Abstract
Infection with various human papillomaviruses (HPVs) induces cervical cancers. Cell surface heparan sulfates (HS) have been shown to serve as primary attachment receptors, and molecules with structural similarity to cell surface HS, like heparin, function as competitive inhibitors of HPV infection. Here we demonstrate that the N,N′-bisheteryl derivative of dispirotripiperazine, DSTP27, efficiently blocks papillomavirus infection by binding to HS moieties, with 50% inhibitory doses of up to 0.4 μg/ml. In contrast to short-term inhibitory effects of heparin, pretreatment of cells with DSTP27 significantly reduced HPV infection for more than 30 h. Using DSTP27 and heparinase, we furthermore demonstrate that HS moieties, rather than laminin 5, present in the extracellular matrix (ECM) secreted by keratinocytes are essential for infectious transfer of ECM-bound virions to cells. Prior binding to ECM components, especially HS, partially alleviated the requirement for cell surface HS. DSTP27 blocks infection by cell-bound virions by feeding into a noninfectious entry pathway. Under these conditions, virus colocalized with HS moieties in endocytic vesicles. Similarly, postattachment treatment of cells with heparinase, cytochalasin D, or neutralizing antibodies resulted in uptake of virions without infection, indicating that deviation into a noninfectious entry pathway is a major mode of postattachment neutralization. In untreated cells, initial colocalization of virions with HS on the cell surface and in endocytic vesicles was lost with time. Our data suggest that initial attachment of HPV to HS proteoglycans (HSPGs) must be followed by secondary interaction with additional HS side chains and transfer to a non-HSPG receptor for successful infection.
Due to the association of human papillomaviruses (HPV) with development of multiple carcinomas, especially cervical carcinomas, early diagnosis and prevention of infection with HPV are of great medical and economic importance. Knowledge of the early steps of papillomavirus infection, which results in infectious entry, will help develop means to prevent HPV-induced lesions. Since HPV are difficult to propagate in cell culture, surrogate infection systems with marker-encoding viral capsids, called HPV pseudovirions, have been developed and successfully used in investigating the HPV entry pathway as well as in testing of substances interfering with HPV infection (2, 33). These studies have led to the identification of specifically modified heparan sulfate proteoglycans (HSPGs) as primary attachment receptors for papillomaviruses (13, 15) and to heparin and other sulfated polysaccharides as inhibitors of HPV infection (1, 7, 13). Recently, carrageenan, an unbranched sulfated polysaccharide from algae with saccharide linkages reminiscent of galactosaminoglycans, has been reported to inhibit HPV infection primarily by preventing the binding of virions to the cell (4). Dispirotripiperazine (DSTP) derivatives represent another substance class with proven antiviral potential. DSTP27 (an N,N′-bisheteryl derivative of DSTP), one of the most active derivatives of this new class of low-molecular-weight antiherpetic compounds, interacts with specific forms of cell surface HSPGs (26). In addition to the inhibition of herpes virus attachment and infection, DSTP27 efficiently blocks the attachment and uptake of members from other virus families that depend on HSPGs as primary attachment molecules (25). In contrast to the HS analogs such as heparin and pentosan polysulfate that have short-lived effects, pretreatment of cells with DSTP27 induces a long-lasting antiviral effect. Based on computer modeling, DSTP27 possibly interacts with two O-sulfate groups located on neighboring saccharides of the HS chain (27). Using the octosaccharide essential for HS-mediated entry of herpes simplex virus type 1 (HSV-1) into host cells (20), these computational studies further show that DSTP27 may additionally interact with a carbonyl group, thus increasing the strength of compound binding.
Since HPV bind specifically to sulfated polysaccharide residues of cell surface HSPGs, particularly 2-O- and 6-O-sulfated HS chains in addition to N-sulfated residues (27), DSTP27 was predicted to work as a potent inhibitor of HPV infection. In this report we demonstrate that DSTP27 efficiently prevents HPV infection when applied several hours pre- or postinfection of cells. This is achieved by two putatively different mechanisms: by blocking virus binding in the preattachment mode and by inducing uptake of virions into a noninfectious pathway in the postattachment mode. We furthermore demonstrate that heparinase treatment and neutralizing antibodies induce a noninfectious uptake of HPV as well, suggesting that unproductive internalization is a common mode of postattachment inhibition of HPV infection. We also use DSTP27 as a novel tool for investigating the role of HS moieties present in the extracellular matrix (ECM) in HPV infection.
MATERIALS AND METHODS
Cell lines, plasmids, antibodies, reagents, VLPs, and pseudovirions.
HEK 293TT cells and expression plasmids for codon-optimized HPV type 18 (HPV18) L1 and L2 (plasmids pel1 and pel2) as well as bovine papillomavirus type 1 (BPV1) L1 and L2 (pSHELL) proteins were provided by Chris Buck, Bethesda, MD (2, 3). Codon-optimized HPV16 L1 and L2 expression plasmids were a kind gift from Martin Muller, Heidelberg, Germany (17). Virus-like particles (VLPs) were produced in Sf9 insect cells as described previously (34). Rat monoclonal antibodies against the hemagglutinin (HA) tag were obtained from Roche (clone 3F10), and HS-specific monoclonal immunoglobulin M (IgM) antibody (F58-10E4) was from Seikagaku. Laminin 5 (LN5) rabbit polyclonal antibodies were purchased from Abcam (ab14509). HPV16-specific rabbit polyclonal antisera K75 and mouse monoclonal antibody H16.56E have been described previously (23). HPV33-specific monoclonal antibody H33.J3 was a kind gift of Neil Christensen, Hershey, PA (6). AlexaFluor-labeled secondary antibodies were purchased from Molecular Probes. Cytochalasin D (30385) and heparinase I (H2519) were obtained from Sigma Aldrich. HPV16, HPV18, and BPV1 pseudovirions were generated and purified by Optiprep gradient centrifugation following published procedures (2). The generation of hybrid HPV16:33BC pseudoviruses harboring the HPV33 BC loop in an HPV16 backbone have been described previously (24). HEK 293TT, COS-7, and HeLa cells were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM), whereas HaCaT cells were maintained in low-glucose DMEM, both supplemented with 10% fetal bovine serum, 1% nonessential amino acids, and 1% Glutamax at 37°C in humidified CO2 (5%) incubators. Chinese hamster ovary K1 (CHO-K1) cells and their derivatives pgsA-745 (12) and pgsD-677 (19) were grown in Ham's F12 medium supplemented with 10% fetal bovine serum and 1% Glutamax (Invitrogen).
Inhibition of papillomavirus infection.
DSTP27 was serially diluted in DMEM in 96-well plates. Pseudovirus was added to HEK 293TT cells suspended in DMEM, and the mixture was dispensed into the DSTP27-containing wells. Infectivity was scored by counting green fluorescent protein (GFP)-expressing cells at 72 h postinfection (hpi). For CHO-K1 derivatives, HeLa, and HaCaT cells, infectivity was scored by immunological staining with AlexaFluor 488-coupled rabbit anti-GFP antibody (Invitrogen).
Kinetic of DSTP27 action.
HEK 293TT cells were grown overnight in 96-well plates. For preattachment neutralization, DSTP27 treatment (5 μg/ml) in a total volume of 50 μl was carried out for 2 h at 37°C at indicated time points, and cells were washed three times with complete DMEM. Subsequently, pseudovirus suspended in 50 μl of DMEM was added, and incubation was continued for 2 h at 37°C. Unbound pseudovirions were removed by two washing steps, and infectivity was scored 72 hpi. For postattachment neutralization, pseudovirions were first bound for 2 h at 37°C, and unbound pseudovirions were removed by two washes. At the time indicated in the figures, DSTP27 treatment (5 μg/ml) was carried out for 2 h at 37°C, cells were washed three times, and infectivity was scored by counting GFP-expressing cells at 72 hpi.
Immunofluorescence.
HeLa or COS-7 cells were grown on coverslips and treated with DSTP27 (50 μg/ml), heparinase I (5 U), or antibodies (1:25) for 1 h pre- or postattachment of pseudovirions. At the indicated time points, cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 15 min at 4°C. After cells were washed with PBS, they were permeabilized with 0.2% Triton X-100 in PBS for 2 min, washed again, and blocked with 1% bovine serum albumin (BSA) in PBS for 30 min, followed by a 1-h incubation with primary antibodies at 37°C. After extensive washing with PBS, cells were blocked again with 1% BSA in PBS for 10 min and then incubated with AlexaFluor-tagged secondary antibodies (Invitrogen) for 1 h. After extensive washing with PBS, cells were mounted in Fluoprep (BioMerieux) and examined by fluorescence microscopy. For surface staining, cells were not permeabilized by Triton X-100 but were processed as described above. Images were captured by confocal microscopy (Zeiss/Bio-Rad Radiance 2000 Confocal Microscope operated by LaserSharp 2000 software) or by deconvolution microscopy (Zeiss Axiovert 200 M) operated by AxioVision software (Zeiss) using a 100× (numerical aperture, 1.25) oil immersion objective. Images were captured in z series. The fluorescence data sets were processed by three-dimensional deconvolution with the inverse filter method.
ECM.
HaCaT cells were grown to confluence in 96-well plates for 2 days. Cells were removed by EDTA (0.5 mM in Dulbecco's PBS [DPBS]) to obtain ECM-coated wells. ECM was treated for 2 h at 37°C with DSTP27 at the indicated concentrations, heparinase I (5 U), anti-LN5 antibody (1:10 or 1:25), or combinations thereof, dispensed in 50 μl of DMEM. For combinatorial treatments, a 1:25 dilution of LN5 antiserum was used. Untreated ECM-containing wells and wells without ECM served as controls. Reagents were removed by three washes, and HPV18 pseudovirus was bound for 2 h at 37°C. Unbound pseudovirions were removed by three washes, and HEK 293TT or HaCaT cells dispensed in 100 μl of DMEM were added. Incubation was continued at 37°C, and infectivity was scored by counting GFP-expressing cells 72 h later. For immunofluorescence, HaCaT cells were grown on coverslips for 48 h and subsequently removed by EDTA treatment (0.5 mM in DPBS) to obtain ECM-coated coverslips. Following treatment for 2 h at 37°C with DSTP27 (5 μg/ml), heparinase I (5 U), rabbit control antibody (1:25) or anti-LN5 antibody (1:25) or combinations thereof, excess reagents were removed by three washes, and HPV16 pseudovirus was bound. Unbound pseudovirions were removed by three washes. Pseudovirions were fixed to coverslips with methanol-20 mM EGTA and detected using HPV16-specific monoclonal antibody H16.56E and AlexaFluor 488-labeled goat anti-mouse secondary antibodies. Bound rabbit antibodies were detected using AlexaFluor 546-labeled goat anti-rabbit secondary antibodies.
Flow cytometry.
For preattachment neutralization, COS-7 cells dispensed in 24-well plates were treated with the indicated reagent for 1 h at 37°C, 1 μg of HPV16 VLPs was added, and incubation was continued for an additional 1 h. For postattachment neutralization, 1 μg of VLPs was bound to COS-7 cells for 1 h at 37°C, and unbound VLPs were removed by three washes. Cells were then treated for 90 min at 37°C with the reagents indicated in the figures. The reagents were removed by three washes with DMEM, and cells were incubated for the times indicated in the figures, detached in DPBS-25 mM EDTA, and fixed for 10 min with 3.7% formaldehyde-DPBS. Cells were incubated with the K75 antiserum (1:1,000; 30 min at 0°C), followed by a 30-min incubation with anti-rabbit IgG AlexaFluor 488 (1:250) and three washes with PBS-1% BSA-5 mM EDTA-0.01 NaN3, pH 6.8; cells were subsequently analyzed in a FACSCalibur (Becton-Dickenson) flow cytometer and evaluated using either Cell Quest or WinMDI, version 2.8, software.
RESULTS
Determination of IC50 values.
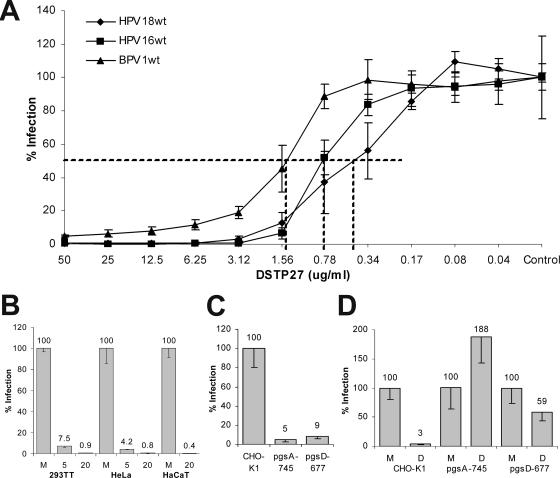
DSTP27 has recently been shown to block infection by various viruses that utilize 2-O-, 6-O-, and/or 3-O- as well as N-sulfated HS moieties as receptors (25, 26). Since we have previously demonstrated that infectivity of many HPV types is dependent on N- and O-sulfation of HS (28), we tested DSTP27 for inhibition of papillomavirus infection. As shown in Fig. 1A, DSTP27 efficiently blocked HPV16, HPV18, and BPV1 infection of HEK 293TT cells with 50% inhibitory doses (IC50s) of approximately 0.8, 0.4, and 1.5 μg/ml corresponding to 1.4, 0.7, and 2.6 μM, respectively. DSTP27 also efficiently blocked infection of cell lines derived from keratinocytes, like HeLa and HaCaT cells (Fig. 1B). Preincubation of pseudovirions with DSTP27 (5 μg/ml) for 1 h and subsequent 100-fold dilution during cell binding (final concentration, 0.05 μg/ml) did not reduce infection, demonstrating that DSTP27 does not directly bind to virions but, rather, blocks cell surface molecules (data not shown). This is supported by our not observing a capsid-dose influence on the IC50 (data not shown), which has previously been reported for carrageenan, a competitive inhibitor of HPV infection that directly binds to viral capsids (4). The importance of HS moieties for DSTP27-mediated inhibition of HPV infection was confirmed by using HS- and glycosaminoglycan (GAG)-deficient CHO-K1 derivatives, psgD-677 and psgA-745. Both cell lines could also be infected by HPV18 pseudovirions, albeit at much reduced levels of 9% and 5%, respectively, compared to parental CHO-K1 cells (Fig. 1C), which is consistent with previous reports (4). HPV18 infection of Gag-deficient cells was not sensitive to DSTP27 treatment, and HPV18 infection of HO-deficient cells was only slightly sensitive to DSTP27 (Fig. 1D). These data suggest that DSTP27 inhibition requires the presence of HSPG. Other GAGs can only partially substitute for HS. It also indicates that the drug does not generally block the intracellular trafficking machinery.
FIG. 1.
Determination of IC50. (A) HEK 293TT cells were infected with HPV16, HPV18, and BPV1 pseudovirions in the presence of decreasing concentrations of DSTP27. (B) HPV18 infection of HEK 293TT, HeLa, and HaCaT cells in the absence (M) or presence (5 or 20) of 5 and 20 μg/ml DSTP27. (C) HPV18 infection of GAG-deficient (psgA-745) and HS-deficient (psgD-677) as well as parental CHO-K1 cells. (D) Infection of CHO-K1 derivative with HPV18 pseudovirions in the absence (M) or presence (D) of DSTP27 (20 μg/ml). Infection was scored 72 hpi by counting GFP-expressing cells. wt, wild type.
Pre- and postattachment inhibition by DSTP27.
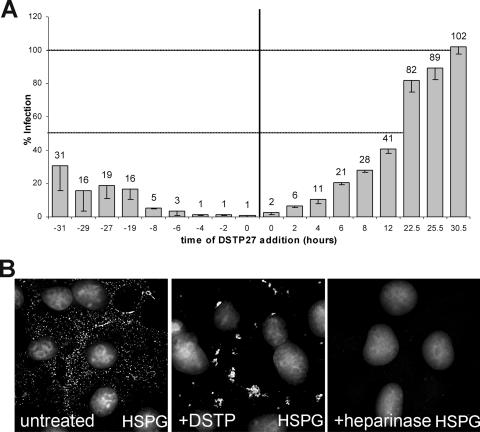
Because of the direct interaction of DSTP27 with a specific subset of cell surface HS (25), we speculated it to be inhibitory if cells are pre- or posttreated with regard to infection. To determine the window of activity, cells were exposed to DSTP27 (5 μg/ml) at various times before or after contact with HPV18 pseudovirus. A representative experiment is shown in Fig. 2A, demonstrating that DSTP27 significantly reduced infection when given more than 30 h before and up to 12 h after the addition of pseudovirions. We assumed that DSTP27 treatment after binding may directly reflect the uptake kinetic of pseudovirions. Indeed, the calculated half-time of 14 h is very similar to previously published data on HPV internalization (4, 13, 28, 31). To determine whether the long-lasting protective effect of DSTP27 prior to infection reflects the synthesis rate of HSPG, we performed a similar time course experiment using heparinase I instead of DSTP27 to remove cell surface-exposed HS prior to HPV18 pseudoinfection. Heparinase treatment reduced infection, but, in contrast to DSTP27, cells became completely sensitive to infection within 6 h after treatment. HS-specific immunofluorescence confirmed that this time period is long enough to translocate sufficient amounts of HSPG to the plasma membrane to facilitate infection (data not shown). These data suggest that DSTP27 either prevents synthesis of new HSPG or that cell-bound DSTP27 is able to further inactivate newly surface-exposed HSPG. Evidence for inactivation of newly translocated HSPG came from immunofluorescence studies. Using the HS-specific antibody F58-10E4 for detection of HSPG, we observed that DSTP27 treatment resulted in strong clustering of cell surface HSPG (Fig. 2B). Staining for HSPG following heparinase treatment in nonpermeabilized cells confirmed the complete removal of cell surface HS (Fig. 2B).
FIG. 2.
Kinetics of DSTP27 action. (A) HEK 293TT cells were treated with DSTP27 (5 μg/ml) at indicated times pre- and postattachment of HPV18 pseudovirions, and infection was scored 72 hpi. (B) Untreated COS-7 cells and cells treated with DSTP27 (50 μg/ml; 1 h) or heparinase (5 U; 90 min) at 37°C were fixed and stained for HS using antibody F58-10E4. Cells were surface stained for HS after heparinase treatment. Pictures were taken using a Zeiss Axiovert 200 M microscope. Deconvoluted images are shown. DNA staining was pasted into the images using Adobe Photoshop.
Transfer of pseudovirions from ECM-located HS moieties to cells.
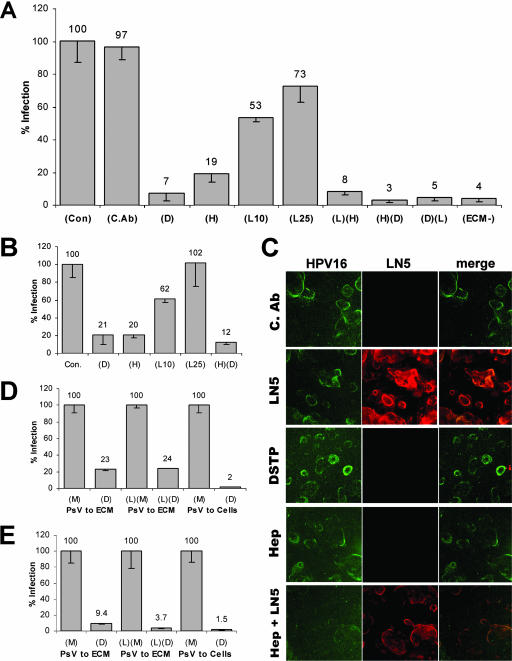
So far, using DSTP27 as an HS-specific probe, we have confirmed the importance of cell surface HSPG for papillomavirus infection. It was recently demonstrated that HPV virions bind to components, especially LN5, of the ECM of keratinocytes and suggested that transfer of virions from LN5 to adjacent cells may facilitate infection (8). Since DSTP27 binds to HS rather than to the virus itself, it is ideally suited to test a possible role of HS in virion binding to the ECM and subsequent transfer to cells. Following the experimental design developed by Culp et al. (8), keratinocytes (HaCaT cells) were grown for 48 h in 96-well plates, and subsequently cells were removed with EDTA. The empty microtiter wells with residual ECM were then treated with DSTP27, heparinase, anti-LN5 antibody, or combinations thereof for 2 h and washed twice. Pseudovirions were bound to ECM, unbound particles were removed, and 293TT detector cells were plated into these wells. As shown in Fig. 3A, treatment of ECM with DSTP27 almost completely blocked infection of overlaying 293TT detector cells by ECM-bound virions (7%), reducing infectivity to levels obtained in uncoated control wells (4%). Heparinase treatment reduced infection to 19% whereas treatment with anti-LN5 alone reduced infection to 73% and 53% when applied at 1:25 and 1:10 dilutions, respectively. Combined treatment of the ECM with heparinase and anti-LN5 (1:25) resulted in a cooperative effect and reduced infectivity to 8%. Combined treatment with DSTP27 and heparinase or DSTP27 and anti-LN5 reduced infectivity to levels obtained in uncoated control wells. This demonstrates that the HS moieties in the ECM play an important role for binding and transferring pseudovirions from ECM to 293TT cells. DSTP27 and heparinase treatment of ECM also blocked infectious transfer of virions to HaCaT detector cells (Fig. 3B). Pretreatment with anti-LN5 antibody only slightly reduced infectivity at a 1:10 dilution, demonstrating that our finding also holds true for keratinocytes, the natural host cell of HPV. DSTP27, but not heparinase, treatment of ECM also slightly reduced infection in a concentration-dependent manner when pseudovirions were preattached to reporter cells (HaCaT or 293TT) and subsequently added to DSTP27-treated ECM (data not shown), suggesting that ECM-bound DSTP27 may affect cell surface HSPG either directly, e.g., by clustering of cell surface HSPG at the interface to the ECM, or indirectly by dissociation from ECM.
FIG. 3.
Transfer of pseudovirions from ECM-located HS moieties to cells. (A and B) HaCaT cells were grown for 2 days in 96-well plates and subsequently removed by treatment with EDTA. Remaining ECM was left untreated (Con) or treated with DSTP27 (D; 5 and 20 μg/ml for HEK 293TT and HaCaT cells, respectively), heparinase I (H; 5 U), anti-LN5 antisera diluted 1:10 (L10) or 1:25 (L25), combinations thereof, or control antibody (C.Ab.). Anti-LN5 antiserum was used at a 1:25 dilution in combinatorial treatments. HPV16 pseudovirions were added, unbound virions were removed, HEK 293TT (A) or HaCaT cells (B) were added, and infection was scored 72 h later. (C) HaCaT cells were grown on coverslips for 48 h and subsequently detached. Remaining ECM was treated with rabbit control antibody, anti-LN5 antibody (1:25), DSTP27 (5 μg/ml), heparinase I (5 U), or heparinase I and anti-LN5 antibody combined. HPV16 pseudovirions were added after removal of the reagents, and coverslips were stained for pseudovirions with H16.56E and for anti-LN5 antibody. (D and E) HEK 293TT (D) or HaCaT (E) cells were mock treated (M) or DSTP27 treated (D; 20 μg/ml) in the presence (PsV to cell) or absence (PsV to ECM) of HPV18 pseudovirions and subsequently seeded into ECM-coated wells with (PsV to ECM) or without (PsV to cell) prebound pseudovirions. ECM was also treated with anti-LN5 (1:25) (L) prior to pseudovirus binding. Infection was scored 72 h later.
We followed binding of HPV16 pseudovirions to the ECM by immunofluorescence and found that neither treatment alone completely prevented virion binding (although some reductions were observed in the individual treatments). Combined treatment with heparinase and anti-LN5 reduced virion attachment to the ECM components (Fig. 3C), even though complete blockage was never achieved, despite almost complete inhibition of infectious transfer (Fig. 3A and B). Since it was recently suggested that interaction with HS induces a conformational change in L1 protein and that this may be required for transfer to putative secondary receptors (28), we wondered if interaction of pseudovirions with HS moieties present in ECM might alleviate the need for cell surface-associated HS. To test this, 293TT cells were treated with an excess of DSTP27 (20 μg/ml) and subsequently added to pseudovirus-loaded but otherwise untreated or anti-LN5-treated ECM. We observed a reduction in infection to 23% and 24% of controls, respectively (Fig. 3D). In contrast, only 2% infectivity was observed when pseudovirions were directly bound to cells in the presence of DSTP27 (20 μg/ml). Similar results, albeit not as pronounced, were obtained using HaCaT instead of 293TT cells as reporter cells (Fig. 3E). Taken together, these data strongly suggest that HPV binds to HS as well as other components of the ECM, probably LN5. However, more interestingly, the interaction with HS moieties in the ECM, rather than binding to LN5, is required for facilitating transfer of the virus from the ECM to cells for initiating the infectious uptake. The observed cooperativity of anti-LN5 antibodies and heparinase or DSTP27 suggests that a transfer from LN5-bound virions to cells is possible but less likely to occur than transfer from HS components. The data also indicate that pseudovirus interaction with ECM-resident HS moieties partially alleviates the requirement for cell surface HSPG.
Effect of DSTP27 on cell-bound virions.
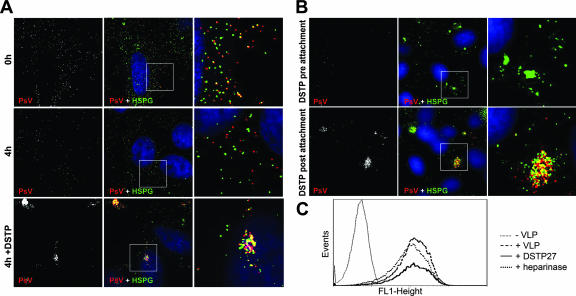
Next, we determined the effects of DSTP27 on HPV16 pseudovirions prebound to cell surface HSPG using HS-specific IgM antibody and HPV16-specific rabbit K75 antiserum by immunofluorescence (Fig. 4). In untreated HeLa cells, we observed partial colocalization of HS moieties with HPV16 pseudovirions but no HSPG clustering (Fig. 4A, 0 h). Colocalization was lost with continued incubation at 37°C (4 h). Postattachment treatment with DSTP27 (50 μg/ml) did not detach pseudovirions and induced prominent clustering of HSPG and pseudovirions (Fig. 4A, 4 h+DSTP). When pseudovirions were prebound, we always noticed a reduction of HS-specific signals, suggesting a sterical hindrance for IgM antibody binding. Pretreatment of cells with DSTP27 (50 μg/ml) significantly reduced binding of virions and also induced clustering of HSPG, as shown for COS-7 cells in Fig. 4B. Treatment of COS-7 cells postbinding induced clustering of HSPG as well as pseudovirions, with colocalization of HSPG and pseudovirus clusters (Fig. 4B). Clustering and colocalization of HSPG and pseudovirions was also observed when HaCaT cells were similarly treated with DSTP27 (data not shown). We also determined cell binding of viral particles using HPV16 VLPs and flow cytometry (Fig. 4C). DSTP27 treatment did not detach cell-bound virus particles, and heparinase treatment only marginally affected the number of cell surface-bound VLPs. The experiments were done under conditions that reduced infection to 0.1% and 24%, respectively (Fig. 5A). These data suggest that HSPG molecules occupied by viral particles cannot be targeted by either reagent and demonstrate that DSTP27 and heparinase neutralize prebound virions without dissociating them from cells.
FIG. 4.
Effect of DSTP27 on prebound pseudovirions. (A) HeLa cells were mock treated or DSTP27 treated (50 μg/ml) after binding HPV16 pseudovirions. Cells were stained for bound pseudovirions (PsV; red), HSPG (green), and DNA (blue) at indicated times. Left panels display black and white images for pseudovirus staining only, middle panels show colored merged images, right panels display enlarged sections. (B) COS-7 cells were mock treated or DSTP27 treated pre- and postattachment of HPV16 pseudovirions (PsV) and stained as described above. (C) HPV16 VLPs were bound to COS-7 cells and subsequently treated with heparinase I (5 U) or DSTP27 (50 μg/ml) or left untreated. VLPs were detected by flow cytometry using K75 antiserum. Cell-bound virions were not removed by treatment with either DSTP27 or heparinase.
FIG. 5.
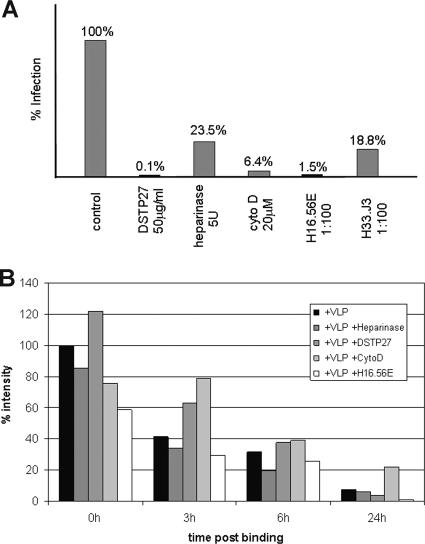
Noninfectious entry of HPV16 pseudovirions. (A) HEK 293TT cells were treated with DSTP27 (5 μg/ml), heparinase I (5 U), cytochalasin D (20 μg/ml), H16.56E (1:100), or H33.J3 (1:100) postattachment, and infection was scored at 72 hpi. (B) Internalization of HPV16 VLPs bound to COS-7 cells treated postattachment as described in panel A and visualized by flow cytometry using 16VLP-specific rabbit antiserum K75. Mean fluorescence intensities normalized to controls are plotted. Cyto D, cytochalasin D.
Uptake of virions into a noninfectious pathway in presence of DSTP27.
Since DSTP27 does not inhibit infection of cell-bound virions by detachment, blockage must occur at subsequent steps of infection. This could include preventing uptake of virus particles or feeding them into a noninfectious entry pathway. To distinguish between these possibilities, we measured cell surface-exposed, prebound VLPs over time by flow cytometry (Fig. 5B). Disappearance of capsids from the cell surface was observed when cells were treated with DSTP27 or heparinase under conditions that prevented and significantly reduced infection, respectively (Fig. 5A), indicative of HPV particle internalization. Both treatments inactivate unoccupied or newly synthesized HSPG and, presumably, feed particles into a noninfectious pathway. Thus, we conclude that infection by papillomavirus requires a secondary interaction with HS moieties following initial attachment to HSPG. Since DSTP27 does not block residual infection of GAG-deficient cells (Fig. 1D) and pseudovirus binding to ECM increases infection of DSTP27-treated reporter cells (Fig. 3D and E), a general effect of the drug on intracellular trafficking is highly unlikely. We also observed internalization in the presence of the actin-depolymerizing drug cytochalasin D under conditions that blocked HPV infection (Fig. 5). This suggests that intact actin filaments are required in early events of infection and, importantly, that the noninfectious entry is independent of actin filaments.
Colocalization of pseudovirions with HS in endocytic vesicles of DSTP27-treated but not untreated cells.
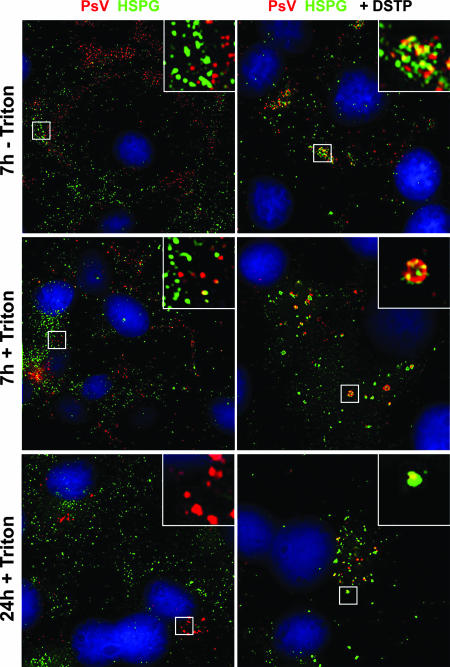
To address the question of whether HSPGs are cointernalized with virions or whether virions are transferred to a secondary non-HSPG receptor in the presence or absence of DSTP27, we followed the internalization of prebound HPV16 pseudovirions by immunofluorescence studies using HPV VLP antisera and HS-specific antibody. We again found that initial colocalization, as observed directly after attachment (Fig. 4A), was almost completely lost on the cell surface of mock-treated but not DSTP27-treated cells at 7 hpi (Fig. 6, 7h −Triton). DSTP27 but not mock-treated cells displayed colocalization of virions with HSPG in intracellular vesicles up to 24 hpi. Taken together, these data strongly suggest a transfer of virions from HSPG to non-HS cell surface molecules prior to internalization in untreated cells. This transfer is blocked by DSTP27, resulting in the noninfectious uptake of virions presumably still complexed with primary HSPG molecules.
FIG. 6.
Cointernalization of HSPG and pseudovirions (PsV) in the presence of DSTP27. HPV16 pseudovirions were bound to COS-7 cells grown on coverslips. Cells were subsequently mock treated or DSTP27 (50 μg/ml) treated and incubated further for the indicated times at 37°C. Cells were surface stained (−Triton) or stained overall (+Triton) using HPV16-specific K75 antiserum and the HS-specific F58-10E4 antibody. Inserts display enlarged sections.
FIG. 7.
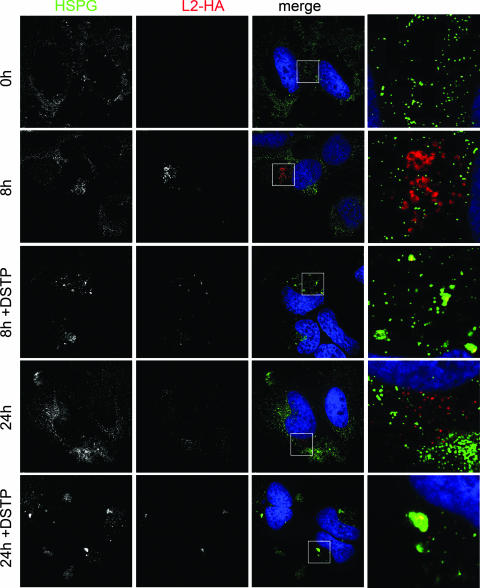
Effect of DSTP27 on pseudovirus uncoating. HPV16 pseudovirus was bound to HeLa cells, mock treated or DSTP27 (50 μg/ml) treated, and incubated at 37°C for the indicated times. Cells were subsequently stained using HS- and HA-specific antibody to detect HSPG (green) and HA-tagged L2 protein (red), respectively.
We also tested if the noninfectious uptake interferes with viral uncoating by detecting the HA-tagged minor capsid protein L2, which becomes accessible to antibody binding only after uncoating (9). As shown in Fig. 7, no HA-tag-specific signal was observed directly after binding (0 h), confirming that the HA tag is not accessible to antibody binding in pseudovirions. The C-terminal HA tag was detected at 8 hpi in untreated HeLa cells, and no colocalization with HS was evident. At 8 hpi, the HA tag was also detectable in DSTP27-treated cells but at a reduced level. However, in contrast to untreated controls, the HA tag completely colocated with HS. At 24 hpi, colocalization was still observed in DSTP27-treated but not in untreated HeLa cells. These data suggest that DSTP27 does not block viral disassembly completely but that the observed uncoating may not occur in compartments that allow viral DNA to escape from endosomes.
Antibody-mediated postattachment neutralization of HPV.
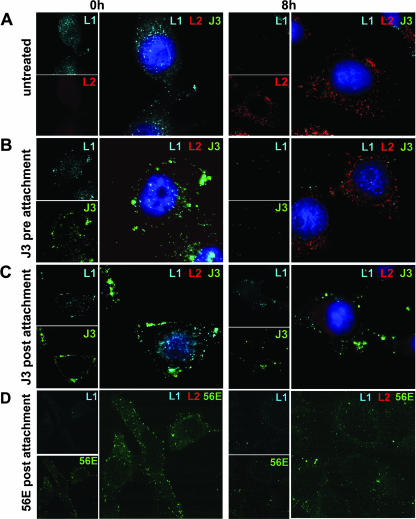
HPV-specific antibodies have been shown to neutralize pre- and postattachment. Preattachment neutralization is largely achieved by preventing binding of virions to target cells. Two recent reports suggest that postattachment neutralization by antibody blocks virus internalization (11, 31). Our analysis with DSTP27 and heparinase led us to wonder if postattachment neutralization can also be achieved by inducing a nonproductive internalization. To test this, we used two mouse monoclonal antibodies. The binding-neutralizing HPV16-specific H16.56E binds the FG loop, with some contributions from the DE loop, and neutralizes pre- and postattachment (Fig. 5A). The HPV33-specific H33.J3 recognizes the BC loop without preventing binding and neutralizes only postattachment (Fig. 5A) (24, 28). HPV16 (recognized by H16.56E) or hybrid HPV16:33BC pseudovirions (recognized by H33.J3) were bound to cells, and antibodies were added. As a control, hybrid pseudovirus was preincubated with H33.J3 prior to cell binding, which did not neutralize (28). Internalization was followed by immunofluorescence by detecting neutralizing antibody and conformational L1 epitopes using HPV16 VLP-specific rabbit polyclonal antiserum K75. In addition, viral uncoating was analyzed by detecting HA-tagged L2. Cells were processed for immunofluorescence either before shifting to 37°C or after an additional 8 h of incubation to allow internalization. Control cells infected with HPV16:33BC pseudovirus in the absence of antibody H33.J3 are shown in Fig. 8A. At 0 hpi, pseudovirus was detected on the cell surface by staining for L1, but no HA tag signal was observed. At 8 hpi, the L1 signal was strongly reduced due to the loss of conformational epitopes recognized by K75, and HA-tagged L2 was detected inside the cell, demonstrating the internalization and uncoating of virions. A similar picture was obtained using H33.J3 for preattachment neutralization (Fig. 8B). At 0 hpi, colocalization of L1 and H33.J3 was observed on the cell surface, confirming that H33.J3 does not block pseudovirus binding (28). At 8 hpi, again, HPV16 L2-HA was detected, and the L1 signal was reduced. Additionally, H33.J3 signal was strongly reduced, indicating that this antibody is degraded concomitantly with L1. At 0 hpi of postattachment neutralization with H33.J3, cell-bound virions were not displaced (Fig. 8C). At 8 hpi, however, intracellular L1 and H33.J3 signals were stabilized. In addition, HA tag could not be detected, indicative of failure in uncoating. This suggests that virus disassembly, but not internalization, is affected in postattachment neutralization. Similar results were obtained with H16.56E to neutralize prebound virions (Fig. 8D). The results strongly suggest that postattachment neutralization with H33.J3 and H16.56E involves virion internalization by a pathway that does not allow uncoating.
FIG. 8.
Cointernalization of pseudovirions with neutralizing antibodies. HeLa cells were infected with HPV16:33BC (A to C) or HPV16 (D) pseudovirions containing HA-tagged L2 in the presence or absence of antibodies, fixed at 0 and 8 hpi, and subjected to immunofluorescence analysis. (A) In the absence of neutralizing antibodies, internalization and virus uncoating are observed at 8 hpi. (B) Preattachment treatment of pseudovirus with H33.J3 does not prevent binding, internalization, and virus uncoating. (C and D) Postattachment neutralization of pseudovirions with antibody H33.J3 (C) or H16.56E (D) does not prevent internalization but blocks viral uncoating. Blue, L1; red, L2; green, antibodies H33.J3 and H16.56E. The nonspecific diffused red signal in panel C is due to over exposure. The weak L1-specific signal in panel D is due to partial H16.56E competitive blocking of K75 binding.
DISCUSSION
We have demonstrated in this study that DSTP27 inhibits papillomavirus infection over an extended period of time, both pre- and postbinding of virions. Unlike previously described inhibitors of HPV infection, such as heparin and carrageenan, which bind to the virus capsid, DSTP27 binds to cell surface HS (25). Since DSTP27 is not cytotoxic (up to 400 μg/ml) (26) and also inhibits infection by other viruses that require HS for infection (e.g., HSV1, HSV2, pseudorabies virus, certain strains of human immunodeficiency virus, and cytomegalovirus) (25), it may be a good candidate for a broad-spectrum antiviral agent. Because papillomaviruses have a very slow internalization kinetic (half-time of up to 14 h) and DSTP27 can inhibit infection postattachment, it may be especially useful in preventing papillomavirus-induced lesions. Testing DSTP27 in animal models should establish its effectiveness in vivo.
We confirmed the importance of cell surface HS for papillomavirus infection using DSTP27. Additionally, we demonstrated that HS moieties also contribute to virion binding to the ECM and that the interactions of HPV virions with HS side chains are of high importance for infectious transfer from the ECM to cells. Pretreatment of ECM with DSTP27 or heparinase strongly impaired infection without completely preventing binding of virions. This also confirms the presence of additional binding partners in the ECM, possibly LN5 (8), although this interaction seems to be of secondary importance, as treatment with LN5 antibody only marginally reduced infection. Therefore, transfer from LN5 to cells is possible but seems to be less likely than the transfer from HS moieties, which is in line with the observation that virion binding to LN5 is of higher affinity than binding to HS (8). Our findings are in line with a recent report demonstrating that heparin does not block binding of HPV16 pseudovirions to the ECM (11).
Similar to results recently reported by Buck et al. (4), we observed a low level of infection in the absence of cell surface GAG or HS. DSTP27 did not block infection of GAG-deficient psgA-745 cells, though it slightly reduced infection of HS-deficient psgD-677 cells, suggesting either that the cells are leaky for HS expression or that non-HS GAGs may be used by HPV, even though inefficiently. We also observed that HPV infection of HEK 293TT cells and keratinocytes cannot be completely inhibited by DSTP27. Therefore, it seems possible that HPV binds to secondary low-affinity binding sites on the cell surface that cannot be blocked by DSTP27. An alternate explanation is that a minority of viral particles in the pseudovirus preparation display a conformation allowing direct binding to the putative non-HSPG receptor. Indeed, we observed a linear relationship between residual infectivity and the quantity of pseudovirions in the presence of high concentrations of DSTP27 (data not shown). Several pieces of evidence published in recent years suggest that primary attachment of virions to cells induces a conformational change in both capsid proteins, which is required for infectious uptake (28, 35). This structural change could be required for transfer of virions to non-HSPG receptors. The presence of such activated conformations in cell-free virions would alleviate the need for interaction with cell surface HSPG and may explain the controversy regarding the need of HSPG (21). Our observation that interaction of virions with the ECM lessens the requirement of cell surface HS for infection supports this notion. Taken together, these data also demonstrate that inhibition by DSTP27 requires cell surface HS and rule out a general effect of the drug on the intracellular trafficking machinery.
The experiments presented in this paper show that induction of a noninfectious uptake is an important mechanism of postattachment inhibition employed by such diverse agents as DSTP27, heparinase, cytochalasin D, and neutralizing antibodies. Our results suggest that the common feature among these agents is that they all prevent interaction of virus with secondary cell surface receptors. Heparinase and DSTP27 achieve this by blocking free HS molecules on cell surface, whereas neutralizing antibodies probably occupy binding sites on the viral capsid, which are required for secondary interactions. The effect of cytochalasin D can be explained by the requirement of intact actin filaments for the processes occurring at the plasma membrane, which may include virus cell “surfing” toward the cellular body (18). Taken together, these results allow us to draw two major conclusions. First, in addition to the primary interaction of HPV with HSPGs, the secondary interaction partners of HPV virions must include further HSPG molecules, since heparinase and DSTP27, which both affect heparan sulfate chains, prevent transfer from the primary attachment receptor to a non-HSPG uptake receptor. Whereas the effect of DSTP27 could be explained by DSTP27-induced clustering of HSPG and consequently clustering and immobilization of virions, it cannot be true for heparinase. Second, the involvement of a non-HSPG receptor is supported by the observed loss of initial colocalization of HS and viral capsid with time, both on the cell surface and in endocytic vesicles of untreated, but not DSTP27-treated, cells. Taken together, these data suggest that the essential steps of HPV infection are the following: (i) binding to primary HSPG receptor, which may occur in the ECM; (ii) transfer to or recruitment of secondary HSPG receptor; (iii) and subsequent transfer to a presumably non-HSPG receptor. Most likely, the receptor binding site on viral capsid involved in primary interaction is not identical to the site mediating the secondary interaction, even though we cannot rule out that it is the same binding site located on a neighboring capsomere.
In contrast to untreated cells, capsid uncoating is severely impaired by neutralizing antibodies, as observed by stabilized L1 protein and the inability to detect L2 protein. DSTP27 does not completely block uncoating, but in contrast to untreated cells, L2 is detected in large vesicles colocating with HSPG (Fig. 7). L1 protein also localizes to the HSPG-containing compartment in DSTP27-treated cells (Fig. 6), suggesting that segregation of L1 and L2, which is observed in untreated cells (9, 16), cannot take place. We assume that detection of L2 in the presence of DSTP27 is due to lysosomal degradation rather than coordinated uncoating events. This suggests that the observed noninfectivity is due to the uptake pathway's inability to promote timely uncoating and/or release of L2 and DNA from endosomes. Whether this is due to a failure to induce conformational changes in viral capsid (28, 35), the bypassing of appropriately acidified compartments (10, 29), or a lack of host cell factors required for uncoating, e.g., furin convertase (22), is unclear and requires further experimentation. For antibody-mediated neutralization, cross-linking of capsomeres may also contribute to the observed phenotype. This is supported by our finding that Fab fragments of H33.J3 display a fourfold-decreased neutralization capacity (data not shown).
Even though postattachment inhibition of HPV infection using antibodies (5, 13, 28), heparin (13), and carrageenan (4) has been reported, the mechanisms have not been described. Postattachment neutralization of dengue-2 virus using antibodies and carrageenan has been demonstrated to occur by inducing a noninfectious entry (14, 30, 32). In such a case, instead of fusing with plasma membrane, antibody-coated virions were internalized following ruffling of virions by cellular pseudopodia and membrane invagination. As observed also in our study, viral uncoating was impaired by both antibodies and carrageenan, and the internalized virions were degraded (30, 32). This suggests that induction of noninfectious entry pathways by virus-neutralizing agents may be a common mechanism developed by organisms to avoid productive viral infections.
In summary, we have described a novel inhibitor of HPV infection that differs in its mode of action from previously identified inhibitors and may be useful as a broad-spectrum antiviral agent. We have demonstrated that transfer of virions from a primary HSPG attachment receptor to a non-HSPG receptor requires additional HSPG molecules. Interference with this transfer induces internalization by a pathway that does not favor infection.
Acknowledgments
We are grateful to Malgorzata Bienkowska-Haba for help with confocal microscopy; Chris Buck, NIH, and Martin Muller, DKFZ, for providing reagents for the pseudovirus system; and Neil Christensen for the H33.J3 antibody.
This work was supported by grants to M.S. from Deutsche Forschungsmeinschaft (SFB490.E2) and by the National Center for Research Resources, a component of the National Institutes of Health (grant P20-RR018724, entitled Center for Molecular and Tumor Virology).
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Bousarghin, L., A. Touze, A. L. Combita-Rojas, and P. Coursaget. 2003. Positively charged sequences of human papillomavirus type 16 capsid proteins are sufficient to mediate gene transfer into target cells via the heparan sulfate receptor. J. Gen. Virol. 84:157-164. [DOI] [PubMed] [Google Scholar]
- 2.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck, C. B., C. D. Thompson, Y. Y. S. Pang, D. R. Lowy, and J. T. Schiller. 2005. Maturation of papillomavirus capsids. J. Virol. 79:2839-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, C. B., C. D. Thompson, J. N. Roberts, M. Muller, D. R. Lowy, and J. T. Schiller. 2006. Carrageenan is a potent inhibitor of papillomavirus infection. PLOS Pathog. 2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, N. D., N. M. Cladel, and C. A. Reed. 1995. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology 207:136-142. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, N. D., C. A. Reed, T. D. Culp, P. L. Hermonat, M. K. Howett, R. A. Anderson, and L. J. Zaneveld. 2001. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 45:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culp, T. D., L. R. Budgeon, M. P. Marinkovich, G. Meneguzzi, and N. D. Christensen. 2006. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J. Virol. 80:8940-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, P. M., C. C. Baker, D. R. Lowy, and J. T. Schiller. 2004. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 101:14252-14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day, P. M., D. R. Lowy, and J. T. Schiller. 2003. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 307:1-11. [DOI] [PubMed] [Google Scholar]
- 11.Day, P. M., C. D. Thompson, C. B. Buck, Y. Y. Pang, D. R. Lowy, and J. T. Schiller. 2007. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 81:8784-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giroglou, T., L. Florin, F. Schäfer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung, S. L., P. L. Lee, H. W. Chen, L. K. Chen, C. L. Kao, and C. C. King. 1999. Analysis of the steps involved in dengue virus entry into host cells. Virology 257:156-167. [DOI] [PubMed] [Google Scholar]
- 15.Joyce, J. G., J.-S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 16.Kämper, N., P. M. Day, T. Nowak, H. C. Selinka, L. Florin, J. Bolscher, L. Hilbig, J. T. Schiller, and M. Sapp. 2006. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J. Virol. 80:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Müller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 75:9201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann, M. J., N. M. Sherer, C. B. Marks, M. Pypaert, and W. Mothes. 2005. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 170:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lidholt, K., J. L. Weinke, C. S. Kiser, F. N. Lugemwa, K. J. Bame, S. Cheifetz, J. Massague, U. Lindahl, and J. D. Esko. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA 89:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., Z. Shriver, R. M. Pope, S. C. Thorp, M. B. Duncan, R. J. Copeland, C. S. Raska, K. Yoshida, R. J. Eisenberg, G. Cohen, R. J. Linhardt, and R. Sasisekharan. 2002. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein D. J. Biol. Chem. 277:33456-33467. [DOI] [PubMed] [Google Scholar]
- 21.Patterson, N. A., J. L. Smith, and M. A. Ozbun. 2005. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J. Virol. 79:6838-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards, R. M., D. R. Lowy, J. T. Schiller, and P. M. Day. 2006. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 103:1522-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rommel, O., J. Dillner, C. Fligge, C. Bergsdorf, X. Wang, H. C. Selinka, and M. Sapp. 2005. Heparan sulfate proteoglycans interact exclusively with conformationally intact HPV L1 assemblies: basis for a virus-like particle ELISA. J. Med. Virol. 75:114-121. [DOI] [PubMed] [Google Scholar]
- 24.Roth, S. D., M. Sapp, R. E. Streeck, and H. C. Selinka. 2006. Characterization of neutralizing epitopes within the major capsid protein of human papillomavirus type 33. Virol. J. 3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidtke, M., A. Karger, A. Meerbach, R. Egerer, A. Stelzner, and V. Makarov. 2003. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 311:134-143. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtke, M., O. Riabova, H. M. Dahse, A. Stelzner, and V. Makarov. 2002. Synthesis, cytotoxicity and antiviral activity of N,N′-bis-5-nitropyrimidyl derivatives of dispirotripiperazine. Antivir. Res. 55:117-127. [DOI] [PubMed] [Google Scholar]
- 27.Schmidtke, M., P. Wutzler, and V. Makarov. 2004. Novel opportunities to study and block interactions between viruses and cell surface heparan sulfates by using dispirotripiperazines. Lett. Drug Des. Discov. 1:35-44. [Google Scholar]
- 28.Selinka, H. C., T. Giroglou, T. Nowak, N. D. Christensen, and M. Sapp. 2003. Further evidence that papillomavirus particles exist in two distinct conformations. J. Virol. 77:12961-12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selinka, H. C., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 30.Se-Thoe, S. Y., A. E. Ling, and M. M. Ng. 2000. Alteration of virus entry mode: a neutralisation mechanism for dengue-2 virus. J. Med. Virol. 62:364-376. [DOI] [PubMed] [Google Scholar]
- 31.Smith, J. L., S. K. Campos, and M. A. Ozbun. 11 July 2007. Human papillomavirus type 31 uses a caeolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J. Virol. doi: 10.1128/JVI.00988-07. [DOI] [PMC free article] [PubMed]
- 32.Talarico, L. B., and E. B. Damonte. 2007. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 363:473-485. [DOI] [PubMed] [Google Scholar]
- 33.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpers, C., P. Schirmacher, R. E. Streeck, and M. Sapp. 1994. Assembly of the major and the minor capsid protein of human papillomavirus type 33 into virus-like particles and tubular structures in insect cells. Virology 200:504-512. [DOI] [PubMed] [Google Scholar]
- 35.Yang, R., P. M. Day, W. H. T. Yutzy, K. Y. Lin, C. F. Hung, and R. B. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]