Abstract
Natural killer (NK) cell recognition of influenza virus-infected cells involves hemagglutinin (HA) binding to sialic acid (SA) on activating NK receptors. SA also acts as a receptor for the binding of influenza virus to its target host cells. The SA binding properties of H3N2 influenza viruses have been observed to change during circulation in humans: recent isolates are unable to agglutinate chicken red blood cells and show reduced affinity for synthetic glycopolymers representing SA-α-2,3-lactose (3′SL-PAA) and SA-α-2,6-N-acetyl lactosamine (6′SLN-PAA) carbohydrates. Here, NK lysis of cells infected with human H3N2 influenza viruses isolated between 1969 and 2003 was analyzed. Cells infected with recent isolates (1999 to 2003) were found to be lysed less effectively than cells infected with older isolates (1969 to 1996). This change occurred concurrently with the acquisition of two new potential glycosylation site motifs in HA. Deletion of the potential glycosylation site motif at 133 to 135 in HA1 from a recent isolate partially restored the agglutination phenotype to a recombinant virus, indicating that the HA-SA interaction is inhibited by the glycosylation modification. Deletion of either of the recently acquired potential glycosylation sites from HA led to increased NK lysis of cells infected with recombinant viruses carrying modified HA. These results indicate that alterations in HA glycosylation may affect NK cell recognition of influenza virus-infected cells in addition to virus binding to host cells.
Influenza A virus is an important human respiratory pathogen belonging to the family Orthomyxoviridae. Multiple subtypes of influenza A virus are defined based on the antigenic nature of the virion surface hemagglutinin (HA) and neuraminidase (NA) glycoproteins; viruses with at least 16 subtypes of HA are known to circulate in aquatic birds. Genetic reassortment between avian and human influenza viruses can lead to the creation of pandemic viruses. The influenza pandemics that have occurred in the last century all have been caused by viruses bearing an HA protein of a subtype distinct from that of the contemporary human viruses, illustrating the importance of HA in determining the disease potential of influenza virus. HA is the virion attachment protein, which binds to sialic acid (SA)-containing receptors on the host cell surface, and also acts as the viral fusion protein. It is the major antigen of the virus against which neutralizing antibodies are directed. Regular epidemics result from antigenic drift of the HA protein as virus of a given subtype circulates in humans. Viruses of the H3N2 subtype have been circulating in humans since 1968 and are known to have undergone considerable genetic and antigenic evolution during this time (32).
We and others have observed changes in the receptor binding properties of the HA protein of H3N2 viruses during the 38 years that they have been circulating in humans (2, 21, 34). These changes, including a loss of the ability to agglutinate chicken red blood cells (RBC), suggest a recent reduction in the affinity of binding of the HA protein to its receptor, SA. Terminal SA can be attached to lactose or lactosamine on glycoproteins or glycolipids by α-2,6 or α-2,3 linkages (e.g., SA-α-2,6-Gal-β-1,4-Glc, SA-α2,6-Gal-β-1,4-GlcNAc, or SA-α-2,3-Gal-β-1,4-Glc). These different carbohydrate species can be represented by synthetic glycopolymers in binding assays. Epithelial cells in the human respiratory tract carry both α-2,6- and α-2,3-linked SA, although the α-2,6-linked form is more abundant and is expressed predominantly in certain cell types (20, 35). α-2,6-linked SA is the receptor predominantly used by human influenza viruses in human hosts. By contrast, avian influenza viruses preferentially bind to α-2,3-linked SA, and HA proteins derived from avian viruses rapidly mutate after introduction into humans to enhance their α-2,6-linked SA binding capacity (19).
There may be several explanations for the observation that H3N2 viruses appear to be evolving in humans to lose affinity for their receptor. First, the viruses may not actually be compromised in their binding to human airway cells; instead, the receptors in chicken RBC may not be good models for those used in the human host. Second, the mutations responsible for reduced receptor binding may have been selected during antigenic drift because they result in decreased antibody recognition, and the selective advantage gained by evasion of antibody neutralization may outweigh the costs of reduced receptor binding. The addition of glycosylation sites on HA is thought to be a viral mechanism to evade antibody neutralization, and it is clear that oligosaccharide chains positioned in the vicinity of the receptor binding site can decrease the HA-SA interaction (25, 26). Third, other selective forces acting on HA may drive a decrease in receptor affinity. The latter may include inhibition of virus-cell interactions by soluble and tethered receptor analogues such as mucins. In addition, natural killer (NK) cell activation can be triggered by binding of natural cytotoxicity receptors, including NKp46 and NKp44, to viral proteins expressed on the surface of infected cells (5, 18). Recently, it has been shown that NKp46 is specifically required for the eradication of influenza virus infection by NK cells in vivo (13). Notably, the binding of influenza virus HA to NKp46 is mediated mainly via SA carried on NKp46 (4), raising the possibility that the evolutionary changes observed in the HA receptor binding properties of human H3N2 viruses may be associated with a reduction in their ability to activate NK cells.
This study compared NK cell lysis of target cells infected with a panel of H3N2 influenza virus isolates representative of viruses circulating in the human population between 1969 and 2003, which display changes in HA leading to altered receptor binding (34). Recent virus isolates with low SA affinity were found to sensitize target cells for NK lysis less efficiently than older virus isolates that retained the ability to agglutinate chicken RBC. NA treatment of NK cells resulted in the reduction of NK lysis of target cells infected by both old and recent viruses, indicating that SA plays an important role in the interaction of NK cells with influenza virus-infected cells. Furthermore, the acquisition of new potential glycosylation site motifs in HA in close proximity to the receptor binding site was found to be a factor not only in the changing receptor binding properties but also in the efficiency of NK cell lysis of infected cells.
MATERIALS AND METHODS
Viruses.
Influenza A (England) virus H3N2 human clinical isolates from 1969 to 2003 were obtained from the Health Protection Agency Centre for Infections, Colindale, London, United Kingdom. A panel of viruses (Table 1) was selected from archived strains that had been passaged no more than three times in MDCK cells from the original respiratory sample prior to storage at −80°C. Viruses were amplified and titrated as previously described (34).
TABLE 1.
Antigenic properties, agglutination characteristics, and ability to infect 143BTK− target cells of H3N2 influenza A viruses isolated from 1969 to 2003
| Virus isolate | Isolate with the most similar antigenic propertiesa | Chicken RBC hemagglutinationb | % Target cell infectionc |
|---|---|---|---|
| A/England/878/69 | A/Aichi/2/68 | ++ | 98.1 |
| A/England/401/85 | A/Mississippi/1/85 | ++ | 95.6 |
| A/England/327/90 | A/Beijing/359/89 | + | 98.6 |
| A/England/289/93 | A/Beijing/32/92 | + | 98.6 |
| A/England/41/96 | A/Johannesburg/33/94 | + | 98.1 |
| A/England/356/96 | A/Wuhan/359/95 | + | 99.4 |
| A/England/26/99 | A/Sydney/5/97 | − | 96.8 |
| A/England/919/99 | A/Sydney/5/97 | − | 99.2 |
| A/England/24/00 | A/Panama/2007/99 | − | 98.6 |
| A/England/367/03 | A/Moscow/10/99 | − | 74.7 |
Antigenic characteristics of A/England/878/69 were described by Both et al. (8). The influenza A virus strain likely showing closest antigenic similarity to A/England/401/85 is A/Missippi/1/85. Antigenic characteristics of strains from 1990 onwards were confirmed using specific ferret antisera.
Data are from Thompson et al. (34). ++, >10 HAU; +, 2 to 10 HAU; −, <2 HAU.
Mean percentage of target cells positive for HA expression.
Hemagglutination assays.
Hemagglutination assays were performed in V-bottomed microtiter plates using 50 μl of 0.5% suspensions in phosphate-buffered saline (PBS) of turkey or chicken RBC (Harlan Sera-Lab Ltd., Loughborough, United Kingdom) added to 50 μl virus serially diluted in PBS. Assays were read as the reciprocal dilution of the last well showing hemagglutination following 1 h of incubation on ice.
Synthesis of sialyl oligosaccharide glycoconjugates.
Glycoconjugates SA-α-2,3-lactose (3′SL-PAA) and SA-α-2,6-N-acetyl lactosamine (6′SLN-PAA) conjugated to a supporting polymer, poly(acrylic acid) (PAA), were chemically (3′SL-PAA) or enzymatically (6′SLN-PAA) synthesized. 3′SL-PAA and 6′SL-PAA were prepared synthetically by glycosylation of selectively protected lactose acceptors. Deprotection of the resulting trisaccharide and subsequent conjugation yielded the desired glycoconjugates (28). Enzymatic synthesis of 6′SLN-PAA from biotin-labeled Gal-β-1,4-GlcNAc-PAA (Glycotech, Gaithersburg, MD) followed the method described by Wu and Air (6, 37).
Sialyl glycoconjugate binding assay.
A solid-phase binding assay based on previously described methods (19, 22) was employed to measure virus binding affinity for SA. Standard concentrations of purified virus particles were adsorbed to a 96-well microtiter plate, biotin-labeled glycopolymers were titrated against the virus particles in the presence of 1 μM NA inhibitor GG167 (a gift from GlaxoSmithKline, Stevenage, United Kingdom), and the binding was detected with streptavidin-horseradish peroxidase. Absorbance data (measured as the optical density at 420 nm) were transformed to Scatchard plots, and relative binding affinities (Kass) were determined from the gradient of the resulting line, a method that has been used successfully to differentiate binding of human and avian HAs (19).
Influenza virus infection of target cells.
143BTK− cells (a human osteosarcoma line obtained from the ATCC, Manassas, VA) were infected with influenza viruses at a multiplicity of infection of 1 for 30 min in serum-free medium and then were washed and incubated overnight in minimal essential medium (Invitrogen Life Technologies, Paisley, United Kingdom) supplemented with 7% fetal calf serum (FCS; PAA Laboratories, Linz, Austria).
Flow cytometric analysis of HA expression in infected cells.
Influenza virus-infected 143BTK− cells were stained with a rabbit anti-HA antibody (R372) (Health Protection Agency Centre), which was detected using an Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G (Molecular Probes, Leiden, Holland). After being stained, cells were fixed in 1% paraformaldehyde. Data were acquired using a FACSCalibur flow cytometer (Becton Dickinson, Oxford, United Kingdom) and were analyzed using CellQuest software (Becton Dickinson).
PBMC and NK cell preparation.
Blood samples were withdrawn with written informed consent from healthy adult Caucasian volunteers at The Edward Jenner Institute for Vaccine Research. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on histopaque-1077 (Sigma Aldrich Ltd., Poole, United Kingdom). NK cells were purified from PBMCs by negative selection using an NK cell isolation kit (Miltenyi Biotech, Bisley, United Kingdom). The resulting preparations routinely contained >70% CD56+ CD3− NK cells.
Chromium release assays.
Target cells were 51Cr labeled by incubation for 1 h with 50 μCi of Na51CrO4 (Amersham Biotech, Little Chalfont, United Kingdom) and then were washed three times in phenol red-free RPMI medium (Invitrogen Life Technologies) supplemented with 10% FCS and added to 96-well plates at 2 × 104 cells/well. Effector cells were added at a range of effector:target (E:T) ratios. Wells to which medium only or 2% (vol/vol) Triton X-100 (Sigma Aldrich Ltd.) was added allowed measurement of spontaneous or maximum 51Cr release, respectively, from each target population. All variables were assayed in triplicate. Plates were incubated for 5 h at 37°C, and then supernatants were harvested and counted in a Trilux 1450 Microbeta counter (Wallac, Turku, Finland). The percentage of specific lysis was calculated with the following formula: (mean test counts − mean spontaneous counts)/(mean maximum counts − mean spontaneous counts) × 100.
To determine the statistical significance of the difference in the increase in the percentage of specific lysis induced by infection of cells with older and more recent influenza virus isolates, data were transformed to empirical logits to stabilize variance, and analysis of variance was performed using a linear model.
Analysis of SA-α-2,3-Gal and SA-α-2,6-Gal moieties on NK cells.
PBMCs (106) were incubated for 1 h at 4°C with 1 μg/ml digoxigenin (DIG)-labeled Maackia amurensis agglutinin (MAA; Roche Diagnostics Ltd., Lewis, United Kingdom), which recognizes SA-α-2,3-Gal, or 0.6 μg/ml DIG-labeled Sambucus nigra agglutinin (SNA; Roche Diagnostics Ltd.), which recognizes SA-α-2,6-Gal. Control cells were incubated without lectins. After being washed in fluorescence-activated cell sorter buffer (PBS supplemented with 2% FCS and 0.2% sodium azide), cells were incubated with a fluorescein isothiocyanate-conjugated anti-DIG antibody (Roche Diagnostics Ltd.), which was diluted 1:200, phycoerythrin-conjugated anti-CD3 antibody (clone UCHT1; PharMingen BD, Oxford, United Kingdom), and R-phycoerythrin-cyanin 5.1-conjugated anti-CD56 antibody (clone N901; Beckman Coulter, High Wycombe, United Kingdom) diluted 1:100 in fluorescence-activated cell sorter buffer. NK cells were identified as CD56+ CD3− cells. Data were acquired using a FACSCalibur and were analyzed using CellQuest software.
Treatment of PBMCs with NAs.
PBMCs were desialylated by incubation with a mixture of NA from Vibrio cholerae (Roche Diagnostics Ltd.) at 0.03 U/ml and NA from Arthrobacter ureafaciens (Roche Diagnostics Ltd.) at 0.003 U/ml or with medium (control) only for 1 h at 37°C. After being washed in phenol red-free RPMI medium, cells were used in chromium release assays. The efficiency of desialylation was tested by staining cells with lectins specific for the SA-α-2,3-Gal or SA-α-2,6-Gal linkage, as described above.
PCR amplification and cloning of A/England/26/99 HA and NA.
Viral RNA was extracted from 150 μl of tissue culture fluid using guanidinium thiocyanate (Severn Biotech Ltd., Kidderminster, United Kingdom) (7). cDNA synthesis was performed using an influenza A virus universal primer, RT-F (5′-AGCAAAAGCAGG-3′). HA was amplified using PCR primers H3-F (5′-CTGCAGGCTCTTCGACCCAGCAAAAGCAGGGGATAATTC-3′) and H3-R (5′-CTGCAGGCTCTTCTTATTAGTAGAAACAAGGGTGTTTT-3′). NA was amplified using PCR primers N2-F (5′-TATTGGCGTCTCACCCAGCAAAAGCAGGAGT-3′) and N2-R (5′-ATATGCCGTCTCTTATTAGTAGAAACAAGGAGTTTTTT-3′). Viral genes were cloned into the SapI or BsmBI site of the pPolI-RT vector, kindly supplied by Thomas Zurcher (GlaxoSmithKline).
Site-directed mutagenesis to delete potential glycosylation site motifs in HA.
Plasmid 26/99-HA-PolI containing HA from virus A/England/26/99 cloned into the SapI site of pPolI-RT was mutated using the QuikChange site-directed mutagenesis kit (Stratagene Europe, Amsterdam, The Netherlands) according to the manufacturer's instructions. The potential glycosylation site motif at positions 122 to 124 was changed from NES to TEG (G1Δ) using complementary primers G122-F (5′-GGCACACTGGAGTTTAACACTGAAGGCTTCAATTGGACTGG-3′) (the nucleotide changes introduced to generate the required amino acid sequence are underlined) and G122-R (5′-CCAGTCCAATTGAAGCCTTCAGTGTTAAACTCCAGTGTGCC-3′). The potential glycosylation site motif at positions 133 to 135 was changed from NGT to NGG (G2Δ) using complementary primers G133-F (5′-GGAGTCGCTCAGAATGGGGGAAGCTCTGCTTGC-3′) and G133-R (5′-GCAAGCAGAGCTTCCCCCATTCTGAGCGACTCC-3′). The resulting plasmids were sequenced and used to produce recombinant viruses as described below.
Reverse genetics.
Virus rescue was carried out using A/Victoria/3/75 plasmids kindly provided by Thomas Zurcher (GlaxoSmithKline) using a method adapted from one previously described (10, 11). To generate recombinant viruses, plasmid 26/99-HA-PolI or one of the mutants described above, paired with a plasmid containing NA from A/England/26/99 (26/99-NA-PolI), was substituted in place of the equivalent A/Victoria/3/75 HA and NA plasmids.
Western Blot analysis of HA proteins.
Recombinant influenza viruses grown in MDCK cells and concentrated by spinning through 30% sucrose were lysed in radioimmunoprecipitation assay buffer as previously described. Proteins in the lysate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel and were transferred to an Immobilon P membrane. HA proteins were detected using a monoclonal antibody to the HA tag epitope (Abcam, Cambridge, United Kingdom) followed by an anti-mouse horseradish peroxidase conjugate and were visualized by chemiluminescence.
RESULTS
Recent H3N2 viruses show reduced affinity for α-2,3- and α-2,6-linked SA.
A unique panel of H3N2 influenza isolates representative of viruses circulating in the human population between 1969 and 2003 were utilized in this study. As summarized in Table 1, these viruses previously had been shown to exhibit differences in receptor binding, with the most recent virus isolates being unable to hemagglutinate chicken RBC due to accumulation of mutations in the HA protein (34).
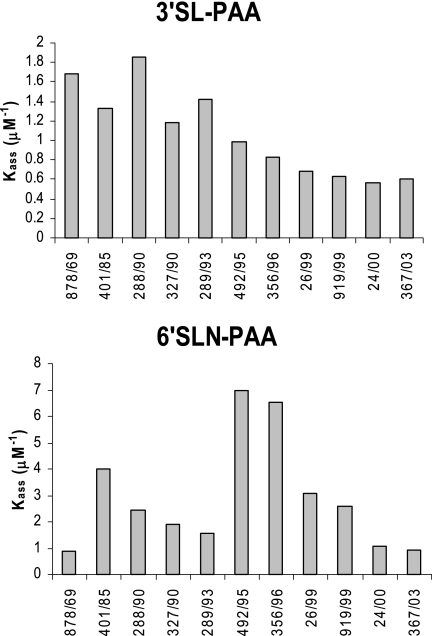
The assays with chicken RBC provided a broad indication of a changing trend in SA binding, suggesting that older viruses may have bound α-2,3-linked SA with higher affinity than recent viruses. However, as the SA content of animal RBC is heterogeneous and generally poorly defined, we used specific glycoconjugates to more accurately characterize the nature of the receptor binding change in the influenza viruses over time. Both chemical and enzymatic methods were employed to produce biotin-labeled glycoconjugates containing defined saccharides, namely, SA-α-2,3-lactose (3′SL-PAA) and SA-α-2,6-N-acetyl lactosamine (6′SLN-PAA), representative of the avian (3′SL) and human (6′SLN) virus receptors, respectively. We chose to use 6′SLN to probe for binding to human receptors because the significance of the asialo components of the carbohydrate chain has been recognized (12, 22), and a structural study identified the conformation of 6′SLN rather than 6′SL as a more likely representative of the influenza virus receptor (9). The binding affinity of all influenza viruses for the receptor analogs (3′SL-PAA and 6′SLN-PAA) was determined in a solid-phase binding assay based on previously described methods (6, 19, 22). The experiments were repeated on several occasions. The absolute values determined for individual HA-glycopolymer affinities were quite variable, but the trends observed were consistent, and representative data are shown here. Relative binding affinities for 3′SL-PAA (Fig. 1, upper panel), representative of the avian receptor, were high for older viruses (Kass of >1 μM−1) but decreased to low levels, particularly with viruses isolated after 1995 (Kass of <1 μM−1), consistent with the observed trend showing loss of agglutination of chicken RBC (34), which are thought to display predominantly α-2,3-linked SA (15, 21, 24). Binding of viruses to 6′SLN-PAA showed marked variation (Fig. 1, lower panel). Binding was initially low (Kass of <1 μM−1), rose to a high level in viruses isolated in the mid-1990s (Kass of 6.5 to 7 μM−1), and then declined for recent isolates (Kass of <3 μM−1). Thus, for both 3′SL and 6′SLN, H3N2 human influenza viruses isolated after 1999 had low binding affinities.
FIG. 1.
Recent H3N2 influenza viruses show reduced binding to synthetic α-2,3- and α-2,6-linked SA. Kass for H3N2 influenza viruses (1968 to 2003) binding to 3′SL-PAA and 6′SLN-PAA were determined as a function of the gradient of the slope defined in Scatchard plots. Binding assays were run on different days, and an overall trend was observed. Representative data sets are shown.
NK lysis of target cells infected by recent H3N2 influenza viruses is less than that of cells infected with older virus isolates.
To gain insight into the effects of evolutionary changes in HA on NK cell recognition of virus-infected cells, we analyzed the extent to which infection with each virus enhanced target cell lysis by NK cells. We used the 143BTK− cell line in these studies, which is lysed only poorly by NK cells when uninfected, rather than a more conventional and readily lysed target cell such as K562, because it was important for us to be able to differentiate small differences in infection-associated lysis following infection with different influenza virus strains.
As shown in Table 1, 143BTK− cells were infected efficiently by all the viruses in the panel, with surface HA expression typically being detected on more than 95% of cells at 24 h postinfection. Exceptions were cells infected with the most recent virus, A/England/367/03, where surface HA expression could be detected on only 74.7% of cells; however, this was probably attributable to differences in the ability of the rabbit antiserum (which was raised against an antigenic variant from 1994, A/England/24/94) to recognize the HA protein of this virus.
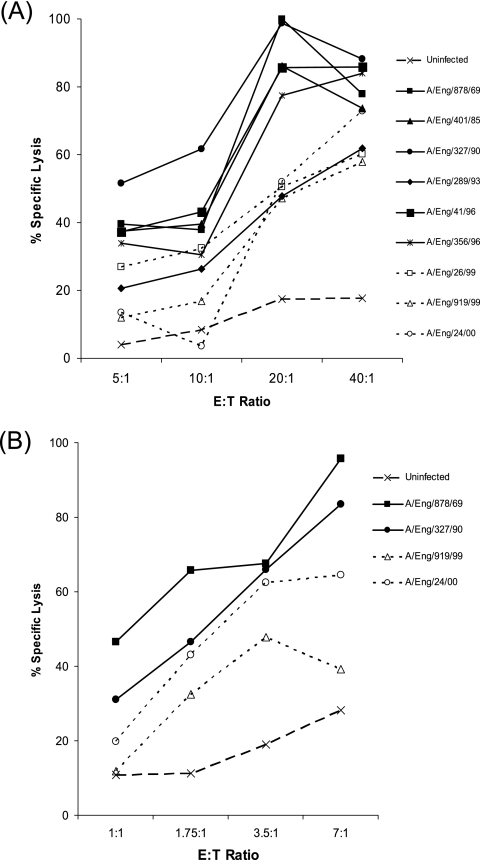
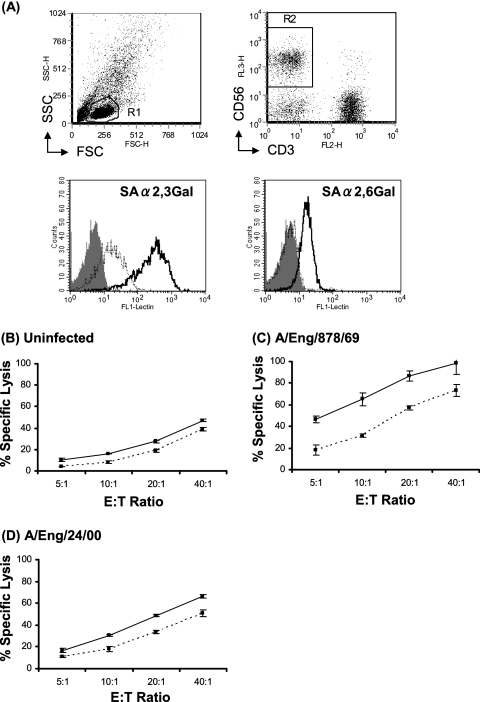
NK cell lysis of cells infected with the different influenza viruses was assessed using 51Cr release assays. The effector cells were either CD56+ CD3− NK cells isolated from human peripheral blood or unseparated cells utilized at a higher range of E:T ratios; as illustrated in Fig. 2A and B, both gave similar results. Uninfected 143BTK− cells activated only a low level of NK cell lysis, but when the cells were infected with influenza viruses, a much higher level of lysis was observed. For example, in the experiment whose results are shown in Fig. 2A, only 17% specific lysis of uninfected cells was mediated at an E:T ratio of 20:1, whereas the specific lysis of cells infected with different influenza viruses ranged from 47 to 100%. The infection-associated increase in target cell lysis was not due to major histocompatibility complex (MHC) down-regulation, as antibody staining revealed that MHC class I expression was retained in the majority of influenza virus-infected 143BTK− cells (data not shown).
FIG. 2.
H3N2 influenza viruses isolated between 1969 and 1996 sensitize target cells for NK lysis more efficiently than recent virus isolates (1999 to 2000). Representative results from 51Cr release assays comparing the percentage of specific lysis of uninfected 143BTK− cells (dashed line) to that of cells infected with different influenza virus isolates by unseparated PBMC effectors (A) or isolated CD56+ CD3− NK cells (B) at a range of E:T ratios. Solid lines and filled symbols are used to denote target cells infected with older virus isolates (1969 to 1996), and dotted lines and open symbols denote target cells infected with more recent viruses.
Although cells infected with all influenza viruses tested were better targets for NK cell lysis than uninfected cells, the increase in target cell lysis associated with infection with different influenza virus isolates varied widely. Notably, in all experiments carried out, the viruses causing the greatest increase in target cell lysis were older (1969 to 1996) influenza virus isolates, while more recent virus isolates (1999 to 2003), which lacked the ability to hemagglutinate chicken RBC, typically induced a more modest increase in target cell lysis. This is exemplified by the representative results in Fig. 2A and B. In Fig. 3, the mean infection-associated increase in target cell lysis induced at different E:T ratios by older (1969 to 1996) and more recent (1999 to 2003) virus isolates from data pooled from three independent experiments is summarized. A statistical analysis of variance using these data confirmed that the difference in the mean infection-associated increase in target cell lysis induced by the two groups of viruses was significant (P = 0.008).
FIG. 3.
Summary of NK lysis of target cells infected with H3N2 influenza viruses isolated between 1969 and 1996 and recent virus isolates (1999 to 2003). Each panel shows the increase in the percentage of specific lysis at different E:T ratios induced by infection of target cells with older (1969 to 1996; squares) and more recent (1999 to 2003; triangles) virus isolates. The mean increase in the percentage of specific lysis induced by each group of viruses is indicated by the horizontal lines. Statistical analysis of the pooled data from all E:T ratios indicated that the difference in the increase in the percentage of specific lysis induced by the two groups of viruses was significant (P = 0.008).
These results show that the evolutionary changes occurring in H3N2 influenza viruses during their circulation in humans are associated with a reduction in the level of NK cell lysis stimulated by infected cells.
NA treatment of NK cells reduces lysis of influenza virus-infected target cells.
NKp46 is a major activating receptor that is expressed exclusively in NK cells, and the ligation of this receptor triggers natural cytotoxicity of target cells (27, 30). It has been reported that the recognition of viral HA by NK cells is mediated through SA expressed on NKp46 and NKp44 on NK cells and that these interactions activate NK cells (4, 5, 18). To gain insight into the role played by SA on the surface of NK cells in their interaction with influenza virus-infected target cells, NK cell expression of α-2,3-linked and α-2,6-linked SA was examined, the efficiency of desialylation by bacterial NAs was tested, and the effect of desialylation on NK lysis of target cells was investigated.
As Fig. 4A shows, CD56+ CD3− NK cells expressed both α-2,3-linked and α-2,6-linked SA on their surface. It may be that the basal level of α-2,3-linked SA expression is higher than that of α-2,6-linked SA. However, since the sensitivities of MAA and SNA lectins recognizing each SA may be different, this cannot be conclusively established. Treatment of PBMCs with a combination of NA from V. cholerae and A. ureafaciens desialylated NK cells (Fig. 4A). The majority of α-2,6-linked SA was removed from NK cells, while some α-2,3-linked SA remained on the surface even after high concentrations of NAs were added.
FIG. 4.
NA treatment of NK cells reduces lysis of influenza virus-infected target cells. PBMCs were treated with NAs or were left untreated. The cells then were stained with DIG-labeled MAA or SNA followed by a fluorescein isothiocyanate-conjugated anti-DIG antibody to determine the expression of SA-α-2,3-Gal or SA-α-2,6-Gal, respectively. NK cells were identified by costaining with antibodies against CD3 and CD56. (A) The upper left panel shows the forward scatter (FSC) versus side scatter (SSC) profile of total PBMCs and the R1 gate set to identify the lymphocyte population. The upper right panel shows CD3 versus CD56 staining of the R1 population and the R2 gate set to identify the CD3− CD56+ NK cell population. In the lower two panels, the gray-shaded histograms represent staining of gated NK cells without lectins, the solid lines represent NK cells without NA treatment, and the dotted lines represent NK cells with NA treatment. The results shown are representative of findings from three independent experiments using PBMCs from different donors. 51Cr release assays also were performed to investigate the percentage of specific lysis of uninfected 143BTK− cells (B), 143BTK− cells infected with A/England/878/69 (C), or 143BTK− cells infected with A/England/24/00 (D) by control and NA-treated PBMCs at a range of E:T ratios. In each graph, the solid line represents the lysis mediated by control PBMCs, and the dotted line represents the lysis mediated by PBMCs treated with NAs. The data are means of triplicate values, and the results shown are representative of findings from three different experiments.
Notably, NA treatment was found to reduce the level of NK cell lysis of 143BTK− cells infected with influenza virus A/England/878/69 or A/England/24/00, with lysis of target cells infected with each virus being reduced by a similar proportion (Fig. 4C and D). This indicates a role for SA on the NK cell surface in the interaction of these cells with influenza virus-infected targets. The level of lysis of virus-infected 143BTK− cells by NA-treated NK cells was still higher than that of lysis of uninfected 143BTK− cells. This may have been due to NK-target cell interaction mediated by the residual SA on the desialylated NK cells, or it may reflect NK cell activation on contact with influenza virus-infected cells via SA-independent pathways. NA treatment also slightly reduced NK cell lysis of uninfected 143BTK− cells (Fig. 4B), suggesting that SA binding proteins expressed by this cell line contribute to its basal recognition by NK cells.
Deletion of a potential glycosylation site motif on HA increases the ability to agglutinate chicken RBC.
As NK cell killing involves an interaction with viral HA, we analyzed the HA sequences of our H3N2 virus panel in an effort to understand the molecular basis for the reduced NK cell lysis. Over 38 years of evolution in humans, H3N2 viruses have acquired four or five additional potential glycosylation site motifs on the globular head of HA1 not found in the prototype H3N2 strain A/Aichi/2/68 (1, 17, 36). Potential glycosylation site motifs were first observed at positions 122 to 124 and 133 to 135 in viruses isolated during the late 1990s and were found to be conserved in viruses from subsequent seasons. To test the hypothesis that the described HA changes in these recent viruses may interfere with the interaction with SA on NK cells, we used reverse genetics to produce viruses with each new potential glycosylation site motif individually deleted.
The following criteria were used to select virus A/England/26/99 from the larger panel of isolates for further analysis: (i) this virus was found in the group of recent isolates that showed reduced triggering of NK cell killing; (ii) the virus had lost the ability to agglutinate chicken RBC; and (iii) it had acquired notable sequence motifs, such as additional potential glycosylation site motifs on the globular head of HA in the region of the receptor binding site. The HA and NA genes of A/England/26/99 were amplified by reverse transcription-PCR. Plasmid-based reverse genetics was used to produce a recombinant virus with the HA and NA genes of A/England/26/99 and the remaining six gene segments provided by A/Victoria/3/75 (6 + 2 recombinant). The resulting recombinant virus was designated 26/99 HA/NA.
Site-directed mutagenesis was used to individually delete each novel potential glycosylation site motif in 26/99 HA and to restore the amino acid found in the prototype H3N2 isolate (Fig. 5A). For the glycosylation site at positions 122 to 124 (G1Δ), two coding nucleotide changes were introduced such that the motif NES was back mutated to TEG, as found in early H3N2 isolates. At positions 133 to 135 (G2Δ), NGT was back mutated to NGG. The mutated HA genes were introduced into recombinant viruses together with the corresponding NA gene from A/England/26/99 in an A/Victoria/3/75 background and were named G1Δ and G2Δ, respectively (Table 2). The mobility of the HA protein expressed following infection with mutant G2Δ was faster than that for virus with wild-type A/England/26/99 HA, implying a change in glycosylation status had occurred as a result of the mutation (Fig. 5B). Interestingly, no difference in mobility was observed for the G1Δ mutant.
FIG. 5.
Manipulation of potential glycosylation site motifs in the HA of a recent virus. (A) Sequence analysis of HA from A/England/26/99 determined that it contained two new potential glycosylation site motifs at positions 122 to 124 and 133 to 135 that were not found in strains from the previous influenza season or from older viruses such as A/Victoria/3/75. Using site-directed mutagenesis, the new potential glycosylation site motif at 122 to 124 was destroyed by mutating the motif from NES to TEG to create G1Δ. Similarly, the new potential glycosylation site motif at 133 to 135 was destroyed by mutating the motif from NGT to NGG to create G2Δ. (B) Western blot analysis of HA proteins from purified virions obtained following infection of MDCK cells with recombinant influenza viruses bearing HA and NA genes of A/England/26/99 or HA modified as illustrated in panel A to alter the glycosylation motifs at amino acids 122 to 124 (G1Δ) or 133 to 135 (G2Δ).
TABLE 2.
Derivation of surface glycoproteins, agglutination characteristics, and ability to infect target cells of recombinant viruses
| Recombinant virus | HA origin | NA origin | Chicken RBC hemagglutinationa | % Target cell infectionb |
|---|---|---|---|---|
| A/Victoria/3/75 | A/Victoria/3/75 | A/Victoria/3/75 | ++ | 90.2 |
| 26/99 HA/NA | A/England/26/99 | A/England/26/99 | − | 89.5 |
| G1Δ | A/England/26/99, glycosylation motif at positions 122 to 124 mutated | A/England/26/99 | − | 86.5 |
| G2Δ | A/England/26/99, glycosylation motif at positions 133 to 135 mutated | A/England/26/99 | + | 94.8 |
++, >10 HAU; +, 2 to 10 HAU; −, <2 HAU.
Mean percentage of target cells positive for HA expression.
The receptor binding properties of the recombinant viruses were tested in a hemagglutination assay. All the viruses agglutinated turkey RBC, which display abundant SA. Viruses were standardized to 128 hemagglutination units (HAU) using these RBC (data not shown). Recombinant virus containing all gene segments from A/Victoria/3/75 agglutinated chicken RBC (64 HAU). Recombinant viruses with recent surface antigens, such as 26/99 HA/NA, behaved similarly to the wild-type virus A/England/26/99 and did not agglutinate chicken RBC (Table 2). Interestingly, virus G2Δ recovered some ability to agglutinate chicken RBC (8 HAU), suggesting that additional glycosylation on the HA of wild-type A/England/26/99 at positions 133 to 135 (HA1) inhibit receptor interaction. Mutation of the motif at 122 to 124 (G1Δ) did not restore agglutination of chicken RBC.
Removal of glycosylation site motifs on HA from a recent virus isolate increases NK cell lysis of infected cells.
Acquisition of new potential glycosylation site motifs in HA appears to correlate with the inability of recent viruses to agglutinate chicken RBC. To investigate whether these sequence changes in HA altered the ability of NK cells to kill virus-infected target cells, we carried out cytotoxicity experiments using the recombinant viruses with the mutations in potential glycosylation site motifs in HA described above.
As shown in Table 2, the majority of 143BTK− target cells were efficiently infected by all recombinant viruses. The mean fluorescence intensity of HA expression on the target cells infected by 26/99 HA, 26/99 HA/NA, G1Δ, and G2Δ also was similar (Fig. 6A); hence, differences in NK cell lysis of target cells are not due to variation in the intensity of HA expression on the surface of infected cells.
FIG. 6.
Glycosylation on HA reduces the efficiency of lysis of influenza virus-infected cells. (A) Cell surface expression of modified HA proteins following infection of 143BTK− cells with recombinant influenza viruses. 143BTK− cells were infected with the viruses indicated and were incubated overnight, and then surface HA expression was detected using a polyclonal anti-H3-specific rabbit serum and fluorescent anti-rabbit secondary (2ry) antibody. The mean fluorescence intensity (MFI) of cells stained with the secondary antibody only (white bars) or with both the anti-HA antiserum and secondary antibody (black bars) is shown. (B) Data from a 51Cr release assay comparing the percent specific lysis of uninfected 143BTK− cells (dashed line, filled squares) to those of cells infected with the A/Victoria/3/75 influenza virus isolate (solid line, filled squares) or recombinant influenza viruses 26/99 HA/NA (dashed line, filled triangles), G1Δ (solid line, open circles), or G2Δ (solid line, open squares) by PBMC effectors at a range of E:T ratios. These results are representative of findings from two independent experiments. Ab, antibody.
Results from cytotoxicity experiments indicate that infection of target cells with A/Victoria/3/75 caused a substantial increase in their lysis compared to that of uninfected cells (Fig. 6B). Cells infected with the 26/99 HA/NA recombinant virus also were lysed more effectively than uninfected target cells; however, cells infected with this virus were lysed relatively poorly compared to those infected with A/Victoria/3/75, as had previously been observed for the wild-type virus A/England/26/99, suggesting that this property was conferred by the viral glycoproteins. Support for the hypothesis that the glycosylation status of HA affects NK cell triggering by influenza virus-infected cells was provided by the observation that mutation of either of the targeted glycosylation sites from HA led to an increase in the killing of infected cells. Cells infected with the G2Δ recombinant were consistently lysed more efficiently than those infected with the G1Δ recombinant. However, removal of the glycosylation site of HA1 at positions 133 to 135 (G2Δ) was not sufficient to induce as much target cell lysis as was mediated by the HA of A/Victoria/3/75, indicating that additional mutations are likely to be involved in the decreased affinity of HA-SA interaction in recent influenza viruses that affects the efficiency of target cell lysis by NK cells.
DISCUSSION
In this study, we analyzed NK lysis of target cells infected with a panel of H3N2 influenza virus isolates representative of viruses circulating in humans between 1969 and 2003, and we showed that cells infected with older (1969 to 1996) influenza virus isolates were lysed more efficiently than cells infected with recent (1999 to 2003) influenza virus isolates.
Mapping of the genetic and antigenic evolution of H3N2 influenza A viruses from 1968 to 2003 has revealed that strains group into antigenic clusters, and minimal genetic change can produce disproportionate changes in antigenicity (32). Our broadly defined groups of older (1969 to 1996) and recent (1999 to 2003) viruses encompass antigenic types from several separate clusters defined by Smith et al. (32) and together represent continuous genetic evolution during antigenic drift (Table 1). Additionally, we have shown that individual mutations that create potential glycosylation site motifs can produce considerable change in both HA binding to SA and efficiency of NK cell activation.
NK cell activation is regulated by the balance of signals received through activating and inhibitory surface receptors (23). The latter interact with MHC class I molecules, allowing NK recognition of target cells with decreased MHC class I expression. In agreement with findings from other studies (3), we found no decrease in MHC class I expression in influenza virus-infected cells, suggesting that the infection-associated increase in target cell lysis probably did not result from a reduction of signaling through inhibitory NK receptors. Instead, it was likely due to triggering of activating NK receptors.
Activating NK receptors include NKG2D and the natural cytotoxicity receptors NKp46, NKp44, and NKp30. The influenza virus HA protein is known to be among the ligands recognized by both NKp46 and NKp44 (5, 18), and this interaction may also contribute to NK activation by influenza virus-infected cells. The binding of influenza virus HA to NKp46 was reported to be mediated mainly via SA carried by NKp46 (4). This suggested to us that HA proteins that bind with differing affinities to SA may have a differential ability to trigger NK cell activation. In line with this hypothesis, we found that cells infected with recent H3N2 influenza virus isolates that exhibit a reduced affinity of binding to both α-2,3- and α-2,6-linked SA activated a lower level of NK cell lysis than cells infected with older virus isolates. Notably, a previous study also found variability in binding of recombinant NKp44-immunoglobulin and NKp46-immunoglobulin to cells infected with different strains of influenza virus: cells infected with H3N2 influenza viruses were recognized less well than those infected with H1N1 viruses. In addition, A/Moscow/99-like H3N2 influenza virus-infected cells were bound less effectively than those infected with an A/Sydney/97-like H3N2 isolate (4). Whether this correlated with agglutination properties or HA-SA affinity differences was not reported.
The HA proteins of recent influenza virus isolates have a reduction in affinity for both α-2,3-linked SA in the form of 3′SL and α-2,6-linked SA in the form of 6′SLN. Older isolates such as A/England/878/69 bind poorly to 6′SLN but well to 3′SL. We have recently reported a changing tropism of the panel of H3N2 influenza viruses whereby recent viruses are more likely to infect nonciliated cells in a human airway epithelial model, whereas older viruses infect more of the ciliated cells (35). Since we also found that nonciliated cells express more α-2,6-linked SA and ciliated cells more α-2,3-linked SA at their surface, this also suggests that recent influenza viruses bind less effectively to α-2,3-linked SA on ciliated cells than older strains. In the present study, our NA treatment protocol removed the majority of α-2,6-linked SA from NK cell surfaces, but some α-2,3-linked SA remained (Fig. 3A). Although this treatment reduced NK cell lysis of target cells infected with both old and new influenza viruses, lysis of target cells infected with both isolates still was greater than that seen for uninfected cells after NA treatment. Since there was residual expression of SA-α-2,3-Gal on the cell surface after NA treatment, this implies that NK cells can be activated via α-2,3-linked SA (although it could potentially reflect NK cell activation by non-SA-dependent pathways). Arnon et al. have suggested that the binding of influenza virus HA to NKp46 is mediated mainly via α-2,6-linked SA carried by NKp46 (4). We suggest that NK cells might also be activated by HA binding to SA-α-2,3-Gal. This would be particularly relevant for older H3N2 human influenza virus isolates and avian influenza viruses that infect humans, since these viruses display high affinity for α-2,3-linked SA receptors.
We also investigated whether potential glycosylation sites acquired during evolution of HA affect the recognition of infected cells by NK cells. In particular, we analyzed the effect of removing two potential glycosylation sites that have recently been acquired at residues 122 to 124 and 133 to 135 of the H3 HA protein. Abe et al. (1) and Vigerust et al. (36) have demonstrated that the motif at residues 133 to 135 does indeed act as a site for glycosylation. Accordingly, we observed a change in HA mobility for the G2Δ mutant concordant with a loss of glycosylation, and this is the most probable explanation for the effects we observed for receptor binding and NK lysis. In contrast, neither we nor Abe et al. (1) were able to demonstrate a shift in mobility of HA proteins altered at the motif at residues 122 to 124, which suggests that this site may not in fact be glycosylated. Interestingly, mutation of the motif at 122 to 124 did not restore chicken red blood cell agglutination, and the effect on NK lysis was less than that for the G2Δ mutant. Nonetheless, mutations at either positions 122 to 124 or positions 133 to 135 partially restored the ability of A/England/26/99 to agglutinate chicken RBC and enhanced NK recognition of infected cells, so the G1Δ mutation presumably resulted in a subtle enhancement in HA-SA interaction that we did not detect using the HA test but that could be picked up in the NK assay. Although NK activation was not fully restored by either mutation to the level seen for the older influenza virus isolate, it is likely that other sequence differences between the HA genes of A/Victoria/3/75 and A/England/26/99 affect their binding to SA and hence the efficiency of NK lysis of cell infected with these strains.
Using a reverse genetic approach, we were able to study differences in NK activation between isolates based solely on changes in the surface genes for HA and NA. It is also plausible that influenza viruses utilize a mechanism(s) involving other viral genes to evade NK cell activation, and these might vary between isolates. Recent studies have shown that the NKG2D ligand MICB is up-regulated in influenza virus-infected macrophages and that this can mediate NK cell activation (29). Secretion of α/β interferon from infected macrophages contributed to the MIC upregulation. We have recently demonstrated variability in the ability of different strains of influenza virus to control the interferon response (14). It may be that some influenza virus isolates up-regulate MICA/MICB and activate NK cells via NKG2D, whereas other influenza viruses are able to suppress the upregulation of stress-inducible molecules such as MIC.
In summary, our results indicate that evolutionary changes occurring in H3N2 influenza viruses during their circulation in humans may be associated with a reduction in the affinity of HA binding to SA that diminishes their interaction with activating NK receptors. This suggests that NK cells are among the forces driving influenza virus evolution in humans. There is evidence from both murine models and human studies to show that NK cells are rapidly activated following influenza virus infection and make an important contribution to control of early virus replication as well as promoting the induction of the adaptive immune response (16, 31, 33). Moreover, the recent demonstration that NKp46 knockout mice become exquisitely sensitive to challenge with influenza virus demonstrates the importance of this early innate response in controlling influenza virus infection (13). Influenza viruses bearing HA proteins that have a reduced affinity of binding to activating NK receptors may thus have an in vivo replicative advantage and gradually may come to dominate the viral population circulating in humans. It would be of interest to extend similar studies to other panels of influenza virus isolates to determine whether the antigenic drift occurring in H1N1 viruses during their circulation in humans also was accompanied by an HA evolutionary path that evaded both antibody and NK recognition.
Acknowledgments
This work was supported by core funding from The Edward Jenner Institute and by the University of Reading Research Endowment Trust Fund (L.J.P.).
We thank Sandro Leidi (University of Reading, United Kingdom) for help with statistical analysis of data, and we are grateful to Thomas Zurcher (GlaxoSmithKline) for providing GG167 and the A/Victoria/3/75 plasmid-based reverse genetics system.
We have no associations that pose a conflict of interest.
This is manuscript number 106 from the Edward Jenner Institute.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Abe, Y., E. Takashita, K. Sugawara, Y. Matsuzaki, Y. Muraki, and S. Hongo. 2004. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J. Virol. 78:9605-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abed, Y., A. M. Bourgault, R. J. Fenton, P. J. Morley, D. Gower, I. J. Owens, M. Tisdale, and G. Boivin. 2002. Characterisation of two influenza A (H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the haemagglutinin gene. J. Infect. Dis. 186:1074-1080. [DOI] [PubMed] [Google Scholar]
- 3.Achdout, H., T. I. Arnon, G. Markel, T. Gonen-Gross, G. Katz, N. Lieberman, R. Gazit, A. Joseph, E. Kedar, and O. Mandelboim. 2003. Enhanced recognition of human NK receptors after influenza virus infection. J. Immunol. 171:915-923. [DOI] [PubMed] [Google Scholar]
- 4.Arnon, T. I., H. Achdout, N. Lieberman, R. Gazit, T. Gonen-Gross, G. Katz, A. Bar-Ilan, N. Bloushtain, M. Lev, A. Joseph, E. Kedar, A. Porgador, and O. Mandelboim. 2004. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood 103:664-672. [DOI] [PubMed] [Google Scholar]
- 5.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. [DOI] [PubMed] [Google Scholar]
- 6.Barclay, W. S., I. M. Jones, H. M. I. Osborn, L. J. Phillipson, J. Ren, G. A. Talevera, and C. I. Thompson. 2007. Probing the receptor interactions of an H5 avian influenza virus using a baculovirus expression system and functionalised poly(acrylic acid) ligands. Bioorg. Med. Chem. 15:4038-4047. [DOI] [PubMed] [Google Scholar]
- 7.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Both, G. W., M. J. Sleigh, N. J. Cox, and A. P. Kendal. 1983. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J. Virol. 48:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen, M. B., S. Sabesan, J. J. Skehel, and D. C. Wiley. 1997. Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography. Virology 232:19-31. [DOI] [PubMed] [Google Scholar]
- 10.Elleman, C. J., and W. S. Barclay. 2004. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321:144-153. [DOI] [PubMed] [Google Scholar]
- 11.Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. Garcia-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:9679-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambaryan, A., S. Yamnikova, D. Lvov, A. Tuzikov, A. Chinarev, G. Pazynina, R. G. Webster, M. Matrosovich, and N. Bovin. 2005. Receptor specificity of influenza viruses from birds and mammals: new data on involvement of the inner fragments of the carbohydrate chain. Virology 334:276-283. [DOI] [PubMed] [Google Scholar]
- 13.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 14.Hayman, A., S. Comely, A. Lackenby, S. Murphy, J. McCauley, S. Goodbourn, and W. S. Barclay. 2006. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology 347:52-64. [DOI] [PubMed] [Google Scholar]
- 15.Ito, T., Y. Suzuki, L. Mitnaul, A. Vines, H. Kida, and Y. Kawaoka. 1997. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 227:493-499. [DOI] [PubMed] [Google Scholar]
- 16.Leung, K. N., and G. L. Ada. 1981. Induction of natural killer cells during murine influenza virus infection. Immunobiology 160:352-366. [DOI] [PubMed] [Google Scholar]
- 17.Macken, C., H. Lu, J. Goodman, and L. Boykin. 2001. The value of a database in surveillance and vaccine selection, p. 103-106. In A. D. M. E. Osterhaus, N. Cox, and A. W. Hampson (ed.), Options for the control of influenza IV. Elsevier Science, Amsterdam, The Netherlands.
- 18.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055-1060. [DOI] [PubMed] [Google Scholar]
- 19.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H.-D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros, R., N. Escriou, N. Naffakh, J. C. Manuguerra, and S. van der Werf. 2001. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 289:74-85. [DOI] [PubMed] [Google Scholar]
- 22.Mochalova, L., A. Gambaryan, J. Romanova, A. Tuzikov, A. Chinarev, D. Katinger, H. Katinger, A. Egorov, and N. Bovin. 2003. Receptor-binding properties of modern human influenza viruses primarily isolated in Vero and MDCK cells and chicken embryonated eggs. Virology 313:473-480. [DOI] [PubMed] [Google Scholar]
- 23.Moretta, L., C. Bottino, D. Pende, M. Vitale, M. C. Mingari, and A. Moretta. 2004. Different checkpoints in human NK-cell activation. Trends Immunol. 25:670-676. [DOI] [PubMed] [Google Scholar]
- 24.Nobusawa, E., H. Ishihara, T. Morishita, K. Sato, and K. Nakajima. 2000. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 278:587-596. [DOI] [PubMed] [Google Scholar]
- 25.Ohuchi, M., R. Ohuchi, A. Feldmann, and H. D. Klenk. 1997. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J. Virol. 71:8377-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohuchi, M., R. Ohuchi, and A. Matsumoto. 1999. Control of biological activities of influenza virus hemagglutinin by its carbohydrate moiety. Microbiol. Immunol. 43:1071-1076. [DOI] [PubMed] [Google Scholar]
- 27.Pessino, A., S. Sivori, C. Bottino, A. Malaspina, L. Morelli, L. Moretta, R. Biassoni, and A. Moretta. 1998. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 188:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillipson, L. J. 2005. Sialyl oligosaccharide glycopolymers: their synthesis and use as probes of influenza A H3N2 virus evolution. The University of Reading, Reading, United Kingdom.
- 29.Sirén, J., T. Sareneva, J. Pirhonen, M. Strengell, V. Veckman, I. Julkunen, and S. Matikainen. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J. Gen. Virol. 85:2357-2364. [DOI] [PubMed] [Google Scholar]
- 30.Sivori, S., D. Pende, C. Bottino, E. Marcenaro, A. Pessino, R. Biassoni, L. Moretta, and A. Moretta. 1999. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 29:1656-1666. [DOI] [PubMed] [Google Scholar]
- 31.Skoner, D. P., T. L. Whiteside, J. W. Wilson, W. J. Doyle, R. B. Herberman, and P. Fireman. 1996. Effect of influenza A virus infection on natural and adaptive cellular immunity. Clin. Immunol. Immunopathol. 79:294-302. [DOI] [PubMed] [Google Scholar]
- 32.Smith, D. J., A. S. Lapedes, J. C. de Jong, T. M. Bestebroer, G. F. Rimmelzwaan, A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2004. Mapping the antigenic and genetic evolution of influenza virus. Science 305:371-376. [DOI] [PubMed] [Google Scholar]
- 33.Stein-Streilein, J., and J. Guffee. 1986. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J. Immunol. 136:1435-1441. [PubMed] [Google Scholar]
- 34.Thompson, C. I., W. S. Barclay, and M. C. Zambon. 2004. Changes in in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host. J. Antimicrob. Chemother. 53:759-765. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, C. I., W. S. Barclay, M. C. Zambon, and R. J. Pickles. 2006. Infection of human airway epithelium by human and avian strains of influenza A virus. J. Virol. 80:8060-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigerust, D. J., K. B. Ulett, K. L. Boyd, J. Madsen, S. Hawgood, and J. A. McCullers. 2007. N-linked glycosylation attenuates H3N2 influenza viruses. J. Virol. 81:8593-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, W., and G. M. Air. 2004. Binding of influenza viruses to sialic acids: reassortant viruses with A/NWS/33 hemagglutinin bind to α2,8-linked sialic acid. Virology 325:340-350. [DOI] [PubMed] [Google Scholar]