Abstract
To generate a null UL49 gene mutant of herpes simplex virus 1 (HSV-1), we deleted from the viral DNA, encoded as a bacterial artificial chromosome (BAC), the UL49 open reading frame and, in a second step, restored it. Upon transfection into Vero cells, the BAC-ΔUL49 DNA yielded foci of degenerated cells that could not be expanded and a few replication-competent clones. The replication-competent viral clones derived from independent transfections yielded viruses that expressed genes with some delay, produced smaller plaques, and gave lower yields than wild-type virus. A key finding is that the independently derived replication-competent viruses lacked the virion host shutoff (vhs) activity expressed by the RNase encoded by the UL41 gene. One mutant virus expressed no vhs protein, whereas two others, derived from independent transfections, produced truncated vhs proteins consistent with the spontaneous in-frame deletion. In contrast, cells infected with the virus recovered upon transfection of the BAC-UL49R DNA (R-UL49) accumulated a full-length vhs protein, indicating that in the parental BAC-ΔUL49 DNA, the UL41 gene was intact. We conclude that expression of the vhs protein in the absence of UL49 protein is lethal, a conclusion bolstered by the evidence reported elsewhere that in transfected cells vhs requires both VP16 and VP22, the product of UL49, to be neutralized.
Of the 84 different known proteins encoded by herpes simplex virus 1 (HSV-1), at least 4 proteins, all located in the tegument of the virion, interact with mRNAs. Of these, the proteins encoded by the US11, UL47, and UL49 open reading frames (ORFs) bind RNAs, whereas the fourth, encoded by the UL41 ORF, acts as an RNase (reviewed in reference 44). Apart from potential regulatory functions, interest in the RNA binding proteins stemmed from the observation that virions package mRNAs (40, 42). Moreover, in the course of studies of this phenomenon, our laboratories reported that VP22, the product of the UL49 ORF, transports the mRNA from infected to uninfected cells for expression prior to viral infection (41). The studies reported here were initially designed to determine the contribution of VP22 to the packaging of mRNAs in the virion. We show that a series of ΔUL49 mutants derived from independent transfections of viral DNA lacking the UL49 ORF yielded recombinant viruses defective in the UL41 gene. On the basis of the results reported in this article and in parallel studies published by Taddeo et al. (52), we conclude that VP22 and VP16 are both required for the replication of viruses encoding functional UL41 protein. Relevant to this report are the following.
The shutoff of cellular protein synthesis, a function designated virion host shutoff (vhs), was first identified and mapped to the UL41 ORF by Frenkel and colleagues (25, 26, 47). Intensive studies carried out over 2 decades demonstrated that the UL41 product is a γ2 protein, that it is packaged in the virion, and that it mediates the degradation of mRNAs during the early phases of infection by endonucleolytic cleavage (reviewed in reference 44). Furthermore, several lines of evidence indicate that vhs degrades mRNA in a selective manner (15, 16, 49). In recent studies, this laboratory unambiguously demonstrated that the UL41 protein is an endoribonuclease with a substrate specificity similar to that of RNase A (50, 51). At late stages of infection the UL41 protein is no longer active, even though it accumulated in large amounts in the course of synthesis of the late protein. Studies carried out primarily by Smiley and associates demonstrated that the UL41 product binds VP16, giving rise to the speculation that VP16 neutralizes the RNase activity at late times after infection (27, 39, 43). Attempts to express and accumulate the UL41 protein by transfection of cells in the absence of both VP16 and VP22 failed. In contrast, a plasmid encoding a UL41 ORF in which 3 codons were replaced to inactivate the enzymatic activity was readily expressed in the absence of both UL48 and UL49. These studies also demonstrated that the UL41 protein binds VP22, but only in the presence of VP16 (52). The studies presented here extend this observation by showing that ΔVP22 viruses contain disabling mutations in the UL41 ORF.
VP22 is a 301-residue γ1 protein capable of forming higher-order structures consisting of dimers or tetramers (31). The protein is nucleotidylylated by casein kinase II (3) and phosphorylated by other enzymes, although the isoform incorporated into the virion is hypophosphorylated (11, 12, 19, 36). The many functions attributed to VP22 include (i) binding to chromatin, microtubules, and membranes (24, 29, 57); (ii) interaction with template activating factor 1 (TAF-1) and impairment of nucleosome assembly on entering viral DNA (53); (iii) mediation of hyperacetylation and bundling of microtubules to render them more resistant to depolymerization (9); (iv) interaction with membranes, and more specifically with membranes of the acidic compartments of cells (5); (v) interaction with several tegument and envelope proteins, including VP16 and gD (6, 13, 21, 54). Although it has been postulated that VP22 plays a key role in virus assembly, the ΔUL49 mutants reported to date do not appear to be defective in this function (34). Perhaps the single most important function attributed to VP22 is the ability to spread to adjacent cells after infection or transfection solely with a plasmid encoding VP22. With regard to recipient cells, VP22 was detected by immunofluorescence in the nuclei of cells fixed with paraformaldehyde or organic fixatives but to a much reduced level in cells fixed with methanol.
The transport of chimeric proteins consisting of VP22 covalently bound to green fluorescent protein (GFP) or other proteins from cell to cell has been disputed (1, 2, 4, 10, 17, 20, 22, 28, 33, 38, 55, 58, 59). The conflicting reports remain to be resolved, but it should be pointed out that some proteins are readily extracted during methanol fixation of cells and that covalently bound polypeptides of a size nearly equivalent to or larger than VP22 could be expected to change the properties of the protein.
The significance of the studies reported here stems from three observations: (i) none of the isolated viruses expressed functional vhs activity; (ii) at least two different kinds of mutations were responsible for the loss of activity; and (iii) the mutants were selected and amplified after transfection of the DNA.
MATERIALS AND METHODS
Cells and viruses.
Vero, HeLa, and HEp-2 cell lines (American Type Culture Collection) were propagated in Dulbecco's modified Eagle's medium supplemented with 5% newborn calf serum. HSV-1(F) is a limited-passage prototype HSV-1 used in our laboratories (8). The ΔUL41 mutant virus has been reported elsewhere (35). Virus stocks were titered on Vero cells. Serial 10-fold dilutions of the lysates were assayed on monolayers of Vero cells in 6-well dishes. After 1 h, cell monolayers were covered with Dulbecco's modified Eagle's medium containing 0.3% methylcellulose. After 3 days of incubation at 37°C, the medium was aspirated from the wells, and the cells were then fixed, stained with crystal violet, and visualized at 10× magnification with an inverted microscope for plaque detection.
Antibodies.
The HSV-1 proteins were detected with the anti-ICP0 monoclonal antibody purchased from the Goodwin Institute (Plantation, FL), an anti-glutathione S-transferase (GST)-US3 polyclonal antibody (32), an anti-VP16 mouse monoclonal antibody (LP1; a kind gift of A. Minson), an anti-GST-UL49 polyclonal antibody (3), an anti-US11 monoclonal antibody (37), and anti-GST-UL41 rabbit polyclonal antiserum (51). A mouse monoclonal antibody against actin was purchased from Sigma (St. Louis, MO).
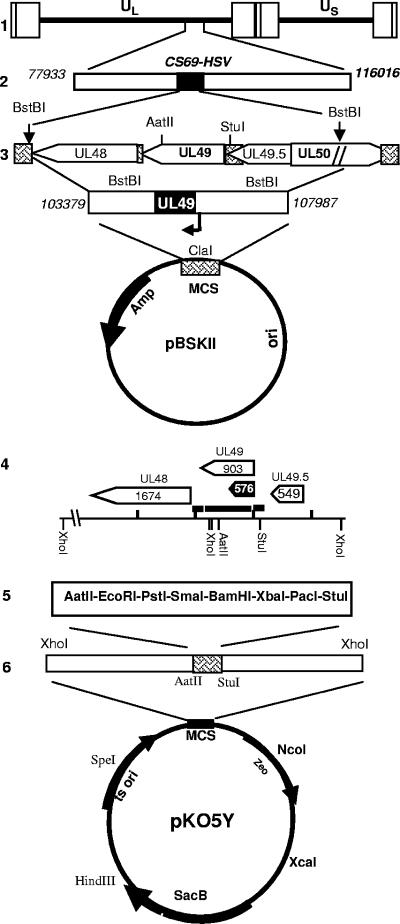
Construction of the BAC HSV-1 DNA from which the UL49 gene was deleted and subsequently restored.
The construction and properties of the BAC-HSV-1(F) DNA have been described elsewhere (23, 56, 60). The HSV-1 mutant lacking the UL49 gene was constructed by using the HSV-1(F) bacterial artificial chromosome (BAC-HSV-1) with transfer plasmid pKo5Y (pRB5708), as schematically illustrated in Fig. 1. To construct the pMTS2 transfer plasmid, cosmid CS69, containing the HSV-1 sequence spanning nucleotides 77933 to 116016, was digested with BstBI restriction enzyme, generating a 4,615-bp fragment containing the UL48, UL49, and UL49.5 ORFs and a portion of the UL50 ORF. The 4,615-bp fragment was subcloned into pBluescript II KS(1) at a compatible ClaI restriction site to yield pMTS2. pMTS2 was cleaved with AatII/StuI to remove an 862-bp fragment encompassing amino acids 1 to 266 of the UL49 ORF. Removal of this fragment generated a frame shift of the residual 35 codons of UL49. The 862-bp fragment was replaced with a polylinker containing the following restriction sites: StuI, PacI, XbaI, BamHI, SmaI, PstI, EcoRI, and AatII (pMTS3). The plasmid obtained was then partially digested with XhoI, and the resulting 3,653-bp fragment was cloned into the pKo5Y shuttle vector to yield pMTS4. The procedure for construction of the mutant virus has been described elsewhere (56). Briefly, RR1 competent cells that harbored the BAC-HSV-1 (RR1-HSV-1) were transformed with 0.6 μg of transfer plasmid pMTS3 DNA, plated onto zeocin (Zeo; 20 μg/ml)-plus-chloramphenicol (Cm; 20 μg/ml) plates, and incubated overnight at 43°C. After incubation, 8 colonies were picked, plated onto Cm-10% sucrose (Suc) Lennox broth (LB) plates, and further incubated at 30°C overnight. To confirm the loss of the replacement vector, 20 Cmr Sucr colonies were restreaked in duplicate onto Cm Suc Zeo LB plates separately and then incubated at 30°C overnight. The Sucr Cm Zeor colonies were further screened by PCR (95°C for 4 min; then 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min). The primers used were Δ49 forward (5′-CCCACATTGGCTCCTGTCACG-3′; from nucleotide 105326 to 105346) and Δ49 reverse (5′-CCTTCCTCGCGGAAACCGAGAC-3′; from nucleotide 106665 to 106644). PCR-confirmed colonies were grown in LB Cm medium, and the recombinant BAC-HSV-ΔUL49 DNA was prepared with a high-speed QIAGEN (Chatsworth, CA) plasmid purification kit. For the generation of UL49-repaired virus (R-UL49), the above-described 4,615-bp fragment derived from CS69 digestion with BstBI restriction enzyme was directly cloned into the pKo5Y shuttle vector to yield pMTS5. RR1 competent cells harboring BAC-ΔUL49 DNA were transformed with 0.6 μg of pMTS5 transfer plasmid DNA, and the recombinant BAC-UL49R DNA was obtained as described above.
FIG. 1.
Schematic representation of cosmid and plasmid DNAs. Line 1, linear representation of the HSV-1 genome. Rectangles represent the inverted repeats flanking the unique sequences (UL and US, represented by thin lines). Line 2, linear representation of cosmid CS69 DNA containing the insertion of HSV-1 sequence spanning nucleotides 77933 to 116016. Line 3, pMTS2, a 4.5-kb BstBI fragment of cosmid CS69 containing the UL48, UL49, and UL49.5 genes and a portion of the UL50 gene. Arrowheads indicate the direction of transcription; hatched boxes, noncoding regions. Line 4, details of the ORFs present in the BstBI fragment and the relevant restriction en- donuclease sites used for construction of the deletion mutants. Black arrow indicates the position of the probe used for this study. Line 5, pMTS2 was cleaved with AatII/StuI to remove an 862-bp fragment containing the coding sequence for amino acids 1 to 266 of UL49. The UL49 sequence was replaced with a polylinker containing the following restriction sites: StuI, PacI, XbaI, BamHI, SmaI, PstI, EcoRI, and AatII (pMTS3). Line 6, the XhoI fragment derived from partially digested pMTS3, resulting in a 3,653-bp fragment, was transferred to shuttle plasmid pKo5Y at the XhoI site (pMTS4).
Viral DNA extraction from RR1 bacterial cells.
BAC-HSV-1, BAC-ΔUL49, and BAC-UL49R DNAs were extracted from RR1-HSV-1, RR1-ΔUL49, and RR1-UL49R bacterial cells, respectively, by using a QIAGEN Max extraction kit according to the manufacturer's instructions.
Transfection of cell lines with recombinant BAC-HSV DNAs.
Subconfluent cultures of Vero, HEp-2, or HeLa cells were transfected with 1.6 μg of the recombinant BAC-HSV-ΔUL49 or BAC-HSV-UL49R DNA by use of Lipofectamine reagent according to the manufacturer's instructions (Life Technologies, Grand Island, NY). BAC-derived ΔUL49 viruses obtained from three independent transfections of Vero cells were identified as V0, V1, and V3, respectively. One ΔUL49 mutant virus obtained from HEp-2 transfected cells was identified as H-3. No replicating ΔUL49 virus was obtained from HeLa cells. The same procedure was also used to generate UL49R virus. The viruses were collected and titered as described above.
Southern blot analyses of viral DNA.
Equal amounts (10 μg) of BAC-HSV-1, BAC-ΔUL49, and BAC-UL49R DNAs, purified from RR1 bacterial cells, were digested with EcoRV enzyme, electrophoretically separated on a 1% agarose gel, and transferred to a nylon membrane (Bio-Rad, Hercules, CA). The hybridization procedures were carried out as recommended by the manufacturer (Bio-Rad). Plasmid pAc-NH2, containing the amino-terminal sequence of UL49 spanning nucleotides 1 to 576 (data not shown), was used to generate a biotin-16-UTP-labeled (Roche Diagnostics, Germany) probe by using a nick translation kit (Roche Diagnostics).
Immunoblotting of electrophoretically separated proteins from cell lysates.
Confluent Vero cell monolayers were either mock infected or infected with 10 PFU/cell of HSV-1(F), ΔUL41 mutant virus, or a ΔUL49 BAC-derived virus and were collected 18 h after infection. The procedures for harvesting, solubilization, protein quantification, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transfer to nitrocellulose membranes were performed as previously reported (49). The membrane was probed for vhs, VP22, US3, ICP0, US11, VP16, and actin using the antibodies listed above.
Immunoblotting of proteins from purified virions.
Wild-type and recombinant virus virions were purified as described elsewhere (40, 45). Briefly, Vero cells grown in 300-cm2 flasks were exposed to 5 PFU of virus per cell. The cells were harvested 22 to 24 h after infection, resuspended in 1 mM phosphate buffer, and disrupted in a glass homogenizer with four strokes. Cytoplasmic fractions were individually layered in dextran-10 gradients (1.04 to 1.09 g/cm3) in 1 mM phosphate buffer. The gradients were centrifuged, and virion-containing bands were collected and diluted in 10 mM phosphate buffer. Purified virions were concentrated and resuspended in 100 μl of 10 mM phosphate buffer and stored at −20°C before processing. The purified virions were resuspended in 4× loading buffer (50 mM Tris-HCl [pH 6.8], 10 mM dithiothreitol, 2% sodium dodecyl sulfate, 0.1% bromphenol blue, 10% glycerol), subjected to electrophoresis on a 10% denaturing polyacrylamide gel, and then transferred to a nitrocellulose membrane. The membrane was blocked for 1 h with 5% nonfat dry milk and reacted with the appropriate primary antibody overnight at 4°C. The monoclonal antibody to US11 and polyclonal antibodies to vhs and VP22 were diluted 1:500 in phosphate-buffered saline containing 1% bovine serum albumin and 0.05% Tween 20. The blots were then washed and incubated with alkaline phosphatase (AP)-conjugated anti-mouse immunoglobulin G (Calbiochem) and AP-conjugated anti-rabbit immunoglobulin G (Santa Cruz Biotechnology), respectively. To develop AP-conjugated secondary antibodies, the immunoblots were reacted with the AP buffer 5-bromo-4-chloro-3-indolylphosphate-tetranitroblue tetrazolium (BCIP/TNBT; Calbiochem).
Analysis of shutoff of protein synthesis in infected cells.
Replicate 25-cm2 cultures of Vero cells were either mock infected or infected with 20 PFU of HSV-1(F), ΔUL41, or ΔUL49 isolates per cell in the presence of actinomycin D (10 μg/ml) and incubated at 37°C. At 3 h after exposure to virus, the cells were labeled for 1 h with [35S]methionine as previously described (35); then they were harvested, solubilized, resolved in a 12% denaturing polyacrylamide gel, dried, and subjected to autoradiography.
VHS sequence analysis of PCR products derived from viral RNA and DNA extracted from cells infected with HSV-1(F), ΔUL49-V0, ΔUL49-V1, or ΔUL49-V3 virus.
Vero cells were infected with HSV-1(F) or with ΔUL49-V0, ΔUL49-V1, or ΔUL49-V3 mutant virus and were harvested 22 h after infection. Total RNA was extracted with TRIzol reagent according to the manufacturer's instructions (Life Technologies). DNase I treatment, phenol-chloroform extraction, and ethanol precipitation (Fisher Scientific, Houston, TX) were carried out to remove possible DNA contamination. Total RNA (2.5 μg) was then reverse transcribed with 60 U of avian myeloblastosis virus (Promega, Madison, WI) in a total reaction volume of 30 μl. The reverse transcription was primed with an oligo(dT)15 primer and performed using a pool of nucleotides consisting of dGTP, dATP, dTTP, and dCTP (Promega) at 1 mM each. Forty units of RNasin (Promega) was added to each reaction mixture. The mixture containing only the RNA template and the oligo(dT)15 was first heated at 70°C for 10 min, then chilled on ice, and, after the addition of the other components, incubated at 42°C for 45 min, shifted to 52°C for 45 min, and then heat inactivated at 95°C for 5 min.
Viral DNAs from HSV-1(F), ΔUL49-V0, ΔUL49-V1, and ΔUL49-V3 viruses were extracted as described elsewhere (30) and used as templates for PCR amplification.
cDNAs obtained from reverse-transcribed viral RNAs and viral DNAs extracted from infected cells were amplified by PCR under the following conditions: 1 min at 95°C, 45 s at 60°C, and 2 min at 72°C. The primers used were VHS forward (5′-ATGGGTTTGTTCGGGATGATGAAG-3′) and VHS reverse (5′-CTACTCGCTCCAGAATTTGGCCAG-3′). The PCR products were then cloned into the pGEM-T Easy vector according to the manufacturer's instructions (Promega) and sequenced.
RESULTS
Isolation of ΔUL49 mutant viruses.
The construction of the HSV-1-BAC ΔUL49 mutant virus is illustrated in Fig. 1 and described in detail in Materials and Methods. The ΔUL49 mutant virus was isolated by transfection of Vero cells with plasmids containing the BAC-ΔUL49 DNA. Of the numerous independent transfections, several yielded foci of degenerating cells, but virus could not be passaged to uninfected cells. In three instances, transfections in Vero cells yielded viruses capable of transmission from cell to cell. These were designated V0, V1, and V3. One isolate, designated H-3, was obtained from transfected HEp-2 cells. In the initial studies, V1 was designated as the prototype virus. This virus was plaque purified, grown in Vero cells, and extensively characterized (data not shown).
During the course of these studies, it became apparent that the ΔUL49-V1 virus carries an additional mutation in the UL41 ORF. At that point we analyzed all viruses isolated from transfectants with respect to the UL41 ORF sequence. As described below, viruses V0 and V1 carry an identical mutation. V3 carries a different mutation that blocks the expression of the UL41 ORF. Finally, the H-3 mutant virus, less extensively characterized, was found to be phenotypically similar to V3.
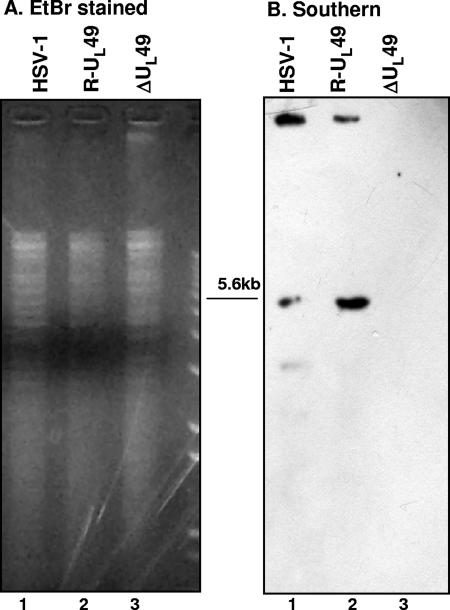
Verification of the structure of BAC-ΔUL49 DNA.
Equal amounts of BAC-HSV-1, BAC-UL49R, and BAC-ΔUL49 plasmid DNAs extracted from RR1 bacteria were separately digested with EcoRV, electrophoretically separated on an agarose gel, and hybridized with a biotinylated DNA probe containing the amino-terminal sequence of UL49. As expected, and as shown in Fig. 2, the probe hybridized with a 5.6-kb fragment derived from digested BAC-HSV-1 or BAC-R-UL49 DNA but not with BAC-ΔUL49 DNA. Identical results were obtained by hybridizing viral DNA extracted from cells infected by wild-type virus or the ΔUL49 mutant (data not shown).
FIG. 2.
Verification of the structure of BAC-ΔUL49 DNA. DNAs (10 μg) from BAC-HSV-1, BAC-UL49R, and BAC-ΔUL49, purified from RR1 bacterial cells, were separately digested with EcoRV. DNA was separated by agarose gel electrophoresis, and the blots were analyzed by hybridization with a biotinylated probe encompassing the amino-terminal region of UL49. (A) Ethidium bromide (EtBr) staining; (B) Southern blotting.
Growth properties of the ΔUL49-V1 mutant virus.
Two series of experiments were done to characterize the growth properties of the ΔUL49-V1 mutant virus. In the first, replicate cultures of Vero, HEp-2, and HeLa cells were exposed to 1 PFU/cell of ΔUL49-V1 virus. At different times after infection, as indicated in Table 1, the cells were harvested and the virus yield was titered on Vero cells. The fundamental conclusion of these titrations was that the yields of the mutant virus differ significantly from those of the wild-type virus. In particular, we noted that in both Vero and HeLa cells and, to a lesser extent, in HEp-2 cells, the amounts of mutant virus detected at 12 h after infection were lower than those of wild-type virus. This difference was still evident with the passing of time, i.e., at 24 h and 48 h after infection, in HeLa and HEp-2 cells. In contrast, in Vero cells, the yields of the mutant viruses at the later time points were similar to those of wild-type HSV-1 (Table 1).
TABLE 1.
Virus yieldsa in different cell lines infected with wild-type HSV-1 or ΔUL49-V1
| Cell line | Virus | Yield at the following time (h) after infection:
|
||||
|---|---|---|---|---|---|---|
| 3 | 12 | 18 | 24 | 48 | ||
| Vero | HSV-1(F) | 2.0 × 103 | 1.2 × 107 | 2.0 × 108 | 2.2 × 107 | 2.0 × 107 |
| ΔUL49-V1 | 6.0 × 102 | 5.7 × 105 | 5.6 × 107 | 1.4 × 107 | 1.2 × 107 | |
| HEp-2 | HSV-1 | 8.4 × 104 | 7.3 × 106 | 4.7 × 107 | 1.0 × 108 | 2.0 × 108 |
| ΔUL49-V1 | 1.2 × 104 | 3.6 × 105 | 1.4 × 106 | 3.1 × 106 | 1.0 × 107 | |
| HeLa | HSV-1 | 2.0 × 103 | 8.5 × 105 | 7.5 × 106 | 1.3 × 107 | 1.2 × 107 |
| ΔUL49-V1 | 2.0 × 103 | 8.7 × 104 | 5.6 × 105 | 1.3 × 106 | 1.7 × 106 | |
PFUs were determined by a plaque assay on Vero cell monolayers as described in Materials and Methods.
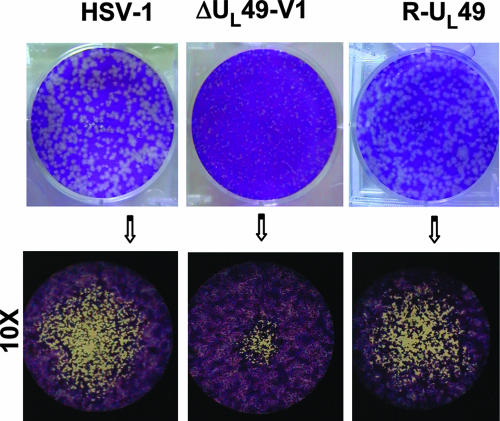
Another noteworthy observation was that the plaque size of the mutant virus was significantly smaller than that of the wild-type virus or the repaired virus (Fig. 3).
FIG. 3.
Growth properties of wild-type HSV-1 and ΔUL49 mutant viruses. Plaque formation in Vero cells infected with wild-type HSV-1, R-UL49, or the ΔUL49 deletion mutant is shown. Following infection, cells were overlaid with a methylcellulose-containing medium for 3 days in duplicate plates for each dilution. Cells were then fixed, stained with crystal violet, and visualized with an inverted microscope (higher magnification, ×10).
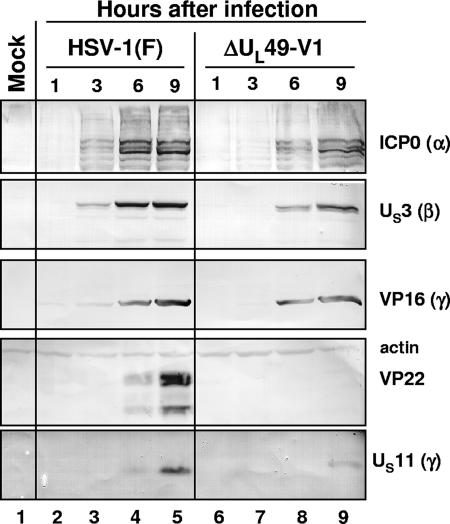
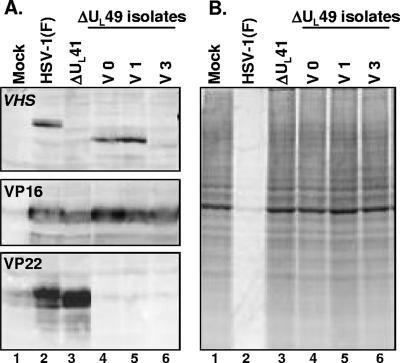
In the second series of experiments, we measured the accumulation of proteins belonging to different kinetic classes. Replicate cultures of Vero cells were exposed to 1 PFU of wild-type or mutant virus per cell. The cells were harvested at 1, 3, 6, or 9 h after infection, solubilized, subjected to electrophoresis in denaturing gels, and reacted with antibodies to ICP0, US3 protein kinase, VP16, VP22, US11, and actin as described in Materials and Methods. The results shown in Fig. 4 indicated the following: (i) as expected, the cells infected with the mutant failed to accumulate VP22 protein (lanes 6 to 9); (ii) the accumulation of ICP0, US3, VP16, and US11 in lysates of mutant-virus-infected cells lagged by at least 3 h the accumulation of the corresponding protein in wild-type-virus-infected cells (compare lane 7 with lane 3).
FIG. 4.
Viral protein synthesis in Vero cells infected with the ΔUL49 mutant virus. Confluent cell monolayers were either mock infected (lane 1) or infected with 10 PFU/cell of either HSV-1(F) (lanes 2 to 5) or the ΔUL49 mutant virus (isolate V1) (lanes 6 to 9). The cells were harvested at the indicated times and processed as described in Materials and Methods. Equal amounts of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against representative α (ICP0), β (US3), and γ (γ1, VP16 and VP22; γ2, US11) proteins. An anti-actin antibody was used as a control.
All independently isolated ΔUL49 mutants fail to express functional vhs protein.
The impetus for the next series of experiments stemmed from two observations. First, the studies described above showed a delay in the accumulation of viral protein in infected cells—a phenotype reminiscent of that of vhs minus mutants. Second, in parallel experiments reported elsewhere, we noted that the accumulation of vhs protein required the presence of VP22 and VP16 (52). The failure of accumulation of vhs protein in the absence of VP22 or VP16 was most likely the result of the high toxicity of vhs protein, inasmuch as a mutated protein lacking RNase activity accumulated even in the absence of VP16 or VP22. To test the hypothesis that the ΔUL49 mutants are also defective in the UL41 gene, four series of experiment were conducted.
In the first, we tested whether the ΔUL49-V1, -V0, and -V3 mutant viruses expressed vhs protein and whether it was active. Specifically, confluent cell monolayers were either mock infected or infected with 10 PFU of HSV-1(F), ΔUL41, or ΔUL49 mutant virus isolates per cell. The cells were harvested 18 h after infection and processed as described in Materials and Methods. Equal amounts of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against vhs, VP16, and VP22. The key finding shown in Fig. 5A is that the vhs protein did not appear to accumulate in cells infected with the V3 mutant (lane 6), whereas in lysates of both the V1 and V0 mutants, the accumulated vhs protein migrated faster than the wild-type protein (compare lanes 4 and 5 with lane 2).
FIG. 5.
vhs accumulation and shutoff of protein synthesis in Vero cells infected with ΔUL49 mutant isolates. (A) Confluent cell monolayers were either mock infected (lane 1) or infected with 10 PFU per cell of HSV-1(F) (lane 2), ΔUL41 (lane 3), or ΔUL49 mutant virus isolates (lanes 4 to 6). The cells were harvested 18 h after infection and processed as described in Materials and Methods. Equal amounts of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against vhs, VP16, and VP22. (B) Virion host shutoff activity. Replicate 25-cm2 cultures of Vero cells were either mock infected (lane 1) or infected with 20 PFU per cell of HSV-1(F) (lane 2), ΔUL41 (lane 3), or ΔUL49 mutant virus isolates (lanes 4 to 6) in the presence of actinomycin D (10 μg/ml) and then incubated at 37°C. At 3 h after exposure to the virus, the cells were labeled for 1 h with [35S]methionine; then they were harvested, solubilized, resolved on a 12% denaturing polyacrylamide gel, dried, and subjected to autoradiography.
In the second series of experiments, replicate 25-cm2 flask cultures of Vero cells were either mock infected or exposed to 20 PFU of HSV-1(F), ΔUL41, or ΔUL49 mutant virus isolates per cell in the presence of actinomycin D (10 μg/ml) and then incubated at 37°C. At 3 h after exposure to virus, the cells were labeled for 1 h with [35S]methionine; then they were harvested, solubilized, and resolved on a 12.5% denaturing polyacrylamide gel. The gel was then dried and subjected to autoradiography. This is a classic test of vhs activity, and as shown in Fig. 5B, vhs was active in cells infected with the wild-type virus (lane 2) but not in cells infected with any of the three ΔUL49 mutants tested (lanes 4 to 6) or in cells infected with the ΔUL41 mutant virus (lane 3).
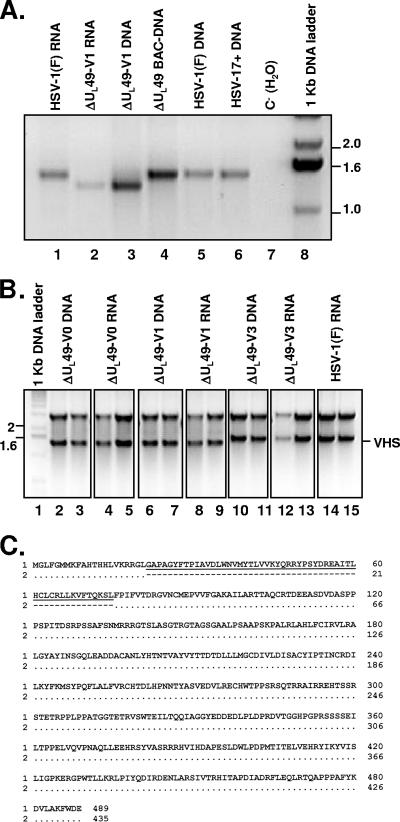
The objective of the third series of experiments was to test the integrity of the UL41 ORF in the BAC-ΔUL49 DNA clone and in the viruses isolated by transfection of BAC DNAs. In this series of experiments, the UL41 ORF was first amplified from either RNA or DNA purified from ΔUL49-V1-infected cells and then compared to those obtained from RNA and DNA extracted from cells infected by wild-type virus (either strain F or strain 17+) as well as to BAC-ΔUL49 plasmid DNA. The results shown in Fig. 6A were as follows. The UL41 ORF amplified from the BAC-ΔUL49 plasmid DNA (lane 4) was identical to those recovered from cells infected with wild-type virus (lanes 1, 5, and 6), and sequence analysis later confirmed that indeed the BAC-ΔUL49 DNA contained a wild-type UL41 ORF. However, the UL41 ORF amplified from either DNA or RNA purified from Vero cells infected with the ΔUL49-V1 isolate appeared to be shorter. The UL41 ORFs of isolates V0 and V3 were also amplified in parallel, and the PCR products were cloned into a T-vector (Fig. 6B, lanes 2 to 5 and 10 to 13, respectively). At least four clones from each sample were sequenced. As shown in Fig. 6C, the UL41 ORF in isolate V1, compared to that amplified from BAC-ΔUL49 plasmid DNA, lacked a 162-bp segment close to the N terminus, resulting in a protein product with an in-frame deletion of 54 codons. The V0 UL41 ORF contained an identical deletion (data not shown). In contrast, the UL41 ORF from isolate V3, even though it appeared to be full length (Fig. 6B, lanes 10 to 13), contained a deletion of 2 nucleotides toward the 3′ end that resulted in a frame shift and early stop codon formation. However, based on the predicted amino acid sequence, the 414-amino-acid protein product should share the first 286 amino acids with wild-type vhs protein. We conclude that all of the independently derived viable ΔUL49 mutant viruses isolated in the course of this study failed to express an intact, functional vhs protein. Furthermore, analyses of the UL41 ORF indicate that the isolates exhibited at least two different mutations.
FIG. 6.
Expression of the vhs ORF in Vero cells infected with ΔUL49 deletion mutant viruses. (A) The UL41 ORF was amplified from either RNA (lane 2) or DNA (lane 3) purified from ΔUL49-V1-infected cells and was compared to the UL41 ORF obtained from RNA (lane 1) or DNA (lane 5) extracted from cells infected by HSV-1(F) or from DNA from HSV-17+-infected cells (lane 6). Lane 4, ΔUL49 BAC DNA used as a template; lane 8, 1-kb DNA ladder. PCR products were resolved on a 2% agarose gel and stained with ethidium bromide. The image was acquired with an Eagle Eye II still-video system (Stratagene, La Jolla, CA) and is shown inverted for clarity. (B) The UL41 ORFs of HSV-1(F), ΔUL49-V0, ΔUL49-V1, and ΔUL49-V3 viruses were amplified from DNA and RNA, and the PCR products were cloned into a T-vector. Two different clones for each sample were analyzed by EcoRI restriction. The image of the ethidium bromide-stained agarose gel was acquired and shown as for panel A. The upper band in panel B represents the T vector, and the lower band is the VHS ORF (VHS). DNA was used as a template for lanes 2, 3, 6, 7, 10, and 11; RNA was used as a template for lanes 4, 5, 8, 9, and 12 to 15. Lane 1, 1-kb DNA ladder; lanes 2 to 13, RNA and DNA obtained from Vero cells infected with either ΔUL49-V0 (lanes 2 to 5), ΔUL49-V1 (lanes 6 to 9), or ΔUL49-V3 (lanes 10 to 13); lanes 14 and 15, RNA obtained from Vero cells infected with HSV-1(F). (C) Predicted vhs protein sequence from the consensus nucleotide sequence generated from the ΔUL49-V1 virus (sequence 2) compared to that amplified from BAC-ΔUL49 DNA (sequence 1). A deletion of a 162-bp segment close to the N terminus results in an in-frame deletion of 54 amino acids (amino acids 22 to 75).
Virions derived from transfected BAC-UL49R DNA contain a full-length vhs protein.
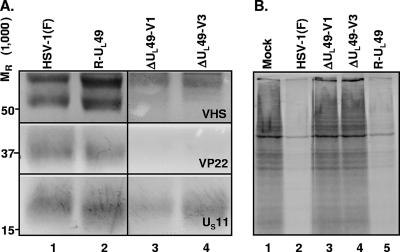
In the studies described above, we noted that all of the progeny derived from independent transfections of BAC-ΔUL49 DNA either failed to express the vhs protein or encoded a truncated form. Even though the BAC-ΔUL49 DNA encoded an intact UL41 ORF (Fig. 6A, lane 4, and C), the question arose whether the UL41 ORF contained in the transfected DNA was defective or whether the only virus that could replicate was that which spontaneously mutated in the course of transfection. The experimental design of our studies included the rescue of the UL49 gene in the BAC-ΔUL49 DNA. The rationale was that if the defect occurred in the course of the manipulation of the BAC-HSV-1 DNA, it would be conserved in the repaired virus. Hence the expression of an intact UL41 ORF by the repaired virus would signify that the defects in the UL41 genes in viral progeny of BAC-ΔUL49 DNA resulted after transfection of the viral DNAs into mammalian cells. In this experiment, purified virions prepared from Vero cells that had been infected with either wild-type virus (HSV-1), the virus derived from transfection of BAC-UL49R DNA (R-UL49), or ΔUL49 isolate V1 or V3 were solubilized, and 10 μg of viral proteins was electrophoretically separated on a denaturing gel, transferred to a nitrocellulose sheet, and probed with polyclonal antibodies to the VP22 and vhs proteins or with a monoclonal antibody to the US11 protein. As shown in Fig. 7, the vhs band contained in the R-UL49 virions could not be differentiated from that contained in wild-type virions (Fig. 7A, compare lanes 1 and 2). The higher-molecular-weight band present in all the samples (Fig. 7A, top, lanes 1 to 4) is a result of cross-reactivity of the anti-vhs polyclonal antiserum. Furthermore, the vhs in R-UL49 virions was active, inasmuch as the protein synthesis shutoff observed in cells infected by R-UL49 (Fig. 7B, lane 5) was identical to that caused by wild-type HSV-1 (lane 2). We conclude that the BAC DNAs transfected into cells contained a UL41 gene capable of being expressed and that the mutant selection occurred after transfection.
FIG. 7.
(A) Immunoblot analysis of virion lysates of wild-type HSV-1 and ΔUL49 viruses. Purified virions derived from Vero cells infected with wild-type HSV-1(F) (lane 1), R-UL49 (lane 2), ΔUL49-V1 (lane 3), or ΔUL49-V3 (lane 4) virus at a multiplicity of infection of 5 PFU/cell and collected 24 h after infection were solubilized and processed for blotting. Equal amounts (10 μg) of viral proteins were electrophoretically separated, transferred to a nitrocellulose sheet, and probed with polyclonal antibodies to VP22 and vhs or with a monoclonal antibody to US11, as described in Materials and Methods. (B) Virion host shutoff activity. Replicate 25-cm2 cultures of Vero cells were either mock infected (lane 1) or infected with 20 PFU per cell of HSV-1(F) (lane 2), ΔUL49-V1 (lane 3), ΔUL49-V3 (lane 4), or R-UL49 (lane 5) virus in the presence of actinomycin D (10 μg/ml) and were incubated at 37°C. At 3 h after exposure to the virus, the cells were labeled for 1 h with [35S]methionine; then they were harvested, solubilized, resolved on a 12% denaturing polyacrylamide gel, dried, and subjected to autoradiography. Lane 1, mock-infected control.
DISCUSSION
The initial objective of the studies described in this report was to produce a ΔUL49 mutant in the genetic background of the viruses used in our studies. The UL49 gene is not an essential gene for virus replication in cell culture systems in vitro (e.g., Vero or HEp-2 cells), and indeed mutants that either lack the UL49 gene or express a mutated gene whose products are not packaged into virions have been reported (7, 14, 34). The ΔUL49 mutant characterized in detail in the studies reported here shares characteristics with other ΔUL49 mutants. Specifically, viral gene expression is delayed and the yields are lower than those of the wild-type virus. The unexpected finding, however, was the observation that these mutants do not exhibit vhs activity. Moreover, analyses of the cell lysates for vhs protein revealed that cells infected with one mutant (V3) do not appear to accumulate vhs protein, whereas cells infected with either of two other, independently derived virus isolates (V1 and V0) accumulate truncated vhs proteins. Whereas V3 encodes a UL41 ORF with a deletion of 2 nucleotides and consequent frame shifting, the ORFs contained in the V0 and V1 isolates exhibit an in-frame deletion of 54 codons.
One hypothesis that could explain our results is that the UL41 ORF was spontaneously mutated in the course of the manipulation of the BAC DNA in order to produce the mutant. If this were the case, it would be expected that the mutation would also be present in the BAC DNA in which the UL49 ORF was restored. The results presented in this report show that the R-UL49 mutant virus expresses a full-length vhs protein, unlike the V0, V1, and V3 mutant viruses derived from the parental BAC-ΔUL49 DNA, and therefore this hypothesis is not tenable.
A alternative hypothesis is that the viable, replication-competent ΔUL49 viruses were selected specifically on the basis of a loss of vhs activity and therefore that ΔUL49 mutants exhibiting vhs activity are replication incompetent. This hypothesis is supported by three series of observations. (i) As noted in Results, in the course of analyzing the products of transfection, we noted the presence of foci of cells exhibiting cytopathic effects, but these did not yield replication-competent viruses upon passage. (ii) As reported above, the products of independent transfections exhibited at least two different mutations that precluded the expression of the vhs activity. In addition to the three Vero cell isolates, we also isolated a replication-competent virus from transfected HEp-2 cells, which also failed to express vhs activity, although the basis of the failure is not known. The data suggest that the genetic basis of the failure to express vhs varies, rendering it highly unlikely that these viruses are derived from a single mutation of the BAC DNA. (iii) In parallel studies carried out in our laboratories and reported elsewhere (52), we noted that VP22 interacts with vhs, but only in the presence of VP16. Moreover, expression of vhs protein in transfected cells required the expression of both VP16 and VP22. In contrast, a mutated form of vhs in which three amino acids were substituted to inactivate the RNase activity was readily expressed in the absence of VP22 or VP16. The necessary conclusion is that both VP16 and VP22 are necessary to neutralize vhs. The implication of the present studies is that vhs activity in the absence of VP22 is lethal.
vhs minus mutants fail to replicate in animal systems, indicating that in natural infections vhs plays an essential role (18, 46, 48). At the same time, the RNase activity—the only activity identified to date for this protein—poses obvious risks to the virus in that it could degrade key viral mRNAs. It is not surprising that the virus has evolved means to constrain the activity of this protein.
Acknowledgments
We thank Weiran Zhang for excellent technical support.
These studies were aided by grants from the Italian Ministry of University and Research (Research Projects of National Interest) and the University of Messina (PRA) and by National Cancer Institute grants CA87661, CA83939, CA71933, CA78766, and CA88860.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Aints, A., M. S. Dilber, and C. I. Smith. 1999. Intercellular spread of GFP-VP22. J. Gene Med. 1:275-279. [DOI] [PubMed] [Google Scholar]
- 2.Beerens, A. M., M. G. Rots, E. F. De Vries, and H. J. Haisma. 2007. Fusion of herpes simplex virus thymidine kinase to VP22 does not result in intercellular trafficking of the protein. Int. J. Mol. Med. 19:841-849. [DOI] [PubMed] [Google Scholar]
- 3.Blaho, J. A., C. Mitchell, and B. Roizman. 1994. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J. Biol. Chem. 269:17401-17410. [PubMed] [Google Scholar]
- 4.Brewis, N., A. Phelan, J. Webb, J. Drew, G. Elliott, and P. O'Hare. 2000. Evaluation of VP22 spread in tissue culture. J. Virol. 74:1051-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brignati, M. J., J. S. Loomis, J. W. Wills, and R. J. Courtney. 2003. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 77:4888-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi, J. H. I., C. A. Harley, A. Mukhopadhyay, and D. W. Wilson. 2005. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 86:253-261. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, C., J. H. Lavail, A. N. Tauscher, E. G. Wills, J. A. Blaho, and J. D. Baines. 2006. Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J. Virol. 80:8664-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, G., and P. O'Hare. 1998. Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J. Virol. 72:6448-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott, G., and P. O'Hare. 1999. Intercellular trafficking of VP22-GFP fusion proteins. Gene Ther. 6:149-151. [DOI] [PubMed] [Google Scholar]
- 11.Elliott, G., D. O'Reilly, and P. O'Hare. 1996. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology 226:140-145. [DOI] [PubMed] [Google Scholar]
- 12.Elliott, G., D. O'Reilly, and P. O'Hare. 1999. Identification of phosphorylation sites within the herpes simplex virus tegument protein VP22. J. Virol. 73:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a macromolecular assembly in coexpressing cells. J. Virol. 69:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott, G., W. Hafezi, A. Whiteley, and E. Bernard. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J. Virol. 79:9735-9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esclatine, A., B. Taddeo, and B. Roizman. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. USA 101:18165-18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 101:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang, B., B. Xu, P. Koch, and J. A. Roth. 1998. Intercellular trafficking of VP22-GFP fusion proteins is not observed in cultured mammalian cells. Gene Ther. 5:1420-1424. [DOI] [PubMed] [Google Scholar]
- 18.Geiss, B. J., T. J. Smith, D. A. Leib, and L. A. Morrison. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 74:11137-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiss, B. J., J. E. Tavis, L. M. Metzger, D. A. Leib, and L. A. Morrison. 2001. Temporal regulation of herpes simplex virus type 2 VP22 expression and phosphorylation. J. Virol. 75:10721-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green, K. L., T. D. Southgate, K. Mulryan, L. J. Fairbairn, P. L. Stern, and K. Gaston. 2006. Diffusible VP22-E2 protein kills bystander cells and offers a route for cervical cancer gene therapy. Hum. Gene Ther. 17:147-157. [DOI] [PubMed] [Google Scholar]
- 21.Hafezi, W., E. Bernard, R. Cook, and G. Elliott. 2005. Herpes simplex virus tegument protein VP22 contains an internal VP16 interaction domain and a C-terminal domain that are both required for VP22 assembly into the virus particle. J. Virol. 79:13082-13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakkarainen, T., T. Wahlfors, O. Merilainen, S. Loimas, A. Hemminki, and J. Wahlfors. 2005. VP22 does not significantly enhance enzyme prodrug cancer gene therapy as a part of a VP22-HSVTk-GFP triple fusion construct. J. Gene Med. 7:898-907. [DOI] [PubMed] [Google Scholar]
- 23.Horsburgh, B. C., M. M. Hubinette, D. Qiang, M. L. MacDonald, and F. Tufaro. 1999. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 6:922-930. [DOI] [PubMed] [Google Scholar]
- 24.Kotsakis, A., L. E. Pomeranz, A. Blouin, and J. A. Blaho. 2001. Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of VP22, a major virion tegument protein. J. Virol. 75:8697-8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam, Q., C. A. Smibert, K. E. Koop, C. Lavery, J. P. Capone, S. P. Weinheimer, and J. R. Smiley. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15:2575-2581. [PMC free article] [PubMed] [Google Scholar]
- 28.Lemken, M. L., C. Wolf, W. A. Wybranietz, U. Schmidt, I. Smirnow, H. J. Buhring, A. F. Mack, U. M. Lauer, and M. Bitzer. 2007. Evidence for intercellular trafficking of VP22 in living cells. Mol. Ther. 15:310-319. [DOI] [PubMed] [Google Scholar]
- 29.Martin, A., P. O'Hare, J. McLauchlan, and G. Elliott. 2002. Herpes simplex virus tegument protein VP22 contains overlapping domains for cytoplasmic localization, microtubule interaction, and chromatin binding. J. Virol. 76:4961-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morse, L. S., T. G. Buchman, B. Roizman, and P. A. Schaffer. 1977. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 × HSV-2) recombinants. J. Virol. 24:231-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouzakitis, G., J. McLauchlan, C. Barreca, L. Kueltzo, and P. O'Hare. 2005. Characterization of VP22 in herpes simplex virus-infected cells. J. Virol. 79:12185-12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, A. L., and S. L. Murphy. 1999. Catch VP22: the hitch hiker's ride to gene therapy? Gene Ther. 6:4-5. [DOI] [PubMed] [Google Scholar]
- 34.Pomeranz, L. E., and J. A. Blaho. 2000. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length VP22. J. Virol. 74:10041-10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon, A. P. W., and B. Roizman. 1997. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant γ134.5 genes of herpes simplex virus 1. Virology 229:98-105. [DOI] [PubMed] [Google Scholar]
- 36.Potel, C., and G. Elliott. 2005. Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0. J. Virol. 79:14057-14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy, V., J. Qiao, P. de Campos-Lima, and M. Caruso. 2005. Direct evidence for the absence of intercellular trafficking of VP22 fused to GFP or to the herpes simplex virus thymidine kinase. Gene Ther. 12:169-176. [DOI] [PubMed] [Google Scholar]
- 39.Schmelter, J., J. Knez, J. R. Smiley, and J. P. Capone. 1996. Identification and characterization of a small modular domain in the herpes simplex virus host shutoff protein sufficient for interaction with VP16. J. Virol. 70:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciortino, M. T., M. Suzuki, B. Taddeo, and B. Roizman. 2001. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75:8105-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sciortino, M. T., B. Taddeo, A. P. Poon, A. Mastino, and B. Roizman. 2002. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc. Natl. Acad. Sci. USA 99:8318-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenk, T. 2002. Might a vanguard of mRNAs prepare cells for the arrival of herpes simplex virus? Proc. Natl. Acad. Sci. USA 99:8465-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein VHS. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 70:5665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzutani, T., M. Nagamine, T. Shibaki, M. Ogasawara, I. Yoshida, T. Daikoku, Y. Nishiyama, and M. Azuma. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 81:1763-1771. [DOI] [PubMed] [Google Scholar]
- 49.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 77:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taddeo, B., and B. Roizman. 2006. The virion host shutoff protein UL41 of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 80:9341-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taddeo, B., W. Zhang, and B. Roizman. 2006. The UL41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 103:2827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taddeo, B., M. T. Sciortino, W. Zhang, and B. Roizman. 2007. Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA. Proc. Natl. Acad. Sci. USA 104:12163-12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Leeuwen, H., M. Okuwaki, R. Hong, D. Chakravarti, K. Nagata, and P. O'Hare. 2003. Herpes simplex virus type 1 tegument protein VP22 interacts with TAF-1 proteins and inhibits nucleosome assembly but not regulation of histone acetylation by INHAT. J. Gen. Virol. 84:2501-2510. [DOI] [PubMed] [Google Scholar]
- 54.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 79:9566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wybranietz, W. A., F. Prinz, M. Spiegel, A. Schenk, M. Bitzer, M. Gregor, and U. M. Lauer. 1999. Quantification of VP22-GFP spread by direct fluorescence in 15 commonly used cell lines. J. Gene Med. 1:265-274. [DOI] [PubMed] [Google Scholar]
- 56.Ye, G. J., and B. Roizman. 2000. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proc. Natl. Acad. Sci. USA 97:11002-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yedowitz, J. C., A. Kotsakis, E. F. M. Schlegel, and J. A. Blaho. 2005. Nuclear localizations of the herpes simplex virus type 1 tegument proteins VP13/14, vhs, and VP16 precede VP22-dependent microtubule reorganization and VP22 nuclear import. J. Virol. 79:4730-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zavaglia, D., M. C. Favrot, B. Eymin, C. Tenaud, and J.-L. Coll. 2003. Intercellular trafficking and enhanced in vivo antitumour activity of a non-virally delivered P27-VP22 fusion protein. Gene Ther. 10:314-325. [DOI] [PubMed] [Google Scholar]
- 59.Zavaglia, D., E. H. Lin, M. Guidetti, O. Pluquet, P. Hainaut, M. C. Favrot, and J. L. Coll. 2005. Poor intercellular transport and absence of enhanced antiproliferative activity after non-viral gene transfer of VP22-P53 or P53-VP22 fusions into p53 null cell lines in vitro or in vivo. J. Gene Med. 7:936-944. [DOI] [PubMed] [Google Scholar]
- 60.Zhou, G., G. J. Ye, W. Debinski, and B. Roizman. 2002. Genetic engineering of a herpes simplex virus 1 vector dependent on the IL13Rα2 receptor for entry into cells: interaction of glycoprotein D with its receptors is independent of the fusion of the envelope and the plasma membrane. Proc. Natl. Acad. Sci. USA 99:15124-15129. [DOI] [PMC free article] [PubMed] [Google Scholar]