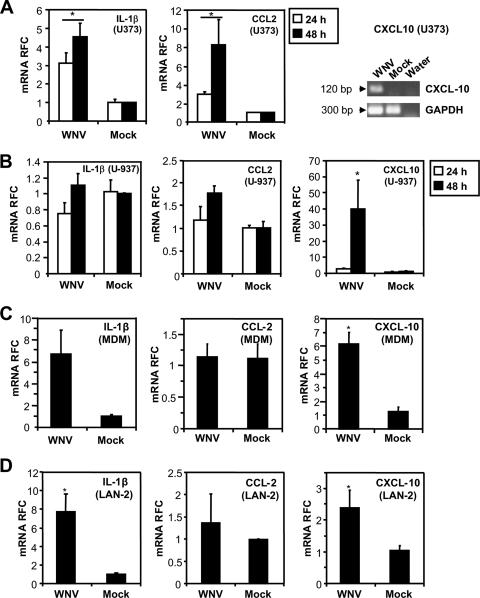
Since our immunohistochemical analyses of autopsied tissues revealed activation of astrocytes and macrophage/microglia cells, we explored the neuroinflammatory gene induction in monocytoid (U-937) and astrocytic (U373) cells after WNV infection (MOI = 1.0) at 24 h postinfection (Fig. 3A and B). WNV infection resulted in a significantly increased (P < 0.05) induction of CCL2 and IL-1β gene expression in cultured astrocytic cells (Fig. 3A) with no significant induction of these proinflammatory genes in the monocytoid cells (Fig. 3B). In both cell lines, CXCL10 was increased upon WNV infection compared to mock-infected controls. Similar to monocytoid (U-937) cells, monocyte-derived macrophages (MDM) were also infected at a substantially lower level (2.9 ± 0.37 log10) than human neuronal (LAN-2) cells at a matched input titer (MOI = 1.0 [data not shown]). In MDM, an upregulation of both IL-1β and CXCL10 was observed (P < 0.05) (Fig. 3C). Since neurons are also sources of neuroinflammatory molecules such as IL-1β or CXCL10 (19, 30), the RNA expression levels of CCL2, CXCL10, and IL-1β were also assessed for comparison in WNV-infected (MOI = 1.0) neuronal cells (Fig. 3D). An increase in CXCL10 and IL-1β RNA expression levels was observed in neuronal cells (P < 0.05). These results indicated that WNV infection of multiple neural cell types resulted in a significant differential induction of genes associated with neuroinflammation, similar to our in vivo observations, despite comparatively low levels of concurrent infection. In particular, the upregulation of CXCL10 in astrocytes was of interest since CXCL10 has been reported to play a significant protective role in WNV pathogenesis in animal models (30), while CXCL10 production by astrocytes has been implicated in the neuropathogenesis of other viral infections (18, 59, 60, 64).
FIG. 3.
Induction of neuroinflammatory responses in WNV-infected cells. (A) Infection of astrocytic cells resulted in the induction of IL-1β, CCL2, and CXCL10 mRNA expression. (B) In monocytoid cells (U-937) only CXCL10 mRNA expression was induced after WNV infection. (C) In MDM, both IL-1β and CXCL10 RNA expression were significantly upregulated, a result similar to what was observed in neuronal cells (LAN-2) (D), which is indicative of differential neuroinflammatory gene induction in individual cell types upon WNV infection. *, P < 0.05 (Dunn's multiple-comparison test).
WNV infection causes neuronal death after neuronal and glia infection.
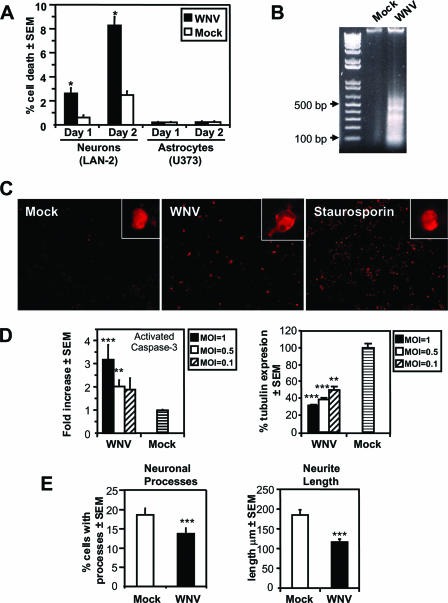
Since WNV induces cell death in multiple cell types, including neurons, and neuroinflammation was a cardinal feature of WNVE (17, 47), we investigated the extent and mechanism(s) by which WNV-NY99 caused cell death in different human neural cell types. At low viral input titers (MOI = 0.01) in infections of human neuronal cells (LAN-2), cell death occurred 1 and 2 days after infection (Fig. 4A). Neuronal death showed an increase from day 1 to day 2, although cell death was not evident in WNV-infected astrocytic cells (Fig. 4A) or monocytoid cells (data not shown), probably due to the restricted infection of both of these cell types by WNV. The presence of laddering (Fig. 4B) in chromosomal DNA isolated from WNV-infected neuronal (LAN-2) cells, together with immunofluorescent detection of activated caspase-3 (Fig. 4C), suggested that the mechanism of neuronal death was primarily apoptotic. Indeed, infection of LAN-2 cells at higher MOIs resulted in diminished cell viability and caspase-3 activation, as determined by in-cell quantitative immunocytochemistry (Fig. 4D).
FIG. 4.
Induction of neuronal apoptosis and neuronal damage by WNV ex vivo. (A) Infection of neural cells with WNV at an MOI of 0.1 resulted in neuronal death, but not astrocyte death, as determined by trypan blue exclusion. WNV-induced neuronal death was apoptotic, as indicated by DNA laddering (B), and induction of activated caspase-3 (C) in neuronal (LAN-2) cells, using mock-infected and staurosporin-treated cells as negative and positive controls, respectively. (D) The level of cell death was dependent on input titer, as reflected by MOI-dependent activation of caspase-3 and reduction of β-tubulin expression. (E) Cell death was preceded by reduction in the number of neuronal cells with processes and a reduction in neurite length, which is indicative of WNV-induced neuronal damage. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Dunn's multiple-comparison test).
In many neurodegenerative diseases, including those induced by viruses, neuronal structural perturbations precede neuronal dysfunction and death (62). Since WNV-infected neuronal (LAN-2) cultures at an MOI of 0.01 displayed limited cell death 1 day after infection, these cultures were ideally suited to investigate early evidence of neuronal damage due to WNV infection. MAP-2 immunostaining of LAN-2 cultures infected for 1 day with WNV disclosed a reduction in the number of cells with neurites, as well as a reduction in neurite length (Fig. 4E). Analyses of neuronal process numbers and neurite length (Fig. 4E) revealed a significant reduction in the number of cells with processes and average neurite length after WNV infection (P < 0.001), a finding indicative of neuronal damage preceding cell death.
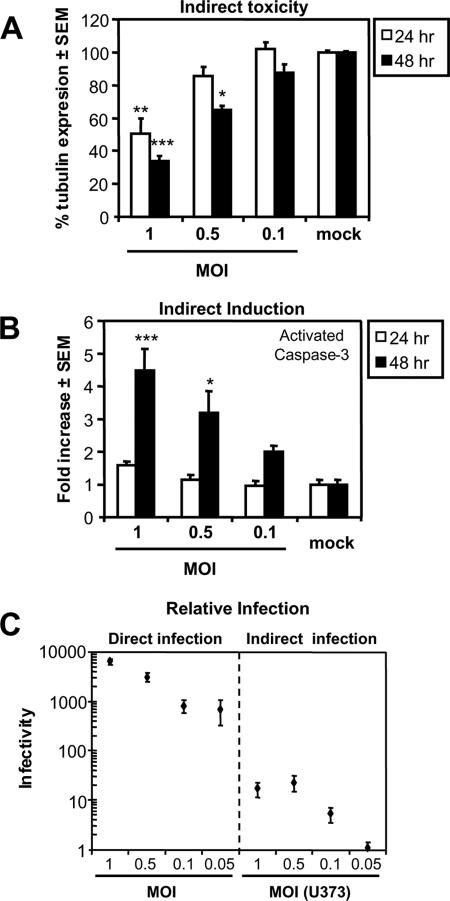
Since astrocytes represent the most abundant cell population in the brain (reviewed in reference 39) and they exhibited the greatest and most consistent immune responses after WNV infection (Fig. 3), we elected to focus on these glial cells in subsequent studies of WNV's pathogenic effects on glia. In addition to the induction of neuroinflammatory responses, exposure to or infection of astrocytes by a virus can result in the release of neurotoxic molecules (50). To investigate the release of soluble neurotoxins after WNV infection of astrocytes, supernatants were collected from WNV-infected astrocytic (U373) cells 24 h after infection and applied to neuronal (LAN-2) cells, using supernatants from mock-infected cells as controls. Supernatants from U373 cells infected with WNV at various MOIs induced significant cell death in LAN-2 cultures after both 24 and 48 h of exposure compared to supernatants of mock-infected cells (Fig. 5A), again showing the activation of caspase-3 in an MOI-dependent manner after 48 h, as assessed by quantitative immunocytochemistry (Fig. 5B). We subsequently compared the level of WNV infection of LAN-2 cells by U373-derived supernatants to the direct infection at various MOIs by WNV (Fig. 5C). The purpose of this experiment was to investigate whether the cell death induced by supernatants from U373 infected with WNV was the result of residual infectious virions in the supernatants or neurotoxins secreted by glia. Viral infectivity in neuronal (LAN-2) cultures treated with conditioned media from infected U373 cells was 100-fold lower compared to a direct infection of LAN-2 cells with a matching MOI, indicating that the amount of virus released from U373 and passed on to the neuronal cells was 100-fold lower compared to the direct infection. The level of neuronal death at 24 h induced by supernatants from the WNV-infected astrocytic cells was similar to that of direct infection by WNV (Fig. 4D). Hence, WNV infection of the astrocytic cells induced the release of neurotoxic molecules, leading to neuronal death with limited contemporaneous infection of neurons.
FIG. 5.
Secretion of neurotoxins by WNV-infected glia. Supernatants from U373 cells infected at various MOIs with WNV reduced cell death in neuronal (LAN-2) viability at both 24 and 48 h of exposure compared to controls (A), which involved activation of caspase-3 (B). (C) Semiquantitative analysis using real-time RT-PCR revealed that the amount of viral RNA present in the LAN-2 cultures treated with supernatants from astrocytic (U373) cells was ∼100-fold lower than a a direct infection of LAN-2 cells at matching MOIs. Thus, only a small amount of virus is transferred to the LAN-2 cultures, implying that infection of the U373 cells by WNV resulted in the release of molecules with a neurotoxic action. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Dunn's multiple-comparison test).
WNV induces differential ER stress gene expression in neurons and glia.
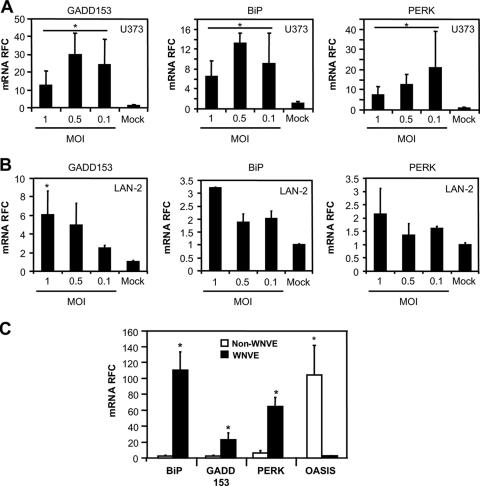
The role of ER stress in viral infections as both a protective and a pathogenic mechanism in neurological infections has received increasing attention (20, 21). The ER stress signaling cascade has variable effects on cellular survival depending on the cell type, the duration of the ER stress, and the comparative abundance of the individual ER stress regulatory molecules (67). Hence, we investigated the ability of WNV to induce the expression of genes associated with ER stress in both astrocytic (U373) and neuronal (LAN-2) cells (Fig. 6). In U373 cells, WNV infection induced the expression of three established ER stress-related genes (BiP, PERK, and GADD153) with no definitive relationship between MOI and level of ER stress gene expression (Fig. 6A). Of interest was the marked reduction upon WNV infection in transcript levels for the early astrocyte-specific stress sensor related protein, OASIS (25; data not shown), which has recently been demonstrated to be involved in ER stress gene expression regulation in astrocytes (31). OASIS expression levels were very low at both high and low MOIs compared to uninfected astrocytes, such that an OASIS-specific PCR product was detectable, but we were unable to quantify it in a reliable fashion (data not shown). Conversely, in LAN-2 cells the expression of the proapoptotic gene, GADD153, was induced in an increasing MOI-dependent manner (Fig. 6B), while OASIS was again not detected in both infected and uninfected LAN-2 cells (data not shown). None of the ER stress genes were induced in LAN-2 cells treated with supernatants from WNV-infected astrocytes (data not shown), implying a different mechanism of neuronal death mediated by the neurotoxins released from WNV-infected astrocytic cells. To extend these ex vivo findings, we investigated the expression of these ER stress genes in autopsied CNS material from WNVE and neuroinflammatory non-WNVE cases (HIV/AIDS and MS). Increased ER stress gene RNA expression was detected for both the HIV/AIDS and MS cases (data not shown). However, a substantially greater upregulation of GADD153, PERK, and BiP RNA expression (P < 0.01 and P < 0.001, respectively) was observed in the WNVE samples compared to non-WNVE samples (HIV/AIDS and MS). In contrast, OASIS expression was markedly suppressed in WNVE compared to the non-WNVE cases (P < 0.05) (Fig. 6C). Thus, the marked suppression of OASIS in astrocytic cells, together with the upregulation of proapoptotic ER stress transducers, such as GADD153, might contribute to WNV neurovirulence ex vivo and in vivo, underscoring the potential role of ER stress in WNV neuropathogenesis.
FIG. 6.
Activation of ER stress genes by WNV infection of neurons and glia. (A) Infection of astrocytic cells (U373) by WNV resulted in a variable induction of mRNA expression for the ER stress genes GADD153, BiP, and PERK compared to uninfected cells at all MOIs tested. (B) In neuronal cells, GADD153 BiP, and PERK transcript levels were induced in an MOI-dependent fashion, indicating differential induction of ER stress gene expression in the different cell types. (C) Similar to the present ex vivo observations in neuronal cells, GADD153, BiP, and PERK mRNA expression was markedly elevated in WNVE brains, whereas OASIS expression was suppressed in WNVE compared to levels observed in other inflammatory brain disorders (HIV and MS). *, P < 0.05 (Dunn's multiple-comparison test).
WNV capsid protein induces neuroinflammatory genes and neuronal death.
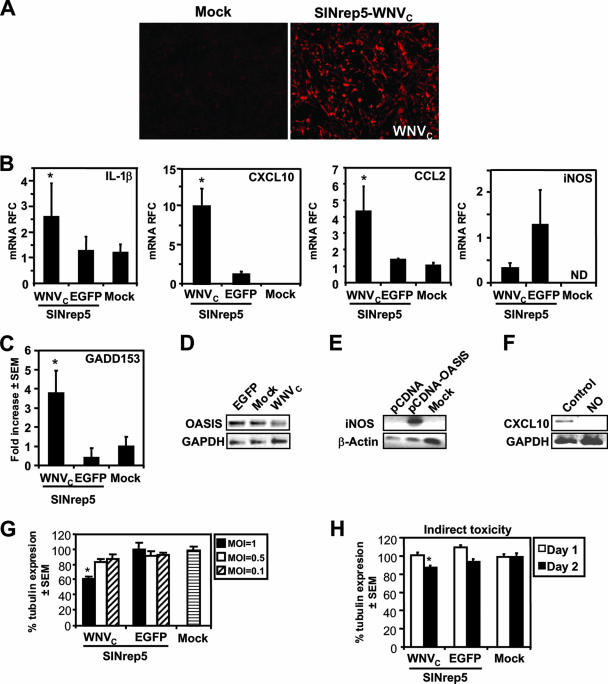
The WNV capsid protein is considered to play an important role in WNV-induced neuronal death (66). To extend the understanding of the pathogenic properties of the WNV capsid protein, we examined the effects of the expression of the capsid protein in neural cells by cloning the ORF encoding the capsid protein of WNV-NY99 into a neurotropic viral vector derived from Sindbis virus (SINrep5-WNVC) (10, 63). WNV capsid protein was efficiently expressed by the SINrep5-WNVC (Fig. 7A). Infection of astrocytes with SINrep5-WNVC resulted in a marked increase in IL-1β, CCL2, and CXCL10 RNA expression (Fig. 7B) compared to SINrep5-EGFP- and mock-infected cells. However, iNOS transcript levels were reduced in astrocytes infected with SINrep5-WNVc compared to SINrep5-EGFP-infected cells (Fig. 7B). These observations indicated that the WNV capsid protein might be responsible for inducing a subset of the inflammatory genes in neural cells. However, the induction of the inflammatory gene expression was associated with induction of GADD153, but not BiP or PERK gene expression, in the same cells (Fig. 7C). Nonetheless, OASIS transcript levels were markedly suppressed after infection of astrocytes by SINrep5-WNVC compared to SINrep5-EGFP- or mock-infected cells (Fig. 7C), a finding similar to observations in WNV-infected astrocytes (data not shown) and in vivo observations (Fig. 6C). We hypothesized that the suppression of iNOS in WNV-infected astrocytes and human brain tissue might be due to the low levels of OASIS. Indeed, transfection of astrocytes with an OASIS-containing vector induced iNOS transcript levels (Fig. 7C). Since nitric oxide (NO), as a product of iNOS expression, is a potent suppressor of CXCL10 (22), we hypothesized that the high levels of CXCL10 observed in WNV-infected brain tissue and cultured astrocytes might be due to the low levels of NO. Treatment of CXCL10-producing human fetal astrocytes with an NO donor, sodium nitroprusside (100 nM), resulted in the reduction of CXCL10 mRNA expression to undetectable levels as determined by RT-PCR (Fig. 7C), suggesting that the WNV capsid influences CXCL10 expression by suppressing iNOS and NO production, perhaps through the suppression of OASIS expression. To investigate the effects of the WNV capsid protein on cell viability, neuronal (LAN-2) cells were infected at different MOIs with SINrep5-WNVC. Indeed, infection of LAN-2 cells with SINrep5-WNVC resulted in decreased cell survival in an MOI-dependent manner, which was not observed in mock- and SINrep5-EGFP-infected cells (Fig. 7D). To determine whether the WNV capsid protein might influence the release of neurotoxins from infected glia, U373 cells were infected with SINrep5-WNVC, and supernatants were harvested at 24 h were applied to LAN-2 cells by using supernatants from SINrep5-EGFP- and mock-infected cells as controls. After 24 and 48 h, cell death in the LAN-2 cells was assessed (Fig. 7E). The supernatant from SINrep5-WNVC-infected astrocytes (MOI = 1.0) was neurotoxic after 48 h of incubation (P < 0.05), albeit in a limited manner. Thus, the WNV capsid protein induced an inflammatory response in astrocytes while also suppressing OASIS expression, together with the release of low-level release of neurotoxic molecules.
FIG. 7.
Expression of WNVC and induction of neuronal death and host responses by the SINrep5-WNVc vector. (A) WNV capsid protein was efficiently expressed by the SINrep5-WNVC in BHK-21 cells. (B) Infection of astrocytes with SINrep5-WNVC resulted in increased IL-1β, CCL2, and CXCL10 RNA expression compared to SINrep5-EGFP- and mock-infected controls. WNV capsid protein suppressed iNOS expression, although not significantly relative to SINrep5-EGFP-infected human fetal astrocytes. (C) Infection of U373 with SINrep5-WNVC induced GADD153, but OASIS transcript levels were suppressed to undetectable levels after infection of astrocytes by SINrep5-WNVC compared to SINRep5-EGFP- or mock-infected cells. Transfection of astrocytes with an OASIS-expressing vector induced expression of iNOS compared to mock transfections. Treatment of human fetal astrocyte cells with the nitric oxide (NO) donor, sodium nitroprusside (100 nM), resulted in the reduction of CXCL10 mRNA expression to undetectable levels, suggesting that the WNV capsid affected CXCL10 expression by suppressing iNOS and NO production, possibly through concurrent OASIS downregulation. (D) Infection of the neuronal LAN-2 cell line at various MOIs with SINrep5-WNVC induced an MOI-dependent decrease in cell viability 2 days after infection that was not observed in mock- and SINrep5-EGFP-infected cells. (E) The supernatant from SINrep5-WNVC-infected cells (MOI = 1.0) was neurotoxic after 2 days of incubation but not at 1 day, indicating that the WNV capsid protein expression caused the release of molecules with neurotoxic actions, albeit to a much lower extent than direct infection by WNV in the same cell types. *, P < 0.05 (Dunn's multiple-comparison test).
WNV capsid mediates in vivo neurovirulence.
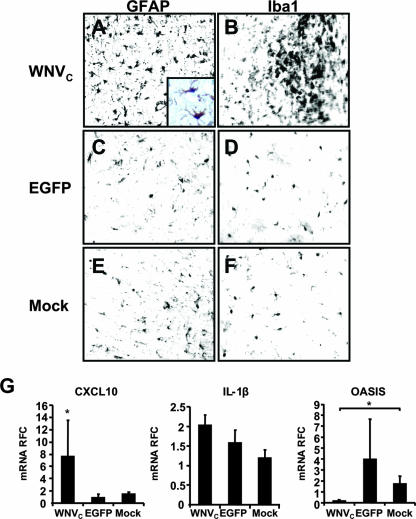
The expression of the WNV capsid protein has been implicated in WNV in vivo neurovirulence, based on the implantation of a nonspecific eukaryote vector encoding the WNV capsid protein (66). Using the present Sindbis virus vector expression system, which in addition to neurons infects both astrocytes and cells of macrophage lineage (63), thus resembling the natural infection and tropism by WNV, we studied the effects of WNV capsid protein expression in vivo after striatal implantation of SINrep5-WNVC, SINrep5-EGFP, or supernatants from mock-transduced cultures. Immunohistochemical analyses indicated the activation of both astrocytes (Fig. 8A) and microglia (Fig. 8B) at day 14 postinfection, evidenced by glial hypertrophy and increased numbers of activated glia in the proximity of the implantation site in SINrep5-WNVC-infected animals, compared to animals receiving SINrep5-EGFP (Fig. 8C and D) or mock infection (Fig. 8E and F). In addition, immunohistochemical detection of WNV capsid protein was evident in cells also expressing the astrocyte protein, GFAP (Fig. 8A, inset). To evaluate the pathogenic properties of the WNV capsid protein, in terms of neuroinflammatory gene induction, transcript levels were assessed by using real-time RT-PCR analysis of RNA derived from tissue proximal to the site of implantation in the striatum, as previously reported (49) (Fig. 8B). These studies revealed a marked induction of CXCL10 in the SINrep5-WNVC-implanted animals (P < 0.05), whereas the transcript levels of IL-1β (Fig. 8G), CCL2, IDO, and iNOS (data not shown) were not altered by SINrep5-WNVC implantation relative to SINrep5-EGFP- or mock-infected animals. However, there was a marked reduction in OASIS expression among the SINrep5-WNVC-infected animals compared to the mock-infected animals, whereas SINrep5-EGFP-implanted animals exhibited a variable (nonsignificant) increased OASIS expression. In these latter animals the trend toward increased OASIS expression might reflect a host response to the vector but also emphasizes the observation of OASIS downmodulation by the WNV capsid. Of note, GADD153, BiP, and PERK expression levels also did not significantly differ among groups (data not shown), in keeping with our ex vivo findings of SINrep5-WNVC infection of astrocytic cells. These studies indicated that in vivo the WNV capsid protein was responsible for the induction of only a subset of neuroinflammatory responses, particularly CXCL10, similar to what was observed in our ex vivo experiments. Moreover, these studies also showed that downregulation of OASIS is a consistent feature of WNV neuropathogenesis and that the WNV capsid protein appears to play a key role in suppressing this astrocyte-specific neuroprotective ER stress response.
FIG. 8.
Immunohistopathological changes and neuroinflammatory gene expression induced by in vivo WNV capsid protein expression. Immunohistochemical analysis revealed activation of astrocytes (GFAP) (A) and microglia (Iba-1) (B) in areas surrounding the SINrep5-WNVC (WNVC), which was not observed in SINrep5-EGFP (EGFP) (C and D) or mock-implanted animals (E and F), respectively. In addition, colocalization of GFAP and WNV capsid protein immunoreactivity was also observed (see panel A, inset: brown = GFAP, blue = WNV capsid), recapitulating the infection of astrocytic cells by WNV in the human CNS. Real-time RT-PCR analysis of the neuroinflammatory gene expression at day 14 after implantation indicated significant induction of CXCL10 mRNA expression in the SINrep5-WNVC implanted animals (G) but not of IL-1β. WNV capsid protein expression suppressed OASIS gene expression levels in astrocytes relative to controls. *, P < 0.05 (Dunn's multiple-comparison test).
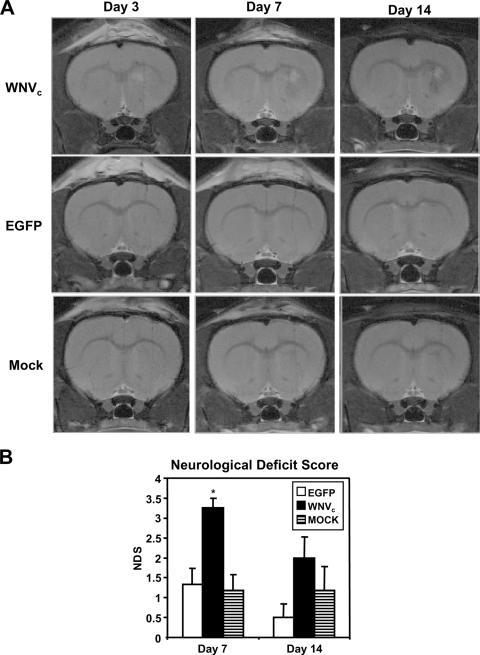
To verify the in vivo neuropathogenic properties of SINrep5-WNVC described above, we examined the neuroimaging and neurobehavioral outcomes after viral vector implantation (Fig. 9). MR images revealed areas of increased T2-weighted signal, surrounded by reduced signal in the basal ganglia of SINrep5-WNVC-infected animals compared to SINrep5-EGFP- and mock-infected animals, which were evident at day 3 postinfection but increased over the subsequent experimental period. Indeed, these findings, indicative of edema and tissue injury, were evident at both days 7 and 14 postinfection (Fig. 9A). Among control animals (SINrep5-EGFP and mock infected) there was, apart from the needle track, minimal tissue damage visible (Fig. 9A). Complementing these findings, a battery of neurobehavioral tests performed at 3, 7, and 14 days (Fig. 9B) revealed significant behavioral deficits in the SINrep5-WNVC-implanted animals, indicated by a significant increase in the mean NDS at day 7, compared to SINrep5-EGFP- and mock-infected animals, while there were no differences at day 3 postinfection among experimental groups (data not shown). The NDS score for the SINrep5-WNVC implanted animals at day 14 was also higher, but this difference was not statistically significant, probably due to animals recovering from the injury and due to the limited infection (i.e., one round of infection) of the SINrep5 vector system. Thus, the WNV capsid protein-encoding virus exerted both ex vivo and in vivo neurovirulent properties, highlighting its central role in WNV-mediated neuropathogenesis.
FIG. 9.
MRI and neurobehavioral analysis in rats after striatal implantation of the SINrep5 expression vectors. (A) MRI analysis revealed edema at the site of implantation, starting at day 3, which increased over time and was most pronounced at day 14, in the SINrep5-WNVC (WNVC)-implanted animals. In the control animals (SINrep5-EGFP [EGFP] and Mock) no sign of tissue injury, apart from the needle track, was visible, a finding consistent with our immunohistopathological analyses. (B) The MRI findings were further corroborated by neurobehavioral analysis at days 3, 7, and 14 after implantation. A significantly increased NDS at day 7 was observed in the SINrep5-WNVC (WNVC)-implanted animals compared to SINrep5-EGFP (EGFP)- and control (Mock)-implanted animals. Increases in the NDS were also observed for SINrep5-WNVC, although this difference was not statistically significant. *, P < 0.05 (Dunn's multiple-comparison test).
DISCUSSION
Activation of glial cells (astrocytes and microglia) in WNV neuropathogenesis has been reported and, together with neuronal death, is considered a key pathogenic feature (26, 28). However, the extent to which infection of glia contributes to WNV-induced neurological disease has received limited attention. In the present study we found evidence of infection of non-neuronal cells in the CNS of fatal human WNVE cases and that the infection by WNV of these cells, in particular astrocytes, contributed to neuronal death by releasing molecules with neurotoxic actions. Moreover, infection of astrocytes by WNV resulted in induction of neuroinflammatory genes. The WNV capsid protein was responsible for a subset of the neuroinflammatory genes induced and was a particularly potent activator of CXCL10 ex vivo and in vivo. Moreover, we observed dysregulation of the ER stress response pathway by downmodulating the expression of the astrocyte-specific protective ER stress gene regulator, OASIS, both in vivo and in vitro by WNV, which was also mediated by the WNV capsid protein.
WNV infects non-neuronal cells and induces neuroinflammation.
WNV infection of non-neuronal CNS cells such as astrocytes ex vivo has been reported (15, 37, 38). However, to our knowledge, this study shows for the first time in the human CNS that, apart from neurons, astrocytes and leukocytoid cells are also infected by WNV. Astrocytes appeared to be more permissive to WNV infection than monocytoid cells. In vivo, 3.3% of GFAP-positive cells exhibited WNV immunoreactivity, but GFAP immunopositivity likely under-represents the actual astrocyte numbers. The pathogenic relevance of the infection of these cells is underscored by the induction of neuroinflammatory gene expression ex vivo in monocytoid and astrocytic cell lines upon WNV infection. Our ex vivo data showed that the induction of the neuroinflammatory genes in the astrocytic and monocytoid cells occurred at low levels of infection. WNV-infected MDM showed low levels of infection, similar to the monocytoid (U-937) cells, but displayed IL-1β induction upon infection. IL-1β induction in MDM might reflect a higher level of differentiation and inherent responsiveness, which recapitulates microglial and perivascular macrophage infection by WNV in vivo. In fact these inflammatory responses resembled the proinflammatory gene profile observed in the CNS of human WNVE and indicated that both astrocytes and cells of a monocytoid/macrophage lineage contribute differentially to WNV-induced neuroinflammation after infection or exposure to WNV. These observations support the notion that limited in vivo infection of glia (and neurons) might significantly contribute to the neuroinflammatory response observed in WNV-associated neurological disease (15). In turn, neuronal cell injury after infection can activate glia and further drive neuroinflammation, a finding similar to what has been suggested for glial cell activation in hypoxia (2, 58).
WNV infection of astrocytes induces the release of neurotoxins.
For many neurological diseases, including those induced by viruses, it is clear that innate immunity in terms of glial cell activation with the subsequent release of neurotoxic molecules is a key component of the pathogenic cascade (9, 50). Our observations suggest that the same circumstances might apply to WNV-induced neurological disease. The conditioned media of astrocytic cells infected by WNV induced apoptotic neuronal death. The neurotoxicity of astrocyte conditioned media is primarily the consequence of molecules with neurotoxic actions that are released from infected astrocytic cells. The levels of glial infection were ∼100-fold lower, and the subsequent amount of virus released into the media was also ∼100-fold lower. Despite this low level of infection, the severity of cell death induced by these supernatants was similar to a direct infection of neurons with a high titer of WNV, indicating that the observed cell death was not the result of the residual WNV released from astrocytic cells. These findings emphasize the importance of both direct killing of neuronal cells by WNV virus and indirect neuronal death induced by neurotoxic molecules released from non-neuronal cells infected by WNV in WNV neuropathogenesis.
The nature of the neurotoxin(s) released from the astrocytic cells remains to be determined. We and others have shown that in the context of HIV type 1 infection of the brain, CXCL10 can act as a neurotoxin (59, 64). CXCL10 induces apoptotic cell death by increasing intracellular Ca2+ levels (60). As demonstrated here, WNV is a potent inducer of CXCL10 gene expression, a finding that has also been reported by other researchers (15, 30). Our results extend these studies by showing that the WNV capsid protein induces CXCL10 both ex vivo and in vivo, as well as a subset of other inflammatory genes, including CCL2. In vivo the WNV capsid protein could also induce the expression of CXCL10 mRNA, combined with neuroinflammation and neuronal injury, as evidenced by histopathological, MRI, and behavioral analyses. CXCL10 has been reported to have a protective function in WNV infection by recruiting immune cells into the CNS to clear infection (30), arguing against CXCL10 as the a major neurotoxin. Indeed, our ex vivo studies revealed limited neurotoxicity caused by supernatants derived from astrocytes expressing WNV capsid protein, suggesting that the induction of neurotoxins by astrocytes acutely plays a minor direct role in WNV capsid-mediated neuronal death, although over time there might be cumulative adverse effects on neurons. Moreover, CXCL10 produced in these cells contributes in a limited manner to the observed neurotoxicity mediated by WNV-infected astrocytes. However, synergistic interactions of CXCL10 with other molecules induced by WNV cannot be excluded. Regardless of the pathogenic role CXCL10 plays in WNV neuropathogenesis, our data suggested that the WNV capsid protein affects CXCL10 mRNA expression through downregulation of iNOS. This suppression would result in lowering the production of NO. A previous study (22), as well as our present observations, revealed that NO reduces the expression of CXCL10 mRNA in astrocytic cells. By disrupting this negative-feedback loop of CXCL10 gene expression, WNV might actually increase CXCL10 expression. Further studies are warranted of this possible mode of action by which WNV and the WNV capsid protein affect CXCL10 expression.
Direct and indirect induction of apoptotic neuronal cell death by WNV.
Similar to previous studies (15, 17, 47, 56), our results indicated that direct infection of neurons or astrocytes led to apoptotic neuronal death but not astrocyte death. Both in vivo and ex vivo studies of the WNV capsid protein have reported the induction of neuronal apoptosis through the activation of caspase-9 and subsequent cleavage of caspase-3 (66). The expression of the WNV capsid protein using our Sindbis virus-based vector in neuronal cells caused neuronal cell death but did not result in the activation of caspase-3, suggesting that the WNV capsid protein might not activate caspase-3 to the same extent in neuronal cells compared to infection by wild-type WNV. Alternatively, the cell death induced by the WNV capsid protein might also involve additional signaling pathways. Although WNV-induced apoptotic neuronal death has been recognized, it has been shown that the mechanism of cell death upon WNV infection is dependent on the initial infective dose, and high-titer infections will result in necrotic rather than apoptotic cell death (17). Since Sindbis virus vectors transiently express large amounts of protein (1, 10), the neuronal cell death observed in the WNV capsid protein-expressing cultures may be necrotic due to the high level of capsid expression. Both necrotic cell death and apoptotic cell death have been reported in WNV-infected tissues in both humans and animals (13, 52, 54, 55). This titer-dependent mechanism of cell death has been proposed to play an important role in WNV pathology (17, 57). Hence, CNS and systemic viral loads may determine pathogenic outcome and explain the diversity in WNV-induced disease (57). Our data further extend these findings by showing that during infections at very low MOIs, with limited neuronal death, the number of cells with neurites, as well as average neurite length, was reduced. These morphological changes suggest that at low-input titers WNV caused neuronal damage, which might be clinically relevant and explain the diversity of neurological phenotypes observed in WNV-infected individuals.
The ER stress response and downregulation of OASIS is a feature of WNV neuropathogenesis and mediated by the WNV capsid protein.
The impact of ER stress, resulting from accumulation of unfolded or misfolded proteins, on the induction of neuroinflammatory responses in glial cells and neuronal death has been proposed to play an important role in various models of neuronal injury and neurological diseases, ranging from Alzheimer's disease, to prion diseases, to retroviral infections (20, 21, 35, 67). Among mouse murine leukemia retrovirus strains, which induce neurological disease, the role of ER stress has been well described (20, 21). Infection of microglial cells and accumulation of the unfolded or misfolded envelope protein in the ER is the chief trigger for the pathogenic cascade and is one of the features that distinguish particular neurovirulent strains from non-neurovirulent retroviral strains (20). Our observations suggest that WNV also appears to induce genes associated with ER stress, such as GADD153, PERK, and BiP. In contrast, the WNV capsid protein alone did not activate these genes, apart from GADD153. The latter observation is plausible because in our WNV capsid expression vectors, the capsid protein is expressed as a single ORF and not as the WNV polypeptide and thus does not accumulate in the ER. Induction of ER stress genes is a normal cellular response to accumulation of large amounts of unfolded proteins, which protects against cell dysfunction or death (35). Recent studies have identified OASIS to be an important sensor of ER stress (31, 42). OASIS is an astrocyte-specific ER-related transcription factor that, upon activation by accumulation of unfolded or misfolded proteins in the ER, is translocated to the nucleus. BiP expression is regulated in part through OASIS by binding to its promoter (31). In models of neuronal damage such as stab injuries, OASIS expression is increased and considered to be part of a protective response of the astrocytes that diminishes neuronal damage (31, 42, 45). Indeed, in our study, OASIS mRNA expression was upregulated in neuroinflammatory diseases, i.e., HIV-associated dementia and MS, which might reflect a protective response. In contrast, for WNVE we did not observe upregulation of OASIS mRNA levels despite upregulation of ER stress genes to levels higher than those observed in AIDS and MS brain samples. Our present ex vivo studies and in vivo animal model indicated that WNV suppresses OASIS mRNA expression. Furthermore, our experiments showed that although the WNV capsid protein does not induce ER stress by itself, it does affect the ER stress response by downregulating OASIS. OASIS is a transcription factor with striking similarities to the activating transcription factor ATF-6, another ER stress transducer in both its overall structure and its mode of activation (31, 42). OASIS binds to cyclic AMP responsive element-like sequences in the BiP promoter (31). Similar elements are present in many other genes, including iNOS (6), and thus the downregulation of OASIS potentially dysregulates the ER stress response, rendering it no longer protective, which might manifest as the increases in BiP, PERK, and GADD153 mRNA expression observed here. Although we assessed the expression of only a subset of ER stress genes, the consistent observation of OASIS downregulation with its protective effects suggests that ER stress may be an important pathway, which is perturbed in WNV-associated neuropathogenesis.
In conclusion, our observations emphasize the pathogenic role of WNV infection of non-neuronal cells in the CNS and extend our knowledge of the role the capsid protein plays as a determinant of WNV neurovirulence. Our study also adds WNV to the growing list of pathogens in which ER stress exerts a pathogenic effect. The prospects of a WNV vaccine are promising (23, 40), but development of anti-inflammatory and neuroprotective strategies remain necessary for cases in which the vaccine is not fully protective. Future therapeutic strategies considered for WNV-induced neurological disease will need to focus on targeting multiple neuroinflammatory pathways. Given the potential key role of ER stress in WNV-induced neuroinflammation suggested by the present study, further study of this response might permit identification of molecular components in the pathogenic cascade leading to neuronal injury and death. These efforts might also aid in determining common regulators of ER stress, which could be targeted in other neuroinflammatory diseases.
Acknowledgments
We thank David George for helpful comments. We thank Neda Shariat and Martine Ooms for technical assistance. We gratefully acknowledge Peter Bredenbeek and Charles Rice for the pSINrep5 vector and the Provincial Laboratory (Edmonton, Alberta, Canada) for the WNV.
The Canadian Institutes of Health Research (C.P. and T.H.) and the Natural Science and Engineering Research Council (C.P. and T.H.) supported these studies. C.P. is an Alberta Heritage Foundation for Medical Research (AHFMR) Senior Scholar and holds a Canada Research Chair (T1) in Neurological Infection and Immunity. T.H. is an AHFMR Scientist.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Agapov, E. V., I. Frolov, B. D. Lindenbach, B. M. Pragai, S. Schlesinger, and C. M. Rice. 1998. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl. Acad. Sci. USA 95:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Saleh, S. S., C. Kaur, and E. A. Ling. 2003. Response of neurons and microglia/macrophages in the area postrema of adult rats following exposure to hypobaric hypoxia. Neurosci Lett. 346:77-80. [DOI] [PubMed] [Google Scholar]
- 3.Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2002. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 296:17-23. [DOI] [PubMed] [Google Scholar]
- 4.Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2001. West Nile virus strains differ in mouse neurovirulence and binding to mouse or human brain membrane receptor preparations. Ann. N. Y. Acad. Sci. 951:332-335. [DOI] [PubMed] [Google Scholar]
- 5.Beasley, D. W., M. C. Whiteman, S. Zhang, C. Y. Huang, B. S. Schneider, D. R. Smith, G. D. Gromowski, S. Higgs, R. M. Kinney, and A. D. Barrett. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 79:8339-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat, N. R., D. L. Feinstein, Q. Shen, and A. N. Bhat. 2002. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells: roles of nuclear factors, nuclear factor κB, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J. Biol. Chem. 277:29584-29592. [DOI] [PubMed] [Google Scholar]
- 7.Biernaskie, J., and D. Corbett. 2001. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J. Neurosci. 21:5272-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boven, L. A., N. Vergnolle, S. D. Henry, C. Silva, Y. Imai, J. Holden, K. Warren, M. D. Hollenberg, and C. Power. 2003. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J. Immunol. 170:2638-2646. [DOI] [PubMed] [Google Scholar]
- 9.Bradl, M., and R. Hohlfeld. 2003. Molecular pathogenesis of neuroinflammation. J. Neurol. Neurosurg. Psychiatry 74:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredenbeek, P. J., I. Frolov, C. M. Rice, and S. Schlesinger. 1993. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J. Virol. 67:6439-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinton, M. A. 2002. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu. Rev. Microbiol. 56:371-402. [DOI] [PubMed] [Google Scholar]
- 12.Burton, J. M., R. Z. Kern, W. Halliday, D. Mikulis, J. Brunton, M. Fearon, C. Pepperell, and C. Jaigobin. 2004. Neurological manifestations of West Nile virus infection. Can. J. Neurol. Sci. 31:185-193. [DOI] [PubMed] [Google Scholar]
- 13.Cantile, C., F. Del Piero, G. Di Guardo, and M. Arispici. 2001. Pathologic and immunohistochemical findings in naturally occurring West Nile virus infection in horses. Vet. Pathol. 38:414-421. [DOI] [PubMed] [Google Scholar]
- 14.Chambers, T. J., and M. S. Diamond. 2003. Pathogenesis of flavivirus encephalitis. Adv. Virus Res. 60:273-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheeran, M. C., S. Hu, W. S. Sheng, A. Rashid, P. K. Peterson, and J. R. Lokensgard. 2005. Differential responses of human brain cells to West Nile virus infection. J. Neurovirol. 11:512-524. [DOI] [PubMed] [Google Scholar]
- 16.Chu, J. J., and M. L. Ng. 2003. Characterization of a 105-kDa plasma membrane associated glycoprotein that is involved in West Nile virus binding and infection. Virology 312:458-469. [DOI] [PubMed] [Google Scholar]
- 17.Chu, J. J., and M. L. Ng. 2003. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J. Gen. Virol. 84:3305-3314. [DOI] [PubMed] [Google Scholar]
- 18.Cinque, P., A. Bestetti, R. Marenzi, S. Sala, M. Gisslen, L. Hagberg, and R. W. Price. 2005. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J. Neuroimmunol. 168:154-163. [DOI] [PubMed] [Google Scholar]
- 19.Delhaye, S., S. Paul, G. Blakgori, M. Minet, F. Weber, P. Staeheli, and T. Michiek. 2006. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA 103:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimcheff, D. E., M. A. Faasse, F. J. McAtee, and J. L. Portis. 2004. Endoplasmic reticulum (ER) stress induced by a neurovirulent mouse retrovirus is associated with prolonged BiP binding and retention of a viral protein in the ER. J. Biol. Chem. 279:33782-33790. [DOI] [PubMed] [Google Scholar]
- 22.Giustizieri, M. L., C. Albanesi, C. Scarponi, O. De Pita, and G. Girolomoni. 2002. Nitric oxide donors suppress chemokine production by keratinocytes in vitro and in vivo. Am. J. Pathol. 161:1409-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, R. A., and A. A. Khromykh. 2004. West Nile virus vaccines. Expert Opin. Biol. Ther. 4:1295-1305. [DOI] [PubMed] [Google Scholar]
- 24.Hayes, E. B. 2005. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 11:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honma, Y., K. Kanazawa, T. Mori, Y. Tanno, M. Tojo, H. Kiyosawa, J. Takeda, T. Nikaido, T. Tsukamoto, S. Yokoya, and A. Wanaka. 1999. Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Brain Res. Mol. Brain Res. 69:93-103. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, R. T. 2002. West Nile virus in the US and abroad. Curr. Clin. Top. Infect. Dis. 22:52-60. [PubMed] [Google Scholar]
- 27.Johnston, J. B., Y. Jiang, G. van Marle, M. B. Mayne, W. Ni, J. Holden, J. C. McArthur, and C. Power. 2000. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J. Virol. 74:7211-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley, T. W., R. A. Prayson, A. I. Ruiz, C. M. Isada, and S. M. Gordon. 2003. The neuropathology of West Nile virus meningoencephalitis: a report of two cases and review of the literature. Am. J. Clin. Pathol. 119:749-753. [DOI] [PubMed] [Google Scholar]
- 29.Kesson, A. M., and N. J. King. 2001. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-κB activation. J. Infect. Dis. 184:947-954. [DOI] [PubMed] [Google Scholar]
- 30.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo, S., T. Murakami, K. Tatsumi, M. Ogata, S. Kanemoto, K. Otori, K. Iseki, A. Wanaka, and K. Imaizumi. 2005. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol. 7:186-194. [DOI] [PubMed] [Google Scholar]
- 32.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 33.Lee, E., and M. Lobigs. 2002. Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J. Virol. 76:4901-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindenbach, B. D., and C. M. Rice. 2003. Molecular biology of flaviviruses. Adv. Virus Res. 59:23-61. [DOI] [PubMed] [Google Scholar]
- 35.Lindholm, D., H. Wootz, and L. Korhonen. 2006. ER stress and neurodegenerative diseases. Cell Death Differ. 13:385-392. [DOI] [PubMed] [Google Scholar]
- 36.Liu, W. J., X. J. Wang, D. C. Clark, M. Lobigs, R. A. Hall, and A. A. Khromykh. 2006. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J. Virol. 80:2396-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1989. Flavivirus infection up-regulates the expression of class I and class II major histocompatibility antigens on and enhances T-cell recognition of astrocytes in vitro. J. Neuroimmunol. 21:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1988. West Nile virus infection modulates the expression of class I and class II MHC antigens on astrocytes in vitro. Ann. N. Y. Acad. Sci. 540:483-485. [DOI] [PubMed] [Google Scholar]
- 39.Mahesh, V. B., K. M. Dhandapani, and D. W. Brann. 2006. Role of astrocytes in reproduction and neuroprotection. Mol. Cell Endocrinol. 246:1-9. [DOI] [PubMed] [Google Scholar]
- 40.Monath, T. P., J. Liu, N. Kanesa-Thasan, G. A. Myers, R. Nichols, A. Deary, K. McCarthy, C. Johnson, T. Ermak, S. Shin, J. Arroyo, F. Guirakhoo, J. S. Kennedy, F. A. Ennis, S. Green, and P. Bedford. 2006. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. USA 103:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami, T., S. Kondo, M. Ogata, S. Kanemoto, A. Saito, A. Wanaka, and K. Imaizumi. 2006. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J. Neurochem. 96:1090-1100. [DOI] [PubMed] [Google Scholar]
- 43.Ni, H., and A. D. Barrett. 1996. Molecular differences between wild-type Japanese encephalitis virus strains of high and low mouse neuroinvasiveness. J. Gen. Virol. 77(Pt. 7):1449-1455. [DOI] [PubMed] [Google Scholar]
- 44.Nickells, M., and T. J. Chambers. 2003. Neuroadapted yellow fever virus 17D: determinants in the envelope protein govern neuroinvasiveness for SCID mice. J. Virol. 77:12232-12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikaido, T., K. Iseki, T. Mori, H. Takaki, S. Yokoya, S. Hagino, J. Takeda, Y. Zhang, M. Takeuchi, S. Kikuchi, and A. Wanaka. 2002. Expression of OASIS, a CREB/ATF family transcription factor, in CNS lesion and its transcriptional activity. Brain Res. Mol. Brain Res. 108:129-138. [DOI] [PubMed] [Google Scholar]
- 46.Noorbakhsh, F., N. Vergnolle, J. C. McArthur, C. Silva, M. Vodjgani, P. Andrade-Gordon, M. D. Hollenberg, and C. Power. 2005. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J. Immunol. 174:7320-7329. [DOI] [PubMed] [Google Scholar]
- 47.Parquet, M. C., A. Kumatori, F. Hasebe, K. Morita, and A. Igarashi. 2001. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 500:17-24. [DOI] [PubMed] [Google Scholar]
- 48.Peeling, J., H. J. Yan, D. Corbett, M. Xue, and M. R. Del Bigio. 2001. Effect of FK-506 on inflammation and behavioral outcome following intracerebral hemorrhage in rat. Exp. Neurol. 167:341-347. [DOI] [PubMed] [Google Scholar]
- 49.Power, C., S. Henry, M. R. Del Bigio, P. H. Larsen, D. Corbett, Y. Imai, V. W. Yong, and J. Peeling. 2003. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann. Neurol. 53:731-742. [DOI] [PubMed] [Google Scholar]
- 50.Power, C., and R. T. Johnson. 2001. Neuroimmune and neurovirological aspects of human immunodeficiency virus infection. Adv. Virus Res. 56:389-433. [DOI] [PubMed] [Google Scholar]
- 51.Power, C., P. A. Kong, T. O. Crawford, S. Wesselingh, J. D. Glass, J. C. McArthur, and B. D. Trapp. 1993. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann. Neurol. 34:339-350. [DOI] [PubMed] [Google Scholar]
- 52.Sampson, B. A., C. Ambrosi, A. Charlot, K. Reiber, J. F. Veress, and V. Armbrustmacher. 2000. The pathology of human West Nile Virus infection. Hum. Pathol. 31:527-531. [DOI] [PubMed] [Google Scholar]
- 53.Sampson, B. A., and V. Armbrustmacher. 2001. West Nile encephalitis: the neuropathology of four fatalities. Ann. N. Y. Acad. Sci. 951:172-178. [PubMed] [Google Scholar]
- 54.Senne, D. A., J. C. Pedersen, D. L. Hutto, W. D. Taylor, B. J. Schmitt, and B. Panigrahy. 2000. Pathogenicity of West Nile virus in chickens. Avian Dis. 44:642-649. [PubMed] [Google Scholar]
- 55.Shieh, W. J., J. Guarner, M. Layton, A. Fine, J. Miller, D. Nash, G. L. Campbell, J. T. Roehrig, D. J. Gubler, and S. R. Zaki. 2000. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg. Infect. Dis. 6:370-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrestha, B., D. Gottlieb, and M. S. Diamond. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77:13203-13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solomon, T., and D. W. Vaughn. 2002. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr. Top. Microbiol. Immunol. 267:171-194. [DOI] [PubMed] [Google Scholar]
- 58.Stys, P. K. 2004. White matter injury mechanisms. Curr. Mol. Med. 4:113-130. [DOI] [PubMed] [Google Scholar]
- 59.Sui, Y., R. Potula, N. Dhillon, D. Pinson, S. Li, A. Nath, C. Anderson, J. Turchan, D. Kolson, O. Narayan, and S. Buch. 2004. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am. J. Pathol. 164:1557-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sui, Y., L. Stehno-Bittel, S. Li, R. Loganathan, N. K. Dhillon, D. Pinson, A. Nath, D. Kolson, O. Narayan, and S. Buch. 2006. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur. J. Neurosci. 23:957-964. [DOI] [PubMed] [Google Scholar]
- 61.Tsutsui, S., J. Schnermann, F. Noorbakhsh, S. Henry, V. W. Yong, B. W. Winston, K. Warren, and C. Power. 2004. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 24:1521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vajda, F. J. 2002. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 9:4-8. [DOI] [PubMed] [Google Scholar]
- 63.van Marle, G., J. Ethier, C. Silva, B. A. MacVicar, and C. Power. 2003. Human immunodeficiency virus type 1 envelope-mediated neuropathogenesis: targeted gene delivery by a Sindbis virus expression vector. Virology 309:61-74. [DOI] [PubMed] [Google Scholar]
- 64.van Marle, G., S. Henry, T. Todoruk, A. Sullivan, C. Silva, S. B. Rourke, J. Holden, J. McArthur, M. J. Gill, and C. Power. 2004. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology 329:302-318. [DOI] [PubMed] [Google Scholar]
- 65.Wicker, J. A., M. C. Whiteman, D. W. Beasley, C. T. Davis, S. Zhang, B. S. Schneider, S. Higgs, R. M. Kinney, and A. D. Barrett. 2006. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 349:245-253. [DOI] [PubMed] [Google Scholar]
- 66.Yang, J. S., M. P. Ramanathan, K. Muthumani, A. Y. Choo, S. H. Jin, Q. C. Yu, D. S. Hwang, D. K. Choo, M. D. Lee, K. Dang, W. Tang, J. J. Kim, and D. B. Weiner. 2002. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg. Infect. Dis. 8:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, K., and R. J. Kaufman. 2006. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:S102-S109. [DOI] [PubMed] [Google Scholar]