Abstract
Cytotoxic T lymphocytes (CTLs) are important for the control of virus replication during respiratory infections. For human metapneumovirus (hMPV), an H-2d-restricted CTL epitope in the M2-2 protein has been described. In this study, we screened the hMPV F, G, N, M, M2-1, and M2-2 proteins using three independent algorithms to predict H-2d CTL epitopes in BALB/c mice. A dominant epitope (GYIDDNQSI) in positions 81 to 89 of the antitermination factor M2-1 and a subdominant epitope (SPKAGLLSL) in N307-315 were detected during the anti-hMPV CTL response. Passive transfer of CD8+ T-cell lines against M2-181-89 and N307-315 protected Rag1−/− mice against hMPV challenge. Interestingly, diversification of CTL targets to include multiple epitopes was observed after repetitive infections. A subdominant response against the previously described M2-2 epitope was detected after the third infection. An understanding of the CTL response against hMPV is important for developing preventive and therapeutic strategies against the virus.
Human metapneumovirus (hMPV) is an important agent of lower respiratory illness in infants, young children, adults, and the elderly (25, 26). The virus infects over 70% of children before the age of 5 years and subsequently causes repetitive infections throughout life (25, 26). In fact, in many studies, hMPV is second only to respiratory syncytial virus (RSV) as a cause of pediatric respiratory disease, and its clinical manifestations range from rhinorrhea, cough, and viral pneumonia to asthmatic exacerbations (14, 26).
hMPV is an enveloped single-stranded negative RNA virus with eight genes encoding nine viral proteins: three membrane glycoproteins (fusion [F], attachment [G], and small hydrophobic [SH]), a matrix protein (M), two regulatory proteins associated with transcription and replication (M2-1 and M2-2), and a nucleoprotein complex (N, P, and L) (6). Two genotypes of hMPV, genotypes A and B, with 80 to 88% homology, have been described by sequence analysis (6). As with another respiratory virus, RSV, neutralizing antibodies against the F protein confer protection against challenge, and cytotoxic T-lymphocyte (CTL) responses are thought to play an important role in the control of viral replication (1, 5, 10, 16, 28). CD8+ T cells recognize major histocompatibility complex class I molecules carrying 8- to 10-amino-acid peptides and control infection by the direct destruction of infected cells or by the release of antiviral cytokines (20).
However, little is known about the hMPV-specific CTL response. A single H-2d-restricted epitope in positions 56 to 64 of the M2-2 protein (M2-256-64) has been described in BALB/c mice, a second CTL epitope has been reported in H-2b-restricted rodents, and responses against a human HLA A*0201 epitope have been shown to protect transgenic mice from viral challenge (10). A role for CD8+ T cells in viral clearance is supported by these findings and by increased hMPV titers in T-cell-depleted mice (1). Importantly, the hierarchical role of the recently described H-2d-restricted M2-2 epitope and the breadth of the CTL response during primary and anamnestic responses to infection are unknown. Understanding anamnestic responses is of particular interest for respiratory viruses, as these agents elicit recurrent infections throughout life, partially overcoming the host's immune response. In this paper, using different inoculation routes, we identify the dominant and subdominant H-2d primary CTL response against hMPV. In addition, we describe a wide diversification of the memory response after secondary infection and characterize the hierarchical role of 12 H-2d-restricted epitopes, including a subdominant role for the memory CTL response against the previously described M2-256-64.
MATERIALS AND METHODS
Epitope prediction and peptide synthesis.
Identification of candidate H-2 Kd/Ld-binding epitopes from hMPV F, G, N, M, M2-1, and M2-2 proteins was conducted using three predictive algorithms: BIMAS (National Institutes of Health), which ranks potential 8-mer, 9-mer, or 10-mer peptides based on a predicted half-time of dissociation to HLA class I molecules; SYFPETHI (Heidelberg University), which takes into consideration the amino acids in the anchor and auxiliary anchor positions as well as other frequent amino acids; and PREDEP (Jerusalem University). The six hMPV proteins were chosen by analogy to those associated with CTL responses in RSV. The hMPV polymerase (P and L proteins) was not mapped for epitopes due to its large size. Sequences with high major histocompatibility complex binding scores were preselected from within each protein. Twelve candidate nonamers (with the highest scores) were selected for further evaluation, and corresponding peptides were synthesized by Sigma-Genosys with ≥90% purity. Peptides were analyzed by high-performance liquid chromatography and dissolved in 10% dimethyl sulfoxide in distilled water.
Infection of mice.
Four- to six-week-old BALB/c and Rag1−/− mice were purchased from The Jackson Laboratories and housed in a pathogen-free facility at Johns Hopkins University. BALB/c mice were inoculated intraperitoneally (i.p.) or intranasally (i.n.) with 1 to 3 doses of 106 PFU of hMPV (Can83) previously grown in LLC-MK2 cells. Inoculations were performed every 30 days. To characterize the kinetics of anti-hMPV CTL responses, mice were euthanized 7, 10, and 14 days after primary infection and 10 days after the second or third dose of virus. All experimentation was approved by the Animal Care and Use Committee at Johns Hopkins University.
Immunospot assay.
Nitrocellulose-based 96-well microtiter plates (Millititer HA; Millipore) were coated with 10 μg/ml of anti-gamma interferon (IFN-γ) monoclonal antibody (catalog no. 551309; BD, Bioscience) overnight at room temperature. For i.p. inoculations, splenocytes (5 × 105 cells/well) were incubated in the coated plates for 18 h with irradiated A-20 cells (ATCC TIB-208) pulsed with 10−6 M of each of the 12 peptides in Dulbecco's modified Eagle's medium with 1.2% recombinant interleukin-2 (IO523; Sigma) and 10% fetal bovine serum. Obtention of pulmonary mononuclear cells (105 cells/well) to assess the pulmonary CTL responses was performed as previously described (4). Spots corresponding to individual IFN-γ-producing cells were detected using biotinylated anti-IFN-γ monoclonal antibody (catalog no. 551506; BD Bioscience) followed by streptavidin peroxidase and 3,3′-diaminobenzidine tetrahydrochloride dehydrate (D-4293; Sigma). All assays were performed in triplicate.
Generation of primary hMPV-specific CD8+ T-cell lines against M2-181-89 and N307-315 epitopes.
To generate epitope-specific CD8+ T-cell lines, we inoculated BALB/c mice with 106 PFU of hMPV i.p. on days 0 and 30. Ten days after the second inoculation, splenocytes were obtained and stimulated in vitro with irradiated A-20 cells pulsed with peptide (M2-181-89 or N307-315) using 10−6 M per 106 cells. Cells were maintained at 37°C in 10% Dulbecco's modified Eagle's medium with 1.2% recombinant interleukin-2 and 10% fetal bovine serum and restimulated weekly (8). M2-181-89 or N307-315 hMPV CTL lines were characterized by flow cytometry using anti-mouse CD3 (fluorescein isothiocyanate, catalog no. 553067; BD Bioscience), CD4 (PerCP, catalog. no 553052; BD Bioscience), and CD8 (phycoerythrin, catalog no. 553041; BD Bioscience) antibodies. Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson).
Protection assay.
To examine the protective efficacy of the M2-181-89 and N307-315 responses, 7 × 106 cells from each T-cell line were passively transferred through the tail vein into 4- to 6-week-old Rag1−/− mice (BALB/c background with no CD3+ and T-cell-receptor alpha-beta-positive cells). Control groups of Rag1−/− mice received a control cell line directed against an irrelevant peptide (Y26 from Plasmodium berghei) or no cells. One hour after cell transfer, Rag1−/− mice were inoculated with 106 PFU of hMPV i.n. Four days after challenge, lungs were collected, and virus titers were estimated by plaque assay using LLC-MK2 cells. Determination of titers was performed using primary rabbit polyclonal anti-hMPV (1:1,000) and goat anti-rabbit (1:1,000, 074-1506; KPL) secondary antibodies. Titers were calculated per gram of tissue.
RESULTS
Identification of H-2d-restricted epitopes in hMPV proteins.
The hMPV proteins F, G, N, M, M2-1, and M2-2 were screened for H-2d-restricted epitopes using three independent online algorithms (Table 1). Twelve epitopes were selected based on scores, and synthetic peptides comprising these sequences were synthesized to determine their role during the CD8+ T-cell response against hMPV. Of these candidate epitopes, three were located in each of the F and N proteins, two were located in the M and M2-1 proteins, and one was located in each of the M2-2 and G proteins (Table 1).
TABLE 1.
Twelve selected H-2d-restricted predictopes and their scores using the three online algorithms, SYFPEITHI, BIMAS, and PREDEP
| Protein | Restriction (positions) | Sequence |
|---|---|---|
| F | Ld (63-71) | GPSLKTEL |
| Ld (97-105) | NPRQSRFVL | |
| Kd (384-392) | CYKGVSCSI | |
| G | Kd (47-55) | IYLIINYTI |
| N | Kd (251-259) | AYGAGQTML |
| Ld (318-326) | CPNFASVVL | |
| Ld(307-315)a | SPKAGLLSL | |
| M | Ld (28-36) | LPASLTIWF |
| Kd (128-136) | KFVSSAKPV | |
| M2-1 | Ld (139-147) | TPASLINNL |
| Kd(81-89)a | GYIDDNQSI | |
| M2-2 | Kd/Db (56-64) | CYLENIEII |
The dominant M2-181-89 and subdominant N307-315 epitopes are highlighted with boldface type.
Screening and identification of dominant and subdominant hMPV H-2d epitopes.
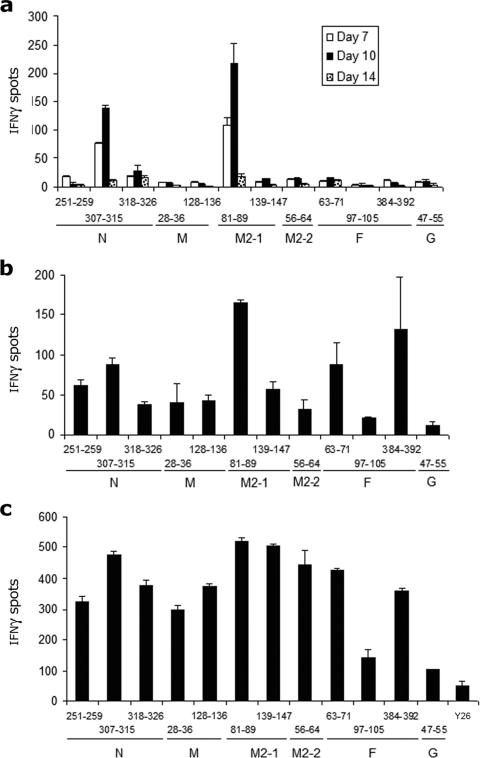
To determine if the 12 selected predictopes elicited CTL responses, we measured IFN-γ production against candidate peptides by CTLs from H-2d-restricted mice (BALB/c) infected i.p. with hMPV (Fig. 1). The H-2d-restricted primary response against 106 PFU of hMPV was dominated by CTLs directed against an epitope (GYIDDNQSI) spanning amino acids 81 to 89 of M2-1 and a second epitope (SPKAGLLSL) encompassing amino acids 307 to 315 in N. Both the dominant M2-181-89 and the subdominant N307-315 CTL responses were detectable 7 days after infection, peaked on day 10, and decreased significantly by day 14 (Fig. 1a). Interestingly, primary cytotoxicity against the other 10 predictopes remained low in comparison to responses against M2-181-89 and N307-315.
FIG. 1.
Identification of dominant and subdominant hMPV H-2d-restricted epitopes after intraperitoneal inoculation. Splenocytes isolated after primary (a), secondary (b), and tertiary (c) infection with 106 PFU of hMPV i.p. were stimulated with peptide-loaded A-20 target cells, stained for IFN-γ, and analyzed by an immunospot assay. Responses are displayed for days 7, 10, and 14 after primary infection (a) and 10 days after secondary and tertiary infection (b and c). An irrelevant control peptide was used as a negative control. The results are means ± standard errors of the means (SEM) and are representative of two to three independent experiments.
Diversification of the H-2d-restricted CD8+ T-lymphocyte anamnestic response against hMPV.
hMPV infects children and adults repeatedly throughout life (26, 27). Therefore, understanding the anamnestic CTL response against the virus is important. To characterize the breadth of the anamnestic CTL response and the hierarchy between epitope-specific responses, we evaluated IFN-γ secretion in splenocytes of BALB/c mice infected with two or three i.p. doses of hMPV (Fig. 1b and c). Interestingly, in addition to the dominant M2-181-89 and subdominant N307-315 responses detected during primary infection, responses against epitopes in F384-392 and F63-71 gained quantitative relevance during secondary i.p. infection (Fig. 1b). During the third i.p. hMPV infection, the CTL response was extensively diversified, and several codominant responses for specific H-2d-restricted epitopes were observed (Fig. 1c). Among these epitopes, the response against the previously identified M2-256-64 region played a subdominant role (Fig. 1c).
Dominant and subdominant CTL responses in the respiratory tract are directed against the M2-181-89 and N307-315 epitopes.
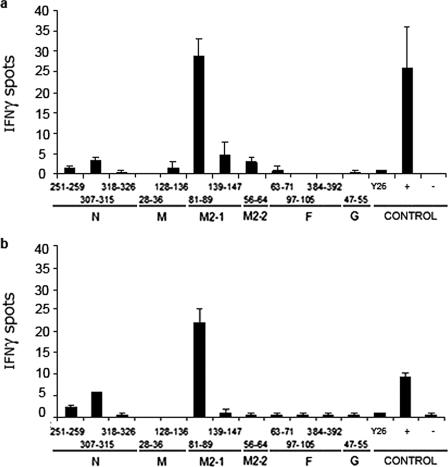
Since the most relevant clinical mode of hMPV infection is respiratory, we examined whether the dominant and subdominant responses identified after i.p. inoculation were also prevalent during i.n. infection (Fig. 2). As described above for i.p. experiments, i.n. hMPV infection elicited a dominant H-2d-restricted CTL response directed against the M2-181-89 epitope. This dominant response was present 10 days after primary i.n. challenge (Fig. 2a). The secondary CTL response against hMPV was also dominated by M2-181-89, while a response against N307-315 emerged in a subdominant role (Fig. 2b).
FIG. 2.
Dominant and subdominant hMPV H-2d-restricted epitopes in the respiratory tract. Pulmonary mononuclear cells isolated after primary (a) and secondary (b) infection with 106 PFU of hMPV i.n. were stimulated with peptide-loaded A-20 target cells, stained for IFN-γ, and analyzed by an immunospot assay. Responses are displayed for day 10 after primary and secondary infection. An irrelevant control peptide was used as a negative control (Y26 from P. berghei). The results are means ± SEM and are representative of two independent experiments.
Evidence of activation in hMPV-specific CD8+ T-cell lines.
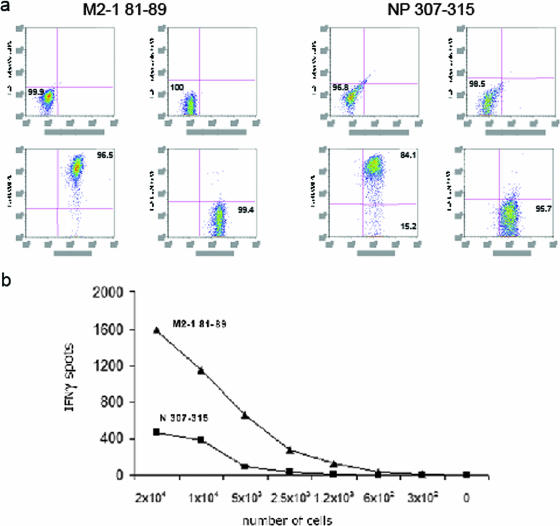
We then generated CD8+ primary T-cell lines against the M2-181-89 or N307-315 region of the virus. The lineage of T-cell lines was evaluated by flow cytometry, and shown, in both cases, to be overwhelmingly CD3+/CD8+ (Fig. 3a). Serial dilution of these cell lines revealed that the number of CD8+ T cells producing IFN-γ was fourfold greater in M2-181-89-specific than in N307-315-specific cells (Fig. 3b). The lytic activity of M2-181-89 and N307-315 was confirmed by examining the killing of peptide-specific target cells, as the cocultivation of epitope-specific cells with peptide-loaded target A-20 cells led to cultures with >90% M2-181-89- or N307-315-specific cells within 5 to 7 days (not shown).
FIG. 3.
Characterization of cytotoxic T-cell lines against hMPV M2-181-89 and N307-315 epitopes. (a) Lineage of hMPV M2-181-89- and N307-315-specific cell lines evaluated for CD3, CD4, and CD8 expression by flow cytometry. (b) Production of IFN-γ after serial dilution of M2-181-89 and N307-315 CD8+ T-cell lines. IFN-γ production was analyzed by an immunospot assay after stimulation with the corresponding peptide-loaded A-20 target cells. Results are representative of two to three independent experiments.
Evidence for CD8+ T-lymphocyte activation after intranasal hMPV inoculation of BALB/c mice.
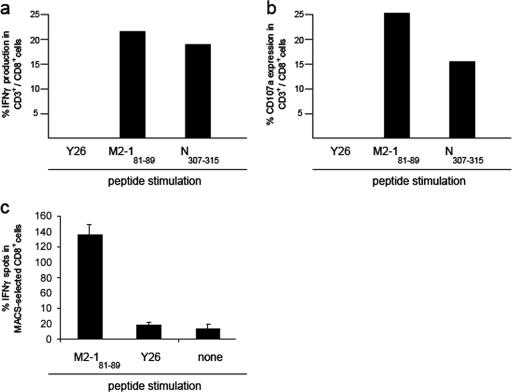
To examine the activation of M2-181-89- and N 307-315-specific CD8+ T lymphocytes after i.n. exposure to hMPV, we studied the intracellular production of IFN-γ and CD107a cell surface expression in CD3+/CD8+ T cells (Fig. 4a and b). The production of IFN-γ and expression of CD107a were detected in pulmonary M2-181-89- and N307-315-specific cells but not in those incubated with an irrelevant control peptide from P. berghei (19). Confirmation of IFN-γ production in CD8+ T lymphocytes from the respiratory tract of infected mice (Fig. 4c) was obtained after the purification of CD8+ cells and stimulation with the M2-181-89 peptide. IFN-γ production was not observed after exposure to an irrelevant control peptide (Fig. 4c). Non-CD8+ T cells exhibited negligible IFN-γ production upon exposure to M2-181-89 or control peptide (not shown).
FIG. 4.
Functional ex vivo studies demonstrating activation of hMPV-specific T lymphocytes in the respiratory tract. Shown is flow cytometry evidence for the ex vivo production of intracellular IFN-γ (a) and cell surface expression of CD107a (b) in pulmonary CD3+/CD8+ T cells after hMPV exposure. (c) shows IFN-γ production in ex vivo magnetic cell sorting (MACS)-purified CD8+ T cells after stimulation with M2-181-89 or an irrelevant control peptide from P. berghei (Y26). Results are representative of two independent experiments.
Passive transfer of CD8+ primary T cells against M2-181-89 or N307-315 reduces hMPV titers in the lungs of mice.
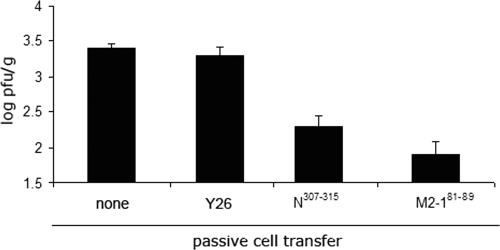
Finally, we evaluated the protective efficacy of M2-181-89 and N307-315 CD8+ T cells by passive transfer of 7 × 106 epitope-specific cells into Rag 1−/− mice followed by i.n. inoculation of 106 PFU of hMPV (Fig. 5). Both the M2-181-89 and N307-315 CD8+ T cells reduced hMPV titers ∼1.5 logs 4 days after challenge compared to titers in two control groups of mice: one infected with hMPV and subjected to transfer with a cell line directed against an irrelevant control epitope (Y26 from P. berghei [19]) and a second group receiving no cells.
FIG. 5.
Protective efficacy of M2-181-89 and N307-315 CD8+ T cells. HMPV titers in lungs of Rag1−/− mice were determined 4 days after i.n. challenge with 106 PFU of wild-type hMPV. Rag1−/− mice received 7 × 106 T cells specific for M2-181-89 or N307-315 epitopes by passive transfer 24 h prior to challenge. Control groups of Rag1−/− mice received cells specific for an irrelevant control peptide (Y26 from P. berghei) or no cells before hMPV infection. Four to five mice per group were used. The results are means ± SEM and are representative of two independent experiments.
DISCUSSION
In this paper, we describe dominant and subdominant H-2d-restricted CTL responses against hMPV, characterize the diversification of the anamnestic CTL response targeting multiple epitopes, and contextualize the role of CTLs against the previously described M2-256-64 region. Our data show that a dominant CTL response against hMPV is directed against amino acids 81 to 89 of the antitermination factor M2-1 and, in addition, identify a subdominant target in N307-315 during primary and secondary infection. Responses against both of these epitopes can decrease the hMPV load in the lungs. Other epitopes, including M2-256-64, are incorporated as targets during memory responses.
HMPV, like other respiratory viruses, causes repetitive infections throughout life (26, 27). Infection in infants and young children is often associated with bronchiolitis, while it causes upper respiratory illness or asthmatic exacerbations in older patients (14, 25-27). The diversification of the anamnestic CTL response provides a broad repertoire of virus-specific CD8+ T cells for viral clearance and protects the host against the emergence of escape mutants. A similar diversification has been described for RSV CTL epitopes in H-2b mice (15), while the extent of the anamnestic H-2d-restricted response after RSV infection, beyond dominant cytotoxicity against a region in RSV M2-1 and subdominant epitopes in RSV F (4, 17, 18), has not been fully characterized. Other viruses, like influenza virus, present a shift in dominance in CTL epitopes between primary and secondary responses (7, 22). Differences in immunodominance during primary and secondary responses against influenza virus have been attributed to differential antigen presentation by dendritic and nondendritic cells (7).
During infections with RSV, CTLs are important for virus clearance (3, 9, 12, 13, 21) and appear to play a critical role in regulating the immune response through the secretion of factors, notably IFN-γ (11, 23, 24). During hMPV infection, a role for T cells in the control of replication is supported by our findings and increased virus titers in mice after T-cell depletion (1). Furthermore, immunization of H-2b mice with hMPV peptides elicited IFN-γ production and cytotoxicity, and HLA A*0201 transgenic mice infected with hMPV had lower virus titers in the lungs when preimmunized with an hMPV-specific CTL peptide than with an irrelevant-peptide vaccine (10). As for immunomodulation, a recent report described CTL-mediated enhancement of Th1 cytokines interleukin-12 and IFN-γ in lungs and regional lymph nodes during hMPV infection (10). The implications of these observations for hMPV pathogenesis remain to be determined.
Despite the protective role described for CTL responses in hMPV, a recent study by Alvarez and Tripp found the primary CTL response against hMPV to be delayed (2). We did not observe a delay in primary CTLs in our study, where vigorous activity against M2-181-89 and N307-315 was detected 7 days after i.p. infection and 10 days after i.n. inoculation. These differences may be explained, at least in part, by differences in predictope selection between studies (2) and differences in design. Alternatively, 51Cr assays may have suboptimal sensitivity when used with target cells infected with live virus (2). Interestingly, our model, using a different inoculation strategy, conferred a minor subdominant role to the previously identified epitope in M2-256-64 (10).
Our study identified H-2d-restricted epitopes in six hMPV proteins. It is possible that additional epitopes are present in the remaining three viral proteins, L, SH, and P, or responses against other epitopes in the six selected proteins were not observed with the current peptide doses but would be detected at higher peptide concentrations. It is also theoretically possible that since epitope-specific CTL selection was based on IFN-γ production, cytotoxic cells producing other cytokines exist.
In summary, we describe a novel dominant and protective CTL epitope in hMPV M2-181-89 and subdominant epitopes in N and F. Our findings demonstrate that cytotoxicity against the virus diversifies extensively during anamnestic responses. Characterization of CD8+ T-cell immunity against hMPV will contribute to the development of vaccines to protect infants and children against this important agent.
Acknowledgments
This work was supported by a National Institute of Environmental Health Sciences contract mechanism with Johns Hopkins University and Fundacion INFANT and AI-054952 (F.P.P.). S.R.K. was supported by the Intramural Research program of the National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services.
We report no commercial or other associations that might pose a conflict of interest.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Alvarez, R., K. S. Harrod, W. J. Shieh, S. Zaki, and R. A. Tripp. 2004. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 78:14003-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, R., and R. A. Tripp. 2005. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J. Virol. 79:5971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev, A., M. E. Serra, F. R. Laham, S. R. Klebereger, P. L. Collins, and F. P. Polack. 2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibility complex class I-restricted epitopes. J. Virol. 80:5854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, J., A. Srikiatkhachorn, and T. J. Braciale. 2001. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J. Immunol. 167:4254-4260. [DOI] [PubMed] [Google Scholar]
- 5.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In B. N. Fields and D. M. Knipe (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 6.Crowe, J. E., Jr. 2004. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr. Infect. Dis. J. 23:S215-S221. [DOI] [PubMed] [Google Scholar]
- 7.Crowe, S. R., S. J. Turner, S. C. Miller, A. D. Roberts, R. A. Rappolo, P. C. Doherty, K. H. Ely, and D. L. Woodland. 2003. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 198:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez, J. A., F. Zavala, and M. Tsuji. 1998. Phenotypic and functional characterization of CD8+ T cell clones specific for a mouse cytomegalovirus epitope. Virology 255:40-49. [DOI] [PubMed] [Google Scholar]
- 9.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herd, K. A., S. Mahalingam, I. M. Mackay, M. Nissen, T. P. Sloots, and R. W. Tindle. 2006. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J. Virol. 80:2034-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussell, T., C. J. Baldwin, A. O'Garra, and P. J. Openshaw. 1997. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 27:3341-3349. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni, A. B., M. Connors, C. Y. Firestone, H. C. Morse III, and B. R. Murphy. 1993. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J. Virol. 67:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni, A. B., H. C. Morse III, J. R. Bennink, J. W. Yewdell, and B. R. Murphy. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J. Virol. 67:4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laham, F. R., V. Israele, J. M. Casellas, A. M. Garcia, C. M. L. Prugent, S. J. Hoffman, D. Hauer, B. Thumar, M. I. Name, A. Pascual, N. Taratutto, M. T. Ishida, M. Balduzzi, M. Maccarone, S. Jackli, R. Passarino, R. A. Gaivironsky, R. A. Karron, N. R. Polack, and F. P. Polack. 2004. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 189:2047-2056. [DOI] [PubMed] [Google Scholar]
- 15.Lukens, M. V., E. A. Claassen, P. M. de Graaff, M. E. van Dijk, P. Hoogerhout, M. Toebes, T. N. Schumacher, R. G. van der Most, J. L. Kimpen, and G. M. van Bleek. 2006. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology 352:157-168. [DOI] [PubMed] [Google Scholar]
- 16.Ma, X., R. Endo, T. Ebihara, N. Ishiguro, H. Ishiko, and H. Kikuta. 2005. Production and characterization of neutralizing monoclonal antibodies against human metapneumovirus F protein. Hybridoma 24:201-205. [DOI] [PubMed] [Google Scholar]
- 17.Nicholas, J. A., K. L. Rubino, M. E. Levely, E. G. Adams, and P. L. Collins. 1990. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J. Virol. 64:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Openshaw, P. J., K. Anderson, G. W. Wertz, and B. A. Askonas. 1990. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J. Virol. 64:1683-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero, P., J. L. Maryanski, G. Corradin, R. S. Nussenzweig, V. Nussenzweig, and F. Zavala. 1989. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341:323-326. [DOI] [PubMed] [Google Scholar]
- 20.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 21.Rutigliano, J. A., M. T. Rock, A. K. Johnson, J. E. Crowe, Jr., and B. S. Graham. 2005. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology 337:335-343. [DOI] [PubMed] [Google Scholar]
- 22.Spencer, J. V., and T. J. Braciale. 2000. Incomplete CD8(+) T lymphocyte differentiation as a mechanism for subdominant cytotoxic T lymphocyte responses to a viral antigen. J. Exp. Med. 191:1687-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang, Y. W., and B. S. Graham. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J. Clin. Investig. 94:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams, J. V., J. E. Crowe, Jr., R. Enriquez, P. Minton, R. S. Peebles, Jr., R. G. Hamilton, S. Higgins, M. Griffin, and T. V. Hartert. 2005. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 192:1149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams, J. V., S. J. Tollefson, J. E. Johnson, and J. E. Crowe, Jr. 2005. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J. Virol. 79:10944-10951. [DOI] [PMC free article] [PubMed] [Google Scholar]