Abstract
Positive transcription elongation factor (P-TEFb), which is composed of CDK9 and cyclin T1, plays an important role in cellular and viral gene expression. Our lab has recently demonstrated that P-TEFb is required for Tax transactivation of the viral long terminal repeat (LTR). P-TEFb is found in two major complexes: the inactive form, which is associated with inhibitory subunits 7SK snRNA and HEXIM1, and the active form, which is associated with, at least in part, Brd4. In this study, we analyzed the effect of Brd4 on human T-lymphotropic virus type 1 (HTLV-1) transcription. Overexpression of Brd4 repressed Tax transactivation of the HTLV-1 LTR in a dose-dependent manner. In vitro binding studies suggest that Tax and Brd4 compete for binding to P-TEFb through direct interaction with cyclin T1. Tax interacts with cyclin T1 amino acids 426 to 533, which overlaps the region responsible for Brd4 binding. In vivo, overexpression of Tax decreased the amount of 7SK snRNA associated with P-TEFb and stimulates serine 2 phosphorylation of the RNA polymerase II carboxyl-terminal domain, suggesting that Tax regulates the functionality of P-TEFb. Our results suggest the possibility that Tax may compete and functionally substitute for Brd4 in P-TEFb regulation.
Human T-lymphotropic virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia (ATL) (37, 50, 51), chronic diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (13, 32, 35), HTLV-1-associated arthropathy (7, 22, 34, 45), uveitis (31), and Sjögren's syndrome (44). The transactivator Tax protein, encoded by the pX region of HTLV-1, is essential for viral replication, transformation, and gene regulation (2, 4, 10, 15, 21, 33, 42). Three highly conserved 21-bp repeat elements located within the long terminal repeat (LTR), Tax-responsive elements (TRE), are critical for Tax-mediated viral transcriptional activation (5, 9, 40, 41, 50). Association of Tax with CREB on the HTLV-1 LTR (1, 43, 52) facilitates recruitment of cellular cofactors CBP (14, 17, 23, 52), p300 (16, 52), and PCAF (19), thereby mediating the activation of HTLV-1 gene expression. Tax also regulates the binding of histone deacetylases, a repressor of transactivation, from the HTLV-1-LTR promoter (25, 27).
P-TEFb is a protein kinase involved in RNA polymerase (Pol) II elongation of many genes in mammalian cells (39). P-TEFb is composed of catalytic subunit CDK9 and either regulatory subunit cyclin T1, -T2, or -K (11, 36). The transcriptional activity of P-TEFb depends on the CDK9 kinase activity, which hyperphosphorylates the Ser 2 residue of the carboxyl-terminal domain (CTD) of the largest subunit of RNA Pol II (28, 53). P-TEFb also phosphorylates negative elongation factors DSIF and NELF, which cooperatively inhibit RNA Pol II processivity, to stimulate the elongation of transcription (3, 12, 38, 39). In a previous study, our lab found that CDK9 was an essential factor for transactivation of the HTLV-1 LTR by Tax (54). Our results further suggested that P-TEFb was recruited to the initiation complex of the HTLV-1 LTR promoter through direct interaction with Tax.
In the cell, approximately one-half of the P-TEFb is sequestered in a high-molecular-weight (HMW) complex in association with 7SK snRNA and HEXIM1 protein (26). The association of 7SK snRNA and HEXIM1 with P-TEFb inhibits the kinase activity of CDK9 and prevents P-TEFb from binding to the transcription template (47-49). The P-TEFb low-molecular-weight (LMW) complex is associated with Brd4, at least in part, and constitutes the active fraction of P-TEFb (18, 46). Brd4 protein is a member of the BET protein family, with two tandem bromodomains and an extra-terminal domain (8). The BET members are mobile in the nucleus and are found associated with acetylated chromatin (8). The bromodomains of Brd4 recognize the acetylated histones H3 and H4 (20, 24, 29). Brd4 recruits P-TEFb to the promoter, where it phosphorylates the RNA Pol II CTD to stimulate transcription elongation (18).
In the present study, we investigated the role of Brd4 in HTLV-1 Tax transactivation. Our studies demonstrate that Brd4 overexpression inhibits Tax transactivation. Interestingly, we found that Tax and Brd4 compete for binding to P-TEFb. Tax interacts with cyclin T1 and competes Brd4 binding in vitro and in vivo by interaction with cyclin T1 at an adjacent or overlapping binding site for Brd4. Tax overexpression results in a decrease in the level of 7SK snRNA associated with P-TEFb, and an increase in Ser 2 CTD phosphorylation, suggesting that Tax increases the functionality of P-TEFb in the cell.
MATERIALS AND METHODS
Cell cultures.
Molt4 and C81 cells were grown in RPMI (Quality Biological, Inc.). HeLa and pA-18G-BHK-21 cells were cultured in Dulbecco modified Eagle medium (Quality Biological, Inc.). The medium was supplemented with 10% fetal bovine serum, 2 mM glutamine (Quality Biological, Inc.), and penicillin-streptomycin in an atmosphere of 5% CO2 chamber at 37°C.
Transfection and reporter assays.
HeLa cells with a confluence of 80% were transfected with Tax or Brd4, 200 ng of pGL3.HTLV-1 LTR-Luc (or pGL3-.HIV-LTR-Luc), and 100 ng of the plasmid pRSV.LacZ using Fugene 6 (Roche). The cells were incubated for 24 h, and extracts were prepared for luciferase activity using the reporter assay system indicated by the manufacturer (Promega). The β-galactosidase activity was determined by using a GalactoLight assay kit (Applied Biosystems) to adjust the transfection efficiency.
Nuclear fraction purification, coimmunoprecipitation, and immunoblotting.
Cells (2 × 107 cells) were collected and lysed in 1 ml of a cytoplasmic fraction buffer containing 10 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor mixture. After centrifugation, nuclear fractions were obtained by extraction with 500 μl of a buffer containing 20 mM HEPES (pH 7.4), 0.3 M KCl, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM PMSF, and protease inhibitors. The nuclear fraction (30 μg) was immunoblotted with specific antibodies against Tax, CDK9 (Santa Cruz), cyclin T1 (Santa Cruz), or Brd4 as described previously (18). For immunoprecipitation, 500 μg of the nuclear extracts in 1 ml of binding buffer (20 mM HEPES [pH 7.4], 0.15 M KCl, 20% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM PMSF, and protease inhibitors) were incubated with 2 μg of CDK9 or Tax antibodies for 4 h at 4°C, followed by incubation with protein G Dynabeads (Invitrogen) for an additional 2 h. The beads were washed five times using lysis buffer, mixed with 2× SDS sample buffer, denatured, and subjected to electrophoresis in a 4 to 20% Tris-glycine (Invitrogen) gel and Western blotting as described above.
Adenovirus transduction and amplification.
Adenovirus expressing Tax (Ad-Tax) was kindly provided by M. Yoshida. Adenovirus encoding green fluorescence protein cDNA (Ad-GFP) was constructed using the Advator adenoviral vector system (Qbiogen). The viruses were amplified, and titers were determined using the PFU counting method in 293 cells. HeLa or pA-18G-BHK-21 cells with 80% confluence were transduced with viruses for 2 h at a multiplicity of infection (MOI) of 100 in the absence of serum. The cells were then incubated for 12 or 24 h in medium containing 10% fetal bovine serum before harvest.
Total cell extract preparation.
pA-18G-BHK-21 cells or HeLa cells (2 × 105 cells) were transduced with adenovirus expressing Tax or GFP for 2 h of an MOI of 100. After 12 h, total cell extracts were prepared in 100 μl of total cell lysis buffer containing 50 mM Tris (pH 7.4), 300 mM NaCl, 0.2 mM EDTA, 1 mM DTT, and 0.5% NP-40. Portions (50 μg) of the whole-cell extracts were immunoblotted with antibodies specific for phosphor-Ser2 (H5), phosphor-Ser5 (H14) of RNA Pol II CTD, RNA Pol II (8WG16), Tax, or actin (Sigma).
In vitro binding assay.
Brd4 (100 ng) and P-TEFb (200 ng) proteins purified from baculovirus-infected cells were incubated with or without His-tagged Tax protein (200 or 400 ng) purified from Escherichia coli in 500 μl binding buffer (25 mM HEPES (pH 7.4), 1 mM DTT, 20 mM NaCl, 0.2 mM EDTA, 0.1% Triton X-100, 20% glycerol, and protease inhibitor cocktail) at 4°C for 2 h. The mixtures were immunoprecipitated with 2 μg of CDK9 antibody, and immunoblot analysis was performed with Tax, CDK9, or Brd4 antibody. For binding assays, 600 ng of glutathione S-transferase (GST)-CDK9, GST-cyclin T1, GST-cyclin T1 truncated mutants, or GST alone was incubated with 400 ng of His-tagged Tax protein. After incubation, GST complexes purified using glutathione beads were assayed for binding.
RNA purification and detection of 7SK snRNA.
Total RNA was purified using TRIzol (Invitrogen) reagent from nuclear extracts of HeLa cells that were mock transfected or transfected with Tax expression plasmid. To analyze the amount of 7SK snRNA associated with the P-TEFb complex, the nuclear extracts were immunoprecipitated with CDK9 antibody. RNA was extracted and reverse transcribed with Superscript II reverse transcriptase (Invitrogen). Semiquantitative PCR was performed by using the Taq DNA polymerase (Applied Biosystems) with 25 cycles amplification reaction.
RESULTS
Brd4 inhibits HTLV-1 LTR transactivation by Tax.
Previous experiments have shown that P-TEFb is essential for Tax transactivation of the HTLV-1 LTR (54). Since Brd4 has been reported to interact with P-TEFb and play a role in P-TEFb activation (18), we were interested in examining the role of Brd4 in HTLV-1 Tax transactivation. HeLa cells were transfected with Tax or Brd4 along with the HTLV-1 LTR-luciferase reporter. As shown in Fig. 1A, the Tax protein showed significant transactivation of the HTLV-1 LTR promoter (45- to 90-fold increase). In contrast, Brd4 failed to activate transcription from the HTLV-1 LTR (Fig. 1A, lanes 4 to 6). To verify the expression of Tax or Brd4 in the transfected cells, Western blot analysis was performed. The results presented in Fig. 1A, bottom panel, demonstrate that Tax and Brd4 were expressed in a dose-dependent manner.
FIG. 1.
Brd4 inhibits Tax transactivation of HTLV-1 LTR. (A) HeLa cells at a confluence of 80% were cotransfected with 200 ng of HTLV-1 LTR-Luc, 100 ng of RSV-lacZ, Tax (10 or 20 ng), or Brd4 (100, 200, or 400 ng) using Fugene 6 transfection reagent (Roche). The cells were incubated for 24 h, cell extracts were prepared, and the luciferase activity was analyzed. Western blot analysis of Tax (Tab 172) or Brd4 was performed to check protein expression. (B) HeLa cells were transfected with 200 ng of HIV-LTR-Luc, 100 ng of RSV-lacZ, and 0, 200, or 400 ng of Brd4. After 24 h, cell lysates were prepared and assayed for luciferase activity. (C) HeLa cells were transfected with 200 ng of HTLV-1 LTR-Luc, 100 ng of RSV-lacZ and 0, 200, and 400 ng of Brd4 (lanes 1 to 3) or 200 ng of HTLV-1 LTR-Luc, 100 ng of RSV-lacZ, 10 ng of Tax and 0, 100, 200, or 400 ng of Brd4 (lanes 4 to 7). The cells were incubated for 24 h, cell extracts were prepared, and the luciferase activity was analyzed. (A to C) To correct for transfection efficiency, all luciferase values were adjusted using RSV-LacZ. Graphs represent the average luciferase activity from three independent experiments with the standard deviation. The panel below the graph shows Western blot analysis using Tax or Brd4 antibody.
In view of the inability of Brd4 to transactivate the HTLV-1 LTR, it was important to demonstrate that the protein was active in the transfected cells. Brd4 has been shown to activate the HIV-LTR promoter (18, 46). HeLa cells were cotransfected with Brd4 and the HIV-LTR-luciferase reporter. The results of this experiment demonstrate that the transfected Brd4 was active and stimulated HIV-LTR transcription (Fig. 1B).
Next, we tested the effect of Brd4 on HTLV-1 LTR promoter activation in the presence of Tax. HeLa cells were cotransfected with Tax and increasing concentrations of Brd4. Although Brd4 alone did not affect HTLV-1 basal transcription, it inhibited Tax transactivation of HTLV-1 LTR in a dose-dependent manner (Fig. 1C, lanes 5 to 7). In the absence of Brd4, Tax induced the HTLV-1 LTR promoter by approximately 20-fold (Fig. 1C, lane 4). In the presence of Brd4, activation of HTLV-1 LTR promoter by Tax was inhibited by approximately 70% (Fig. 1C, lane 7). Western blot analysis of Tax and Brd4 expressed in the cotransfected cells demonstrated that Brd4 expression did not effect Tax expression (Fig. 1C, bottom panel, lanes 4 to 7). We did note, however, that in the presence of Tax, the expression of Brd4 was slightly increased (Fig. 1C, bottom panel, lanes 2 and 6 and lanes 3 and 7).
Tax inhibits the interaction of Brd4 and CDK9.
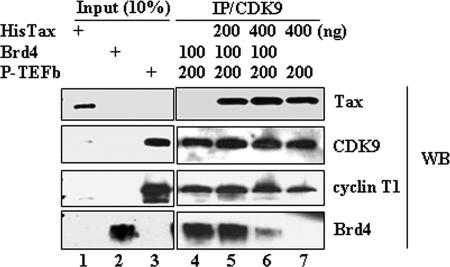
Given the interaction of Brd4 and Tax with P-TEFb and the importance of P-TEFb in HTLV-1 transcription, we were interested in determining whether there was a competitive interaction of Tax and Brd4 with P-TEFb. Brd4 and P-TEFb were purified from baculovirus and His-tagged Tax protein was purified from E. coli. Tax and Brd4 protein, either alone or in combination, were incubated with P-TEFb. After incubation, complexes were immunoprecipitated with anti-CDK9 and analyzed by immunoblotting with Brd4, CDK9, cyclin T1, and Tax antibodies. The results demonstrated that both Tax and Brd4 were able to bind independently to P-TEFb (Fig. 2, lanes 4 and 7). We next tested the ability of Tax and Brd4 to bind to P-TEFb when added together. When Brd4 was incubated along with a low concentration of Tax and P-TEFb, binding of both Brd4 and Tax to P-TEFb was observed (Fig. 2, lane 5). When the concentration of Tax was increased in the binding reaction, an increase in Tax binding and a decrease in Brd4 binding was observed (Fig. 2, lane 6). These results suggest that Brd4 and Tax bind competitively to P-TEFb.
FIG. 2.
Tax inhibits the interaction of Brd4 and CDK9 in vitro. A total of 200 ng of P-TEFb and 100 ng of Brd4 purified proteins were incubated with 0, 200, or 400 ng of His-tagged Tax protein as indicated above the figure. After incubation, the reaction mixtures were immunoprecipitated with CDK9 antibody, and immunoblot analysis was performed with Tax, CDK9, cyclin T1, or Brd4 antibody to detect protein-protein interaction.
Tax interacts with cyclin T1, but not CDK9 like Brd4.
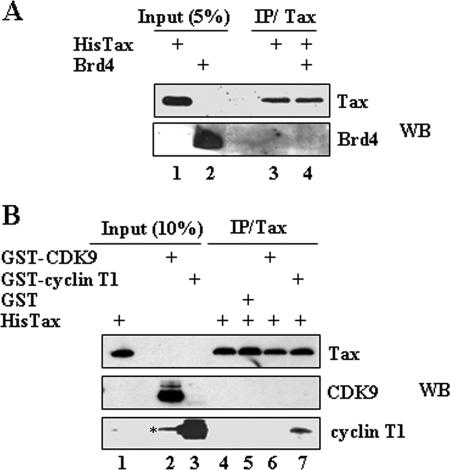
Because Tax could interfere with Brd4 binding to P-TEFb by interaction with either Brd4 or CDK9/cyclin T1, we carried out in vitro binding assays to examine whether Tax interacts with Brd4. His-tagged Tax protein was incubated with baculovirus-purified Brd4 protein. After incubation, the mixture was immunoprecipitated with Tax antibody, followed by Western blot analysis with Brd4 antibody. The results of this experiment demonstrate that Tax did not bind with Brd4 directly in vitro (Fig. 3A). Next, we investigated whether Tax interacted with CDK9 or cyclin T1. GST-CDK9, GST-cyclin T1, or control GST protein purified from E. coli was incubated with His-Tax. After immunoprecipitation with Tax antibody, the immune complex was analyzed by Western blotting with CDK9, cyclin T1, or Tax antibodies, respectively. Figure 3B shows that Tax associates with cyclin T1 (lane 7, bottom panel) but not CDK9 (lane 7, middle panel).
FIG. 3.
Tax interacts with cyclin T1. (A) His-tagged Tax (200 ng) and Brd4 (200 ng) proteins were incubated in binding buffer, immunoprecipitated with Tax antibody, and then analyzed by Western blot analysis with Brd4 and Tax antibodies. (B) Portions (600 ng) of GST, GST-CDK9, or GST-cyclin T1 were incubated with 400 ng of His-Tax protein in binding buffer. After incubation, the reaction mixtures were immunoprecipitated by using Tax antibody, followed by Western blot analysis with Tax, CDK9, or cyclin T1 antibody. The amounts of purified GST, GST-CDK9, or GST-cyclin T1 used for binding assay were equivalent, as determined by Gel-code staining (*, nonspecific band from overloading of lane 3).
Tax interacts with two domains of cyclin T1.
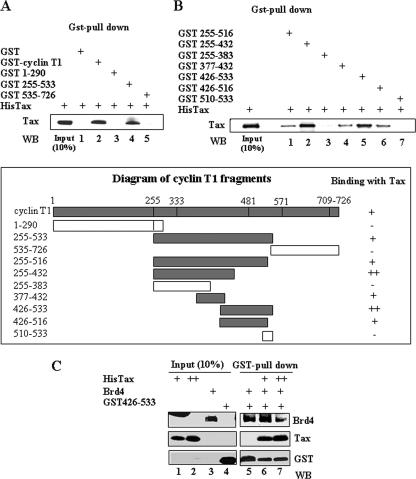
We next performed in vitro GST pull-down assays to identify the domain of cyclin T1 involved in the interaction with Tax. Full-length cyclin T1 (GST cyclin T1) and three deletion mutants (GST 1-290, GST 255-533, and GST 535-726) were fused to GST protein, expressed in E. coli, purified, and incubated with His-tagged Tax protein. After purification of the GST-cyclin T1 complex with glutathione beads, Western blot analysis with anti-Tax antibody was performed. The results presented in Fig. 4A demonstrate that full-length cyclin T1 and the central region amino acids 255 to 533 interacted with Tax (lanes 2 and 4). In contrast, the GST control or GST 1-290 and GST 535-726 failed to interact with Tax. To further define the domain responsible for interacting with Tax, a series of GST fusion proteins scanning the region from amino acids 255 to 533 were constructed. As shown in Fig. 4B, lanes 2 and 5, Tax interacted strongly with the GST 255-432 and the GST 426-533 domains of cyclin T1. Whether Tax interacts with two independent sites, one within amino acids 383 to 426 and the other between amino acids 432 and 533, or whether Tax interacts with the overlapping region between the fragments (amino acids 426 to 432) is not known.
FIG. 4.
Tax interacts with two domains of cyclin T1. (A and B) Portions (600 ng) of GST, GST-cyclin T1, or GST-cyclin T1 fragments were incubated with 400 ng of purified His-Tax protein. After incubation the mixtures were subjected to GST pull-down with glutathione beads, and Western blot analysis was performed using Tax antibody. (C) Portions (200 or 400 ng) of His-tagged Tax protein were incubated with 200 ng of Brd4 and 200 ng of GST 426-533. GST-bound proteins were precipitated with glutathione beads, and immunoblot analysis was performed with Brd4, Tax, or GST antibody to detect protein-protein interaction. Equivalent amounts of GST fusion proteins were added to the reactions as determined by Gel-code staining.
Interestingly, Brd4 has been previously mapped to bind to cyclin T1 domain amino acids 426 to 516 (18). To determine whether Tax binding to the 426-533 domain of cyclin T1 interferes with Brd4 binding, we performed in vitro competition binding assays. Brd4 protein was incubated with GST-cyclin T1 426-533 protein in the absence or presence of Tax. The complexes were pulled down with glutathione beads and analyzed by immunoblotting with Brd4, Tax, or GST antibodies. The results presented in Fig. 4C, lane 5, demonstrate that Brd4 binds to the GST-cyclin T1 426-533 protein. In the presence of increasing amounts of Tax protein, the amount of Brd4 associated with the 426-533 fragment of cyclin T1 was significantly decreased (Fig. 4, lane 7). This result provides further support that Tax and Brd4 bind competitively to cyclin T1.
Tax associates with P-TEFb and inhibits the interaction of Brd4 and CDK9 in vivo.
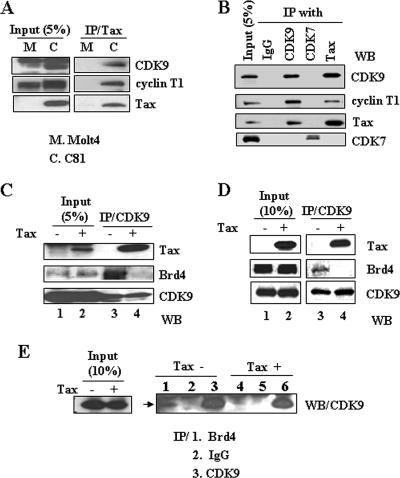
The results presented in Fig. 5A confirm that Tax interacts with the P-TEFb CDK9/cyclin T1 complex in vivo. Nuclear extracts from Molt4 (control lymphocytic cells) or C81 (HTLV-1-transformed Tax-expressing) cells were immunoprecipitated with Tax antibody, followed by Western blot analysis with CDK9 or cyclin T1 antibodies. As shown in Fig. 5A, CDK9 and cyclin T1 were coimmunoprecipitated with Tax in C81 cells. In view of the fact that Tax does not interact directly with CDK9 in vitro (Fig. 3B), we interpret these results to indicate that Tax interacts with P-TEFb through the cyclin T1 subunit. To prove the binding between Tax with P-TEFb is specific, nuclear extracts from C81 were immunoprecipitated with CDK7, CDK9, or Tax antibodies and analyzed by Western blot analysis. As shown in Fig. 5B, Tax associated with CDK9 and cyclin T1 (lanes 2 and 4) but not CDK7 (lane 3).
FIG. 5.
Tax interacts with P-TEFb in vivo and inhibits the interaction of Brd4 with P-TEFb. (A) Nuclear extracts from Molt4 or C81 cells were immunoprecipitated with Tax antibody and then immunoblotted with CDK9, cyclin T1, and Tax antibodies. (B) Nuclear extracts from C81 cells were immunoprecipitated with control immunoglobulin G, CDK9, CDK7, or Tax antibody and then immunoblotted with CDK9, cyclin T1, Tax, or CDK7 antibody. (C) HeLa cells were transfected with either a control or a Tax expression plasmid. At 24 h after transfection, nuclear extracts were prepared and immunoprecipitated with CDK9 antibody. Proteins present in the immunoprecipitates were analyzed by Western blot analysis with Tax, Brd4, and CDK9 antibodies. (D) HeLa cells were infected with Ad-GFP or Ad-Tax at an MOI of 100. At 24 h postinfection, the interaction of Tax and Brd4 with CDK9 was assayed by immunoprecipitation with CDK9 antibody and Western blot analysis with Tax, Brd4, and CDK9 antibodies. (E) Nuclear extracts from HeLa cells transfected with control or Tax expression plasmid were immunoprecipitated with control IgG, Brd4, or CDK9 antibody. Western blot analysis was then performed with CDK9 antibody.
In light of the in vitro competition results presented above, we next investigated whether Tax can affect the interaction between Brd4 and P-TEFb complex in vivo. HeLa cells were transfected with Tax-expressing plasmid or control plasmid, and nuclear lysates were prepared and immunoprecipitated with CDK9 antibody. Western blot analysis with either Brd4 or Tax antibodies was performed to compare the level of Brd4 bound to CDK9 in the presence or absence of Tax protein. The data presented in Fig. 5C show that Brd4 is bound to P-TEFb (CDK9) in the control cells (lane 3, middle panel). In the presence of Tax, the amount of Brd4 associated with P-TEFb was decreased (Fig. 5C, lane 4). In a parallel series of experiments, Tax was overexpressed in HeLa cells by transduction with an Ad-Tax virus. Similar to the results presented above, the association of Brd4 with the P-TEFb complex was significantly decreased in the presence of Tax (Fig. 5D, middle panel, lanes 3 and 4). In another experiment, complexes were immunoprecipitated with Brd4, and then Western blot analysis was performed with anti-CDK9. The results of this experiment confirm that Tax expression decreases the amount of Brd4 in the P-TEFb complex (Fig. 5E, compare lanes 1 and 4). These results are consistent with the in vitro competition between Brd4 and Tax for binding with P-TEFb shown above.
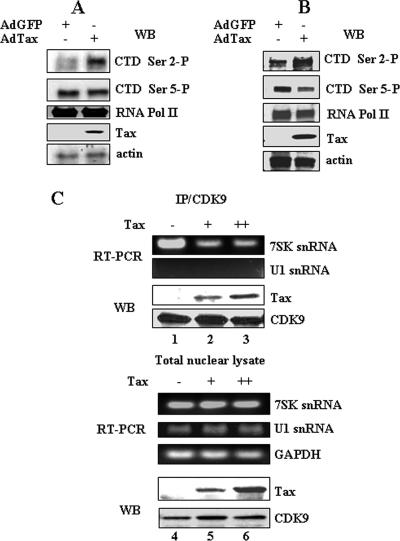
Tax stimulates functionality of P-TEFb.
In view of the fact that Tax competes for Brd4 binding to the P-TEFb complex, it was of interest to examine the effect of Tax on P-TEFb activity. Previous studies have demonstrated that overexpression of Brd4 results in an increase in Pol II CTD phosphorylation at Ser 2, the normal substrate of P-TEFb (18). To determine whether Tax has similar properties to Brd4 in this regard, pA-18G-BHK-21 cells were transduced with adenovirus expressing Tax or GFP. Total cell lysates were prepared to determine the phosphorylation level of RNA Pol II CTD in the presence or absence of Tax protein. As shown in Fig. 6A, overexpression of Tax significantly increased Ser 2 phosphorylation level. The overall level of Pol II protein and CTD Ser 5 phosphorylation in the cell was not affected by Tax (Fig. 6A, third panel). This effect was also investigated with HeLa cells transduced with Ad-Tax or Ad-GFP. Consistent with the result of Fig. 6A, Tax enhanced the Pol II CTD Ser 2 phosphorylation level without affecting CTD Ser 5 phosphorylation (Fig. 6B). These studies suggest that, similar to Brd4, Tax increases the overall activity of P-TEFb in the cell, presumably by increasing the amount of LMW active P-TEFb complexes.
FIG. 6.
Tax stimulates activity of P-TEFb in vivo. pA-18G-BHK-21 cells (A) or HeLa cells (B) were infected at an MOI of 100 with Ad-GFP or Ad-Tax. At 12 h postinfection, total cell extracts were prepared and analyzed by Western blot analysis with antibodies specific for Pol II CTD phospho-Ser2 (H5), Pol II CTD phospho-Ser5 (H14), RNA Pol II (8WG16), Tax, or actin. (C) HeLa cells were transfected with a total of 6 μg of plasmid DNA containing 0, 2, or 6 μg of Tax expression plasmid and 6, 4, or 0 μg of pcDNA3 plasmid. At 24 h posttransfection, nuclear extracts were prepared and immunoprecipitated with CDK9 antibody. Total RNAs from the immunoprecipitates were isolated and used for quantitative reverse transcription-PCR using primers specific for 7SK snRNA or U1 snRNA (upper panel). Total nuclear lysates were used for RNA isolation, and quantitative reverse transcription-PCR for 7SK and U1 snRNA was performed (bottom panel). CDK9 immunoprecipitates and nuclear extracts used for immunoprecipitation were also analyzed by Western blot analysis with Tax and CDK9 antibodies.
If Tax increases the amount of active LMW P-TEFb complex, one might expect a concomitant decrease in the level of HMW P-TEFb complex. To examine whether the Tax could affect the binding of 7SK snRNA to P-TEFb, HeLa cells were transfected with a Tax expression plasmid. At 12 h posttransfection, cell extracts were prepared and immunoprecipitated with anti-CDK9 antibody. Total RNA was purified from the immunoprecipitation, and cDNA was prepared by reverse transcriptase. PCR amplification with primers specific for 7SK snRNA was then performed. To check specific binding of 7SK snRNA with P-TEFb, we used U1 snRNA as a negative control. The results presented in Fig. 6C demonstrate that Tax decreases the amount of 7SK snRNA associated with the P-TEFb complex by approximately 50% (Fig. 6C, upper panel). In contrast, U1 snRNA was not detected in the P-TEFb complex. The decrease in 7SK snRNA bound to P-TEFb was not due to an overall decrease in 7SK snRNA levels since the amount of input 7SK snRNA was equivalent in the control and Tax-transfected cells (Fig. 6C, bottom panel). These results suggest that Tax pushes CDK9/cyclin T1 from the inactive HMW form toward active LMW form, thereby stimulating P-TEFb activity. Based on the results of the competition assays, the LMW complex would be a mixture of Tax/CDK9/cyclin T1 and Brd4/CDK9/cyclin T1, the ratio of which would depend upon the level of Tax and Brd4 expression in the cells.
DISCUSSION
Our lab previously reported that Tax recruited P-TEFb to the HTLV-1 LTR promoter and that P-TEFb played a significant role in HTLV-1 Tax transactivation in vivo and in vitro. Inhibition of CDK9 expression by siRNA treatment or immunodepletion inhibited Tax transactivation (54). In the cell P-TEFb is present in two states, an LMW active-form P-TEFb or an HMW inactive form in which 7SK snRNA and HEXIM1 were found associated with P-TEFb complex. In HeLa cells, the HMW complex constitutes about 50% of the P-TEFb under normal growth state (18, 30, 46). Stress-inducing conditions such as UV irradiation, actinomycin D, or DRB (5,6-dicholoro-1-β-d-ribofuranosylbenzimidazole) treatment cause a shift from the 7SK snRNA/HEXIM-1-containing HMW P-TEFb complex to the LMW kinase-active complex (6, 47, 48). A recent study by Jang et al. (18) suggested that the active P-TEFb is associated with Brd4, a bromodomain motif-containing protein, which binds acetylated histones. Consistent with this interpretation, overexpression of Brd4 increased the phosphorylation of RNA Pol II CTD and enhanced the transactivation of cellular and viral promoters (18). In addition, siRNA treatment of Brd4 leads to decreased RNA Pol II phosphorylation and transactivation. We have analyzed here the interaction of Brd4 and Tax with P-TEFb.
Our results suggest that Brd4 inhibits Tax transactivation of the HTLV-1 LTR promoter in a dose-dependent manner. Consistent with these findings, in vitro or in vivo binding experiments showed that Brd4 and Tax compete for interaction with P-TEFb. Tax did not bind to Brd4 directly but interacts with the cyclin T1 subunit of P-TEFb, similar to Brd4. Using deletion mutants of cyclin T1, we found that Tax interacted with a peptide of cyclin T1 containing amino acids 426 to 533, overlapping the binding site for Brd4. Consistent with these data, in vitro or in vivo binding competition assays showed that Tax and Brd4 competed with each other for binding to the P-TEFb. The inhibitory effect of Brd4 on HTLV-1 LTR Tax transactivation likely was the result of this competitive interaction with P-TEFb.
In view of the ability of Tax to interact with P-TEFb and compete with Brd4 binding, we next examined the amounts of 7SK snRNA bound to P-TEFb in the absence or presence of Tax. In the presence of overexpressed Tax, the level of 7SK snRNA was decreased. Thus, similar to Brd4, Tax pushed the P-TEFb equilibrium toward the active LMW complex. Consistent with this observation, we found that, similar to the overexpression of CDK9, cyclin T1, or Brd4 (18, 54), Tax stimulated Ser 2 phosphorylation of CTD of RNA Pol II without changing Ser 5 phosphorylation, denoting an increase in the functionality of P-TEFb. Interestingly, ChIP assays suggest that Brd4 is associated with the HTLV-1 basal LTR promoter but is decreased in the presence of Tax (data not shown). In contrast, the amount of CDK9 that associates with the HTLV-1 LTR was increased in the presence of Tax. This result suggests that Tax facilitated the binding of the Tax/P-TEFb complex to the active promoter instead of Brd4/P-TEFb, allowing a mechanism to stimulate Tax-dependent transcription.
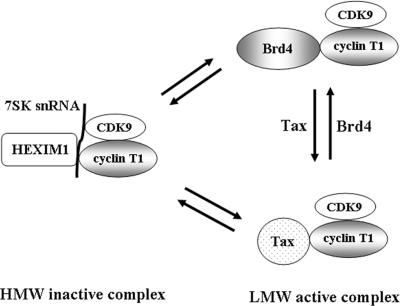
Our results suggest that Tax facilitates the release of P-TEFb from the HMW inactive complex to an active LMW P-TEFb complex composed of Tax/CDK9/cyclin T1. The LMW Tax/P-TEFb complex is in competitive equilibrium with the LMW P-TEFb complex composed of Brd4/CDK9/cyclin T1 (Fig. 7). The relative amounts of Brd4/P-TEFb and Tax/P-TEFb in the cell likely depends upon the concentration of Tax and Brd4 in the cell. Release of P-TEFb from the HMW complex could be due to the direct action of Tax facilitating the release of HEXIM1 and 7SK snRNA. Alternatively, Brd4 released from the LMW P-TEFb complex by Tax competition could feed back and signal the release of HMW P-TEFb to the active fraction. The appearance of Tax in the LMW active fraction suggests that Tax may function, in part, like Brd4, facilitating the interaction of P-TEFb with Tax-responsive promoters. Other parallels between Tax and Brd4 activity with P-TEFb have been observed. We have recently found that both Tax and Brd4 regulate P-TEFb activity through a novel autophosphorylation pathway that regulates CDK9 kinase activity on the Pol II CTD. These findings suggest that Tax, at least in part, mimics the activity of the cellular Brd4 transcriptional factor to facilitate the transcription of Tax-regulated genes.
FIG. 7.
Model for competitive interaction of Tax and Brd4 with the cellular P-TEFb complex. Our results demonstrate that Tax interacts with P-TEFb through interaction with the cylin T1 subunit. The interaction of Tax with P-TEFb inhibits the interaction of Brd4, suggesting that there may be two LMW “active” fractions of P-TEFb in HTLV-1 Tax-expressing cells. Overexpression of Tax causes a decrease in the level of the HMW 7SK snRNA/HEXIM1/P-TEFb complex, with a concomitant increase in P-TEFb activity, as measured by the level of Pol II CTD phosphor-Ser2.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Intramural AIDS Targeted Antiviral Program.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Adya, N., and C. Z. Giam. 1995. Distinct regions in human T-cell lymphotropic virus type I tax mediate interactions with activator protein CREB and basal transcription factors. J. Virol. 69:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi, T., H. Ono, and K. Shimotohno. 1995. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood 86:4243-4249. [PubMed] [Google Scholar]
- 3.Barboric, M., and B. M. Peterlin. 2005. A new paradigm in eukaryotic biology: HIV Tat and the control of transcriptional elongation. PLoS. Biol. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-1 Tax protein. Methods 16:83-94. [DOI] [PubMed] [Google Scholar]
- 5.Brady, J., K. T. Jeang, J. Duvall, and G. Khoury. 1987. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J. Virol. 61:2175-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers, S. A., J. P. Price, J. J. Cooper, Q. Li, and D. H. Price. 2005. HEXIM2, an HEXIM1-related protein, regulates positive transcription elongation factor b through association with 7SK. J. Biol. Chem. 280:16360-16367. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese, L. H., and S. J. Naides. 2005. Viral arthritis. Infect. Dis. Clin. N. Am. 19:963-980. [DOI] [PubMed] [Google Scholar]
- 8.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100:8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-1 is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 10.Franklin, A. A., and J. K. Nyborg. 1995. Mechanisms of Tax regulation of human T cell leukemia virus type I gene expression. J. Biomed. Sci. 2:17-29. [DOI] [PubMed] [Google Scholar]
- 11.Fu, T. J., J. Peng, G. Lee, D. H. Price, and O. Flores. 1999. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 274:34527-34530. [DOI] [PubMed] [Google Scholar]
- 12.Garriga, J., and X. Grana. 2004. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 337:15-23. [DOI] [PubMed] [Google Scholar]
- 13.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and T. G. de. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 14.Giebler, H. A., J. E. Loring, O. K. Van, M. A. Colgin, J. E. Garrus, K. W. Escudero, A. Brauweiler, and J. K. Nyborg. 1997. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol. Cell. Biol. 17:5156-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassmann, R., C. Dengler, I. Muller-Fleckenstein, B. Fleckenstein, K. McGuire, M. C. Dokhelar, J. G. Sodroski, and W. A. Haseltine. 1989. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 86:3351-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrod, R., Y. L. Kuo, Y. Tang, Y. Yao, A. Vassilev, Y. Nakatani, and C. Z. Giam. 2000. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 275:11852-11857. [DOI] [PubMed] [Google Scholar]
- 17.Harrod, R., Y. Tang, C. Nicot, H. S. Lu, A. Vassilev, Y. Nakatani, and C. Z. Giam. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 18:5052-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang, M. K., K. Mochizuki, M. Zhou, H. S. Jeong, J. N. Brady, and K. Ozato. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19:523-534. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanno, T., Y. Kanno, R. M. Siegel, M. K. Jang, M. J. Lenardo, and K. Ozato. 2004. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol. Cell 13:33-43. [DOI] [PubMed] [Google Scholar]
- 21.Kashanchi, F., and J. N. Brady. 2005. Transcriptional and posttranscriptional gene regulation of HTLV-1. Oncogene 24:5938-5951. [DOI] [PubMed] [Google Scholar]
- 22.Kato, T., H. Asahara, M. S. Kurokawa, K. Fujisawa, T. Hasunuma, H. Inoue, M. Tsuda, S. Takahashi, S. Motokawa, T. Sumida, and K. Nishioka. 2004. HTLV-1 env protein acts as a major antigen in patients with HTLV-1-associated arthropathy. Clin. Rheumatol. 23:400-409. [DOI] [PubMed] [Google Scholar]
- 23.Kwok, R. P., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the coactivator CBP. Nature 380:642-646. [DOI] [PubMed] [Google Scholar]
- 24.Ladurner, A. G., C. Inouye, R. Jain, and R. Tjian. 2003. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell 11:365-376. [DOI] [PubMed] [Google Scholar]
- 25.Lemasson, I., N. J. Polakowski, P. J. Laybourn, and J. K. Nyborg. 2004. Transcription regulatory complexes bind the human T-cell leukemia virus 5′ and 3′ long terminal repeats to control gene expression. Mol. Cell. Biol. 24:6117-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Q., J. P. Price, S. A. Byers, D. Cheng, J. Peng, and D. H. Price. 2005. Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing Cdk9 phosphorylated at threonine 186. J. Biol. Chem. 280:28819-28826. [DOI] [PubMed] [Google Scholar]
- 27.Lu, H., C. A. Pise-Masison, R. Linton, H. U. Park, R. L. Schiltz, V. Sartorelli, and J. N. Brady. 2004. Tax relieves transcriptional repression by promoting histone deacetylase 1 release from the human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 78:6735-6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall, N. F., J. Peng, Z. Xie, and D. H. Price. 1996. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem. 271:27176-27183. [DOI] [PubMed] [Google Scholar]
- 29.Matangkasombut, O., and S. Buratowski. 2003. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol. Cell 11:353-363. [DOI] [PubMed] [Google Scholar]
- 30.Michels, A. A., A. Fraldi, Q. Li, T. E. Adamson, F. Bonnet, V. T. Nguyen, S. C. Sedore, J. P. Price, D. H. Price, L. Lania, and O. Bensaude. 2004. Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor. EMBO J. 23:2608-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochizuki, M., T. Watanabe, K. Yamaguchi, K. Tajima, K. Yoshimura, S. Nakashima, M. Shirao, S. Araki, N. Miyata, and S. Mori. 1992. Uveitis associated with human T lymphotropic virus type I: seroepidemiologic, clinical, and virologic studies. J. Infect. Dis. 166:943-944. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, F., H. Kajihara, M. Nakamura, H. Sasaki, T. Kumamoto, and K. Okada. 1989. HTLV-1 associated myelopathy in an HTLV-1 and HBV double carrier family: report of a case and the mode of vertical transmission of both viruses. J. Gastroenterol. Hepatol. 4:387-390. [DOI] [PubMed] [Google Scholar]
- 33.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka, K., I. Maruyama, K. Sato, I. Kitajima, Y. Nakajima, and M. Osame. 1989. Chronic inflammatory arthropathy associated with HTLV-1. Lancet i:441. [DOI] [PubMed] [Google Scholar]
- 35.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-1 associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 36.Peng, J., Y. Zhu, J. T. Milton, and D. H. Price. 1998. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 12:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prelich, G. 2002. RNA polymerase II carboxy-terminal domain kinases: emerging clues to their function. Eukaryot. Cell 1:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price, D. H. 2000. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20:2629-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen, C. A., R. Park, J. G. Sodroski, and W. A. Haseltine. 1987. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc. Natl. Acad. Sci. USA 84:4919-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen, C. A., J. G. Sodroski, and W. A. Haseltine. 1985. The location of cis-acting regulatory sequences in the human T-cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell 41:813-823. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tie, F., N. Adya, W. C. Greene, and C. Z. Giam. 1996. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J. Virol. 70:8368-8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernant, J. C., G. Buisson, J. Magdeleine, T. J. De, A. Jouannelle, C. Neisson-Vernant, and N. Monplaisir. 1988. T-lymphocyte alveolitis, tropical spastic paresis, and Sjogren syndrome. Lancet i:177. [DOI] [PubMed] [Google Scholar]
- 45.Yakova, M., A. Lezin, F. Dantin, G. Lagathu, S. Olindo, G. Jean-Baptiste, S. Arfi, and R. Cesaire. 2005. Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, Z., J. H. Yik, R. Chen, N. He, M. K. Jang, K. Ozato, and Q. Zhou. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19:535-545. [DOI] [PubMed] [Google Scholar]
- 47.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 48.Yik, J. H., R. Chen, R. Nishimura, J. L. Jennings, A. J. Link, and Q. Zhou. 2003. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol. Cell 12:971-982. [DOI] [PubMed] [Google Scholar]
- 49.Yik, J. H., R. Chen, A. C. Pezda, and Q. Zhou. 2005. Compensatory contributions of HEXIM1 and HEXIM2 in maintaining the balance of active and inactive positive transcription elongation factor b complexes for control of transcription. J. Biol. Chem. 280:16368-16376. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao, L. J., and C. Z. Giam. 1992. Human T-cell lymphotropic virus type I (HTLV-1) transcriptional activator, Tax, enhances CREB binding to HTLV-1 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 89:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou, M., M. A. Halanski, M. F. Radonovich, F. Kashanchi, J. Peng, D. H. Price, and J. N. Brady. 2000. Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 20:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou, M., H. Lu, H. Park, J. Wilson-Chiru, R. Linton, and J. N. Brady. 2006. Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription. J. Virol. 80:4781-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]