Abstract
Differentiation and survival defects of human immunodeficiency virus (HIV)-specific CD8+ T cells may contribute to the failure of HIV-specific CD8+ T cells to control HIV replication. It is not known, however, whether simian immunodeficiency virus (SIV)-infected rhesus macaques show comparable defects in these virus-specific CD8+ T cells or when such defects are established during infection. Peripheral blood cells from acutely and chronically infected rhesus macaques were stained ex vivo for memory subpopulations and examined by in vitro assays for apoptosis sensitivity. We show here that SIV-specific CD8+ T cells from chronically SIV infected rhesus macaques show defects comparable to those observed in HIV infection, namely, a skewed CD45RA− CD62L− effector memory phenotype, reduced Bcl-2 levels, and increased levels of spontaneous and CD95-induced apoptosis of SIV-specific CD8+ T cells. Longitudinal studies showed that the survival defects and phenotype are established early in the first few weeks of SIV infection. Most importantly, they appear to be antigen driven, since most probably the loss of epitope recognition due to viral escape results in the reversal of the phenotype and reduced apoptosis sensitivity, something we observed also for animals treated with antiretroviral therapy. These findings further support the use of SIV-infected rhesus macaques to investigate the phenotypic changes and apoptotic defects of HIV-specific CD8+ T cells and indicate that such defects of HIV-specific CD8+ T cells are the result of chronic antigen stimulation.
Simian immunodeficiency virus (SIV)-infected nonhuman primates are the primary animal model for human immunodeficiency virus (HIV) infection of humans, because infection with pathogenic strains of SIV leads to persistent infection and an immunodeficiency syndrome similar to AIDS (6, 12, 13, 22). In this model, CD8+ T cells play a key role in controlling SIV infection (10, 11, 26). HIV-specific CD8+ T cells suffer from intrinsic defects that may impair their ability to control HIV replication (5, 18, 23). These include susceptibility to CD95/Fas-induced apoptosis (18), decreased ex vivo expression of the antiapoptotic molecule Bcl-2 (23), and a skewed memory phenotype, with most cells being CD45RA− CD62L− CCR7− effector memory CD8+ T cells (5, 18). CD95-induced apoptosis of CD8+ T cells may be an immune evasion mechanism of lentiviruses through upregulation of CD95/Fas ligand (FasL) by the viral protein Nef (9, 29). However, it is not clear when these defects are established and why.
For chronically SIV infected rhesus macaques, functional impairment (reduced cytotoxicity, cytokine production) of SIV-specific CD8+ T cells (8) and increased apoptosis of lymphocytes (7), CD4+ T cells, and CD8+ T cells have been shown (3). It is not known, however, whether SIV-specific CD8+ T cells share the apoptotic potential and the skewed memory phenotype with HIV-specific CD8+ T cells. To examine the memory phenotype of SIV-specific and total CD8+ T cells, we used the CD45RA and CD62L markers, because these markers break down CD8+ T cells into four distinct populations (25). A recent study examining the memory phenotype of CD8+ T cells in uninfected rhesus macaques suggested the use of CD95 and CD28 to define memory subpopulations (24). With this combination, however, CD8+ T cells cannot be subdivided into two different effector memory populations and one cannot detect the skewing in these populations. This is important in view of the fact that differences in the effector memory population distribution between HIV- and cytomegalovirus-specific CD8+ T cells in HIV-infected individuals were previously described using these markers (5, 18). More studies are necessary to definitely identify the correlation between different surface markers and cell functions of memory subpopulations in rhesus macaques. Here we present data indicating that SIV-specific CD8+ T cells have intrinsic defects comparable to those described for HIV-specific CD8+ T cells. Most importantly, we show that these defects are established early during SIV infection and that antigen recognition drives these defects. Our findings suggest that defects of SIV-specific and HIV-specific CD8+ T cells are established early during infection and may result from chronic antigenic stimulation.
MATERIALS AND METHODS
Animals.
The Mamu-A*01 Indian rhesus macaques (Macaca mulatta) were housed at the Bioqual animal facility according to the standards and guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (9a) and according to animal care standards deemed acceptable by the Association for the Assessment and Accreditation of Laboratory Animal Care—International. All experiments were performed following institutional animal care and use committee approval. The acute SIV study included 14 animals, and the chronic SIV study included 18 uninfected and 15 SIV-infected animals. Animals were infected intravenously with 100 50% infective doses of SIVmac251. Four of the 15 chronically SIV infected macaques were on antiretroviral therapy (ART) [q-R-(2-phosphonomethoxypropyl)adenine (PMPA) and β-2′,3′-dideoxy-3 thia-5-fluorocytidine (FTC)]. None of these animals had undergone any previous vaccination or treatment. The virus load was determined by Taqman PCR analysis as described previously with a sensitivity of 200 SIV RNA copies/ml of blood (20). Viral loads and CD4 and CD8 cell counts are summarized in Table 1.
TABLE 1.
Viral loads and CD4 and CD8 cell counts of uninfected and infected rhesus macaques
| Group (n) | Median (range)
|
||
|---|---|---|---|
| Viral load (SIV RNA molecules/ml of blood) | CD4 cells/μl | CD8 cells/μl | |
| Chronically infected, ART treated (4) | 380 (200-880) | 981 (784-1,528) | 1,161 (886-1,725) |
| Chronically infected, ART naïve (11) | 17,070 (1,890-801,600) | 564 (187-1,261) | 1,082 (701-4,683) |
| Uninfected controls (18) | 1,601 (535-4,479) | 1,498 (472-3,772) | |
Flow cytometry.
Peripheral blood mononuclear cells (PBMC) were freshly isolated by Percoll density gradient centrifugation (1.075 g/ml; Amersham Biosciences, Uppsala, Sweden) from heparinized venous blood. Blood was processed within 6 h of collection. For surface and intracellular staining, anti-human antibodies cross-reactive with the corresponding rhesus macaque antigens were used: anti-CD3-PerCP-Cy5.5 (clone SP34), anti-CD8-phycoerythrin (PE)-Cy5 (clone RPA-T8), anti-CD8-Alexa Fluor 405 (clone 3B5), anti-CD45RA-fluorescein isothiocyanate (clone 5H9), anti-CD62L-PE (clone SK11), anti-Bcl-2-PE (clone 6C8) and an isotype control (Armenian hamster immunoglobulin G), anti-Bcl-xL-PE (clone 7B2.5) and an isotype control (mouse immunoglobulin G3), and annexin V-Cy5.5. Antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), Caltag (Burlingame, CA), and Southern Biotechnology (Birmingham, AL). SIV-specific CD8+ T cells were identified using tetramers of MamuA*01-β2-microglobulin loaded with SIV Gag 181-189 CM9 (CTPYDINQM) peptide or SIV Tat SL8 28-35 (STPESANL) peptide (2). Briefly, 0.5 × 106 PBMC were stained in fluorescence-activated cell sorter (FACS) wash (Hanks’ balanced salt solution [Cellgro, Herndon, VA], 3% heat-inactivated horse serum [Invitrogen, Carlsbad, CA], 0.02% NaN3) for 30 min on ice, washed twice, and fixed with 1% paraformaldehyde. When annexin V was used for apoptosis detection, 2.5 mM CaCl2 was added to all solutions. For measurement of Bcl-2 and Bcl-xL expression, PBMC were fixed and permeabilized with Cytofix-Cytoperm (BD Biosciences), washed with Perm/Wash (BD Biosciences), incubated with specific antibodies or corresponding isotype controls for 1 h on ice, washed, and fixed with 1% paraformaldehyde. Samples were collected on a FACSCalibur (BD Biosciences) and a FACSAria (BD Biosciences) and were analyzed using FlowJo software (TreeStar, San Carlos, CA). For each sample, 300,000 events were collected, and virus-specific CD8+ T-cell populations were included in the study only when they accounted for more than 0.1% of CD8+ T cells and there were 60 events in the virus-specific CD8+ T-cell population. Levels of Bcl-2 and Bcl-xL expression were calculated by subtracting the mean fluorescence intensity (MFI) for the isotype control from the MFI for the corresponding Bcl2 or Bcl-xL stain.
Apoptosis studies.
Freshly isolated PBMC were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Cellgro) at 37°C in a 5% CO2 incubator at 1 × 106 cells/ml in 24-well plates without or with 10 ng/ml soluble FasL (sFasL; Axxora, San Diego, CA) and 1 μg/ml enhancer (Axxora). To examine the effect of interleukin-15 (IL-15) on apoptosis sensitivity, 5 ng/ml recombinant rhesus macaque IL-15 (a kind gift from F. Villinger, Emory University, Atlanta, GA) was added to the cultures as indicated. After 14 h of incubation, cells were harvested and stained for apoptosis. Specific apoptosis was calculated as (% induced apoptosis − % spontaneous apoptosis)/(100 − % spontaneous apoptosis) × 100.
Statistical analysis.
The Mann-Whitney U test, Student t test, nonparametric Wilcoxon signed-rank test for paired samples, and Shapiro-Wilk W test for normality were used for statistical analysis with the JMP statistical analysis program (SAS, Cary, NC). P values of <0.05 were considered significant.
RESULTS
SIV-specific CD8+ T cells belong predominantly to the CD45RA− CD62L− effector memory subpopulation.
The memory phenotype of SIV-specific CD8+ T cells in chronically infected rhesus macaques was analyzed to examine whether these cells show a skewed effector memory phenotype comparable to that found in HIV-specific CD8+ T cells.
Total CD8+ T cells of uninfected (n = 18) and SIV-infected (n = 15) rhesus macaques showed similar distributions of memory phenotype (Fig. 1B). The distribution was greatly skewed, however, when Gag-specific CD8+ T cells were analyzed (n = 15). Most of these cells were found in the CD45RA− CD62L− effector memory population (74% ± 5%), whereas only 14% ± 4% belonged to the CD45RA+ CD62L− effector memory population (Fig. 1A and B). This skewed phenotype of SIV-specific CD8+ T cells becomes even more evident when the ratio of the CD45RA− CD62L− to the CD45RA+ CD62L− effector memory population is calculated (Fig. 1C). For total CD8+ T cells, this ratio is 0.89 ± 0.09 for uninfected animals and 1.49 ± 0.25 for SIV-infected animals (P < 0.05), whereas for Gag-specific CD8+ T cells from SIV-infected animals, the ratio is 18 ± 5.5, >12-fold higher than the ratio for total CD8+ T cells from SIV-infected animals (P < 0.006) (Fig. 1C).
FIG. 1.
Reduced levels of CD45RA+ CD62L− effector memory SIV-specific CD8+ T cells in chronically SIV infected rhesus macaques. PBMC from uninfected and SIV-infected rhesus macaques were analyzed ex vivo for memory subpopulations. Gag-specific CD8+ T cells from SIV-infected animals were identified using the Gag CM9 tetramer. (A) Representative FACS plots showing memory subpopulations of Gag-specific and total CD8+ T cells from an SIV-infected rhesus macaque after direct ex vivo staining of freshly isolated PBMC. Cells were first gated on lymphocytes using forward scatter and side scatter, then on CD3+ T cells, and subsequently for the indicated antigens. (B) Percentages of memory subpopulations in CD8+ T cells from uninfected control rhesus macaques (n = 18) and in total and Gag-specific CD8+ T cells from SIV-infected rhesus macaques (n = 15). Horizontal lines indicate means. (C) Ratios of CD45RA− CD62L− to CD45RA+ CD62L− effector memory cells for total CD8+ T cells from uninfected animals and for Gag-specific and total CD8+ T cells from SIV-infected animals. Each line indicates a statistically significant difference between two groups. P values are given in the key.
This phenotype distribution of memory SIV-specific CD8+ T cells is similar to that described previously for human HIV-specific CD8+ T cells (5, 18) and suggests that the skewed memory phenotype of lentivirus-specific CD8+ T cells is common in both lentivirus infections.
SIV-specific CD8+ T cells from chronically SIV infected rhesus macaques are highly susceptible to CD95-induced apoptosis, which is inhibited by IL-15.
To examine the apoptosis sensitivities of total and SIV-specific CD8+ T cells from SIV-infected and uninfected rhesus macaques, freshly isolated PBMC were examined overnight for spontaneous and CD95-induced apoptosis in the presence or absence of IL-15.
In uninfected and SIV-infected rhesus macaques, significantly more CD8+ T cells became apoptotic in the presence of sFasL than in its absence (Fig. 2A). IL-15 reduced spontaneous and CD95-induced apoptosis in total CD8+ T cells from SIV-infected macaques but not from uninfected animals (Fig. 2B). The frequency of spontaneous apoptosis of SIV-specific CD8+ T cells was significantly higher than that of total CD8+ T cells from non-SIV-infected animals (P < 0.01) but not from SIV-infected animals (Fig. 2A). SIV-specific CD8 T cells were highly sensitive to CD95-induced apoptosis, the frequency of which was significantly higher than the frequencies of their spontaneous apoptosis (P < 0.0005) and the CD95-induced apoptosis observed in total CD8+ T cells from SIV-uninfected and infected animals (Fig. 2A). The calculated percentage of CD95-specific apoptosis of SIV-specific CD8+ T cells was significantly higher than those of total CD8+ T cells from uninfected and SIV-infected animals (Fig. 2C). This suggests that SIV-specific CD8+ T cells are selectively more sensitive to CD95/Fas-induced apoptosis than total CD8+ T cells in SIV-infected and uninfected rhesus macaques. IL-15 significantly decreased the percentages of spontaneous apoptosis and CD95-induced apoptosis of SIV-specific CD8+ T cells by 30% and 54%, respectively (Fig. 2B). When CD95-specific apoptosis was calculated, IL-15 significantly decreased the percentage of CD95-specific apoptosis in SIV-specific CD8+ T cells by 73% and that in total CD8+ T cells from SIV-infected animals by 60% (Fig. 2C). Therefore, IL-15 potently inhibits the apoptosis of SIV-specific CD8+ T cells.
FIG. 2.
SIV-specific CD8+ T cells are highly sensitive to apoptosis, which is inhibited by IL-15. PBMC from uninfected and SIV-infected rhesus macaques were cultured in the presence or absence of sFasL (10 ng/ml) overnight, and spontaneous and CD95-induced apoptosis was analyzed. Gag-specific CD8+ T cells from SIV-infected animals were identified using the Gag CM9 tetramer. (A) Pooled data showing the percentages of spontaneous and CD95-induced apoptosis for CD8+ T cells from uninfected rhesus macaques (n = 18) and for total (n = 15) and Gag-specific (n = 15) CD8+ T cells from SIV-infected rhesus macaques in overnight cultures of PBMC. Horizontal lines indicate means. (B) Pooled data showing the percentages of spontaneous and CD95-induced apoptosis in the presence and absence of 5 ng/ml IL-15 for CD8+ T cells from uninfected rhesus macaques (n = 18) and for total (n = 15) and Gag-specific (n = 15) CD8+ T cells from SIV-infected rhesus macaques in overnight cultures of PBMC. Horizontal lines indicate means. (C) CD95-specific apoptosis of total CD8+ T cells from uninfected rhesus macaques and of total and Gag-specific CD8+ T cells from SIV-infected rhesus macaques. For calculation of specific apoptosis, see Materials and Methods. Horizontal lines indicate means. Lines with asterisks at the top indicate statistical significance. P values are given in the key. (D) Pooled data showing the percentages of spontaneous and CD95-induced apoptosis of naïve and memory CD8+ T-cell subpopulations of chronically SIV infected rhesus macaques (n = 5). (E) (Left) Pooled data showing MFI of Bcl-2 expression in total CD8+ T cells from 16 uninfected macaques and in Gag-specific and total CD8+ T cells from 15 SIV-infected rhesus macaques. (Right) Bcl-2 MFIs after 14 h in culture in the presence or absence of IL-15 (5 ng/ml) for total CD8+ T cells from uninfected controls (n = 6) and for total (n = 7) and Gag-specific (n = 7) CD8+ T cells from SIV-infected animals. (F) (Left) MFI of Bcl-xL expression in total CD8+ T cells from 16 uninfected macaques and in Gag-specific and total CD8+ T cells from 15 SIV-infected rhesus macaques. (Right) Bcl-xL MFIs after 14 h in culture in the presence or absence of IL-15 (5 ng/ml) for total CD8+ T cells from uninfected controls (n = 6) and total (n = 7) and Gag-specific (n = 7) CD8+ T cells from SIV-infected animals.
When the different memory subpopulations were examined for apoptosis sensitivity (n = 5) (Fig. 2D), CD45RA− CD62L− CD8+ T cells had the highest rates of spontaneous and CD95-induced apoptosis. The apoptotic defects of SIV-specific CD8+ T cells described above and the effect of IL-15 closely resemble those seen in HIV-specific CD8+ T cells (17-19).
SIV-specific CD8+ T cells have reduced Bcl-2 levels that can be restored by IL-15.
Ex vivo Bcl-2 expression in SIV-specific CD8+ T cells was more than 50% lower (MFI, 691 ± 112 [n = 15]) than that in total CD8+ T cells from SIV-infected (MFI, 1,511 ± 176 [n = 15]; P < 0.0005) and uninfected (MFI, 1,705 ± 171 [n = 16]; P < 0.0005) animals (Fig. 2E). Ex vivo Bcl-xL levels, however, were not reduced in SIV-specific or total CD8+ T cells from SIV-infected animals (Fig. 2F).
We next examined whether IL-15 upregulates the expression of Bcl-2 and Bcl-xL, because this could explain the ability of IL-15 to inhibit apoptosis in these cells. Overnight in vitro IL-15 treatment increased the MFI of Bcl-2 significantly (2.1-fold), from 767 ± 161 to 1,596 ± 234, in SIV-specific CD8+ T cells (P < 0.0005; n = 7). Similar increases were also seen in total CD8+ T cells from uninfected (P < 0.05; n = 6) and SIV-infected (P < 0.0005; n = 7) animals (Fig. 2E). IL-15 also increased Bcl-xL expression 1.7-fold in SIV-specific CD8+ T cells and 1.5-fold in total CD8+ T cells (Fig. 2F).
These results show that the reduced expression of Bcl-2 without a concomitant increase in the level of Bcl-xL expression, previously shown for HIV-specific CD8+ T cells (23), is also found in SIV-specific CD8+ T cells from chronically infected animals.
The memory phenotype and apoptosis sensitivity of SIV-specific CD8+ T cells appear early in SIV infection.
Having verified that SIV-specific CD8+ T cells exhibit the same skewed phenotype and apoptosis sensitivity as HIV-specific CD8+ T cells, we were able to determine when these defects are established by following SIV-specific CD8+ T cells longitudinally during the first 20 weeks of SIV infection.
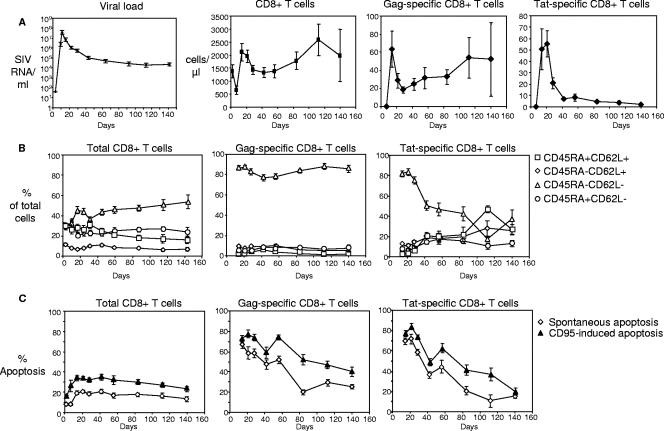
Viral loads and Gag-specific, Tat-specific, and total CD8+ T-cell numbers are shown in Fig. 3A. When the memory phenotype of the Gag-specific CD8+ T cells was examined, we found that at week 2, 86% ± 2.4% of cells had a CD45RA− CD62L− phenotype, and that this proportion did not change over the following weeks (Fig. 3B). Although Tat-specific CD8+ T cells were also mostly CD45RA− CD62L− during weeks 2 and 3 postinfection (86% ± 2.9%), the prevalence of this memory phenotype decreased subsequently, to 40% at week 6, and dropped below 40% at week 20. These changes in the memory phenotype of Tat-specific CD8+ T cells were all due to the loss of absolute numbers of CD45RA− CD62L− cells, which, in turn, could be due to loss of recognition of the Tat peptide (a phenomenon that occurs frequently at week 4 postinfection), as suggested previously (1).
FIG. 3.
Memory phenotype and apoptosis susceptibility of Gag-specific CD8+ T cells in acutely SIV infected rhesus macaques. (A) Pooled data for viral loads and absolute numbers of total, Gag-specific, and Tat-specific CD8+ T cells from week zero up to week 20 post-SIV infection. (B) Distribution of memory cells for total, Gag-specific, and Tat-specific CD8+ T cells from week zero up to week 20 post-SIV infection. (C) Spontaneous and CD95-induced apoptosis for total, Gag-specific, and Tat-specific CD8+ T cells from week zero up to week 20 post-SIV infection. For all panels, data for day zero to day 56 represent 12 animals, and data for day 84 to day 140 represent 5 animals. Means ± standard errors for each time point are shown.
Spontaneous apoptosis sensitivity was high in Gag-specific CD8+ T cells during the first weeks; the level was 67% ± 3.1% at week 2 and then decreased to 25% ± 2.0% at week 20 (Fig. 3C). Although the percentage of CD95-induced cell death was also very high at week 2 (72% ± 3.4%), this was due mostly to the very high level of spontaneous apoptosis. In the following weeks, despite the decrease in the level of spontaneous apoptosis, CD95-induced apoptosis remained high, with 40% ± 4.8% at week 20 (Fig. 3C), a level similar to that found during the chronic phase (Fig. 1A). As seen with the memory phenotype distribution, spontaneous and CD95-induced apoptosis levels of Tat-specific CD8+ T cells were comparable to those of Gag-specific CD8+ T cells during the first 3 to 4 weeks postinfection. However, apoptosis sensitivity then dropped rapidly to 20% (Fig. 3C), suggesting that antigen recognition may be driving apoptosis sensitivity, because SIV escape mutants occur at 4 weeks postinfection (1).
Since Tat-specific CD8+ T cells lost the skewed memory phenotype and apoptosis sensitivity after week 4 postinfection, simultaneously with the previously described appearance of escape mutations in Tat (1), we examined whether reducing the viral load and hence the antigen level by ART would lead to a similar effect on Gag-specific CD8+ T cells in chronically infected animals. The mean viral load for the ART-treated animals (n = 4) was 460 ± 164 SIV RNA molecules/ml, while ART-naïve animals (n = 9) had a mean viral load of 17,239 ± 6,294 SIV RNA molecules/ml. ART-treated animals had a memory phenotype with more CD45RA− CD62L+ central memory and CD45RA+ CD62L− effector memory SIV-specific CD8+ T cells and fewer CD45RA− CD62L− effector memory cells than the group of ART-naïve animals (Fig. 4 A). When the ratio of CD45RA− CD62L− to CD45RA+ CD62L− cells was calculated, a clear difference was observed between ART-treated (5.5 ± 3.3) and ART-naïve (22 ± 6.2) animals (Fig. 4B); however, the difference was not statistically significant (P = 0.067), which could be due to the small number of animals in the ART-treated group. When apoptosis was compared for ART-treated and ART-naïve animals, only a trend of lower CD95-specific apoptosis for the ART-treated animals was found (percentages of CD95-specific apoptosis, 30% ± 13% for ART-treated and 45% ± 7.2% for ART-naïve animals).
FIG. 4.
ART treatment reverses the memory phenotype. (A) Pooled data for memory subpopulations of Gag-specific CD8+ T cells from ART-treated (n = 4) and ART-naïve (n = 11) animals. Lines indicate means. (B) Ratio of CD45RA− CD62L− to CD45RA+ CD62L− Gag-specific CD8+ T cells from ART-treated (n = 4) and ART-naïve (n = 11) animals. Means ± standard errors are shown.
Overall, the data described above show that the skewed memory phenotype and survival defects of SIV-specific CD8+ T cells observed for chronically infected animals are already established during acute infection. Most importantly, these findings indicate that antigen stimulation may be a factor driving these defects.
DISCUSSION
The present study closes a gap between recent studies conducted on HIV-specific CD8+ T cells and the paucity of data for SIV-specific CD8+ T cells in SIV infection of nonhuman primates. Although SIV infection is used as a model for HIV infection, it is unclear whether SIV-specific CD8+ T cells suffer from the same intrinsic defects as those found in HIV-specific CD8+ T cells (5, 18, 23). If they do, this animal model could be used to elucidate the mechanism behind these defects and potential therapeutics targeting them. Of specific interest is the comparison of Gag- and Tat-specific CD8+ T cells, because the Gag epitope mutates infrequently and later in rhesus macaques infected with SIV (4, 21) than the Tat epitope, which has been reported to acquire at least one mutation early in SIV infection in all animals examined (1, 21). This allows us to determine whether loss of recognition of the cognate epitope influences the memory phenotype and apoptosis sensitivity of SIV-specific CD8+ T cells.
Recent studies have shown a skewed memory phenotype, with mostly CD45RA− CD62L− CCR7− effector memory cells, in human HIV-specific CD8+ T cells (5, 18), which may contribute to the failure of the immune system to control HIV infection. In the present study, we found the same memory phenotype skewing of SIV-specific CD8+ T cells toward CD45RA− CD62L− effector memory cells. We used the CD45RA and CD62L markers to study the memory phenotype because these markers break down CD8+ T cells into four distinct populations, including two different effector memory populations (25), revealing skewing that would be overlooked if CD95 and CD28 were used as suggested by a recent study (24). Total CD8+ T cells in the blood and lymph nodes of SIV-infected rhesus macaques are more terminally differentiated CD45RA+ CCR7− effector memory cells in slow progressors compared to more CD45RA− CCR7− effector memory cells in modest progressors (15). SIV-specific CD8+ T cells in the blood and lymph nodes, shown here, and in the gastrointestinal tissues (27) are predominantly of the CD45RA− CD62L− phenotype, suggesting that this skewed memory phenotype of SIV-specific CD8+ T cells is present in all tissues. Although in this study we examined only SIV-specific CD8+ T cells, due to limitations of reagents, and therefore could not directly compare SIV-specific to other virus-specific responses, our data suggest that this is a SIV-specific CD8+ T-cell defect, since the memory phenotype and apoptosis sensitivity of SIV-specific CD8+ T cells differ significantly from those of total CD8+ T cells.
We also found that Gag-specific CD8+ T cells from chronically SIV infected rhesus macaques exhibit increased levels of spontaneous and CD95-induced apoptosis, which is in line with studies of HIV-specific CD8+ T cells from HIV-infected individuals (18). This increased apoptosis was already found very early during acute infection. Such increased apoptosis sensitivity of SIV-specific CD8+ T cells may compromise their antiviral effect by reducing their survival, since SIV-infected cells may kill SIV-specific CD8+ T cells through CD95/CD95L interactions, something we have shown for HIV-specific CD8+ T cells (18). Indeed, blocking of CD95/Fas ligand can restore the in vitro cytotoxicity of SIV-specific CD8+ T cells (29). Like HIV-specific CD8+ T cells (23), SIV-specific CD8+ T cells also have reduced levels of Bcl-2 that are not accompanied by an increase in Bcl-xL expression. Others have shown increases in the levels of the antiapoptotic Bcl-2 family members Bak and Bim but not Bax in total CD8+ T cells in chronically infected rhesus macaques (3). Taken together, the findings discussed above suggest that perturbation of Bcl-2 family members contributes to the apoptosis sensitivity of these cells.
Our previous in vitro studies with the cytokine IL-15 have shown that IL-15 decreases the apoptosis sensitivity of HIV-specific CD8+ T cells and increases their gamma interferon production and cytotoxicity (17). In the present study, in vitro treatment of PBMC from rhesus macaques with IL-15 had a similar inhibitory effect on spontaneous and CD95-induced apoptosis of SIV-specific CD8+ T cells, as reported by others for total CD8+ T cells from SIV-infected rhesus macaques (3).
Although the skewed memory phenotype of HIV-specific CD8+ T cells has been well described (5, 18), the mechanism behind this is unknown. To understand when this differentiation defect occurs and the potential mechanism behind it, we followed the SIV-specific CD8+ T-cell responses during the course of infection. We found that Gag-specific CD8+ T cells were predominantly of the CD45RA− CD62L− phenotype already at their first detection at 2 weeks postinfection, and this did not change when animals progressed to the chronic stage. That this phenotype may be associated with the presence of antigen was supported by our finding that animals at the chronic stage who were treated with ART shifted their Gag-specific CD8+ T cells from CD45RA− CD62L− to CD45RA+ CD62L− and CD45RA− CD62L+ memory cells. Further evidence for a role for antigen comes from our study of acute infection in which Gag- and Tat-specific CD8+ T cells were compared. Tat mutates in all SIV-infected, MamuA*01-positive rhesus macaques by week 4 of infection (1), most probably driven by the cytotoxic T-lymphocyte response. Tat-specific CD8+ T cells are initially CD45RA− CD62L−, but after week 4, they are distributed among all memory phenotypes, whereas Gag-specific CD8+ T cells retain the CD45RA− CD62L− phenotype. This loss of skewing of the memory phenotype of Tat-specific CD8+ T cells was mostly due to the loss of absolute numbers of CD45RA− CD62L− cells after antigen escape. The possibility that chronic antigen exposure can lead to inadequate differentiation of T cells has been suggested for lymphocytic choriomeningitis virus (28) and HIV infection (5). A change in the phenotype of HIV-specific CD8+ T cells, with a decrease in the proportion of CD27− CD11ahigh cells, during highly active ART of HIV-infected individuals has been described (14). The finding that Gag-specific CD8+ T cells are CD45RA− CD62L− suggests that in MamuA*01-positive rhesus macaques that are relatively good controllers of viral replication (16, 30), the antigenic burden is still above a threshold that retains the CD45RA− CD62L− phenotype.
High apoptosis sensitivity of Gag- and Tat-specific CD8+ T cells was found very early during acute infection. However, when antigen recognition is lost, as in the case of the mutated Tat epitope, levels of spontaneous and CD95-induced apoptosis are rapidly reduced. The sensitivity of Gag-specific CD8+ T cells to CD95-induced apoptosis in chronically SIV infected rhesus macaques was reduced when the animals were treated with ART, although this was not significant.
In conclusion, the data presented in this study suggest that chronic SIV infection results in survival and differentiation defects of SIV-specific CD8+ T cells that are very similar to those we have shown before for HIV-specific CD8+ T cells (18). SIV infection leads to increased spontaneous and CD95-induced apoptosis of SIV-specific CD8+ T cells, which is accompanied by a decrease in Bcl-2 expression. In vitro IL-15 treatment inhibits this apoptosis and upregulates Bcl-2 and Bcl-xL expression in these cells. SIV-specific CD8+ T cells are predominantly of the CD45RA− CD62L− effector memory phenotype, like HIV-specific CD8+ T cells (5, 18). This skewed effector memory phenotype and the increased apoptosis of SIV-specific CD8+ T cells are established early during infection. Finally, our data suggest that antigenic stimulation retains the CD45RA− CD62L− phenotype of SIV-specific CD8+ T cells and increases their apoptosis sensitivity. These findings suggest that chronic T-cell receptor stimulation may be driving the survival and differentiation defects of HIV-specific CD8+ T cells.
Acknowledgments
We thank David Weiner and Jean Boyer (Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine) for supplying blood from rhesus macaques chronically infected with SIV.
This work was supported by grants NIH R0I AI62437 and NIH R01 AI46719 to P.D.K.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 3.Arnoult, D., F. Petit, J. D. Lelievre, D. Lecossier, A. Hance, V. Monceaux, B. Hurtrel, R. Ho Tsong Fang, J. C. Ameisen, and J. Estaquier. 2003. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 10:1240-1252. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyerl, S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 6.Geretti, A. M. 1999. Simian immunodeficiency virus as a model of human HIV disease. Rev. Med. Virol. 9:57-67. [DOI] [PubMed] [Google Scholar]
- 7.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9:553-563. [DOI] [PubMed] [Google Scholar]
- 8.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodge, S., F. J. Novembre, L. Whetter, H. A. Gelbard, and S. Dewhurst. 1998. Induction of Fas ligand expression by an acutely lethal simian immunodeficiency virus, SIVsmmPBj14. Virology 252:354-363. [DOI] [PubMed] [Google Scholar]
- 9a.Institute for Laboratory Animal Research, National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [PubMed]
- 10.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 12.Letvin, N. L., M. D. Daniel, P. K. Sehgal, R. C. Desrosiers, R. D. Hunt, L. M. Waldron, J. J. MacKey, D. K. Schmidt, L. V. Chalifoux, and N. W. King. 1985. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 230:71-73. [DOI] [PubMed] [Google Scholar]
- 13.Letvin, N. L., and N. W. King. 1990. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Acquir. Immune Defic. Syndr. 3:1023-1040. [PubMed] [Google Scholar]
- 14.Mollet, L., T. S. Li, A. Samri, C. Tournay, R. Tubiana, V. Calvez, P. Debre, C. Katlama, B. Autran, et al. 2000. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. J. Immunol. 165:1692-1704. [DOI] [PubMed] [Google Scholar]
- 15.Monceaux, V., L. Viollet, F. Petit, R. Ho Tsong Fang, M. C. Cumont, J. Zaunders, B. Hurtrel, and J. Estaquier. 2005. CD8+ T cell dynamics during primary simian immunodeficiency virus infection in macaques: relationship of effector cell differentiation with the extent of viral replication. J. Immunol. 174:6898-6908. [DOI] [PubMed] [Google Scholar]
- 16.Mothé, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller, Y. M., P. M. Bojczuk, E. S. Halstead, A. H. Kim, J. Witek, J. D. Altman, and P. D. Katsikis. 2003. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 101:1024-1029. [DOI] [PubMed] [Google Scholar]
- 18.Mueller, Y. M., S. C. De Rosa, J. A. Hutton, J. Witek, M. Roederer, J. D. Altman, and P. D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity 15:871-882. [DOI] [PubMed] [Google Scholar]
- 19.Mueller, Y. M., V. Makar, P. M. Bojczuk, J. Witek, and P. D. Katsikis. 2003. IL-15 enhances the function and inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells. Int. Immunol. 15:49-58. [DOI] [PubMed] [Google Scholar]
- 20.Mueller, Y. M., C. Petrovas, P. M. Bojczuk, I. D. Dimitriou, B. Beer, P. Silvera, F. Villinger, J. S. Cairns, E. J. Gracely, M. G. Lewis, and P. D. Katsikis. 2005. Interleukin-15 increases effector memory CD8+ T cells and NK cells in simian immunodeficiency virus-infected macaques. J. Virol. 79:4877-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connor, D. H., A. B. McDermott, K. C. Krebs, E. J. Dodds, J. E. Miller, E. J. Gonzalez, T. J. Jacoby, L. Yant, H. Piontkivska, R. Pantophlet, D. R. Burton, W. M. Rehrauer, N. Wilson, A. L. Hughes, and D. I. Watkins. 2004. A dominant role for CD8+ T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 78:14012-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overbaugh, J., P. A. Luciw, and E. A. Hoover. 1997. Models for AIDS pathogenesis: simian immunodeficiency virus, simian-human immunodeficiency virus and feline immunodeficiency virus infections. AIDS 11(Suppl. A):S47-S54. [PubMed] [Google Scholar]
- 23.Petrovas, C., Y. M. Mueller, I. D. Dimitriou, P. M. Bojczuk, K. C. Mounzer, J. Witek, J. D. Altman, and P. D. Katsikis. 2004. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J. Immunol. 172:4444-4453. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz, J. E., R. S. Veazey, M. J. Kuroda, D. B. Levy, A. Seth, K. G. Mansfield, C. E. Nickerson, M. A. Lifton, X. Alvarez, A. A. Lackner, and N. L. Letvin. 2001. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood 98:3757-3761. [DOI] [PubMed] [Google Scholar]
- 28.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, X. N., G. R. Screaton, F. M. Gotch, T. Dong, R. Tan, N. Almond, B. Walker, R. Stebbings, K. Kent, S. Nagata, J. E. Stott, and A. J. McMichael. 1997. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp. Med. 186:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, Z. Q., T. M. Fu, D. R. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]