Abstract
Herpesviruses use gB and gH-gL glycoproteins to execute fusion. Other virus-specific glycoproteins are required for receptor binding and fusion activation. The human cytomegalovirus (HCMV) UL131-128 proteins are essential for the infection of leukocytes, endothelial cells (ECs), and many epithelial cell lines. Here we show that UL131-128 play a role in a chain of events involving gB and gH during HCMV entry into ECs. An HCMV strain bearing the wild-type (wt) UL131-128 locus exhibited a gB transition from a protease-resistant to protease-sensitive form, a conformational change that was suppressed by a thiourea inhibitor of fusion (WY1768); in contrast, gH was susceptible to proteolysis throughout entry. Moreover, gB and gH transiently interacted, and a lipid mixing assay showed that the wt strain had carried out fusion by 60 min postinfection. However, these events were greatly altered when UL131-128-defective strains were used for infection or when there was an excess of soluble pUL128 during wt infection: the gB conformational change became WY1768 resistant, the gB-gH complex was no longer observed, and fusion was prevented. Both gB and gH in this case showed late protease resistance, related to their endocytic uptake. Our data point to the involvement of UL131-128 proteins in driving gB through a WY1768-sensitive fold transition, thus promoting a short-lived gB-gH complex and fusion; they also suggest that HCMV fuses with the EC plasma membrane and that endocytosis ensues only when the virus cannot trigger UL131-128-dependent steps.
Human cytomegalovirus (HCMV), the prototypical member of the Betaherpesvirinae subfamily, is the leading infectious cause of congenital defects, a major opportunist in transplant recipients and immunocompromised patients, and a suspected cofactor for cardiovascular diseases, systemic sclerosis, and gastrointestinal cancer (7, 27, 29, 41, 42, 49). Clinical isolates of HCMV infect various cell types in vitro, including endothelial cells (ECs), thus replicating the broad cell type tropism observed in HCMV infections of immunocompromised subjects (EC-tropic strains). However, laboratory propagation in fibroblasts (FBs) restricts viral tropism (FB-tropic strains), and mutations causing the loss of tropism for ECs, epithelial cells, polymorphonuclear leukocytes, and dendritic cells (DCs) have been mapped to the contiguous UL131, UL130, and UL128 open reading frames (ORFs) (13, 25, 18, 56).
There is considerable indirect evidence indicating that UL131-128 proteins act as regulators of virus-cell fusion: (i) FB-tropic strains fail to transfer tegument pp65-UL83 protein to EC nuclei (18), which suggests that infection stops before virus uncoating; (ii) an HCMV deletion mutant lacking the UL128-UL150 ORFs infects retinal pigment epithelial cells after exposure to polyethylene glycol in order to force fusion between viral and cellular membranes (50); and (iii) EC-tropic strains are syncytiogenic at a high multiplicity of infection (18, 56).
The membrane-spanning glycoproteins gB and gH and the soluble gL form the conserved core of herpesviral fusion machinery (54). The solved structure of herpes simplex virus type 1 (HSV-1) gB resembles that of an established fusion protein, vesicular stomatitis virus glycoprotein G (28), and certain gB mutations confer syncytial phenotypes (16). While gL is an essential chaperone of gH, the gH homologs include elements typical of class I fusion proteins, such as heptad repeats (HRs) and a fusion peptide (20-22, 40, 54). The gH-gL proteins of varicella-zoster virus (10) and HCMV (37) induce syncytia when transfected into selected cells, in the absence of other viral proteins. A putative HR has also been noted in HCMV gB (40). The entry of HCMV and HSV-1 is inhibited by peptides spanning the relevant HRs (14, 17, 21, 40).
Of the three major glycoprotein complexes of the HCMV envelope (24), the gCI complex coincides with gB homo-oligomers (11, 23); the gCII complex is made up of gM and gN glycoproteins, which contribute to the initial attachment of HCMV particles to cell surface heparan sulfate proteoglycans (HSPG) (34, 35, 43, 54); and the gCIII complex contains gH-gL heterodimers as fixed components (12, 36). In FB-tropic strains, gCIII also includes glycoprotein gO (32, 39), which, although variable in sequence across strains (44, 47), is critical for FB infection (30). Importantly, in EC-tropic strains, two of the UL131-128 locus proteins (gpUL130 and pUL128) are incorporated into HCMV virions (45, 57) and interact with gH-gL in alternative to gO (57). UL131 encodes a soluble glycoprotein that is retained intracellularly when singly expressed (our unpublished results). One of two studies failed (57) but the other succeeded (1) in detecting gpUL131 bound to gH-gL in cells and virions. A further function of gpUL131 might be that of chaperoning the other products of the locus (57). EC-tropic HCMV particles therefore expose two gCIII variants: one includes gO, and the other includes gpUL130, pUL128, and (perhaps) gpUL131.
On the basis of the above, it is possible that the UL131-128 locus acts by supplying functions that are essential for the deployment of fusion in permissive cells. However, earlier work identified a postpenetrational arrest for FB-tropic strains infecting ECs (5, 6, 51, 52), and polyethylene glycol treatment induced capsid internalization, but not infection, in ECs (50). Moreover, it has been reported that the entry of an EC-tropic isolate into ECs and epithelial cells is inhibited by lysosomotropic agents, which seemed to indicate that HCMV uses endocytic uptake into a low-pH compartment to trigger fusion in such cells (50); furthermore, this finding raises the possibility that UL131-128 proteins play a role in targeting to the endosomal fusion compartment.
In this study, we show that HCMV entry into human umbilical vein ECs (HUVECs) (a model of ECs) takes place by fusion at the plasma membrane and that mutations in the UL131-128 locus or biochemical interference with locus products arrests the process at a prefusion stage. UL131-128 protein function(s) is required for the transition of gB to a fusion-proficient conformation, which contacts gH to effect fusion. We discuss our results in the light of recent models of how herpesviral glycoproteins mediate fusion activation and execution.
MATERIALS AND METHODS
Cell cultures, viruses, and infections.
Human embryonic lung FBs (HELFs) were cultured in minimal essential medium (Invitrogen), 10% fetal bovine serum (FBS) (Cambrex). HUVECs were cultured in flasks coated with rat tail collagen type I (BD Biosciences) in MCDB-131 medium (Sigma) with 10% FBS, containing 50 μg/ml heparin, 1 μg/ml hydrocortisone, 10 ng/ml epidermal growth factor, and 1 ng/ml fibroblast growth factor b (all from Sigma). HUVECs were used at passage 2 to 5 after isolation.
HCMV clinical isolate VR1814 (48) and the FB-adapted strains Merlin (13), AD169, and Towne were used as EC-tropic and FB-tropic strains, respectively, in our study. Viral preparations were produced and titrated as described previously (45), with modifications. Briefly, only infected cell media were used as virus sources. Viral particles were concentrated by centrifugation (35,000 × g for 55 min at 20°C) and used immediately at multiplicities of infection of 2.8 to 3.2 for synchronized infections. Titers of viral preparations were quantified in parallel to each experiment. The use of freshly prepared virus appeared to be crucial for consistency in infectivity and experimental outcome, possibly because freeze-thaw cycles resulted in preparations with a high proportion of noninfectious, damaged virions. For synchronized infections, prechilled confluent cell monolayers were incubated on ice with viral inocula for 1 h in cold medium. Cells were then washed three times with cold medium containing 200 μg/ml heparin, covered with prewarmed medium, and incubated in a 5% CO2 incubator at 37°C for the indicated times.
Lipid mixing assay.
A total of 1 × 105 viral infectious units, resuspended in 500 μl of Dulbecco's modified Eagle medium without phenol red (Invitrogen), were mixed 1:1 with 10 μg/ml of octadecyl rhodamine B (R18; Invitrogen) in the same medium and incubated in the dark at room temperature for 1 h. Viral particles were separated from unincorporated R18 by centrifugation through a preformed 40, 55, and 70% sorbitol gradient and resuspended in the appropriate infection medium. For synchronized infections R18-labeled virus was absorbed to monolayers and the incubation temperature shifted to 37°C for 60 min. Cells were then transferred on ice and photographed under an Olympus IX71 inverted epifluorescence microscope equipped with a cooled charge-coupled device camera (Roper Scientific Photometrics) controlled by the Metamorph software (Universal Imaging Corporation). Titers of R18-labeled viral samples were evaluated in parallel.
Cloning, expression, and purification of rpUL128.
VR1814 ORF UL128 nucleotides 80 to 516 were amplified from a cDNA template (25) using Pfu polymerase (Promega), with primers 5′-CGGGTGGATCCAGAAGAATGTTGCGAATTCATAAA-3′ (forward) and 5′-CCAAGCTTCAATTGTCACTGCAGCATATAGCCCATTT-3′ (reverse) (the BamHI and MfeI cloning sites are underlined). The amplimer was inserted into pTrcNHisB expression vector (Invitrogen). The resulting recombinant pUL128 (rpUL128) lacks the signal sequence (amino acids 1 to 27), which is replaced by vector polyhistidine and an epitope tag. rpUL128 was expressed in Escherichia coli strain BL21 and purified on Ni-nitrilotriacetic acid resin (QIAGEN). Peak fractions were pooled; dialyzed against 50 mM HEPES (pH 7.0), 1 mM 1,4-dithioerythritol, 1 mM phenylmethylsulfonyl fluoride; and loaded onto a P11 phosphocellulose (Whatman) column equilibrated in the same buffer. The column was eluted with a 0.1 to 0.5 M NaCl gradient, and fractions containing rpUL128 were dialyzed against infection medium without FBS, adjusted to 2% FBS, flash-frozen, and stored at −80°C. Control experiments were performed with the unrelated hepatitis B virus N-terminally His-tagged Pres1-Pres2 protein (urp), expressed, and purified exactly as for rpUL128.
Cell enzyme-linked immunosorbent assay (CELISA).
A total of 3 × 104 cells were seeded in 96 microtiter wells. After 24 h, the cells were treated or mock treated with 5 mIU of heparinase III (Sigma) in DPBS-Ca2+-Mg2+ with 0.01% bovine serum albumin for 1 h in a CO2 incubator. The samples were then washed with cold infection medium and incubated for 1 h on ice with increasing concentrations of rpUL128 in the same medium. Unbound protein was washed out and cells fixed in 3% paraformaldehyde in immunofluorescence (IF) fixation buffer (60 mM PIPES, 30 mM HEPES, 1 mM EGTA, 1 mM MgSO4, pH 7.0). The samples were incubated with anti-epitope tag antibody (monoclonal antibody [MAb] anti-Xpress; Invitrogen) and anti-mouse horseradish peroxidase conjugate diluted in DPBS-Ca2+-Mg2+, 10% FBS. Signals were developed with o-phenylenediamine dihydrochloride (Sigma), and absorbances at 450 nm were measured in a microtiter plate reader.
Inhibition of infection.
The HCMV fusion inhibitor WY1768 was a generous gift of J. D. Bloom (Wyeth Pharmaceuticals, Madison NJ). WY1768 (2 μM) was added to the cells with viral inocula and maintained in subsequent incubations. In infections including rpUL128 or urp, cell monolayers were incubated for 1 h on ice with the indicated concentration of purified protein in cold infection medium. The same concentration was maintained in all subsequent incubations and washes.
Controlled proteolysis.
Confluent HUVEC monolayers were treated for synchronized infections (with additional treatments as stated), shifted to 37°C for the indicated times, and transferred on ice. After three washes with cold DPBS-Ca2+-Mg2+ (Invitrogen), samples were incubated on ice for 30 min with 0.5 mg/ml proteinase K (PrK) (Promega) in the same buffer. An equal volume of cold 3% bovine serum albumin, 0.5 mM phenylmethylsulfonyl fluoride in DPBS was added to stop the digestion. Cells were then lysed in Laemmli sample buffer for Western blotting (WB) assays. Equal loading in all immunoblots was verified with anti-α-tubulin (MAb clone DM1A; Sigma).
Immunoblots, IF, and immunoprecipitations.
Anti-gB and anti-gH MAbs (clones 1.B.223 and 8.F.72, respectively; U.S. Biological) were used. Standard WB analyses were performed. In IF experiments, confluent HUVEC monolayers on collagen-coated glass coverslips were subjected to synchronous infections with the indicated treatments. Next, cells were placed on ice, washed in cold DPBS-Ca2+-Mg2+, fixed with cold 3.7% paraformaldehyde in IF fixation buffer for 20 min, and quenched in 150 mM NH4Cl. Cells were porated or mock porated in PBS, 10% FBS, with or without 0.05% Triton X-100, and stained with anti-gB MAb, followed by fluorescein isothiocyanate-conjugated anti-mouse antibody (Chemicon). Nuclei were counterstained with 200 ng/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS.
For immunoprecipitations, HUVEC monolayers were subjected to synchronous infections. At the indicated times of incubation at 37°C, cells were transferred on ice; lysed in cold 50 mM Tris-Cl (pH 7.5), 300 mM NaCl, 1% Triton X-100, 0.15% Tween 20, and EDTA-free Complete protease inhibitor mix (Roche); and centrifuged at 16,000 × g for 20 min at 4°C. The extracts were precleared by incubation at 4°C for 2 h with an unrelated MAb and GammaBind G Sepharose resin (GE Healthcare Life Sciences) and incubated at 4°C for 16 to 18 h with anti-gB MAb. Immunocomplexes were captured on GammaBind G Sepharose resin for 1 h at 4°C. After three 30-min washes in the same buffer at 4°C, bound proteins were eluted in Laemmli sample buffer and analyzed by WB.
RESULTS
The EC-tropic HCMV envelope fuses with EC membranes, whereas the fusion of UL131-128-defective strains is prevented.
The requirement for UL131-128 proteins in EC, epithelial cell, polymorphonuclear leukocyte, and DC infection was previously established, but their biochemical action remains unknown. In order to investigate whether UL131-128 products are necessary for virion/EC membrane fusion, we directly measured the fusion between the HCMV lipid envelope and EC membranes by labeling the virus with the self-quenching fluorescent lipid probe R18 (31). When HCMV fusion occurs, R18 spreads from the viral envelope to cell membranes, thus giving rise to cell fluorescence (31). The virus was added to the ECs and, in parallel, to HELFs at 4°C to allow attachment. After the removal of the unbound virus, the temperature was increased to 37°C for 1 h to allow the infection to proceed. One EC-tropic (VR1814) and three FB-tropic (the UL131-defective AD169, the UL130-defective Towne, and the UL128-defective Merlin) strains of HCMV were used in the mixing assay. Lipid mixing was observed in the ECs when the EC-tropic virus was used (Fig. 1) but was reduced to near background levels with all three FB-tropic strains (Fig. 1). In contrast, all of the strains led to intense R18 fluorescence in HELFs (Fig. 1).
FIG. 1.
Virion-EC fusion visualization by lipid mixing assay. R18-labeled HCMV particles were absorbed to HELFs or HUVECs in the presence or absence (NT) of 2 μM WY1768, incubated at 37°C for 60 min, and photographed in the red channel of an inverted UV microscope. Magnification, ×10.
The bis-(aryl)thiourea inhibitor WY1768 is a potent inhibitor of HCMV fusion in FBs (3, 33) and retains its activity on VR1814 strain infections of ECs (50% infective dose in ECs, 1.96 ± 0.06 nM [mean ± standard deviation {SD}; n = 4] [data not shown]), suggesting that the affected mechanism is essential for HCMV entry in both cell types. When the lipid mixing assay was performed in the presence of 2 μM WY1768, the VR1814 virus failed to transfer R18 fluorescence to either ECs or HELFs. As expected, the R18-labeled AD169, Towne, and Merlin strains were also prevented from transferring the tracer to HELFs (Fig. 1).
Mixing assay phenotypes show a strict correlation between one strain's ability to transfer R18 to EC and an intact UL131-128 locus. In order to confirm the direct role of UL131-128 products in the assay outcome, we tested the effect of soluble rpUL128. Earlier studies have shown that some soluble versions of herpesviral glycoproteins block entry by tying up the cognate receptors or interfering with the virion counterpart (2, 38). Purified rpUL128 (Fig. 2A) saturably binds to the surface of ECs, while the same recombinant protein adheres to the HELF surface to a lesser extent and its binding is not saturated at the attainable rpUL128 concentrations. Moreover, rpUL128 binding to ECs was found to be insensitive to prior cell treatment with heparitinase III to remove HSPG, while HELF binding was nearly abolished by the same treatment. (Fig. 2B).
FIG. 2.
rpUL128 binds the EC surface in a saturable manner. (A) rpUL128 was purified from soluble E. coli protein by capture on an Ni-nitrilotriacetic acid column and final purification on an ion-exchange (P11) column. Protein identity was verified by WB with a MAb recognizing the rpUL128 N-terminal epitope tag. NI, noninduced cell lysate; I, induced cell lysate. (B) HUVECs (♦) or HELFs (•) were treated ( - - - - ) or mock treated (—) with heparinase III and incubated on ice for 1 h with the indicated concentrations of rpUL128 in culture medium. Cells were then washed three times and assayed by CELISA with antitag (anti-Xpress antibody; Invitrogen). Optical densities at 450 nm (OD450) were plotted (mean ± SD; n = 3).
Furthermore, rpUL128 inhibits VR1814 infection. In fact, when ECs were preincubated with increasing rpUL128 concentrations and the same amounts of protein were maintained during infection, there was a proportional reduction in infected cells, with a more than 80% reduction in immediate-early antigen-positive cells at the highest concentration (Fig. 3A), and a calculated 50% inhibitory concentration of less than 300 nM. The same treatment of HELFs exposed to VR1814 had negligible effects, and an urp subjected to the same purification steps did not inhibit VR1814 infection of either ECs or HELFs (Fig. 3A).
FIG. 3.
rpUL128 inhibits HCMV-EC fusion. (A) HUVECs (♦) or HELFs (▪) were preincubated with either rpUL128 ( —) or urp (- - - - ) and infected with VR1814 virus. At 24 h postinfection, monolayers were stained for immediate-early antigen and positive nuclei plotted as percentages of values for 0 nM samples (mean ± SD; n = 3). (B) Cells, mock treated (NT) or preincubated with 10 μM urp or rpUL128, were exposed to R18-labeled VR1814 particles and treated as for Fig. 1.
Strikingly, in the R18 assay, 10 μM rpUL128 greatly reduced dye transfer to ECs but not to HELFs, whereas the control urp did not affect transfer to either cell host (Fig. 3B).
The successful dye transfer to HELFs from all strains, as well as the observed inhibition of the lipid mixing assays by a chemical inhibitor of HCMV entry, indicates that the R18 data faithfully reflect fusion and that they are not compromised by probe-related virus inactivation or spontaneous probe transfer to cell membranes.
Current theories divide virus-induced membrane fusion into three phases: phase 1 brings the viral envelope and cell membrane into close apposition; phase 2 leads to lipid mixing between the outer membrane leaflets to generate a fusion intermediate (hemifusion); and phase 3 forms and expands a fusion pore, which allows the mixing of virion and cell content (complete fusion) (9). Our data therefore indicate that UL131-128 products are required for HCMV to complete the fusion process in ECs but not in FBs; their absence or inhibition stops the entry process earlier than hemifusion. The data do not rule out any subsequent postentry roles of UL131-128 products, but this issue is beyond the scope of the present study.
EC-tropic HCMV fuses at the plasma membrane, whereas FB-tropic strains are endocytosed.
In order to distinguish two EC entry mechanisms, i.e., fusion at the plasma membrane and fusion in endosomes, we used PrK protection as a measure of endocytic uptake. Glycoproteins in virions that have not entered the cell or from virions that have fused directly with the plasma membrane remain accessible to PrK and are digested. Full-length glycoproteins can be detected only if they are protected from digestion by internalization.
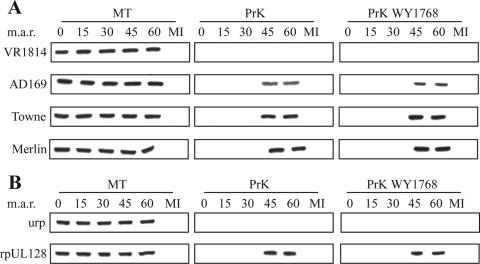
The protection of glycoprotein gH from PrK was measured by WB in replicate experiments comparing the VR1814, AD169, Towne, and Merlin strains. VR1814 gH was found to be 100% sensitive to protease at all of the time points (0 to 60 min of incubation at 37°C) (Fig. 4A). However, the gH of all of the FB-tropic strains showed a different pattern: it was digested by PrK between 0 and 30 min at 37°C but became resistant at the last time points of 45 and 60 min (Fig. 4A). In all cases, mock protease treatment after incubation generated a totally unaffected gH at all time points, which demonstrates that gH degradation by ECs does not contribute to WB signal reduction (Fig. 4A).
FIG. 4.
Viral envelope exposure at EC surface during entry. (A) HUVECs were infected or mock infected (MI) in the presence or absence of 2 μM WY1768. After the indicated times at 37°C (minutes after release [m.a.r.]), cells were treated with PrK or mock treated (MT) and lysates analyzed in WB for gH. (B) HUVECs were preincubated with 10 μM urp or rpUL128, infected with VR1814 particles, and treated as for panel A.
The opposing gH protection patterns of EC-tropic and FB-tropic strains did not change when WY1768 was present during virus adsorption and incubation (Fig. 4A). In contrast, when ECs were preincubated with rpUL128 and the same concentration of recombinant protein was maintained during subsequent washes and incubations, VR1814 changed to the gH protection profile observed for FB-tropic strains (i.e., with substantial resistance after 45 to 60 min of incubation) regardless of the presence or absence of WY1768. In contrast, the urp control did not change the VR1814 gH protection pattern (Fig. 4B).
In non-PrK-untreated controls, the intensity of the VR1814 gH band in the presence of rpUL128 was constant and similar to that obtained with control urp, which makes the additional point that rpUL128 does not inhibit entry by reducing attachment (Fig. 4B).
Surface accessibility of the envelope during entry was further assessed by means of IF. VR1814 or AD169 particles were absorbed at 4°C on ECs and then incubated for 1 h at 37°C. VR1814 strain entry was blocked with WY1768 or rpUL128 in parallel experiments. Aldehyde-fixed cells were stained using an anti-gB MAb as the primary antibody, with or without previous cell poration using a nonionic detergent (Fig. 5). In agreement with the gH proteolysis experiments, it was found that VR1814 gB was accessible to the cognate MAb at the end of entry, generating a specific spotty pattern with and without poration. In contrast, AD169 gB could not be detected on the surface of infected ECs but generated distinctive vesicular staining inside porated cells. Once again, WY1768 treatment did not alter the IF pattern of VR1814, whereas rpUL128 treatment shifted it to the FB-tropic strain intracellular, endosome-like staining.
FIG. 5.
HUVECs were infected with or without additional treatments (10 μM rpUL128 or 2 μM WY1768). At the indicated minutes after release (m.a.r.), monolayers were fixed and incubated with MAb anti-gB with (perm +) or without (perm −) prior permeabilization, followed by anti-mouse-fluorescein isothiocyanate (green fluorescence) and DAPI. Magnification, ×60.
Our data argue that EC-tropic HCMV infects ECs by fusion at the plasma membrane, whereas endocytic uptake reflects the abortive internalization of FB-tropic virus. It seems that defective UL131-128 function lies at the origin of the endocytosis of FB-tropic strains, because a similar effect is produced by adding excess rpUL128 to VR1814 infection. Conversely, the WY1768 chemical inhibitor does not promote VR1814 endocytosis or prevent FB-tropic strain endocytosis. We return to this discrepancy below.
The gB transmembrane subunit switches from a PrK-resistant to a PrK-sensitive form during entry into ECs.
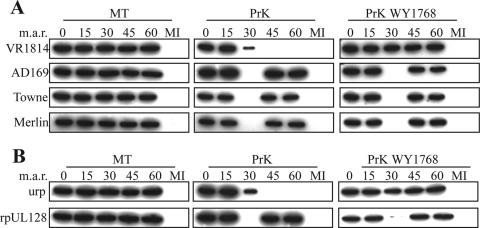
Most herpesviral gB homologs other than HSV-1 gB are matured proteolytically. Each 160-kDa gB monomer in HCMV is cleaved to generate the 116-kDa surface (SU) and the 55-kDa transmembrane (TM) subunits, which remain linked via disulfide bonds (8, 53); the MAb used to detect gB in IF experiments reacts to the TM subunit ectodomain. When the PrK protection analysis was extended to gB, WB with the anti-TM antibody revealed an unexpected finding (Fig. 6A): PrK digestion did not affect the TM on the attached virions of any of the strains (0-min time point). At the beginning of virus entry (0 to 15 min at 37°C), PrK resistance persisted, but from 30 min after the start of entry, VR1814 TM became largely sensitive to proteolysis, and it was completely digested after 45 to 60 min at 37°C. In the FB-tropic strains, TM was digested completely by 30 min and became resistant again 45 to 60 min after the start. Experiments using mock PrK treatments showed that cell-mediated degradation did not contribute to the changes in the TM signal (Fig. 6A).
FIG. 6.
Changes in gB sensitivity to PrK during entry in ECs. (A and B) HUVECs were treated as for Fig. 4A and B, respectively, and analyzed in WB for gB-TM.
Comparison of the gH and gB PrK protection and gB IF data indicates that TM is intrinsically PrK resistant at the start of entry, which could be due to gB oligomerization, masking by the SU subunit or other envelope components, compact TM ectodomain conformation, and/or interaction with cell receptors. However, at 15 to 30 min after the start of entry, TM becomes susceptible to the action of PrK, with slightly accelerated kinetics in FB-tropic strains. Furthermore, while VR1814 TM remains accessible to PrK during the last portion of entry, the TM of FB-tropic strains is protected by endocytosis after 45 min at 37°C.
This picture was altered by WY1768 and rpUL128 in a highly informative manner. The presence of WY1768 totally suppressed VR1814 TM sensitization to PrK (Fig. 6B) but did not change the PrK susceptibility of the TM of FB-tropic strains at 30 min. On the other hand, in the presence of rpUL128, the PrK digestion phenotype of VR1814 TM was changed to that of its FB-tropic virus counterpart: resistant for 15 min at 37°C, susceptible after 30 min, and resistant again after 45 to 60 min.
These data suggest that UL131-128 products are necessary to constrain (although not to activate) a gB transition that leads to an accessible, PrK-sensitive TM subunit. These proteins apparently pilot gB transition through a WY1768-sensitive, PrK-resistant intermediate, and so WY1768 would irreversibly trap VR1814 TM in a PrK-resistant form. This explanation is fully consistent with previous findings that thiourea inhibitors require virus attachment to be effective and that their viral target is gB, because the point mutations that confer WY1768 resistance map within TM pretransmembrane and transmembrane alpha-helical stretches (33).
Our data also suggest that the absence of UL131-128 products, or their perturbation with rpUL128, makes TM switch to a PrK-sensitive form without passage through a WY1768-sensitive stage. This explains why WY1768 effects were epistatically affected by UL131-128 mutations or rpUL128 in all of our PrK protection and IF experiments.
A transient gB-gH interaction is detected in productive EC entry but is prevented by UL131-128 mutations and by the WY1768 and rpUL128 entry inhibitors.
As gH-gL and gB transiently interact at entry in HSV-1 infection (19), we wondered whether gB-gH interaction occurs during the entry of HCMV into ECs and if this is influenced by UL131-128 proteins. ECs infected with VR1814 or the three FB-tropic strains were collected at different times after the start of entry and processed for immunoprecipitation with the anti-TM MAb. The washed immunocomplexes were analyzed by means of WB with the anti-TM and anti-gH MAbs as the primary antibodies; infected cell lysates directly blotted and incubated with anti-gH were used as controls (Fig. 7A). A coprecipitated gH band was detected only with VR1814 infection and only at single time point (50 min) after the start of entry. The effect of adding WY1768 (Fig. 7A) or rpUL128 (Fig. 7B) during VR1814 entry was verified, and both led to the disappearance of coprecipitated gH.
FIG. 7.
gB-gH interaction during entry in ECs. (A) HCMV particles were absorbed on HUVECs in the presence or absence of WY1768. At the indicated minutes after release (m.a.r.), cells were processed for immunoprecipitation (IP) with anti-gB. Immunocomplexes were analyzed in WB for gB-TM and gH. (B) HUVECs were preincubated with urp or rpUL128, exposed to VR1814 particles and analyzed as for panel A. gH input, total cell lysates directly analyzed in WB.
The data indicate that gB and gH form a transient complex late in the HCMV entry process. The gB-gH interaction occurs after gB transition through the WY1768-sensitive stage and is thus prevented by UL131-128 mutations and equally inhibited by rpUL128 and WY1768.
DISCUSSION
Herpesviruses have evolved an especially elaborate strategy of controlling fusion between viral and cell membranes. HSV-1 studies indicate the gD viral glycoprotein as the principal receptor-binding molecule and trigger for fusion activation and indicate gB and gH-gL as fusion executors. The attachment of gD to alternate cell receptors seems to activate a conformational change in gD, which promotes the sequential recruitment of gB and gH-gL in a transient complex that disassembles once fusion is completed (see reference 19 and references therein). A variant model suggests that gH and gL are the main executors of hemifusion, and gB is essential for complete fusion (55).
However, gD is found only in HSV-1 and its closest relatives among Alphaherpesvirinae. The betaherpesvirus human herpesvirus 6A expresses the alternative complexes of gH-gL-gO and gH-gL-gQ1-gQ2. The latter binds the CD46 entry receptor and allows human herpesvirus 6A entry into CD46-positive cell types (see reference 46 and references therein). The gamma-1-herpesvirus Epstein-Barr virus needs the gH-gL-associated glycoprotein gp42 to bind the HLA-II receptor and fuse with B cells, but it infects epithelial cells via a gp42-independent pathway (see reference 60 and references therein).
HCMV virions likewise contain two gH complexes, gH-gL-gO and gH-gL-gpUL130-pUL128 (57); the latter may also contain gpUL131 (1, 57). The UL131-128 products are necessary for the infection of ECs, polymorphonuclear leukocytes, DCs, and many epithelial cell lines (13, 18, 25, 56). This paper describes the function of UL131-128 products in the course of HCMV entry into EC and reports the following observations.
An EC-tropic strain of HCMV (VR1814) binds at 4°C to the EC surface in a heparin-resistant manner, as efficiently as three FB-tropic strains lacking the UL131-128 component on their virions. However, after the temperature is increased to 37°C, only the EC-tropic strain progresses to fusion, which is inhibited by the thiourea inhibitor WY1768 and rpUL128. Interestingly, unlike the case for WY1768, the addition of fusion-inhibitory amounts of rpUL128 to VR1814 infections mimics the genetic lesion in FB-tropic strains by inducing virion endocytosis.
VR1814 entry is accompanied by a conformational change in viral gB, as revealed by the conversion of the gB TM moiety to a protease-sensitive form, which is blocked by WY1768. This inhibitory effect on gB switch is absent in FB-tropic strains and is abolished by rpUL128 in VR1814 infections.
A transient complex containing gB and gH can be immunoprecipitated from ECs exposed to VR1814. This complex did not form in ECs infected with FB-tropic strains or in cells exposed to VR1814 in the presence of either WY1768 or rpUL128.
These findings suggest an outline of HCMV entry into ECs involving the attachment of virions via a mechanism that does not depend on a continued interaction with HSPG. When entry is allowed to initiate, the structure of gB changes so that its TM subunit (which is initially resilient against proteolysis) becomes accessible to protease digestion. Subsequently, gB briefly interacts with gH in a stage that might coincide with fusion execution.
One specific action of UL131-128 proteins seems to be that of driving gB through a distinctive folding intermediate, whose existence is revealed by its sensitivity to WY1768: when UL131-128 function is hampered by genetic causes (null mutations of the locus) or epigenetic causes (an excess of rpUL128), WY1768 loses its ability to trap gB, which proceeds with even accelerated kinetics to a dead-end open conformation. Remarkably, in all strains, all of the TM subunits on the EC-attached virions are switched to the degradable form. As it can be expected that only a limited part of the attached virion envelope touches the cell surface, this en masse conversion suggests the lateral migration of envelope glycoproteins and their convergence at the contact site in a virion-cell adhesion synapsis. Other explanations may entail virion rolling or a “gB conformational wave” that spreads from the site of glycoprotein/cell receptor engagement over the entire envelope surface.
Thiourea inhibitors of HCMV are active against both EC-tropic and FB-tropic strains in FBs (3, 33; this report), and so transition through a WY1768-sensitive gB intermediate seems to be a basic mechanism of HCMV entry. However, in order to be completed properly, this transition requires additional functions provided by UL131-128 proteins in ECs (and presumably the other relevant cell types). Alternatively, gO and UL131-128 proteins may be functionally equivalent factors that are specifically required in subsets of permissive cells. Clarifying this will require further studies.
Two additional questions raised and not answered by our experiments are (i) what triggers gB conversion to the fusion-proficient or abortive open form and (ii) what critical interactions take place between viral proteins and between these and EC receptors. An increasing number of cell surface molecules have been proposed as HCMV entry or signaling receptors interacting with gB or gH (4, 15, 26, 58, 59). Contacts with receptors might trigger gB switch, with the UL131-128 proteins promoting a gB fold that is favorable to gH binding and fusion execution. It is worth noting that a transient association between the gB receptor epidermal growth factor receptor and the gB/gH receptor integrin αVβ3 is promoted by HCMV entry into FBs (58), which parallels the gB-gH interaction reported here.
Our data also provide evidence that of the UL131-128 products, at least pUL128 may be directly involved in receptor binding, because rpUL128 both binds saturably to the surface of ECs and inhibits the fusion process. As rpUL128 does not inhibit virion binding, the putative receptor may be involved in entry but not in attachment. On the other hand, as rpUL128 was present throughout the entry process in our experiments, it is also possible that the inhibition depends on interference with virion pUL128 rather than the inhibitory occupancy of a cell receptor.
Two approaches assaying envelope exposure during VR1814 entry concordantly indicated that gB and gH continuously remain at the cell surface throughout entry. This agrees with the propensity of EC-tropic strains to induce syncytia and evidences the ability of their fusion proteins to work at the cell surface and at neutral pH. We found that endocytosis was actually secondary to impaired UL131-128 function, whereas WY1768 (which inhibits gB opening) did not lead to virion internalization. According to our results, then, endocytosis may be related to the abortive gB transition. However, the potential for the same virus to use alternate receptors and entry mechanisms in a virus strain- and host cell-dependent fashion is a growing theme in the herpesvirus field (54), and our findings do not exclude the possibility that select HCMV strains enter susceptible cell types by an endocytic pathway. Indeed Ryckman et al. (50) recently reported that infection of retinal pigment epithelial cells and immortalized HUVECs with a reconstituted TR isolate bacvirus is inhibited by agents blocking endosome acidification, concluding from this that HCMV infects those cell types using a low-pH-dependent, endocytic pathway. A possible alternative explanation of the data reported by Ryckman et al. is that a cell receptor essential for the EC entry of VR1814 is downregulated as a result of a lysomotropic agent arresting its cell surface recycling.
In summary, our results suggest that UL131-128 proteins are activators of fusion and may be entry receptor ligands (at least pUL128). Our data highlight a sustained cross talk between gCI and gCIII complexes during entry into ECs. We confirm that one basic aspect of the fusion mechanism proposed for HSV-1 (i.e., the recruitment of gB and gH into a transient fusion complex) also exists in HCMV and may be a general trait of herpesviruses. However, measurable gB motion accompanies and is necessary for hemifusion, which differentiates HCMV-EC fusion from, in particular, the HSV-1 sequential activation model (55).
Acknowledgments
We thank F. Baldanti and G. Gerna for helpful discussions of the manuscript and J. D. Bloom for the gift of the WY1768 inhibitor. We also thank F. Rossi and A. Mattevi for technical help with protein expression and purification, G. Maga for assistance with 50% inhibitory concentration calculations, and E. Lesma for assistance with CELISA.
This investigation was funded by MURST PRIN 2005 (protocol N. 2005052977_005) and by Fondazione CARIPLO (grant code 93005).
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. H. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87:2451-2460. [DOI] [PubMed] [Google Scholar]
- 2.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom, J. D., R. G. Dushin, K. J. Curran, F. Donahue, E. B. Norton, E. Terefenko, T. R. Jones, A. A. Ross, B. Feld, S. A. Lang, and M. J. DiGrandi. 2004. Thiourea inhibitors of herpes viruses. 2. N-Benzyl-N′-arylthiourea inhibitors of CMV. Bioorg. Med. Chem. Lett. 14:3401-3406. [DOI] [PubMed] [Google Scholar]
- 4.Boehme, K. W., M. Guerrero, and T. Compton. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094-7102. [DOI] [PubMed] [Google Scholar]
- 5.Bolovan-Fritts, C. A., and J. A. Wiedeman. 2001. Human cytomegalovirus strain Toledo lacks a virus-encoded tropism factor required for infection of aortic endothelial cells. J. Infect. Dis. 184:1252-1261. [DOI] [PubMed] [Google Scholar]
- 6.Bolovan-Fritts, C. A., and J. A. Wiedeman. 2002. Mapping the viral genetic determinants of endothelial cell tropism in human cytomegalovirus. J. Clin. Virol. 25:S97-S109. [DOI] [PubMed] [Google Scholar]
- 7.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 8.Britt, W. J., and L. G. Vugler. 1989. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J. Virol. 63:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernomordik, L. V., and M. M. Kozlov. 2005. Membrane hemifusion: crossing a chasm in two leaps. Cell 123:375-382. [DOI] [PubMed] [Google Scholar]
- 10.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 11.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, and G. L. Smith. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cranage, M. P., G. L. Smith, S. E. Bell, H. Hart, C. Brown, A. T. Bankier, P. Tomlinson, B. G. Barrell, and T. C. Minson. 1988. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J. Virol. 62:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan, A., C. Cunningham, R. D. Hector, A. F. Hassan-Walker, L. Lee, C. Addison, D. J. Dargan, D. J. McGeoch, D. Gatherer, V. C. Emery, P. D. Griffiths, C. Sinzger, B. P. McSharry, G. W. Wilkinson, and A. J. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85:1301-1312. [DOI] [PubMed] [Google Scholar]
- 14.English, E. P., R. S. Chumanov, S. H. Gellman, and T. Compton. 2006. Rational development of beta-peptide inhibitors of human cytomegalovirus entry. J. Biol. Chem. 281:2661-2667. [DOI] [PubMed] [Google Scholar]
- 15.Feire, A. L., Koss, H., and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, T. P., J. M. Melancon, and K. G. Kousoulas. 2001. An alpha-helical domain within the carboxyl terminus of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is associated with cell fusion and resistance to heparin inhibition of cell fusion. Virology 287:8-29. [DOI] [PubMed] [Google Scholar]
- 17.Galdiero, S., M. Vitiello, M. D'Isanto, A. Falanga, C. Collins, K. Raieta, C. Pedone, H. Browne, and M. Galdiero. 2006. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins H and B. J. Gen. Virol. 87:1085-1097. [DOI] [PubMed] [Google Scholar]
- 18.Gerna, G., E. Percivalle, D. Lilleri, L. Lozza, C. Fornara, G. Hahn, F. Baldanti, and M. G. Revello. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86:275-284. [DOI] [PubMed] [Google Scholar]
- 19.Gianni, T., C. Forghieri, and G. Campadelli-Fiume. 2006. The herpesvirus glycoproteins B and H.L are sequentially recruited to the receptor-bound gD to effect membrane fusion at virus entry. Proc. Natl. Acad. Sci. USA 103:14572-14577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus 1 gH, located downstream of the alpha-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianni, T., A. Piccoli, C. Bertucci, and G. Campadelli-Fiume. 2006. Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gretch, D. R., R. C. Gehrz, and M. F. Stinski. 1988. Characterization of a human cytomegalovirus glycoprotein complex (gcI). J. Gen. Virol. 69:1205-1215. [DOI] [PubMed] [Google Scholar]
- 24.Gretch, D. R., B. Kari, L. Rasmussen, R. C. Gehrz, and M. F. Stinski. 1988. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J. Virol. 62:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 27.Harkins, L., A. L. Volk, M. Samanta, I. Mikolaenko, W. J. Britt, K. I. Bland, and C. S. Cobbs. 2002. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557-1563. [DOI] [PubMed] [Google Scholar]
- 28.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 29.Hengel, H., and C. Weber. 2000. Driving cells into atherosclerotic lesions—a deleterious role for viral chemokine receptors? Trends Microbiol. 8:294-296. [DOI] [PubMed] [Google Scholar]
- 30.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoekstra, D., T. de Boer, K. Klappe, and J. Wilschut. 1984. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 23:5675-5681. [DOI] [PubMed] [Google Scholar]
- 32.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, T. R., S. W. Lee, S. V. Johann, V. Razinkov, R. J. Visalli, B. Feld, J. D. Bloom, and J. O'Connell. 2004. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J. Virol. 78:1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kari, B., and R. Gehrz. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kari, B., W. Li, J. Cooper, R. Goertz, and B. Radeke. 1994. The human cytomegalovirus UL100 gene encodes the gC-II glycoproteins recognized by group 2 monoclonal antibodies. J. Gen. Virol. 75:3081-3086. [DOI] [PubMed] [Google Scholar]
- 36.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 37.Kinzler, E. R., and T. Compton. 2005. Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 79:7827-7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirschner, A. N., J. Omerovic, B. Popov, R. Longnecker, and T. S. Jardetzky. 2006. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J. Virol. 80:9444-9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 78:8333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunardi, C., C. Bason, R. Corrocher, and A. Puccetti. 2005. Induction of endothelial cell damage by hCMV molecular mimicry. Trends Immunol. 26:19-24. [DOI] [PubMed] [Google Scholar]
- 42.Lunardi, C., C. Bason, R. Navone, E. Millo, G. Damonte, R. Corrocher, and A. Puccetti. 2000. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat. Med. 6:1183-1186. [DOI] [PubMed] [Google Scholar]
- 43.Mach, M., B. Kropff, P. Dal Monte, and W. J. Britt. 2000. Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 74:11881-11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson, D. A., A. P. Dyer, R. S. Milne, E. Sevilla-Reyes, and U. A. Gompels. 2002. A role for human cytomegalovirus glycoprotein O (gO) in cell fusion and a new hypervariable locus. Virology 293:281-294. [DOI] [PubMed] [Google Scholar]
- 45.Patrone, M., M. Secchi, L. Fiorina, M. Ierardi, G. Milanesi, and A. Gallina. 2005. Human cytomegalovirus UL130 protein promotes endothelial cell infection through a producer cell modification of the virion. J. Virol. 79:8361-8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen, S. M., and P. Hollsberg. 2006. Complexities in human herpesvirus-6A and -6B binding to host cells. Virology 356:1-3. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen, L., A. Geissler, C. Cowan, A. Chase, and M. Winters. 2002. The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates. J. Virol. 76:10841-10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revello, M. G., F. Baldanti, E. Percivalle, A. Sarasini, L. De-Giuli, E. Genini, D. Lilleri, N. Labò, and G. Gerna. 2001. Human cytomegalovirus immediate-early messenger RNA in blood of pregnant women with primary infection and of congenitally infected newborns. J. Gen. Virol. 82:1429-1438.11369888 [Google Scholar]
- 49.Rich, J. D., J. M. Crawford, S. N. Kazanjian, and P. H. Kazanjian. 1992. Discrete gastrointestinal mass lesions caused by cytomegalovirus in patients with AIDS: report of three cases and review. Clin. Infect. Dis. 15:609-614. [DOI] [PubMed] [Google Scholar]
- 50.Ryckman, B. J., M. A. Jarvis, D. D. Drummond, J. A. Nelson, and D. C. Johnson. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80:710-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 52.Slobbe-van Drunen, M. E., A. T. Hendrickx, R. C. Vossen, E. J. Speel, M. C. van Dam-Mieras, and C. A. Bruggeman. 1998. Nuclear import as a barrier to infection of human umbilical vein endothelial cells by human cytomegalovirus strain AD169. Virus Res. 56:149-156. [DOI] [PubMed] [Google Scholar]
- 53.Spaete, R. R., R. M. Thayer, W. S. Probert, F. R. Masiarz, S. H. Chamberlain, L. Rasmussen, T. C. Merigan, and C. Pachl. 1988. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 167:207-225. [DOI] [PubMed] [Google Scholar]
- 54.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, D., and T. Shenk. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 79:10330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 102:18153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 11:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 60.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]