Abstract
Cells carry a variety of molecules, referred to as pathogen recognition receptors (PRRs), which are able to sense invading pathogens. Interaction of PRRs with viral compounds instigates a signaling pathway(s), resulting in the activation of genes, including those for type I interferon (IFN), which are critical for an effective antiviral response. Here we demonstrate that the double-stranded RNA (dsRNA)-dependent protein kinase PKR, which has been shown to function as a PRR in cells treated with the dsRNA mimetic poly(I:C), serves as a PRR in West Nile virus (WNV)-infected cells. Evidence for PKR's role as a PRR was obtained from both human and murine cells. Using mouse embryonic fibroblasts (MEFs), we demonstrated that PKR gene knockout, posttranscriptional gene silencing of PKR mRNA using small interfering RNA (siRNA), and chemical inhibition of PKR function all interfered with IFN synthesis following WNV infection. In three different human cell lines, siRNA knockdown and chemical inhibition of PKR blocked WNV-induced IFN synthesis. Using the same approaches, we demonstrated that PKR was not necessary for Sendai virus-induced IFN synthesis, suggesting that PKR is particularly important for recognition of WNV infection. Taken together, our data suggest that PKR could serve as a PRR for recognition of WNV infection.
West Nile virus (WNV) is a member of the genus Flavivirus, within the family Flaviviridae. This genus contains many important human pathogens, including Japanese encephalitis virus (JEV), dengue virus (DENV), yellow fever virus, and WNV, which are responsible for significant morbidity and mortality worldwide. During the time that WNV has been present in the United States, it has become a major public health problem that is responsible for significant morbidity and mortality, and it has now spread into Canada, Mexico, and the Caribbean. Now reported in every contiguous state within the United States, the total number of documented human cases has reached nearly 23,000, resulting in nearly 900 fatalities over the past seven seasons (http://www.cdc.gov/ncidod/dvbid/westnile/index.htm). The most severe disease manifestations of WNV infection, such as West Nile encephalitis and West Nile meningitis, account for approximately 40% of the documented cases and occur primarily in the immunocompromised and the elderly.
Flaviviruses, like other viruses, are susceptible to the action of interferon (IFN) in vitro (3, 50) and in vivo, as studies with mice have demonstrated that animals with deficits in IFN signaling demonstrate higher mortality due to flavivirus infections than do wild-type (WT) mice (30, 51). Additionally, molecular studies demonstrate that WNV is capable of blocking IFN signaling (24, 39, 54), although cells are still capable of producing IFN in response to WNV infection (19, 54).
The host's ability to produce IFN in response to infection is a key part of the innate immune response. This early, nonspecific immune response is triggered when cellular factors, called pathogen recognition receptors (PRRs), recognize specific pathogen-associated molecular patterns (1, 58, 59). PRRs recognize different pathogen-associated molecular patterns, including carbohydrate components, lipoproteins, specific DNA motifs, double-stranded RNA (dsRNA), and single-stranded RNA, the last two of which are considered to be the most important for recognition of RNA viruses (62). To date, the following three classes of PRRs which recognize dsRNA have been identified: dsRNA-dependent protein kinase R (PKR) (10), the RNA helicases retinoic acid-inducible gene I (RIG-I) (66) and melanoma differentiation-associated gene 5 (mda-5) (31), and Toll-like receptor 3 (TLR3) (2, 64). Additionally, two TLRs, TLR7 and TLR8, have been shown to recognize single-stranded RNA and may be particularly important in inducing high levels of IFN-α early in infection in immune system cells, such as plasmacytoid dendritic cells (4, 12, 27).
Recognition of extracellular or cytoplasmic dsRNA is thought to occur primarily via two different classes of PRRs, i.e., TLR3 and RIG-I/mda-5. Activation of TLR3, which is present on the cell surface or within endocytic vesicles (20, 42, 46, 57), results in the recruitment of the adaptor molecule TRIF (TIR domain-containing adaptor inducing IFN) (48, 64). TRIF subsequently recruits and activates two kinases, IκB kinase-ɛ (IKK-ɛ) and TANK-binding kinase-1 (TBK-1), which phosphorylate IRF3 (17, 56). Phosphorylated IRF3 forms a homodimer and translocates into the nucleus, where it, along with the coactivator CBP/p300, induces the expression of multiple antiviral genes, including that for IFN-β (14, 37, 67). RIG-I and mda-5, on the other hand, are intracellular RNA helicases which also utilize IRF3 as a transcriptional activator. Once activated by intracellular dsRNA, RIG-I/mda-5 utilizes the adaptor protein IPS-1 (also called VISA, MAVs, or Cardif) to recruit a linker kinase(s) to phosphorylate IRF3 (25, 33, 44, 55, 63). As with TLR3-mediated signaling, activation of IRF3 via RIG-I induces the expression of multiple antiviral genes, including that for IFN-β. The importance of TLR3 and RIG-I/mda-5 as PRRs for WNV infection is implied by studies indicating that the downstream mediator of their activity, IRF3, is an important part of WNV infection. Reports have shown that WNV activates IRF3 late in infection, and cells derived from mice lacking IRF3 demonstrated larger plaque sizes as well as prolonged WNV particle release (19). In addition, WNV infection can suppress TLR3-mediated IRF3 phosphorylation early in infection, suggesting that blocking IRF3 activity could be beneficial for the virus (54). A role for RIG-I-mediated signaling in response to flavivirus infections has also been established. A recent report indicated a role for RIG-I-mediated signaling during JEV (7, 32) and DENV (7) infections, and another report indicated that cells which lacked RIG-I demonstrated a delay in the induction of the ISG56 and ISG54 genes, two IRF3 target genes, in response to WNV infection (18).
In comparison to TLR3 and RIG-I/mda-5, PKR has been studied much less as a PRR. PKR is best known as an IFN-stimulated gene (ISG) which phosphorylates eukaryotic initiation factor 2α (eIF-2α), resulting in a block of translation of both viral and cellular RNAs (9, 43). Work from the late 1980s and early 1990s indicates that PKR, which is activated by cytoplasmic dsRNA, could also be important for IFN-β production (26, 41, 70), and several recent reviews have described PKR as a PRR for cytoplasmic dsRNA (29, 45). Recently, Diebold et al. demonstrated that PKR was required for IFN production in bone marrow-derived dendritic cells (BM-DCs) following transfection with the dsRNA mimetic poly(I:C) (13), suggesting that in these cells, PKR could act as a PRR.
In this report, we utilized three different methods to demonstrate that PKR is a potential PRR for recognition of WNV. First, mouse embryonic fibroblast (MEF) cells obtained from PKR null mice were shown to produce less IFN than WT MEF cells following infection with WNV-derived virus-like particles (VLPs). Second, treatment of MEFs or three different human cell lines with a PKR inhibitor blocked IFN production following VLP infection. Third, posttranslational silencing of PKR in human and mouse cells abrogated IFN production following WNV VLP infection. Interestingly, Sendai virus (SeV)-induced IFN levels were not affected by the inhibition of PKR activity, indicating that the ability of PKR signaling to induce IFN is especially important for WNV infection.
MATERIALS AND METHODS
Cell lines, virus, and VLPs.
Two different wild-type MEF cell lines (WT MEF-G, provided by I. Frolov, University of Texas Medical Branch [UTMB], and WT MEF-D, provided by M. Gale, UT Southwestern), PKR null MEF cells (65), PKR-RNase L null MEF cells (69) (both kindly provided by I. Frolov, UTMB), and STAT1 null MEF cells (provided by J. Durbin, Ohio State University) (15) were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 1 mM sodium pyruvate, antibiotics, and nonessential amino acids. Hec1B cells (provided by K. Narayanan, UTMB) were propagated in DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, antibiotics, and nonessential amino acids. MRC-5 cells (American Type Culture Collection) were propagated in modified Eagle's medium (MEM) supplemented with 10% FBS, 1 mM sodium pyruvate, antibiotics, and nonessential amino acids. A549 cells (provided by K. Narayanan, UTMB) were propagated in DMEM/F-12 50/50 medium supplemented with 10% FBS and antibiotics. Huh7 cells were propagated in DMEM supplemented with 10% FBS and antibiotics (50). The generation of WNV VLPs by utilizing a Venezuelan equine encephalitis virus replicon encoding the WNV structural proteins has been described previously (16). For these studies, we generated WNV VLPs by utilizing a WNV replicon (WNR-CNS1-5) containing a full capsid-encoding region, as this replicon has been shown to replicate to high titers (16; unpublished data). In most of the studies, WNR-CNS1-5 VLPs were produced in a packaging cell line expressing an E protein mutated at position 138 (Glu to Lys), a mutation shown to confer heparan sulfate (HS)-mediated infections (36).WNV VLPs were titrated on each of the cell lines described above, and multiplicities of infection (MOIs) were calculated based on the specific infectivity of each cell line. In most cases, HS-binding VLPs (VLPHS) were used due to their enhanced infectivity on many cell types (P. W. Mason, unpublished data). As expected, we noted no difference in antigen expression levels in HS-binding VLPs versus non-HS-binding VLPs. The live WNV used in this study was a low-passage virus recovered from BHK cells electroporated with an infectious cDNA clone of a human 2002 Texas isolate (50) modified as described above to encode an HS-binding E protein. SeV Cantell was obtained from Charles River Laboratories. The vesicular stomatitis virus (VSV) used was the Hazelhurst strain of the New Jersey serotype (obtained from R. B. Tesh, UTMB).
Infections and dsRNA treatments.
Cell monolayers were infected with WNV VLPs or VLPHS at an MOI of 3. VLPs (or medium alone for mock infections) were diluted in DMEM containing 1% FBS, 1% antibiotics, and 1 mM HEPES, added to the cell monolayer, and incubated for approximately 1 h at 37°C to allow for attachment and entry. Following incubation, the VLPs were removed from the cells and replaced with DMEM containing 1% FBS, 1% antibiotics, and 1 mM HEPES, and the cells were incubated for approximately 24 h at 37°C prior to being harvested. For SeV infections, cell monolayers were infected with 40 hemagglutination units/ml of SeV as described above. Following infection, the SeV-infected cells were incubated for approximately 8 h at 37°C prior to being harvested. WNV VLPs were incubated under a UV fluorescent lamp (254 nm, 4 W, 10 cm) for 2 min to inactivate infectious VLPs and then diluted in DMEM containing 1% FBS, 1% antibiotics, and 1 mM HEPES. Cells were infected with UV-inactivated WNV VLPs as described above. VSV infections were performed as described above, except that infected cells were incubated for 8 h prior to being harvested.
Poly(I:C) treatments.
For intracellular stimulation with poly(I:C) (EMD Biosciences), 40 μg of poly(I:C) was transfected into cell monolayers in 48-well plates. Briefly, 40 μg poly(I:C) was incubated with 0.5 μl DharmaFECT-1 transfection reagent (Dharmacon) diluted in 50 μl DharmaFECT cell culture reagent (Dharmacon) for approximately 10 min. The poly(I:C)-DharmaFECT-1 complexes were added to the cell monolayers (50 μl/1-cm2 well), and the volume was raised to 250 μl with DMEM containing 1% FBS, 1% antibiotics, and 1 mM HEPES. Transfected monolayers were incubated for 24 h at 37°C. For extracellular stimulation with poly(I:C), 250 μl of DMEM supplemented with 1% FBS, antibiotics, 1 mM HEPES, and 40 μg/ml poly(I:C) was added to cell monolayers and incubated for approximately 24 h at 37°C.
siRNA treatments.
Monolayers of cells were transfected with either 30 nM Dharmacon plus Smartpool PKR-specific small interfering RNA (siRNA) (murine or human specific), 30 nM Dharmacon plus Smartpool nontargeting siRNA negative control (NEG siRNA; Dharmacon), RIG-I-specific siRNA (targeted to GGAAGAGGTGCAGTATATT [Ambion]), or medium alone, using DharmaFECT-1 transfection reagent. Briefly, siRNAs were incubated with 0.5 μl DharmaFECT-1 diluted in 50 μl DharmaFECT cell culture reagent for approximately 10 min. The siRNA-DharmaFECT-1 complexes were added to the wells (50 μl/1-cm2 well), and 200 μl of cells at a concentration of 10,000 cells/ml was added to the complexes and incubated for approximately 72 h at 37°C. Following the incubation period, the transfected cells were treated with poly(I:C) or infected with WNV VLPs as described above.
PKR inhibition.
Cells were treated with 100 μM PKR inhibitor (PKR-I; CalBiochem) or PKR-I negative control (PKR-N; CalBiochem) for 1 h. The medium was removed, and cells were infected or treated with poly(I:C) as described above.
IFN bioassay.
Supernatants harvested from treated cells were clarified and serially diluted in DMEM supplemented with 1% FBS, 1% antibiotics, and 1 mM HEPES side by side with either a human IFN-α standard (NIAID) or a murine IFN-β standard (NIAID). The murine IFN-β standard was produced from mouse L cells, and the human IFN-α standard was produced from FS-4 human foreskin fibroblast cells. Both standards were acquired through the NIAID Reference Reagent Repository operated by either Braton Biotech, Inc. (human IFN-α), or KamTek, Inc. (murine IFN-β). Either WT MEF-G cells or Huh7 cells were treated with the IFN standard or supernatant dilutions for approximately 24 h and then infected with firefly luciferase (Fluc)-expressing WNV VLPs (WNV VLP-319) (16). At 24 hours postinfection, Fluc expression was assayed by lysing the cell monolayers with a Fluc substrate as previously described (16). The IFN concentration (U/ml) of each sample was determined by plotting the dilution giving 50% inhibition of WNV VLP Fluc activity and multiplying this value by the number of units of the IFN standard (human or mouse) that produced 50% inhibition in side-by-side assays. The limits of detection for these assays were approximately 2 and 5 U/ml of human and mouse IFN, respectively. Units of activity were expressed in terms of U/mg of protein in the treated monolayers (typically within 0.5 to 1.5 mg of cellular protein per ml of cell lysate analyzed), as determined by protein assay (see below).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis.
Monolayers of treated cells were rinsed in phosphate-buffered saline, harvested in 75 μl lysis buffer (0.1% Triton X-100, 300 mM NaCl, 50 mM Tris, pH 7.69) containing 100 nM calyculin A (Upstate Cell Signaling Solutions), clarified, and stored at −20°C. Protein concentrations were determined by the Bio-Rad DC protein assay (Bio-Rad). Equal amounts of protein from each cell lysate (2 μg) were resolved in NuPAGE 4 to 12% Bis-Tris gels (Invitrogen). Following electrophoresis, proteins were transferred to an Immobilon polyvinylidene difluoride transfer membrane (Millipore) and blocked with phosphate-buffered saline containing 0.1% Tween 20 (Sigma) and 5% instant nonfat dry milk (Nestle) for at least 1 h at room temperature. Membranes were probed using the following monoclonal and polyclonal antibodies: goat anti-actin (Sigma), rabbit anti-PKR (D-20; Santa Cruz), rabbit anti-phospho-PKR threonine 451 (Cell Signaling), rabbit anti-phospho-eIF-2α serine 51 (Cell Signaling), rabbit anti-eIF-2α (Cell Signaling), and anti-IκBα (Santa Cruz). Membranes were then washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG; KPL) or HRP-conjugated goat anti-rabbit IgG (KPL). HRP-decorated protein bands were visualized using an ECL Plus Western blotting detection system (Amersham) followed by exposure on Kodak film.
NF-κB activation.
Monolayers of WT MEF-G or PKR null MEF cells were plated onto poly-l-lysine-coated LabTek chamber slides. The cells were either mock or WNV VLP infected and incubated for 16 or 24 h. Thirty minutes prior to fixation (see Fig. S1 in the supplemental material) or harvest (see above), selected wells of mock-infected cells were treated with either 50 or 500 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma) or 1% ethanol (diluent control). Treated cells utilized for Western blot analysis of IκBα degradation were prepared and run as described above.
Statistical analysis.
Student's t test was used to determine the significance of differences for these studies.
RESULTS
PKR is not critical for the development of an anti-WNV state in MEF cells.
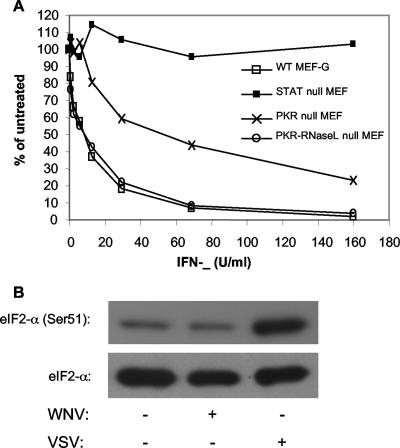
Several reports have indicated that pretreatment with IFN has an inhibitory effect on WNV infection (3, 50, 54). To determine whether PKR is important in this IFN-induced anti-WNV response, we treated monolayers of WT MEF cells, PKR null MEF cells, and PKR-RNase L null MEF cells with various concentrations of murine IFN-β and then tested their ability to be infected by WNV. Following treatment, the cells were infected with WNV for an additional 24 h. The results of this study indicated that while slightly more IFN was needed to protect PKR null cells from WNV infection, PKR-RNase L null MEF cells showed a response indistinguishable from that of WT MEF cells (Fig. 1A). These data indicate that PKR is not a critical factor in mediating the IFN-induced anti-WNV response. Since it seems unlikely that RNase L deletion would make PKR null cells better able to respond to IFN treatment, it seems likely that the difference observed between the two PKR null cell lines resulted from differences in the abilities of these two immortalized cell lines to respond to IFN treatment or to be infected by WNV due to differences independent of their PKR genotype.
FIG. 1.
PKR null MEF cells display no defect in their ability to establish an IFN-induced anti-WNV state. (A) Effect of IFN pretreatment on WNV infection. Cell monolayers were incubated with the indicated concentrations of murine IFN-β for approximately 24 h. The treated cells were infected with approximately 50 focus-forming units and incubated for an additional 48 h. The cells were fixed and assayed for WNV focus formation by immunohistochemistry (54). Data are presented as percentages of the foci formed in untreated, WNV-infected monolayers. Results are representative of data obtained from two independent experiments. (B) Western blot analysis showing levels of phosphorylated eIF-2α and total eIF-2α in WT MEF-G cells following infection with either WNV VLPs (24 h) or VSV (8 h).
To further probe the role of PKR in establishing an anti-WNV environment, we examined the levels of phosphorylated eIF-2α, a functional target of PKR antiviral action, in WNV- or VSV-infected cells. Similar to other reports, VSV infection did trigger eIF-2α phosphorylation in these experiments, whereas WNV VLP infection did not trigger eIF-2α phosphorylation (Fig. 1B), consistent with the fact that flaviviruses do not shut off host protein synthesis (38). Taken together, these data indicate that PKR does not serve as an essential IFN effector molecule during WNV infection of these cells.
PKR acts as a dsRNA PRR.
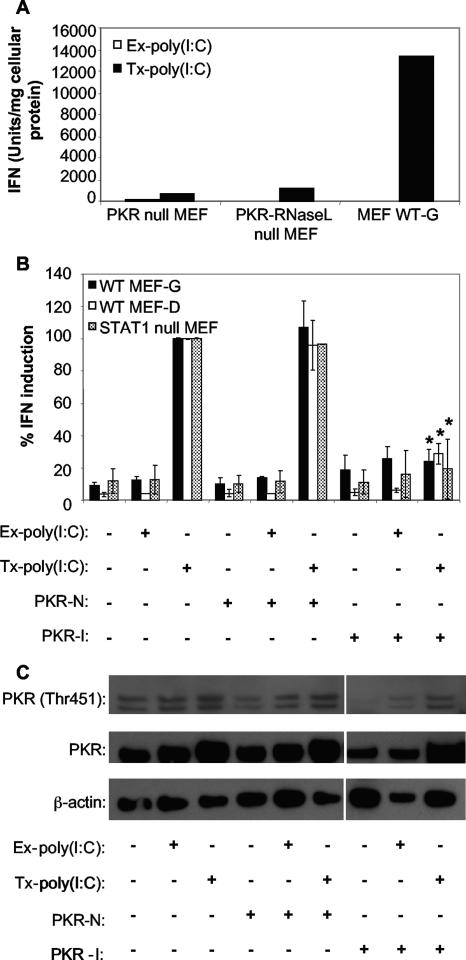
To determine whether PKR had an effect on dsRNA-induced IFN production in our system, poly(I:C) was added to the medium [extracellular dsRNA; Ex-poly(I:C)] or transfected into [intracellular dsRNA; Tx-poly(I:C)] WT MEF-G cells, PKR null MEF cells, or PKR-RNase L null MEF cells. The supernatants from these treated cells were harvested and assayed for IFN production by bioassay. Cells treated with Ex-poly(I:C), which stimulates IFN through TLR3-mediated signaling (64), had very low levels of IFN, regardless of the cell's genotype. However, 24 hours following Tx-poly(I:C) treatment, WT MEF-G cells produced high levels of IFN, whereas MEF cell lines lacking PKR produced 10-fold less IFN (Fig. 2A). These data indicate that the absence of PKR abrogates the ability of the cells to stimulate IFN gene expression following intracellular poly(I:C) stimulation. This suggests that PKR is a possible PRR in these MEF cells and that IFN stimulation is independent of TLR3 signaling.
FIG. 2.
IFN induction in MEF cells treated with poly(I:C) is dependent on PKR. (A) IFN production by WT MEFs and two different lineages of PKR null MEFs treated by the addition of poly(I:C) to their media [Ex-poly(I:C)] or transfected with poly(I:C) [Tx-poly(I:C)] for 24 h. IFN concentrations were determined by bioassay and were normalized to mg of cellular protein in the treated monolayers (see Materials and Methods). Data shown are from one of two experiments showing similar results. (B) Effect of chemical inhibition of PKR activity on the ability of Ex-poly(I:C) and Tx-poly(IC) to induce IFN production in two different WT MEF cell lines (WT MEF-G and WT MEF-D) or STAT1 null MEF cells. PKR-I is a specific inhibitor of PKR, and PKR-N is a closely related compound with no anti-PKR activity. Cells were incubated with the indicated compounds for 1 hour and then treated with poly(I:C) for 24 h (see Materials and Methods). IFN activity was determined as described for panel A and then normalized to IFN levels produced in cells transfected with poly(I:C) and treated without either PKR-I or PKR-N. Error bars represent standard deviations between data obtained in two separate experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for Tx-poly(I:C)-stimulated cells treated with PKR-N. (C) Western blot showing the levels of the phospho-Thr451 form of PKR, total PKR, and β-actin in lysates of MEF WT-G cells treated with the indicated compounds and exposed to poly(I:C) by the indicated methods. The presence of two bands in the Western blot is curious. It is possible that these are two separate forms of PKR, as they were affected identically by treatment with PKR-I. However, it is unclear why there are not two bands in blots probed for total PKR levels.
To confirm these results and to ensure that the differences observed in IFN induction were not due to defects in the clonal knockout cell lines unrelated to the PKR null genotype, we utilized a small-molecule inhibitor of PKR activity. Although 2-aminopurine is widely used as a PKR inhibitor, we were unable to demonstrate an inhibition in PKR autophosphorylation (by monitoring phosphorylation at Thr451). Therefore, we utilized an imidizolo-oxindole compound (PKR-I) shown to act as a potent PKR inhibitor (28). As an added control, we utilized PKR-N, a closely related oxindole compound shown to be ineffective in inhibiting PKR activity (28). To test the effect of PKR-I on dsRNA-induced IFN production, WT MEF-G cells were pretreated with 100 μM PKR-I or 100 μM PKR-N followed by stimulation with either Ex- or Tx-poly(I:C). Poly(I:C)-transfected WT MEF-G cells treated with PKR-I produced lower concentrations of IFN than did poly(I:C)-transfected, PKR-N-treated WT MEF-G cells (Fig. 2B). Consistent with the results shown in Fig. 2A, treatment with Ex-poly(I:C) induced little or no IFN in these cells (Fig. 2B). Identical treatments in a second WT MEF cell line, WT MEF-D, demonstrated similar results, indicating that the observed effect in WT MEF-G cells was not specific for the cell line utilized. Additionally, experiments performed in STAT1 null MEF cells produced similar results (Fig. 2B). Since these MEF cells are deficient in STAT1 (15), a critical component of IFN signaling, these data demonstrate that the role for PKR during IFN induction was not dependent on IFN signaling, which is known to induce PKR expression.
To ensure that the PKR-I treatment was effective at inhibiting PKR activity, Western blot analysis was performed on the cell lysates harvested from WT MEF-G cells. Blots were probed for the presence of PKR phosphorylated at the threonine residue at site 451. This residue is an autophosphorylation site critical for kinase activity (49). Cells treated with PKR-I but not PKR-N had low levels of phosphorylated PKR (Fig. 2C). The abrogation of IFN production following chemical inhibition of PKR implicates PKR as a possible PRR in response to cytoplasmic dsRNA in MEF cells, consistent with our observations from PKR gene knockout MEFs.
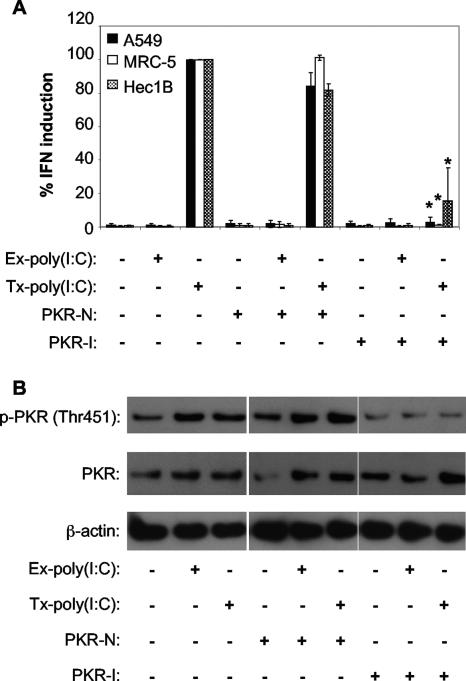
To determine if PKR is important for IFN production in other cell types, we studied several human cell lines, including MRC-5, a human lung fibroblast cell line; A549, a lung epithelial cell line; and Hec1B, a uterine epithelial cell line. All of these cell lines are permissive for WNV infection and produce IFN-α/β in response to WNV infection (data not shown). Additionally, Hec1B cells were included in our studies because the IFN receptor present on these cells binds IFN very inefficiently, resulting in impaired IFN signaling (21, 60).These cells act as the human equivalent of STAT1 null MEFs and can be used to examine the effects of PKR activity in the absence of IFN signaling. In support of the observations with the MEF cell lines, treatment of the MRC-5 cells, A549 cells, and Hec1B cells with PKR-I inhibited their ability to produce IFN following Tx-poly(I:C) treatment (Fig. 3A). Additionally, Ex-poly(I:C) stimulation resulted in little or no detectable IFN production, suggesting that poly(I:C)-induced IFN expression is stimulated via a TLR3-independent pathway in these cells (Fig. 3A).
FIG. 3.
IFN induction in human cell lines treated with poly(I:C) is dependent on PKR. (A) Effect of chemical inhibition of PKR activity on the ability of Ex-poly(I:C) or Tx-poly(I:C) to induce IFN production in three different human cell lines. A549, MRC-5, and Hec1B cells were incubated with the indicated compounds for 1 hour and then treated with poly(I:C) for 24 h (see Materials and Methods). IFN activity was determined as described in the legend to Fig. 1 and then normalized to IFN levels produced in cells transfected with poly(I:C) and treated without either PKR-I or PKR-N. PKR-I is a specific inhibitor of PKR, and PKR-N is a closely related compound with no anti-PKR activity. Error bars represent the standard deviations between data obtained from two independent experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for Tx-poly(I:C)-stimulated cells treated with PKR-N. (B) Western blot showing the levels of the phospho-Thr451 form of PKR, total PKR, and β-actin in lysates of A549 cells treated with the indicated compounds and exposed to poly(I:C) by the indicated methods.
Western blot analysis of cell lysates from PKR-I-treated A549 cells demonstrated lower levels of phosphorylated PKR than those in mock- or PKR-N-treated cells (Fig. 3B). Interestingly, low levels of PKR were induced in cells transfected with poly(I:C), even in the presence of PKR-I. This suggests that IFN is produced at low levels via PKR-independent pathways (see Fig. 8), resulting in autocrine stimulation leading to an increase in the expression of ISGs, including PKR. Analysis of lysates harvested from MRC-5 cells was similar to that with lysates from A549 cells (data not shown). As expected, levels of PKR in Hec1B cells did not increase following intracellular poly(I:C) treatment (data not shown), consistent with the fact that Hec1B cells do not respond to IFN stimulation.
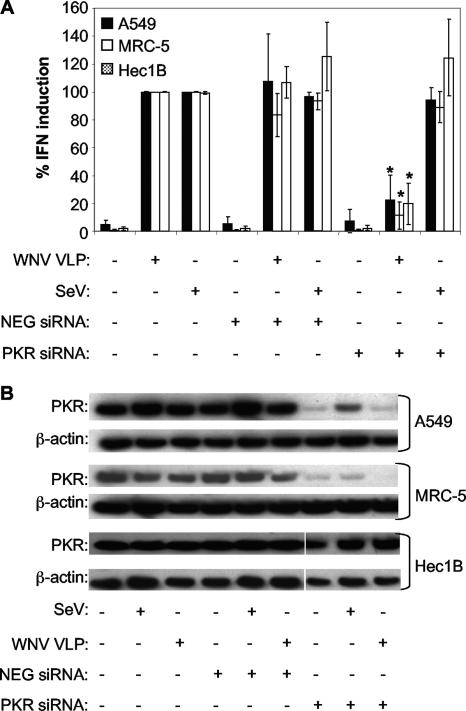
FIG. 8.
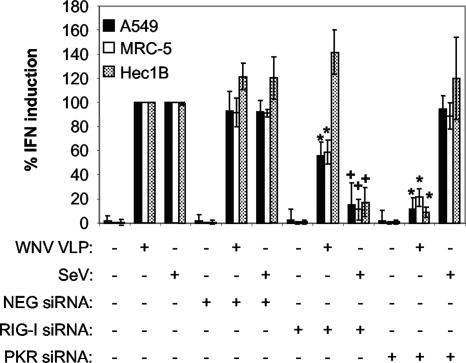
IFN induction in human cells infected with WNV VLPs, but not SeV, is blocked by transfection of PKR-specific siRNA. (A) Effect of siRNA transfection on the ability of WNV VLPs or SeV to induce IFN production in A549, MRC-5, and Hec1B cells. The PKR siRNA is specific for the human PKR transcript, whereas NEG siRNA is a nonsilencing control siRNA. Cells were transfected with the indicated siRNA (or treated with transfection agents alone), incubated at 37°C for 3 days to permit posttranscriptional knockdown of the PKR mRNA, and then infected with WNV VLPs for 24 h or SeV for 8 h (see Materials and Methods). IFN activity was determined as described in the legend to Fig. 1 and then normalized to IFN levels produced in cells infected with WNV VLPs or SeV and treated without any siRNA. Error bars represent standard deviations between data obtained in three separate experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for WNV VLP-infected, NEG siRNA-transfected cells. (B) Western blot showing levels of total PKR and β-actin in lysates of A549, MRC-5, and Hec1B cells following transfection with the indicated siRNAs and infection with WNV VLPs or SeV.
PKR contributes to WNV VLP-induced IFN production.
To study the role of PKR during WNV-induced IFN production, we utilized WNV VLPs (53). These VLPs infect cells and initiate genome replication in a manner indistinguishable from that of WT virus, but they are unable to produce any progeny virions. We utilized VLPs in these studies to prevent the potentially confounding effects of secondary replication cycles obtained with WT WNV. Due to low infectivities with several of our cell lines, many of our experiments were performed using HS-binding VLPs, referred to as WNV VLPHS (see Materials and Methods).
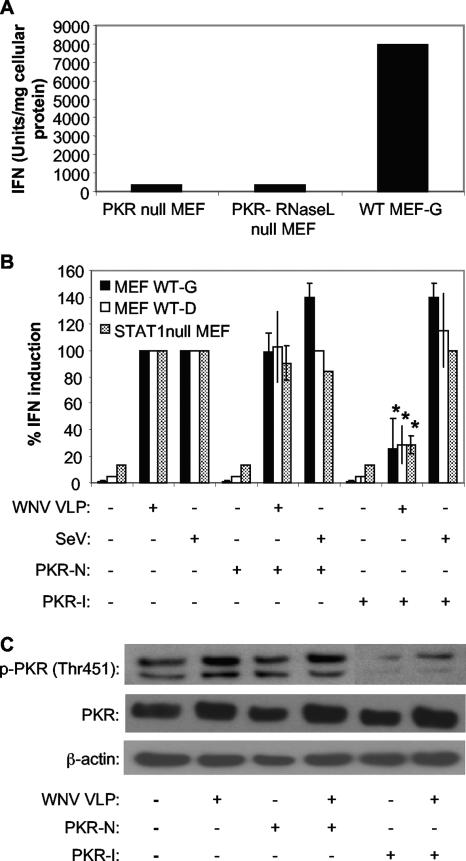
IFN bioassays performed on supernatants from WNV VLP-infected MEF cells revealed that cells lacking PKR produced significantly lower levels of IFN than did WT MEF-G cells (Fig. 4A), implicating PKR as a potential PRR for WNV infection. Additionally, PKR-I-treated WT MEF-G cells infected with WNV VLPHS exhibited lower levels of IFN than did PKR-N- or mock-treated WT MEF-G cells (Fig. 4B). However, SeV-induced induction of IFN was not affected by PKR-I treatment, suggesting that treatment with PKR-I did not generate nonspecific effects on IFN synthesis (Fig. 4B). Incubation with UV-inactivated WNV VLPHS did not induce IFN production (data not shown), consistent with previous reports indicating that WNV replication is necessary for host recognition of WNV (18).
FIG. 4.
IFN induction in MEF cells infected with WNV VLPs is dependent on PKR. (A) IFN production by WT MEFs and two different lineages of PKR null MEFs infected with WNV VLPs for 24 h. Data shown are from one of two experiments showing similar results. IFN concentrations were determined by bioassay as described in the legend to Fig. 1. (B) Effect of chemical inhibition of PKR activity on the ability of WNV VLPs or SeV to induce IFN production in two different WT MEF cell lines (WT MEF-G and WT MEF-D) or STAT1 null MEF cells. Cells were incubated with the indicated compounds for 1 hour and then infected with WNV VLPs for 24 h or SeV for 8 h (see Materials and Methods). IFN activity was determined as described for panel A and then normalized to IFN levels produced in cells infected with WNV VLPs or SeV and treated without either PKR-I or PKR-N. Error bars represent standard deviations between data obtained in two independent experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for WNV VLP-infected, PKR-N-treated cells. (C) Western blot showing the levels of the phospho-Thr451 form of PKR, total PKR, and β-actin in lysates of WT MEF-G cells treated with the indicated compounds and infected with WNV VLPs.
Analysis of activated PKR levels within WT MEF-G cells showed that the inhibitor was quite effective at inhibiting PKR phosphorylation. Additionally, WNV VLPHS infection resulted in an increase in both total PKR and activated PKR levels (Fig. 4C). Interestingly, WNV VLPHS induced low levels of PKR protein in PKR-I-treated WT MEF-G cells, suggesting that IFN produced via PKR-independent mechanisms was stimulating expression of this ISG (Fig. 4C), as described above for Tx-poly(I:C)-stimulated cells treated with this inhibitor.
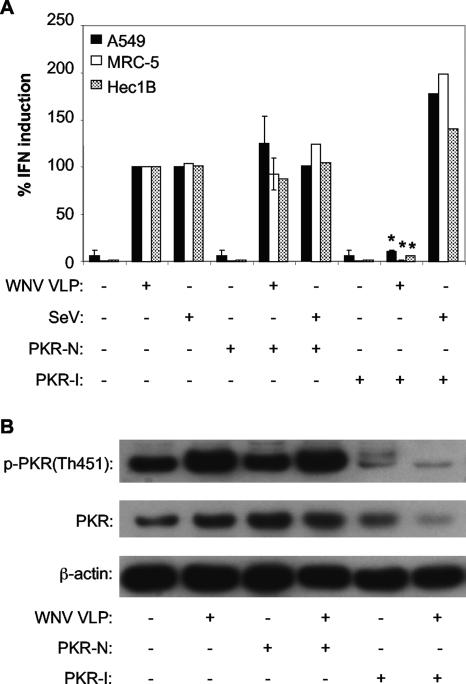
To determine whether PKR was involved in WNV-induced IFN production in human cell lines, A549 cells, MRC-5 cells, and Hec1B cells were pretreated with PKR-I or PKR-N and infected with WNV VLPHS. All of these human cell lines demonstrated a nearly complete abrogation of WNV VLPHS-induced IFN production following PKR-I treatment (Fig. 5A). As with the MEF cell lines, SeV-induced IFN production in these cells was unaffected by PKR-I treatment (Fig. 5A).
FIG. 5.
IFN induction in human cells infected with WNV VLPs, but not SeV, is dependent on PKR. (A) Effect of chemical inhibition of PKR activity on the ability of WNV VLPs or SeV to induce IFN production in A549, MRC-5, or Hec1B cells. Cells were incubated with the indicated compounds for 1 hour and then infected with WNV VLPs for 24 h or SeV for 8 h (see Materials and Methods). IFN activity was determined by bioassay as described in the legend to Fig. 1 and then normalized to IFN levels produced in cells infected with WNV VLPs or SeV and treated without either PKR-I or PKR-N. Error bars represent standard deviations between data obtained in two separate experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for WNV VLP-infected, PKR-N-treated cells. (B) Western blot showing the levels of the phospho-Thr451 form of PKR, total PKR, and β-actin in lysates of A549 cells treated with the indicated compounds and infected with WNV VLPs.
As expected, WNV VLPHS infection induced PKR phosphorylation in A549 cells, and lower levels of phosphorylated PKR were detected following PKR-I treatment (Fig. 5B). However, in these cells, total PKR protein levels were not increased by WNV VLPHS infection, in contrast to the case in MEF cells (Fig. 4C), where low levels of IFN produced in PKR-I-treated cells appeared to increase total levels of PKR, a readily inducible ISG. In fact, in WNV VLPHS-infected, PKR-I-treated A549 cells, only very low levels of PKR protein were detected (Fig. 5B). This is a puzzling point. One possible explanation is that phosphorylated PKR present in normal, non-PKR-I-treated cells self-regulates its expression. PKR-I treatment would be expected to lower total PKR synthesis. However, this phenomenon was not observed in any of the other cell types we tested, so we are unsure if self-regulation can explain the effect of PKR-I down-regulation of PKR expression in A549 cells. Western blot analyses of cell lysates from MRC-5 and Hec1B cells demonstrated that WNV VLP infection produced phosphorylated PKR in all samples that produced high levels of IFN (results not shown) and that PKR-I blocked phosphorylation, consistent with its ability to block IFN synthesis (Fig. 5A).
Inhibition of PKR activity abrogates WNV-induced IFN production.
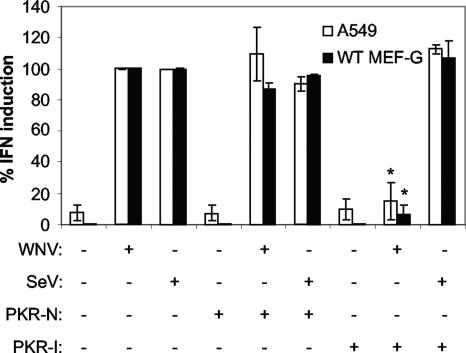
To ensure that the inhibition of IFN production was not an artifact of the VLP preparations, we examined the ability of the PKR-I to disrupt WNV-induced IFN production in both WT MEF-G cells and A549 cells. These experiments demonstrated that PKR-I treatment significantly reduced the levels of WNV-induced IFN produced in both WT MEF-G cells and A549 cells (Fig. 6). In contrast, PKR-I treatment had no inhibitory effect on SeV-induced IFN production in these cells (Fig. 6). These data are consistent with our WNV VLP studies demonstrating the importance of PKR for inducing IFN synthesis following WNV infection and indicate that, as we expected, VLPs mimic live virus in inducing IFN synthesis.
FIG. 6.
Inhibiting PKR abrogates WNV-induced IFN production. The graph shows the effect of PKR-I on WNV-induced IFN synthesis. Monolayers of A549 cells or WT MEF-G cells were treated with PKR-N or PKR-I followed by infection with SeV or HS-binding WNV (MOI = 3). IFN concentrations were determined by bioassay and were normalized to mg of cellular protein in the treated monolayers (see Materials and Methods). Error bars represent standard deviations between data obtained in two separate experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for WNV-infected cells treated with PKR-N.
Silencing PKR expression impairs WNV VLP-induced IFN production.
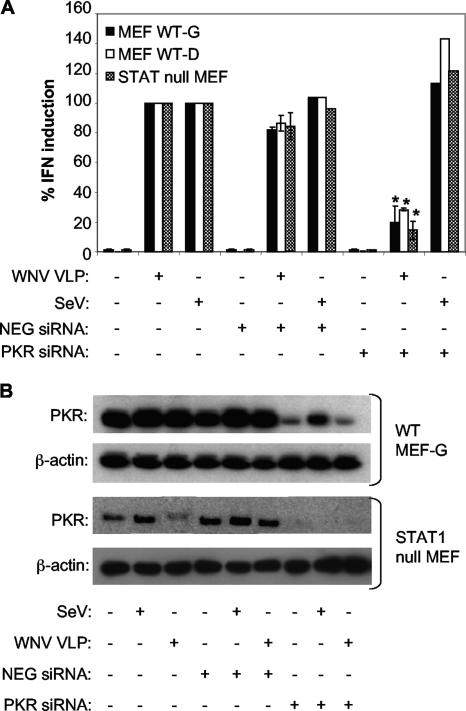
To confirm the role of PKR-mediated IFN production in murine and human cells, WT MEF-G, WT MEF-D, and STAT1 null MEF cells were transfected with either a PKR-specific siRNA or NEG siRNA. To allow for optimum posttranslational gene silencing (data not shown), the transfected cells were incubated for 72 h prior to infection with WNV VLPHS. Following VLP infection, PKR siRNA-transfected MEF cell lines (WT MEF-G, WT MEF-D, and STAT1 null MEFs) produced significantly less IFN than either untransfected cells or cells transfected with NEG siRNA (Fig. 7A). Silencing PKR expression in these cells did not affect SeV-induced IFN production (Fig. 7A), consistent with our previous findings showing that PKR activity was not needed for SeV-induced IFN production. Analysis of PKR protein expression in WT MEF-G cells showed that the PKR siRNA markedly decreased the constitutive level of PKR. However, there was a slight increase in PKR levels in the presence of PKR siRNA following infection with WNV VLPHS (Fig. 7B), consistent with an autocrine ISG response in these cells and subsequent stimulation of PKR mRNA expression in excess of the cell's silencing machinery. STAT1 null MEF cells, on the other hand, did not demonstrate a WNV VLP-induced increase in PKR protein level in the presence of PKR siRNA, as these cells are unable to respond to IFN. Analysis of cell lysates from WT MEF-D cells demonstrated similar levels of PKR compared to those in WT MEF-G cells (data not shown).
FIG. 7.
IFN induction in MEF cells infected with WNV VLPs, but not SeV, is blocked by transfection of PKR-specific siRNA. (A) Effect of siRNA transfection on the ability of WNV VLPs or SeV to induce IFN production in two different WT MEF cell lines (WT MEF-G and WT MEF-D) or STAT1 null MEF cells. The PKR siRNA is specific for the murine PKR transcript, whereas NEG siRNA is a nonsilencing control siRNA. Cells were transfected with the indicated siRNA (or treated with transfection agents alone), incubated at 37°C for 3 days to permit posttranscriptional knockdown of the PKR mRNA, and then infected with WNV VLPs for 24 h or SeV for 8 h (see Materials and Methods). IFN activity was determined as described in the legend to Fig. 1 and then normalized to IFN levels produced in cells infected with WNV VLPs or SeV and treated without any siRNA. Error bars represent standard deviations between data obtained in two separate experiments, in the case of WNV VLP infection, or one experiment, in the case of SeV infection. Asterisks indicate values that are statistically significant (P < 0.05) compared to those for WNV VLP-infected, NEG siRNA-transfected cells. (B) Western blot showing the levels of total PKR and β-actin in lysates of WT MEF-G and STAT1 null MEF cells following transfection with the indicated siRNAs and infection with WNV VLPs or SeV.
A549, MRC-5, and Hec1B cells transfected with PKR-specific siRNAs produced less IFN following stimulation with WNV VLPHS than did cells transfected with NEG siRNA (Fig. 8A). For both the A549 and Hec1B cells, however, the decrease in IFN induction observed was not as pronounced as the decrease observed following PKR-I treatment. This effect appears to be due, in part, to the effectiveness of posttranslational PKR silencing in these cells, since both A549 and Hec1B cells contained detectable levels of PKR following the siRNA treatment (Fig. 8B), whereas MRC-5 cells (like the MEF cells [see above]) had barely detectable levels of PKR (Fig. 8B).
RIG-I also contributes to IFN induction in A549 and MRC-5 cells, but not Hec1B cells.
Since it is thought that RIG-I may play a role in the establishment of an antiviral environment (18), we sought to examine the role of RIG-I-dependent IFN stimulation in our system. To this end, A549, MRC-5, and Hec1B cells were transfected with RIG-I-specific siRNA, PKR-specific siRNA, or NEG siRNA. Following a 72-h incubation, the transfected cells were infected with WNV VLPHS and assayed for IFN production. Although all three of the cell lines transfected with PKR-specific siRNA demonstrated a significant impairment of IFN induction, A549 and MRC-5 cells transfected with the RIG-I-specific siRNA showed only a moderate impairment of this induction (Fig. 9). Interestingly, Hec1B cells transfected with RIG-I siRNA did not demonstrate a reduction in IFN production (Fig. 9), indicating that in these cells the RIG-I pathway does not play a major role in WNV-induced IFN synthesis. Side-by-side studies with SeV infection demonstrated that under the transfection conditions used, RIG-I, but not PKR, siRNA treatment blocked SeV-mediated IFN production.
FIG. 9.
IFN induction in human cells infected with WNV VLPs is blocked more efficiently by transfection of PKR-specific siRNA than by transfection of RIG-I-specific siRNA, but the opposite is true for SeV-induced IFN induction. (A) Effect of siRNA transfection on the ability of WNV VLPs (or SeV) to induce IFN production in A549, MRC-5, and Hec1B cells. The PKR siRNA is specific for the human PKR transcript, the RIG-I siRNA is specific for the human RIG-I transcript, and NEG siRNA is a nonsilencing control siRNA. Cells were transfected with the indicated siRNA (or treated with transfection agents alone), incubated at 37°C for 3 days to permit posttranscriptional knockdown of the PKR and/or RIG-I mRNA, and then infected with SeV for 8 h or WNV VLPs for 24 h (see Materials and Methods). IFN activity was determined as described in the legend to Fig. 1 and then normalized to IFN levels produced in cells infected with WNV VLPs and treated without any siRNA. Error bars represent standard deviations between data obtained in two separate experiments, and asterisks indicate values that are statistically significant (P < 0.05) compared to those for NEG siRNA-transfected, WNV-infected cells. Plus signs represent values that are statistically significant (P < 0.05) compared to those for NEG siRNA-transfected, SeV-infected cells.
NF-κB may be involved in PKR-mediated IFN synthesis in response to WNV infection.
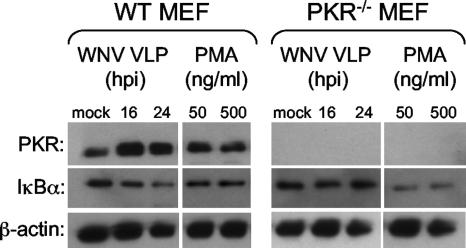
Since several reports have indicated that PKR is involved in a pathway leading to NF-κB signaling (34, 35, 68), we examined the ability of WNV VLPs to induced NF-κB activation in either WT MEF-G cells or PKR null MEF cells. NF-κB activation was examined by two methods, namely, degradation of IκBα (Fig. 10) and nuclear translocation of NF-κB (see Fig. S1 in the supplemental material). Monolayers of WT MEF-G or PKR null MEF cells were plated onto chamber slides and mock or WNV VLP infected for either 16 or 24 h (MOI = 5 based on titrations performed on WT MEF-G cells). Thirty minutes prior to being harvested, selected wells of mock-infected cells were treated with 50 ng/ml or 500 ng/ml of PMA. The treated cell monolayers were then either lysed (Western blotting) or fixed with 4% paraformaldehyde (immunofluorescence assay). Analysis of the cell lysates indicated that in the WT MEF-G cells, WNV VLP infection resulted in a degradation of IκBα; however, less degradation was observed in PKR null MEF cells (Fig. 10). This suggests that NK-κB activation was impaired in the absence of PKR. However, IκBα degradation in PKR null MEF cells did not appear to be affected following PMA treatment, indicating that NF-κB can be activated by a PKR-independent pathway in PMA-treated cells. Consistent with these IκBα degradation data, WNV VLP infection triggered NF-κB nuclear translocation in most of the WT MEF cells, and this translocation was observed at much lower frequencies in the PKR null MEF cells (see Fig. S1 in the supplemental material).
FIG. 10.
Analysis of WNV VLP-induced IκBα degradation. WT MEF-G and PKR null MEF cells were mock or WNV VLP infected for either 16 or 24 h. Approximately 30 min prior to being harvested, selected wells of mock-infected cells were treated with 50 ng/ml or 500 ng/ml PMA. Treated cell monolayers were harvested and assayed by Western blotting as described in Materials and Methods to reveal levels of IκBα. Parallel blots prepared from the same samples show levels of PKR and β-actin.
DISCUSSION
PKR is known primarily for its ability to phosphorylate eIF-2α, resulting in a block in initiation of mRNA translation. It is an important ISG, and its induction and inhibition of translation of both host and viral mRNAs have been shown to be important aspects of the antiviral response to many viral infections. Mice deficient in PKR displayed increased mortality following encephalomyocarditis virus infection, even with IFN treatment (69). PKR activity was also shown to be critical for the cell's antiviral response to VSV, as cells derived from mice containing a targeted deletion in the PKR gene were more susceptible to VSV-induced apoptosis and PKR null mice infected with VSV showed significantly higher mortality than did WT mice (5). The importance of PKR in viral infections is also highlighted by the fact that several viruses, including influenza virus, have evolved mechanisms to inhibit PKR activity (40).
PKR has also been shown to be important in controlling infections by viruses within the Flaviviridae family. Hepatitis C virus (HCV) subgenomic replicons have been shown to replicate more efficiently in PKR null MEF cell lines, suggesting that PKR is involved in the control of HCV replication (6). Other studies have shown that HCV can block the host cell's response to IFN by inhibiting PKR activity (22). In the case of DENV, in vitro studies have shown that PKR null MEFs showed no detectable increase in virus yield (11). Additionally, PKR-deficient cells demonstrated no impairment in IFN-β-mediated inhibition of DENV infection (11), a finding we have reproduced for WNV infection. However, PKR null mice were reported to be significantly more susceptible to WNV infection, demonstrating increased viremia and viral loads in both the peripheral tissues and the central nervous system, than are WT mice (52).
Over the last few years, a number of reports have implicated TLR3, TLR7/8, and RIG-I/mda-5 as critical PRRs for recognizing flaviviral infections (see the introduction). However, there have been reports that PKR activity is essential for virus-induced IFN responses (see the introduction), and Diebold et al. demonstrated that BM-DCs derived from PKR null mice produced lower levels of poly(I:C)-induced IFN-α than did BM-DCs derived from WT mice (13).
In this study, we investigated PKR-mediated IFN induction during WNV infection, using three different experimental methods. First, MEF cells harboring a PKR gene disruption generated lower levels of IFN than did WT MEF cells following challenge with WNV VLPs. Secondly, MEF and human cell lines treated with PKR-I displayed lower poly(I:C)- or WNV VLP-induced IFN levels than did mock-treated cells. SeV-induced IFN production was unaffected by PKR-I, indicating that the inhibitor did not have a nonspecific effect on IFN gene expression. Finally, transfection of cells with PKR siRNAs produced a specific abrogation of IFN induction following challenge with poly(I:C) or WNV VLPs. Additionally, the impairment of PKR activity in these cells had no effect on SeV-induced IFN production, indicating a WNV-specific role of PKR during IFN induction. Consistent with the cytoplasmic location of PKR, neither Ex-poly(I:C) nor UV-inactivated WNV VLPs stimulated IFN production.
To our knowledge, this is the first report that PKR serves to mediate flavivirus-induced IFN synthesis. However, several other PRRs have been investigated in flavivirus-infected cells. Work with JEV and DENV implicated RIG-I-dependent signaling in the initiation of IFN gene expression in cells infected with these two flaviviruses (7). In the case of WNV, recent reports suggest an involvement of both TLR3- and RIG-I-mediated signaling in the induction of an antiviral response. In HeLa cells, WNV genome expression was shown to block IRF3 dimerization and nuclear translocation and to block stimulation of poly(I:C)-induced IFN, suggesting that the virus has evolved a mechanism to block TLR3 recognition of dsRNA (54). Another report indicated that the IRF3 pathway was important in controlling WNV infection (19), and the same group's subsequent work also demonstrated that RIG-I played a role in stimulating ISG56 and ISG54 following WNV infection (18). Furthermore, IRF3 phosphorylation in WNV-infected 293 cells was shown to correlate with IFN-β RNA levels (19).
To directly address the role of RIG-I as a PRR in human cells, we relied on siRNA posttranslational knockdown. These studies showed that transfection with RIG-I siRNA partially blocked VLP-induced IFN in A549 and MRC-5 cells but not in Hec1B cells, suggesting that MRC-5 and A549 cells might utilize both PKR and RIG-I as potential PRRs. For all three cell types, RIG-I siRNA treatment completely blocked SeV-induced IFN synthesis (consistent with published data [32]), indicating that we had achieved sufficient RIG-I knockdown. One explanation for differences between our data indicating that PKR serves as a potential PRR and other's results indicating that RIG-I serves as a PRR in WNV-infected cells may be explained in part by the experimental methods employed in these studies. Fredericksen et al. examined the role of RIG-I in establishing an anti-WNV environment by examining virus-dependent induction of gene products (including ISG56 and ISG54) which are primarily dependent on IRF3 transcriptional activation (18, 23). Our studies, however, examined IFN synthesis, which is driven by a promoter with multiple transcription factor binding sites, including those for NF-κB. Since activated PKR phosphorylates IκB (34, 35, 68), permitting NK-κB to translocate into the nucleus and to activate transcription of IFN-β (61), dsRNA binding to PKR could signal the induction of IFN gene expression, but not ISG56 and ISG54 gene expression, which was examined in detail by Fredericksen and Gale (18). Since WNV infection has been shown to activate NF-κB (8), the link between our data demonstrating that PKR is required for IFN synthesis and NF-κB activation is the subject of ongoing studies. However, initial studies have indicated that PKR null MEF cells show an impairment in WNV VLP-induced IκBα degradation and NF-κB nuclear translocation compared to WT MEF cells, suggesting that this signaling pathway may be involved in PKR-dependent IFN synthesis in response to WNV VLP infection. Interestingly, PKR null MEF cells produce low levels of IFN, indicating, as mentioned above, that some IFN is induced via a PKR-independent pathway(s), possibly via an RNA helicase. It is likely that the importance of various pathways for WNV-induced antiviral action varies from cell to cell, and different PRRs may be important in establishing an effective antiviral environment in different tissue types in vivo.
Our data are particularly interesting in light of a report showing that PKR null mice are more susceptible than WT mice to WNV infection (52). Mice lacking PKR demonstrated increased WNV replication in the periphery and earlier entry into the brain, with no significant difference detected in serum IFN levels. These studies went on to show modest differences in IFN′s ability to reduce the WNV yield from one type of neuronal cell, consistent with a role for PKR as an ISG that inhibits viral spread within the central nervous system (52). In our hands, PKR knockout had no detectable effect on the ability of MEF cells to establish an IFN-induced antiviral state. However, PKR knockout did have a profound effect on the ability to produce IFN in response to WNV infection in our cell lines. Interestingly, preliminary studies performed with primary human monocyte-derived DCs showed that inhibition of PKR activity reduced the amount of IFN-α produced in response to WNV VLP infection in these cultures (data not shown). These preliminary data are consistent with our cell line data and support our supposition that PKR could play a role in WNV-induced IFN synthesis in vivo. It is possible, however, that PKR acts as a downstream kinase for a different PRR. For example, a recent report showed that PKR associates with TRAF3 (47), which is involved in signaling through other PRRs. Regardless, our data suggest that PKR is involved in WNV-induced IFN synthesis and could be important for helping to control WNV infections in animal models of West Nile encephalitis and natural infections.
Supplementary Material
Acknowledgments
We thank all of the researchers who generously supplied reagents necessary for this work. We thank G. Milligan (UTMB) and F. Scholle (NC State) for critical reviews of the manuscript. We also thank K. Li (UTMB) for valuable discussions on signaling pathways. We thank E. Knutson (UTMB) at the UTMB Infectious Disease and Toxicology Optical Imaging Core Facility for the confocal images and G. Boswick (UTMB) for generously providing PMA.
F.D.G. was supported by an NIH Training Grant in Emerging and Tropical Infectious Diseases (AI07526). This work was also supported by a grant from NIAID to P.W.M. through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH grant number U54 AI057156), by NIH grant AI061441, and by the Sealy Center for Vaccine Development.
Footnotes
Published ahead of print on 8 August 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. F., and J. J. Rahal. 2002. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg. Infect. Dis. 8:107-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 6.Chang, K. S., Z. Cai, C. Zhang, G. C. Sen, B. R. Williams, and G. Luo. 2006. Replication of hepatitis C virus (HCV) RNA in mouse embryonic fibroblasts: protein kinase R (PKR)-dependent and PKR-independent mechanisms for controlling HCV RNA replication and mediating interferon activities. J. Virol. 80:7364-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, T. H., C. L. Liao, and Y. L. Lin. 2006. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect. 8:157-171. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, Y., N. J. King, and A. M. Kesson. 2004. The role of tumor necrosis factor in modulating responses of murine embryo fibroblasts by flavivirus, West Nile. Virology 329:361-370. [DOI] [PubMed] [Google Scholar]
- 9.Chong, K. L., L. Feng, K. Schappert, E. Meurs, T. F. Donahue, J. D. Friesen, A. G. Hovanessian, and B. R. Williams. 1992. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 11:1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens, M. J. 1997. PKR—a protein kinase regulated by double-stranded RNA. Int. J. Biochem. Cell. Biol. 29:945-949. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, M. S., and E. Harris. 2001. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289:297-311. [DOI] [PubMed] [Google Scholar]
- 12.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 13.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 14.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 15.Durbin, J. E., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 16.Fayzulin, R., F. Scholle, O. Petrakova, I. Frolov, and P. W. Mason. 2006. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology 351:196-209. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 18.Fredericksen, B. L., and M. Gale, Jr. 2006. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J. Virol. 80:2913-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale, Jr. 2004. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funami, K., M. Matsumoto, H. Oshiumi, T. Akazawa, A. Yamamoto, and T. Seya. 2004. The cytoplasmic ‘linker region’ in Toll-like receptor 3 controls receptor localization and signaling. Int. Immunol. 16:1143-1154. [DOI] [PubMed] [Google Scholar]
- 21.Fuse, A., H. Ashino-Fuse, and T. Kuwata. 1984. Binding of 125I-labeled human interferon to cell lines with low sensitivity to interferon. Gann 75:379-384. [PubMed] [Google Scholar]
- 22.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 23.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo, J. T., J. Hayashi, and C. Seeger. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 79:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiscott, J., R. Lin, P. Nakhaei, and S. Paz. 2006. MasterCARD: a priceless link to innate immunity. Trends Mol. Med. 12:53-56. [DOI] [PubMed] [Google Scholar]
- 26.Hu, Y., and T. W. Conway. 1993. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J. Interferon Res. 13:323-328. [DOI] [PubMed] [Google Scholar]
- 27.Ito, T., Y. H. Wang, and Y. J. Liu. 2005. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 26:221-229. [DOI] [PubMed] [Google Scholar]
- 28.Jammi, N. V., L. R. Whitby, and P. A. Beal. 2003. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem. Biophys. Res. Commun. 308:50-57. [DOI] [PubMed] [Google Scholar]
- 29.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 33.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 34.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, E., and M. Lobigs. 2000. Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J. Virol. 74:8867-8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed.,vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 39.Liu, W. J., X. J. Wang, V. V. Mokhonov, P. Y. Shi, R. Randall, and A. A. Khromykh. 2005. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 79:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 41.Marcus, P. I., and M. J. Sekellick. 1988. Interferon induction by viruses. XVI. 2-Aminopurine blocks selectively and reversibly an early stage in interferon induction. J. Gen. Virol. 69:1637-1645. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154-3162. [DOI] [PubMed] [Google Scholar]
- 43.Meurs, E. F., Y. Watanabe, S. Kadereit, G. N. Barber, M. G. Katze, K. Chong, B. R. Williams, and A. G. Hovanessian. 1992. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 66:5805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 45.Mogensen, T. H., and S. R. Paludan. 2005. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J. Mol. Med. 83:180-192. [DOI] [PubMed] [Google Scholar]
- 46.Nishiya, T., and A. L. DeFranco. 2004. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J. Biol. Chem. 279:19008-19017. [DOI] [PubMed] [Google Scholar]
- 47.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 48.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4:161-167. [DOI] [PubMed] [Google Scholar]
- 49.Romano, P. R., M. T. Garcia-Barrio, X. Zhang, Q. Wang, D. R. Taylor, F. Zhang, C. Herring, M. B. Mathews, J. Qin, and A. G. Hinnebusch. 1998. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol. Cell. Biol. 18:2282-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi, S. L., Q. Zhao, V. K. O'Donnell, and P. W. Mason. 2005. Adaptation of West Nile virus replicons to cells in culture and use of replicon-bearing cells to probe antiviral action. Virology 331:457-470. [DOI] [PubMed] [Google Scholar]
- 51.Samuel, M. A., and M. S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. G. Williams, R. H. Silverman, M. Gale, Jr., and M. S. Diamond. 2006. PKR and RNase L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scholle, F., Y. A. Girard, Q. Zhao, S. Higgs, and P. W. Mason. 2004. trans-Packaged West Nile virus-like particles: infectious properties in vitro and in infected mosquito vectors. J. Virol. 78:11605-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholle, F., and P. W. Mason. 2005. West Nile virus replication interferes with both poly(I:C)-induced interferon gene transcription and response to interferon treatment. Virology 342:77-87. [DOI] [PubMed] [Google Scholar]
- 55.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 56.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 57.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 58.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi, O., H. Hemmi, and S. Akira. 2004. Interferon response induced by Toll-like receptor signaling. J. Endotoxin Res. 10:252-256. [DOI] [PubMed] [Google Scholar]
- 60.Verhaegen, M., M. Divizia, P. Vandenbussche, T. Kuwata, and J. Content. 1980. Abnormal behavior of interferon-induced enzymatic activities in an interferon-resistant cell line. Proc. Natl. Acad. Sci. USA 77:4479-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Visvanathan, K. V., and S. Goodbourn. 1989. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 8:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 65.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 67.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou, A., J. M. Paranjape, S. D. Der, B. R. Williams, and R. H. Silverman. 1999. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258:435-440. [DOI] [PubMed] [Google Scholar]
- 70.Zinn, K., A. Keller, L. A. Whittemore, and T. Maniatis. 1988. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science 240:210-213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.