Abstract
Before 2003, only occasional case reports of human H7 influenza virus infections occurred as a result of direct animal-to-human transmission or laboratory accidents; most of these infections resulted in conjunctivitis. An increase in isolation of avian influenza A H7 viruses from poultry outbreaks and humans has raised concerns that additional zoonotic transmissions of influenza viruses from poultry to humans may occur. To better understand the pathogenesis of H7 viruses, we have investigated their ability to cause disease in mouse and ferret models. Mice were infected intranasally with H7 viruses of high and low pathogenicity isolated from The Netherlands in 2003 (Netherlands/03), the northeastern United States in 2002-2003, and Canada in 2004 and were monitored for morbidity, mortality, viral replication, and proinflammatory cytokine production in respiratory organs. All H7 viruses replicated efficiently in the respiratory tracts of mice, but only Netherlands/03 isolates replicated in systemic organs, including the brain. Only A/NL/219/03 (NL/219), an H7N7 virus isolated from a single fatal human case, was highly lethal for mice and caused severe disease in ferrets. Supporting the apparent ocular tropism observed in humans following infection with viruses of the H7 subtype, both Eurasian and North American lineage H7 viruses were detected in the mouse eye following ocular inoculation, whereas an H7N2 virus isolated from the human respiratory tract was not. Therefore, in general, the relative virulence and cell tropism of the H7 viruses in these animal models correlated with the observed virulence in humans.
Human infections with avian influenza virus have been limited to viruses within the H5, H7, and H9 subtypes. Highly pathogenic avian influenza (HPAI) virus strains of the H5 subtype continue to pose a significant threat to animal health and have resulted in over 300 laboratory-confirmed human cases of H5N1 infection in 12 countries (51a). Prior to 2003, human infections with influenza A H7 viruses were historically rare and largely due to laboratory or occupational exposure (1, 4, 9, 21, 45, 51). H7 viruses of avian origin have mounted productive infections in horse and seal populations (34, 51), and a substantial increase in the number of H7 virus outbreaks and human infections with H7 viruses has occurred in recent years, with the majority of infections resulting in conjunctivitis (5). The largest H7 viral outbreak in humans to date occurred in The Netherlands in 2003, when HPAI H7N7 viruses caused more than 80 human cases, primarily as a result of exposure to infected poultry during culling operations (12, 19). This outbreak resulted in one fatality due to acute respiratory distress syndrome (ARDS), with limited virologic evidence of human-to-human transmission (12). North American lineage avian H7 viruses have also caused human infections. An outbreak of highly pathogenic H7N3 in British Columbia, Canada, in 2004 resulted in two cases of human infection (16, 49). Additionally, a single human case of infection with a low-pathogenic avian influenza (LPAI) H7N2 virus was identified in New York in 2003 (6). Serological evidence of additional human infections with both HPAI and LPAI H7 viruses has been reported (7, 26, 31).
Mouse and ferret models that reflect the severity and outcome of disease observed in humans have been established previously to study the virulence of avian H5 influenza A viruses (11, 13, 24, 25, 53). H5N1 viruses that show a high-pathogenicity phenotype in mice are highly lethal and replicate systemically, including in the brain. Attachment of H5N1 influenza virus to cells within the lower respiratory tract in ferrets closely resembles that observed in human tissue (38, 46, 50), demonstrating an advantage of the ferret model for studying influenza virus pathogenesis. H5N1 viruses that exhibited the high-pathogenicity phenotype in ferrets were highly lethal, replicated systemically, including in the brain, and caused severe lethargy, weight loss, fever, and lymphopenia (25, 53).
Avian influenza viruses that have infected humans have previously been evaluated in animal models, but there are currently only limited data evaluating the H7 subtype. Equine H7N7 influenza viruses isolated between 1956 and 1977 were shown to be lethal to mice without prior adaptation (18), and the H7N7 virus isolated from the only fatal human case in The Netherlands outbreak in 2003 was found to be highly lethal in BALB/c mice (10). However, disease following wild-type H7 influenza virus infection of ferrets has not been reported. To better understand the capacity of H7 viruses to cause human disease, we assessed the pathogenicity of H7 viruses recently isolated from humans and of related avian strains in two animal models. This enabled us to better compare the relative virulence of a number of H7 viruses with the previously well-characterized disease process following H5N1 virus infection. We observed enhanced virulence with Eurasian H7N7 viruses compared to that with North American H7N2 and H7N3 isolates, a finding that correlates with the severities of the respective disease events in humans. These results were observed with both mammalian models examined.
MATERIALS AND METHODS
Viruses.
Influenza A viruses of the H7 subtype used in this study are shown in Table 1. A/Viet Nam/1203/04 (H5N1) and A/Memphis/102/73 (H3N2) were additionally used in ocular studies. Virus stocks were grown in the allantoic cavity of 10-day-old embryonated hens' eggs at 37°C (H7N7, H7N3, and H5N1 viruses) or 35°C (H7N2 and H3N2 viruses) for 26 to 40 h. Allantoic fluid pooled from multiple eggs was clarified by centrifugation and frozen in aliquots at −70°C. The 50% egg infectious dose (EID50) for each virus stock was calculated by the method of Reed and Muench (32) following serial titration in eggs. All experiments with HPAI viruses were conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agent Program (33).
TABLE 1.
Mouse infectivity titers of H7 viruses
| Virus | Name in this study | Subtype | Intravenous pathogenicity index | Patient symptomsa | MID50b | LD50b | % Maximum wt lossc | HA cleavage site sequencea |
|---|---|---|---|---|---|---|---|---|
| A/Netherlands/219/03 | NL/219 | H7N7 | HPAI | Fatal ARDS | 0.76 | 2.5 | 23.7 | PEIPKRRRR/G |
| A/Netherlands/230/03 | NL/230 | H7N7 | HPAI | Conjunctivitis | 2.5 | >7 | 14.5 | PEIPKRRRR/G |
| A/Ck/Netherlands/1/03 | Ck/NL/1 | H7N7 | HPAI | NA | 3.25 | >7 | 12.5 | PEIPKRRRR/G |
| A/Canada/504/04 | Can/504 | H7N3 | HPAI | Conjunctivitis | 2.25 | >7 | 2.5 | PENPKQAYQKRMTR/G |
| A/Canada/444/04 | Can/444 | H7N3 | LPAI | Conjunctivitis | 2.5 | >7 | 2.5 | PENPKQAYQKQMTR/G |
| A/NY/107/03 | NY/107 | H7N2 | LPAI | Respiratory symptoms | 2.25 | >7 | 12.2 | PEKPKPR/G |
| A/Tky/VA/4529/02 | Tky/VA | H7N2 | LPAI | NA | 1.76 | >7 | 3.5 | PEKPKPR/G |
| A/Ck/CT/260413-2/03 | Ck/CT | H7N2 | LPAI | NA | 2.25 | >7 | 10.1 | PEKPKPR/G |
| A/GH/Mass/148081-11/02 | GH/Mass | H7N2 | LPAI | NA | 1.5 | >7 | 16.1 | PEKPKKR/G |
Nucleotide sequencing.
Extraction of viral RNA for sequence analysis was performed using a QIAGEN RNeasy kit (QIAGEN, Valencia, CA). The RNA was used as a template for reverse transcriptase PCR with H7N7 segment-specific oligonucleotide primers (Promega, Madison, WI). Reaction conditions and primer sequences are available upon request. Nucleotide sequencing reactions were performed with a cycle sequencing kit and resolved on an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA). Accession numbers for the A/Netherlands/230/2003 genome can be found at http://flu.lanl.gov (ISDN234173 to -234179). The obtained sequences were assembled, aligned, and edited using DNAStar (Madison, WI) and BioEdit, version 5.0.6 (North Carolina State University), software. The phylogenetic tree was generated with the use of MEGA3.1 software, using the Kimura two-parameter neighbor-joining algorithm (20).
Mouse experiments.
Female BALB/c mice (Harlan Laboratories, Indianapolis, IN) of 6 to 8 weeks of age were lightly anesthetized with CO2 before intranasal (i.n.) inoculation with 50 μl of infectious virus diluted in sterile phosphate-buffered saline (PBS). The 50% mouse infectious dose (MID50) and 50% lethal dose (LD50) were determined as previously described (24). Briefly, mice were infected with 10-fold dilutions (from 107 to 100 EID50) of each virus. Three mice per group were euthanatized on day 3 postinoculation (p.i.), and homogenized lungs were serially titrated in eggs to determine the MID50, calculated by the method of Reed and Muench (32). Five mice per virus were monitored daily for 14 days p.i. for morbidity, as measured by weight loss, and mortality to determine the LD50. Any mouse that lost >25% of its preinfection body weight was euthanatized. Replication and systemic spread of H7 viruses were determined by harvesting lungs, noses, spleens, and brains of mice (three mice per group) on days 3 and 6 p.i. Tissues were homogenized in 1 ml of cold PBS, and clarified homogenates were titrated in eggs to determine virus infectivity, starting at a 1:10 dilution (lungs; limit of detection, 101.5 EID50/ml) or 1:2 dilution (noses, spleens, and brains; limit of detection, 100.8 EID50/ml). Statistical significance for all experiments was determined using Student's t test.
For ocular inoculation, mice were deeply anesthetized with 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma, St. Louis, MO). The right eye of each mouse was lightly scarified by three twists of a 2-mm corneal trephine (Katena Products, Denville, NJ), followed by administration of 5 μl virus diluted in PBS onto the corneal surface and massaged in with the eyelids. For each virus, 5 to 10 mice were monitored daily for 14 days p.i. for morbidity and mortality. Replication and systemic spread of each virus were determined by harvesting the right eye and lung of three to six mice on the indicated days p.i. and titrating tissues in eggs. The limit of detection for all tissues was 100.8 EID50/ml.
Ferret experiments.
Six male Fitch ferrets (Triple F Farms, Sayre, PA), of 7 to 11 months of age and serologically negative by hemagglutination inhibition for currently circulating influenza viruses, were used to assess the virulence of each indicated virus in this study. The pathogenesis of each virus following 107 EID50 i.n. inoculation was determined as previously described (25).
Cytokine quantification.
Clarified tissue homogenates from days 3 and 6 p.i. from mice infected with 106 EID50 of each virus indicated were analyzed by enzyme-linked immunosorbent assay according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Cytokines analyzed were tumor necrosis factor alpha (TNF-α) (assay sensitivity, 5.1 pg/ml), alpha interferon (IFN-α) (assay sensitivity, 12.5 pg/ml), and IFN-β (assay sensitivity, 15.6 pg/ml).
RESULTS
Pathogenicity of H7 viruses in mice.
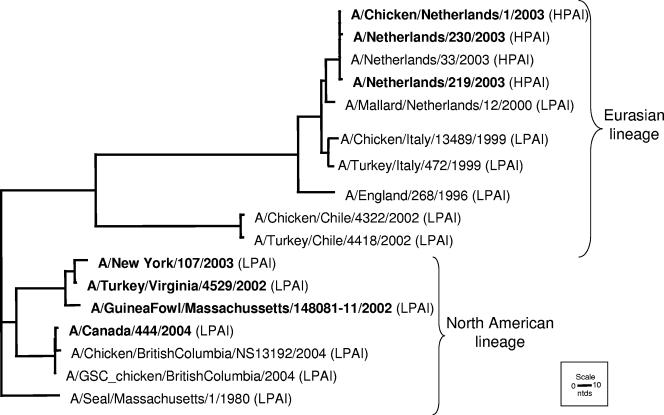
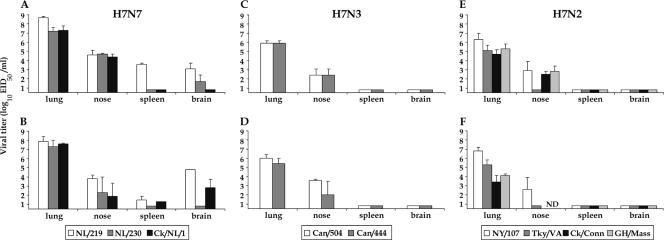
Phylogenetic analysis of the HA genes of HPAI H7 viruses determined that these viruses share common ancestors with LPAI viruses and fall into two geographically distinct lineages, North American and Eurasian (Fig. 1) (2, 34). The BALB/c mouse model was used to assess the virulence of recently isolated H7 viruses from both lineages following i.n. inoculation (Table 1). The representative Eurasian lineage viruses, NL/219, NL/230, and Ck/NL/1, were from an outbreak of HPAI H7N7 in The Netherlands in 2003 (Table 1). Following i.n. inoculation, NL/219-infected mice showed the greatest signs of illness, such as ruffled fur and severe morbidity (23.7% mean maximum weight loss). The LD50 of NL/219 virus was 102.5 EID50, a value similar to those for viruses of the H5N1 subtype which exhibit a high-pathogenicity phenotype in mice (24, 25). Although NL/230 and Ck/NL/1 induced substantial morbidity, all mice recovered, as demonstrated by an LD50 of >107.0 (Table 1). All H7 viruses replicated efficiently (>103.25 EID50/ml) in the mouse lung after i.n. inoculation with 106 EID50 of virus (Fig. 2). The NL/219-inoculated mice had lung titers that were significantly higher than the lung titers of all other viruses examined on day 3 p.i. (P < 0.02) and significantly higher than those of all H7N3 viruses examined on day 6 p.i. (P < 0.05). The Netherlands/03 H7N7 viruses spread systemically during the course of infection, and the brains of mice infected with NL/219 virus had 100-fold higher titers of virus on day 6 p.i. than did those of mice infected with NL/230 and Ck/NL/1 (Fig. 2B). Additionally, NL/219 virus titers in the spleen on day 3 p.i., as well as NL/219 and Ck/NL/1 virus titers in the brain on day 6 p.i., were significantly higher than those in the respective tissues in all mice infected with North American lineage viruses (P < 0.05). In contrast to the lethal outcome of the NL/219 virus, two North American lineage viruses (Can/504 and Can/444) associated with human conjunctivitis during an outbreak of HPAI H7N3 from British Columbia, Canada, caused insignificant morbidity (Table 1) and failed to spread to non-respiratory-tract tissues (Fig. 2C and D).
FIG. 1.
Phylogenetic tree of selected H7 HA1 genes. This tree includes viruses examined in this study (in bold) as well as viruses within the H7 subtype associated with disease in humans prior to 2002 and avian viruses that share high homology with these viruses. Virus lineages are indicated with curved brackets.
FIG. 2.
Comparison of mean titers of influenza A H7 viruses recovered from mouse tissues. BALB/c mice were inoculated i.n. with 106 EID50/50 μl of each virus indicated. Tissues were collected on days 3 (A, C, and E) and 6 (B, D, and F) p.i. from three mice per group. Tissue homogenates were prepared and titrated in eggs. Virus end-point titers are expressed as mean log10 EID50/ml plus standard deviations. The limit of virus detection was 100.8 EID50/ml. ND, not determined.
Four viruses of the North American lineage, all LPAI H7N2 viruses representative of viruses circulating in northeastern U.S. live bird markets, were examined. In November 2003, an influenza virus was isolated from a hospital patient with respiratory symptoms; the patient recovered, and subsequent subtyping tests revealed that the patient had been infected with avian influenza A (H7N2) virus (6). The human NY/107 (H7N2) virus shares 98.4% sequence identity with Tky/VA (C. Pappas, submitted for publication), a virus isolated from an LPAI H7N2 outbreak in Virginia in 2002 in which an individual involved in culling operations had serological evidence of H7N2 virus infection (7). NY/107 virus induced an elevated level of morbidity and replicated efficiently in BALB/c mice. Additionally, mean lung viral titers of NY/107 were at least 10-fold higher than those of all other H7N2 viruses on day 3 p.i. and were significantly higher on day 6 p.i. (P < 0.03) (Fig. 2E and F). None of the LPAI H7N2 viruses tested were detected in non-respiratory-tract tissues.
In summary, the H7N7 viruses from The Netherlands exhibited the highest level of virulence in mice and had lung virus titers that were 10- to 100-fold higher than those in mouse lungs infected with the North American H7 isolates. Furthermore, Netherlands/03 viruses caused systemic infection in mice, a phenotype observed previously with some HPAI H5N1 viruses. Interestingly, both HPAI and LPAI H7 viruses tested possessed low MID50 values (100.76 to 103.25 EID50) (Table 1), demonstrating high infectibility of mice without the need for prior animal adaptation.
Cytokine production following primary infection with H7 viruses in mice.
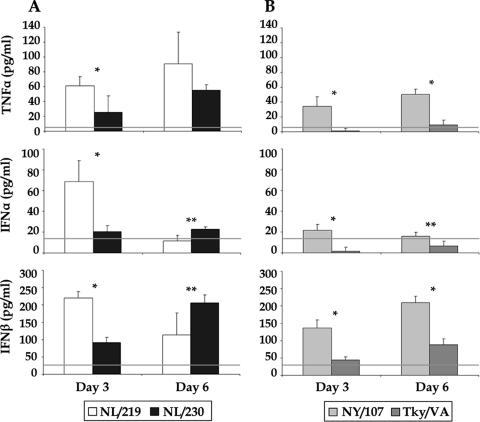
Since both HPAI H7N7 and LPAI H7N2 viruses replicated efficiently in lung tissues but differed in their lethality for mice, we next determined the extents of proinflammatory cytokine production in mouse lungs induced by representative viruses of either group (Fig. 3). We found that the highly lethal NL/219 virus, isolated from a fatal case, induced higher levels of TNF-α, IFN-α, and IFN-β cytokines than did all other viruses examined; the elevated levels of TNF-α and IFN-β were sustained until the death of these mice (Fig. 3A). Interestingly, the nonlethal human H7N2 NY/107 virus induced significantly higher levels of cytokines than those elicited by the turkey H7N2 Tky/VA virus (P < 0.02) (Fig. 3B). This difference in cytokine production observed between the H7N2 viruses may reflect the higher level of replication in the lungs of mice infected with the human NY/107 virus than that of the avian Tky/VA virus (>10-fold higher on days 3 and 6 p.i.) (Fig. 2E and F).
FIG. 3.
Determination of proinflammatory cytokine TNF-α, IFN-α, and IFN-β levels in H7-infected mouse lungs. BALB/c mice (three mice/group) were inoculated i.n. with 106 EID50/50 μl of either H7N7 (A) or H7N2 (B) virus as shown, and lungs were removed at the indicated days p.i. and frozen at −70°C until processed. Clarified cell lysates from lungs homogenized in 1 ml PBS were assayed by enzyme-linked immunosorbent assay. The constitutive cytokine levels (horizontal lines) present in the lung were determined by harvesting normal, uninfected lungs of BALB/c mice. The mean cytokine levels plus standard deviations are shown (*, P < 0.0006; **, P < 0.02).
Pathogenicity of H7 viruses in ferrets.
For each virus examined in this model, six ferrets were inoculated i.n. with 107 EID50; three animals were observed for 14 days p.i. for clinical signs, and three ferrets were euthanatized on day 3 p.i. for assessment of virologic and histopathologic parameters. Both H7N7 viruses caused fever, with a peak mean rise in body temperature of 2.1 to 2.3°C over baseline (body temperature range, 36.7 to 38.7°C) (data not shown). In addition, NL/219-infected ferrets exhibited substantial lethargy, with two of three animals exhibiting anorexia, nasal discharge, diarrhea, dyspnea, and severe weight loss (Table 2). These two animals were euthanatized during the observation period due to the development of neurological symptoms on day 8 or 12 p.i. Postmortem necropsies of these animals revealed severe macroscopic pathology, including hematomas, purpura, and focal areas of pulmonary discoloration. Multiple hemorrhages and hematomas (2 to 3 cm) were observed in adipose tissue throughout the abdominal cavity, and pervasive liver discoloration was found in both animals. The severe illness and gross pathology indicated that the NL/219 virus is highly virulent for ferrets. In contrast, two of three ferrets infected with NL/230 virus exhibited only modest weight loss (maximum mean weight loss, 5%) and respiratory signs (sneezing in one animal on days 6 to 7 p.i.) (Table 2). One NL/230-infected ferret died unexpectedly on day 7 p.i., exhibiting 16% weight loss at death but no other clinical signs or significant gross pathology. Necropsies performed on the surviving NL/230-infected ferrets on day 21 p.i. at the end of the experiment revealed only minor pulmonary lesions. Transient lymphopenia in peripheral blood was observed with both H7N7 viruses, although NL/219 caused significantly more lymphocyte depletion than did NL/230 (P < 0.01) (Table 2).
TABLE 2.
Summary of results for ferrets inoculated with H7 influenza viruses
| Virus | Clinical symptoms through day 14 p.i.
|
Day of death for each animal in group | Virulence in ferrets | ||||
|---|---|---|---|---|---|---|---|
| % Maximum wt lossa | Lethargy (relative inactivity index)b | Respiratory symptomsb | Neurological symptomsb | % Lymphopenia on day 5 p.i.c | |||
| NL/219 | 21.4 | 3/3 (1.6) | 3/3 | 2/3 | 71 | 8,d 12,d survived | High |
| NL/230 | 8.6 | 1/3 (1.0) | 1/3 | 0/3 | 45 | 7, survived, survived | Low |
| NY/107 | 10.8 | 2/3 (1.0) | 2/3 | 0/3 | 58 | All survived | Low |
| Tky/VA | 10.8 | 2/3 (1.0) | 2/3 | 0/3 | 41 | All survived | Low |
Mean percent maximum weight loss (three ferrets/group).
Data are the number of animals with symptom/total number of animals in group.
Percentages of lymphocytes were determined by differential counts of blood smears stained with Hema-3 differential stain.
Euthanatized due to hind limb paralysis.
Ferrets infected with NY/107 or Tky/VA virus exhibited only modest fever, with a peak mean rise in body temperature of 1.4 to 1.5°C over the preinfection baseline temperature (body temperature range, 37.0 to 38.6°C) (data not shown), transient weight loss, and modest respiratory signs, similar to those observed in ferrets infected with NL/230 (Table 2). All H7N2-infected animals survived the 14-day observation period. Necropsies performed on day 21 p.i. at the end of the experiment showed only modest pulmonary discoloration. Moderate transient lymphopenia in peripheral blood was observed with both viruses, similar to what was observed following NL/230 virus infection (Table 2). In summary, the NL/219 virus caused substantial illness and was lethal for ferrets, while infection with NL/230 or LPAI H7N2 virus generally did not result in severe disease.
Replication of H7 viruses in ferrets.
Virus shedding was determined with nasal wash specimens collected on alternate days after infection to evaluate the extent of virus replication in the upper respiratory tract of ferrets. All viruses tested replicated in the upper respiratory tract of ferrets through day 5 p.i. to similar titers to those observed following infection with HPAI H5N1 viruses in ferrets (25; data not shown). The mean viral titer in NL/219-infected ferrets remained high by day 7 p.i.; conversely, ferrets infected with NL/230 virus and both LPAI H7N2 viruses examined essentially cleared the viruses by day 7 p.i. (data not shown).
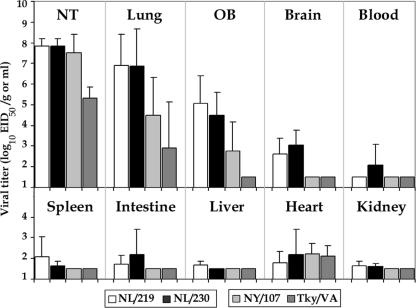
Three animals per group were euthanatized on day 3 p.i. to determine viral titers in nasal turbinates, lungs, and major organs (Fig. 4). Both H7N7 viruses examined replicated to high titers in the nasal turbinates and lungs. NY/107 virus, but not Tky/VA virus, was found at a similarly high titer in the nasal turbinates. Both H7N2 viruses exhibited lung virus titers on day 3 p.i. that were reduced >100-fold compared to those of H7N7 viruses, with no virus detected in the lungs of one of three Tky/VA virus-infected ferrets. All viruses except the poultry Tky/VA isolate were detected in the olfactory bulb of the brain, but only the H7N7 viruses were detected in the brain posterior to the olfactory bulb. Titers in this organ are generally higher for the highly virulent viruses than for low-virulence viruses (25). The H7N7 viruses were also detected in multiple systemic organs of ferrets, whereas neither H7N2 virus was detected in any additional systemic organ, other than at low levels in the heart (Fig. 4). Taken together, these data indicate that the HPAI H7N7 viruses, in general, replicated to higher titers and for a longer duration in the respiratory tract and spread to multiple organs compared with the LPAI H7N2 viruses.
FIG. 4.
Comparison of mean titers of influenza A H7 viruses recovered from ferret tissues. Ferrets were inoculated i.n. with 107 EID50/ml of each virus indicated (three ferrets/group). Tissues were collected on day 3 p.i., with tissue homogenates prepared and titrated in eggs. Virus end-point titers are expressed as mean log10 EID50/g plus standard deviations for all tissues except the nasal turbinates (NT) and blood, whose titers are expressed as log10 EID50/ml. The limit of virus detection was 101.5 EID50/ml of tissue homogenate. OB, olfactory bulb.
Replication and spread of HPAI H7 viruses following ocular inoculation in mice.
The majority of human infections associated with H7 viruses have resulted in ocular, not respiratory, disease (12, 21, 28). To evaluate this potential route of virus entry in a mammalian model, we infected mice with selected H7 influenza viruses by the ocular route following corneal scarification. For comparison, groups of mice were infected with A/Memphis/102/72 (Mem/72), an early H7N3 strain previously shown to infect mice through the i.n. route (17), and with the HPAI H5N1 virus A/Viet Nam/1203/04 (VN/1203). Mice were either observed for 14 days p.i. to determine morbidity and mortality or euthanized on the indicated days p.i. for determination of virus replication in the eye and spread to the lung. Ocular infection with the LPAI H7N2 virus (NY/107), H5N1 virus (VN/1203), or H3N2 virus (Mem/72) showed no detectable virus in this tissue on any day p.i. Strikingly, ocular infection of mice with the HPAI H7 viruses resulted in eye virus titers on days 3 and 6 p.i. (Table 3). In particular, ocular inoculation with NL/219 virus additionally resulted in significant titers of virus in the lung on day 3 p.i., with elevated titers found in this tissue on day 6 p.i., resulting in 30% mortality (P < 0.05) (Table 3). These findings closely mirror the pattern of virus isolation observed for humans following H7 infection (19, 49) and demonstrate the ability of influenza viruses to productively mount an infection following exposure to ocular tissue in a mammalian model.
TABLE 3.
Replication of H7 influenza viruses following ocular inoculation
| Virus | % Mortality | Mean virus titer ± SE in mouse tissuea
|
|||
|---|---|---|---|---|---|
| Day 3
|
Day 6
|
||||
| Eye | Lung | Eye | Lung | ||
| NL/219 | 30 | 1.7 ± 0.5 | 2.9 ± 0.6 | 1.6 ± 0.4 | 4.8 ± 0.7 |
| NL/230 | 0 | 2.4 ± 0.6 | ≤0.8 | 2.1 ± 0.5 | ≤0.8 |
| Can/504 | 0 | 3.5 ± 0.5 | ≤0.8 | 2.7 ± 0.6 | 1.5 ± 0.4 |
| NY/107 | 0 | ≤0.8 | ≤0.8 | ≤0.8 | ≤0.8 |
| VN/1203 | 0 | ≤0.8 | ≤0.8 | ≤0.8 | ≤0.8 |
| Mem/72 | 0 | ≤0.8 | ≤0.8 | ≤0.8 | ≤0.8 |
Mean virus titers in mice infected with 106 EID50/5 μl of virus following corneal scarification. Virus end-point titers are expressed as the mean log10 EID50/ml plus standard errors for three to six mice per tissue. The limit of virus detection was 100.8 EID50/ml.
DISCUSSION
The increase in frequency of human infections with H7 viruses since 2002 highlights the need to better understand the potential of these viruses to cause disease in mammalian species. Such pathogenesis data, which include determinations of MID50 and LD50 values, provide valuable information for the development of H7 influenza vaccines in preclinical testing. In this study, we compared the relative virulence of H7 viruses associated with disease in humans and of closely related viruses isolated from avian species in both mouse and ferret models. In both mammalian models, we found that H7 viruses within the Eurasian lineage replicated to higher titers, spread more systemically, and resulted in more prominent morbidity than did North American H7 isolates. In general, the relative virulence of each virus examined was consistent between both animal models. Furthermore, H7 viruses were also found to replicate well in the mouse eye following ocular inoculation. To our knowledge, this is the first report characterizing ocular replication in mice and disease in ferrets following wild-type H7 virus infection.
Viruses isolated during an HPAI H7N7 outbreak in The Netherlands in 2003 replicated to high titers in the respiratory tracts of both mice and ferrets but were also present in the brain and other extrapulmonary organs in these animal models. The high virulence of NL/219 virus in the mouse model following intranasal inoculation has been documented previously (10). We further demonstrate here that the severe systemic pathology found during postmortem necropsies of NL/219-infected ferrets, coupled with the appearance of severe clinical signs, indicates that NL/219 virus is highly virulent in ferrets. The lethality of NL/219 virus in both mammalian species is consistent with the severity of disease observed in the human case (12). Interestingly, the genetically related H7N7 NL/230 virus mounted a productive infection in ferrets but was not highly virulent in this model. The one ferret found dead during the course of observation following NL/230 virus infection did not exhibit histological or clinical signs consistent with severe disease. NL/219 virus differs from NL/230 virus at 15 amino acids, including the presence of a Lys residue at position 627 of PB2 (Table 4). The importance of the polymerase complex in maintaining the high level of virulence of a recent HPAI H5N1 isolate in both mice and ferrets has been demonstrated (36), and previously, a Lys at position 627 of PB2 of H5N1 viruses was shown to enhance virulence in mice (14, 39). Recent work investigating the PB2 protein of H7N7 viruses has shown the presence of Lys at position 627 to be a major determinant of the highly virulent NL/219 phenotype in mice (27). Thus, PB2 position 627 Lys/Glu disparity is likely to contribute substantially to the difference in virulence for ferrets and mice between the NL/219 and NL/230 viruses, but the precise contributions of the other amino acid substitutions to the lethal phenotype require further study.
TABLE 4.
Amino acid differences between H7N7 influenza viruses
| Virus | Amino acid at position
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2
|
PA 397 | HA
|
NA
|
NS1 137 | |||||||||||
| 79 | 297 | 355 | 563 | 627 | 13 | 127 | 143 | 416 | 308 | 346 | 442 | 458 | |||
| NL/219 | I | I | K | R | K | E | S | D | T | R | S | V | A | S | I |
| NL/230 | S | V | R | Q | E | V | I | N | A | K | N | A | T | P | V |
Compared with the Eurasian lineage viruses examined, the North American HPAI and LPAI viruses tested in this study exhibited a lower-level virulence phenotype in both mammalian models following i.n. inoculation. It is noteworthy that despite the lack of substantial morbidity or mortality observed following infection of mice and ferrets with the North American viruses examined in this study, we observed efficient infection and replication within the respiratory tract in both animal models. Virus titers measured in the respiratory tract of ferrets were even higher than those observed following infection with human H3N2 viruses (40, 53). The isolation of NY/107 virus from tissues of the olfactory bulb of ferrets could be due to the proximity of the high virus titer attained in the nasal turbinates following i.n. inoculation with this virus and may not be an indicator of extrapulmonary spread, as a similar pattern of replication was also demonstrated with ferrets infected with a human H3N2 virus (53).
The increased levels of cytokine expression, also referred to as a “cytokine storm,” in HPAI H5N1 virus-infected human macrophages and in the blood of H5N1 virus-infected patients have been proposed to contribute to the increased severity of the disease caused by this virus subtype (8, 48). In the mouse model, proinflammatory cytokines have been shown to be produced at elevated levels in the lung (15), including substantial increases in TNF-α levels following i.n. inoculation with mouse-adapted A/PR/8/34 virus (30) or with highly pathogenic H5N1 viruses (48). The functions and actions of TNF-α in the lungs or other tissues, such as the brain, remain to be elucidated fully but probably include both beneficial and detrimental effects, the latter of which may contribute to influenza virus pathogenesis. Thus, TNF-α is a key regulator of inflammation and may possess antiviral properties (47); however, this cytokine has been shown to contribute to morbidity during H5N1 virus infection in mice (44), and the local synthesis of TNF-α within the brain may lead to anorexia, weight loss, and death (35). In the current study, we observed the highest levels of the TNF-α cytokine in the lungs of mice infected with either H7N7 virus, especially NL/219, in contrast to those in the lungs of mice infected with the H7N2 viruses (Fig. 3). Inoculation with any of the H7 viruses we examined here did not result in significant production above constitutive levels of TNF-α in the mouse brain (data not shown). Previous work has demonstrated a correlation between high virus titers and increased interferon production by leukocytes in the lung (52), and to support this finding, we observed elevated IFN-α and IFN-β levels following inoculation with NL/219, the virus that replicated to the highest titers in the mouse lung. Further investigations will be needed to determine the functional roles of the TNF-α, IFN-α, and IFN-β cytokines in H7 pathogenesis, using transgenic mice deficient in one or more of these proinflammatory cytokines.
HPAI H7 viruses of both the Eurasian and North American lineages were detected in the mouse eye following ocular inoculation, a finding that was not observed with a human H3N2 virus or an HPAI H5N1 virus. Current studies are under way to determine the ocular pathology and the location of H7 virus replication in the eye. Interestingly, NL/219 virus was able to mount productive lower respiratory tract infections in mice following ocular inoculation and induce severe morbidity and some mortality. A previous study also demonstrated ocular inoculation of a respiratory virus into mice resulting in virus being detected in the lung and postulated that replication-independent travel of virions in lacrimal ducts could be involved (3). This is the first report of lethal disease following exposure of the ocular surface to influenza virus in a mammalian model and demonstrates experimentally the ability of influenza viruses to use this tissue as a portal of entry. The lack of replication in the eye following ocular inoculation of the LPAI H7N2 virus NY/107 is supported by its association with human respiratory disease, not ocular disease (6).
The multibasic-amino-acid hemagglutinin (HA) cleavage site plays a significant role in determining the pathogenicity of an influenza virus, as it enables cleavage of the HA precursor by a broader range of host proteases with a greater tissue distribution (42). Unlike the Eurasian H7 viruses studied here, which all possess identical HA cleavage sites that contain five basic amino acids (12), the North American H7 viruses examined display far more heterogeneity. The cleavage site of viruses from the 2004 H7N3 outbreak in Canada is unusual in that a nonhomologous recombination event between the HA and matrix gene segments of the same virus caused a seven-amino-acid insertion (Table 1) (29). Can/504 and Can/444 are divergent at one amino acid within the insertion, and this change is purported to be responsible for the highly pathogenic phenotype in poultry following inoculation with Can/504 but not Can/444 (16). The only other naturally occurring viruses found to date to have acquired a highly pathogenic phenotype in chickens by nonhomologous recombination near the HA cleavage site are viruses isolated from chickens during an H7N3 outbreak in Chile in 2002 (43). We evaluated a pair of HPAI and LPAI viruses, isolated from chickens during this outbreak, that contained a 10-amino-acid insertion at the HA cleavage site derived from the nucleoprotein gene and found these viruses to be of low virulence in our mouse model (data not shown). These results suggest that insertions in the HA cleavage site of H7 viruses, while often conferring a highly pathogenic phenotype in chickens, do not necessarily correlate with enhanced virulence in mammals. A laboratory variant of A/Seal/Mass/1/80 (H7N7) with a three-arginine-residue insertion at the HA cleavage site resulted in increased pathogenicity in mouse and ferret models that was not observed with the avirulent wild-type strain, which contained a single arginine residue at the HA cleavage site (23, 37). Viruses isolated from northeastern U.S. live bird markets have acquired additional basic amino acids at the HA cleavage site in recent years (41). A recent study demonstrated that insertion of additional basic amino acids at the HA cleavage site, but not mutation of existing amino acids, could result in LPAI H7N2 viruses achieving an HPAI phenotype in chickens (22). Our pathotyping of a virus with four basic amino acids at the cleavage site, namely, GH/Mass, demonstrated a similar phenotype in mice to that conferred by LPAI H7N2 viruses containing three basic amino acids at this location (Ck/Conn, NY/107, and Tky/VA). Additional studies are required to better understand the impact on mammals of this progression towards a cleavage site that resembles that of HPAI viruses.
Recent H7 outbreaks resulting in human infection necessitate well-characterized animal models to better distinguish and evaluate those viruses which pose a serious threat to human health. By comparing viruses with either a high- or low-pathogenicity phenotype in multiple models, we can better understand the molecular and biologic properties of these viruses which confer pathogenic traits in mammals. These findings will be useful in allowing for a more direct comparison of virulence between highly pathogenic avian viruses that could potentially cause disease in humans and mammals.
Acknowledgments
We thank David Swayne (Southeast Poultry Research Laboratory, USDA Agricultural Research Service) and Ron Fouchier (Erasmus Medical Center) for providing some of the viruses used in this study and Lindsay Edwards for technical assistance.
J.A.B. received financial support for this work from the Oak Ridge Institute for Science and Education, Oak Ridge, TN.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Banks, J., E. Speidel, and D. J. Alexander. 1998. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch. Virol. 143:781-787. [DOI] [PubMed] [Google Scholar]
- 2.Banks, J., E. C. Speidel, J. W. McCauley, and D. J. Alexander. 2000. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch. Virol. 145:1047-1058. [DOI] [PubMed] [Google Scholar]
- 3.Bitko, V., A. Musiyenko, and S. Barik. 2007. Viral infection of the lungs through the eye. J. Virol. 81:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, C. H., R. G. Webster, and S. S. Breese, Jr. 1970. Fowl plague virus from man. J. Infect. Dis. 122:513-516. [DOI] [PubMed] [Google Scholar]
- 5.Capua, I., and D. J. Alexander. 2004. Avian influenza: recent developments. Avian Pathol. 33:393-404. [DOI] [PubMed] [Google Scholar]
- 6.CDC. 2004. Update: influenza activity—United States and worldwide, 2003-04 season, and composition of the 2004-05 influenza vaccine. Morb. Mortal. Wkly. Rep. 53:547-552. [PubMed] [Google Scholar]
- 7.CDC. 2004. Update: influenza activity—United States, 2003-04 season. Morb. Mortal. Wkly. Rep. 53:284-287. [PubMed] [Google Scholar]
- 8.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 9.DeLay, P. D., H. L. Casey, and H. S. Tubiash. 1967. Comparative study of fowl plague virus and a virus isolated from man. Public Health Rep. 82:615-620. [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit, E., V. J. Munster, M. I. Spronken, T. M. Bestebroer, C. Baas, W. E. Beyer, G. F. Rimmelzwaan, A. D. Osterhaus, and R. A. Fouchier. 2005. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 79:12401-12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybing, J. K., S. Schultz-Cherry, D. E. Swayne, D. L. Suarez, and M. L. Perdue. 2000. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J. Virol. 74:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 15.Hennet, T., H. J. Ziltener, K. Frei, and E. Peterhans. 1992. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 149:932-939. [PubMed] [Google Scholar]
- 16.Hirst, M., C. R. Astell, M. Griffith, S. M. Coughlin, M. Moksa, T. Zeng, D. E. Smailus, R. A. Holt, S. Jones, M. A. Marra, M. Petric, M. Krajden, D. Lawrence, A. Mak, R. Chow, D. M. Skowronski, S. A. Tweed, S. Goh, R. C. Brunham, J. Robinson, V. Bowes, K. Sojonky, S. K. Byrne, Y. Li, D. Kobasa, T. Booth, and M. Paetzel. 2004. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg. Infect. Dis. 10:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz, J. M., X. Lu, S. A. Young, and J. C. Galphin. 1997. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Infect. Dis. 175:352-363. [DOI] [PubMed] [Google Scholar]
- 18.Kawaoka, Y. 1991. Equine H7N7 influenza A viruses are highly pathogenic in mice without adaptation: potential use as an animal model. J. Virol. 65:3891-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmans, M., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R. Fouchier, A. Osterhaus, and A. Bosman. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in The Netherlands. Lancet 363:587-593. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz, J., R. J. Manvell, and J. Banks. 1996. Avian influenza virus isolated from a woman with conjunctivitis. Lancet 348:901-902. [DOI] [PubMed] [Google Scholar]
- 22.Lee, C. W., Y. J. Lee, D. A. Senne, and D. L. Suarez. 2006. Pathogenic potential of North American H7N2 avian influenza virus: a mutagenesis study using reverse genetics. Virology 353:388-395. [DOI] [PubMed] [Google Scholar]
- 23.Li, S. Q., M. Orlich, and R. Rott. 1990. Generation of seal influenza virus variants pathogenic for chickens, because of hemagglutinin cleavage site changes. J. Virol. 64:3297-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L. M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. Nguyen, J. H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijer, A., A. Bosman, E. E. van de Kamp, B. Wilbrink, R. van Beest Holle Mdu, and M. Koopmans. 2006. Measurement of antibodies to avian influenza virus A (H7N7) in humans by hemagglutination inhibition test. J. Virol. Methods 132:113-120. [DOI] [PubMed] [Google Scholar]
- 27.Munster, V. J., E. de Wit, D. van Riel, W. E. Beyer, G. F. Rimmelzwaan, A. D. Osterhaus, T. Kuiken, and R. A. Fouchier. 2007. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J. Infect. Dis. 196:258-265. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson, S., U. Kumlin, K. Dimock, and N. Arnberg. 2005. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect. Dis. 5:184-188. [DOI] [PubMed] [Google Scholar]
- 29.Pasick, J., K. Handel, J. Robinson, J. Copps, D. Ridd, K. Hills, H. Kehler, C. Cottam-Birt, J. Neufeld, Y. Berhane, and S. Czub. 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J. Gen. Virol. 86:727-731. [DOI] [PubMed] [Google Scholar]
- 30.Peper, R. L., and H. Van Campen. 1995. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog. 19:175-183. [DOI] [PubMed] [Google Scholar]
- 31.Puzelli, S., L. Di Trani, C. Fabiani, L. Campitelli, M. A. De Marco, I. Capua, J. F. Aguilera, M. Zambon, and I. Donatelli. 2005. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J. Infect. Dis. 192:1318-1322. [DOI] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Richmond, J. Y., and R. W. M. McKinney. 2007. Laboratory biosafety level criteria, p. 16-43. In J. Y. Richmond and R. W. McKinney (ed.), Biosafety in microbiological and biomedical laboratories, 5th ed. Centers for Disease Control and Prevention, Atlanta, GA.
- 34.Rohm, C., T. Horimoto, Y. Kawaoka, J. Suss, and R. G. Webster. 1995. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology 209:664-670. [DOI] [PubMed] [Google Scholar]
- 35.Rothwell, N. J. 1999. Cytokines—killers in the brain? J. Physiol. 514:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon, R., J. Franks, E. A. Govorkova, N. A. Ilyushina, H. L. Yen, D. J. Hulse-Post, J. Humberd, M. Trichet, J. E. Rehg, R. J. Webby, R. G. Webster, and E. Hoffmann. 2006. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 203:689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheiblauer, H., A. P. Kendal, and R. Rott. 1995. Pathogenicity of influenza A/Seal/Mass/1/80 virus mutants for mammalian species. Arch. Virol. 140:341-348. [DOI] [PubMed] [Google Scholar]
- 38.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435-436. [DOI] [PubMed] [Google Scholar]
- 39.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 40.Smith, H., and C. Sweet. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10:56-75. [DOI] [PubMed] [Google Scholar]
- 41.Spackman, E., D. A. Senne, S. Davison, and D. L. Suarez. 2003. Sequence analysis of recent H7 avian influenza viruses associated with three different outbreaks in commercial poultry in the United States. J. Virol. 77:13399-13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinhauer, D. A. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1-20. [DOI] [PubMed] [Google Scholar]
- 43.Suarez, D. L., D. A. Senne, J. Banks, I. H. Brown, S. C. Essen, C. W. Lee, R. J. Manvell, C. Mathieu-Benson, V. Moreno, J. C. Pedersen, B. Panigrahy, H. Rojas, E. Spackman, and D. J. Alexander. 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 10:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szretter, K. J., S. Gangappa, X. Lu, C. Smith, W. J. Shieh, S. R. Zaki, S. Sambhara, T. M. Tumpey, and J. M. Katz. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81:2736-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, H. R., and A. J. Turner. 1977. A case report of fowl plague keratoconjunctivitis. Br. J. Ophthalmol. 61:86-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson, C. I., W. S. Barclay, M. C. Zambon, and R. J. Pickles. 2006. Infection of human airway epithelium by human and avian strains of influenza A virus. J. Virol. 80:8060-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tracey, K. J., and A. Cerami. 1989. Cachectin/tumor necrosis factor and other cytokines in infectious disease. Curr. Opin. Immunol. 1:454-461. [DOI] [PubMed] [Google Scholar]
- 48.Tumpey, T. M., X. Lu, T. Morken, S. R. Zaki, and J. M. Katz. 2000. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 74:6105-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tweed, S. A., D. M. Skowronski, S. T. David, A. Larder, M. Petric, W. Lees, Y. Li, J. Katz, M. Krajden, R. Tellier, C. Halpert, M. Hirst, C. Astell, D. Lawrence, and A. Mak. 2004. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10:2196-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2006. H5N1 virus attachment to lower respiratory tract. Science 312:399. [DOI] [PubMed] [Google Scholar]
- 51.Webster, R. G., J. Geraci, G. Petursson, and K. Skirnisson. 1981. Conjunctivitis in human beings caused by influenza A virus of seals. N. Engl. J. Med. 304:911. [DOI] [PubMed] [Google Scholar]
- 51a.World Health Organization. 29 June 2007, posting date. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_06_29/en/index.html.
- 52.Wyde, P. R., M. R. Wilson, and T. R. Cate. 1982. Interferon production by leukocytes infiltrating the lungs of mice during primary influenza virus infection. Infect. Immun. 38:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]