Abstract
Severe inherited retinal diseases, such as retinitis pigmentosa and Leber congenital amaurosis, are caused by mutations in genes preferentially expressed in photoreceptors. While adeno-associated virus (AAV)-mediated gene transfer can correct retinal pigment epithelium (RPE) defects in animal models, approaches for the correction of photoreceptor-specific diseases are less efficient. We evaluated the ability of novel AAV serotypes (AAV2/7, AAV2/8, AAV2/9, AAV2rh.43, AAV2rh.64R1, and AAV2hu.29R) in combination with constitutive or photoreceptor-specific promoters to improve photoreceptor transduction, a limiting step in photoreceptor rescue. Based on a qualitative analysis, all AAV serotypes tested efficiently transduce the RPE as well as rod and cone photoreceptors after subretinal administration in mice. Interestingly, AAV2/9 efficiently transduces Müller cells. To compare photoreceptor transduction from different AAVs and promoters in both a qualitative and quantitative manner, we designed a strategy based on the use of a bicistronic construct expressing both enhanced green fluorescent protein and luciferase. We found that AAV2/8 and AAV2/7 mediate six- to eightfold higher levels of in vivo photoreceptor transduction than AAV2/5, considered so far the most efficient AAV serotype for photoreceptor targeting. In addition, following subretinal administration of AAV, the rhodopsin promoter allows significantly higher levels of photoreceptor expression than the other ubiquitous or photoreceptor-specific promoters tested. Finally, we show that AAV2/7, AAV2/8, and AAV2/9 outperform AAV2/5 following ex vivo transduction of retinal progenitor cells differentiated into photoreceptors. We conclude that AAV2/7 or AAV2/8 and the rhodopsin promoter provide the highest levels of photoreceptor transduction both in and ex vivo and that this may overcome the limitation to therapeutic success observed so far in models of inherited severe photoreceptor diseases.
Inherited retinal diseases, such as retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA), are characterized by progressive impairment of visual function associated with photoreceptor loss (14, 21, 34). RP and LCA are monogenic, highly genetically heterogeneous diseases due to mutations in genes primarily expressed in the retinal pigment epithelium (RPE) or in photoreceptors (14, 21). In fact, the majority of genes associated with retinal inherited diseases are photoreceptor specific. Gene therapy holds great potential for the treatment of inherited retinal diseases for which there currently is no cure. Efficient retinal gene transfer has been achieved with several viral vectors, including those derived from adenovirus, retrovirus, herpesvirus, and adeno-associated virus (AAV) (15). Among these, AAV vectors appear particularly amenable to retinal gene transfer, because they are nonpathogenic and they efficiently transduce nondividing cells potentially for the life of the experimental animal following a single administration (5, 9). Since the generation of the first AAV vector (30, 53) (AAV2/2, in which the first number defines the vector genome and the second the capsid), dozens of AAV variants have been isolated, and some of them have been converted into gene delivery vehicles (11, 12, 23-25, 45). AAV serotypes differ in their compositions of the capsid surface proteins, which affect their tropism and transduction. We and others have demonstrated that it is possible to tailor somatic transgene expression on distinct retinal cell types in the adult and fetal retina by exchanging the AAV capsids among the various AAV serotypes and that this may impact the therapeutic outcome in animal models of retinal diseases (7, 44, 51, 57). While AAVs are suitable for gene transfer to different retinal cell types in vivo, their ability to transduce retinal stem cells and their derivatives has not been characterized yet. This limitation hampers the possibility of genetically modifying retinal stem cells derived from the adult eye (54), which can be used as tools for cell therapy of retinal degenerations.
Among the various AAV vectors tested so far in the retina, AAV2/1 and AAV2/4 are considered ideal vectors for RPE gene transfer (7, 51, 56, 57), and AAV2/5 is considered ideal for photoreceptor transduction (7, 37). AAV2/2, AAV2/1, and AAV2/4 have been used successfully to treat RPE-specific genetic defects, including mucopolysaccharidoses (29, 31), ocular albinism (52), and RP and LCA due to MERTK and RPE65 gene deficiencies, respectively (1, 2, 16, 32a, 35, 39, 41, 50). In particular, the most remarkable results using AAV-mediated gene transfer have been obtained in the Briard dog bearing homozygous mutations in the RPE65 gene, a model of LCA (1, 2, 32a,35, 39). RPE65 gene transfer resulted in stable restoration of visual function, regardless of the AAV vector used, suggesting that, at least in animal models, treatment of RPE-specific diseases does not depend on the capsid used. Various centers are testing the toxicity and tolerability of AAV2/2 vectors in the retinas of patients with RPE65 mutations (10). Gene transfer approaches for photoreceptor-specific diseases have been successful in rare cases (38, 43, 59), probably due to limitations of the vector system, the nature of photoreceptor-specific retinal degenerations, and constraints in photoreceptor accessibility to vectors. The ability of AAV vectors to transduce RPE more efficiently than photoreceptors may be related to the simple monolayer organization of the RPE as opposed to the photoreceptors that are higher in number and architectonically organized in rows (13), which may represent a physical barrier to their transduction. A paradigmatic example of the challenge represented by the rescue of photoreceptor diseases is the attempt to use AAV2/2 vectors to treat mice carrying loss-of-function mutations in the PDE6B gene (rd1 mice) (33). In this case, evidence of prolonged and sustained morphological and functional photoreceptor rescue is lacking, probably due to the combination of low levels of photoreceptor transduction by AAV2/2 vectors and the severity of the rd1 degeneration (33). Similarly, in the rds model of photoreceptor disease due to peripherin deficiency, AAV2/2-mediated gene transfer resulted in short-lived rescue of photoreceptor survival (4, 47, 49). The mechanism of photoreceptor loss suggests that gene transfer efficiency may be a limiting factor for photoreceptor rescue. RP initially affects the peripheral retina, resulting in the degeneration of rods, while cones and central vision are preserved at this stage (21). With the progression of the disease, the cones also degenerate (rod-cone degeneration), suggesting a non-cell-autonomous mechanism of cell death. Nonautonomous patterns of degeneration similarly are observed in those inherited retinal diseases primarily affecting cones, such as cone-rod dystrophies, suggesting a general mechanism of degeneration in inherited retinal diseases (28). Therefore, widespread photoreceptor transduction is desirable to prevent detrimental effects from nontransduced photoreceptors.
We evaluated the efficiency of photoreceptor gene transfer mediated by six novel AAV serotypes and isolates (we refer to them as novel because they have not been previously tested in the retina) in combination with constitutive and photoreceptor-specific promoter elements. We determined their onset, tropism, distribution, and levels of transgene expression and compared them to those of AAV2/5, so far the most efficient vehicle system for photoreceptor targeting (7, 37). The impact of novel AAV capsids and promoter elements on photoreceptor gene transfer was determined with a strategy that allows precise quantification of levels of transgene expression in photoreceptors. We found that the combination of capsids from AAV7 or AAV8 combined with the 800-bp proximal fragment of the rhodopsin (RHO) promoter results in the highest levels of photoreceptor transduction.
MATERIALS AND METHODS
Generation of the plasmid constructs.
For the production of AAV encoding enhanced green fluorescent protein (EGFP) with different promoters, pAAV2.1-CMV-EGFP (6), pAAV2.1-CBA-EGFP, pAAV2.1-RHO-EGFP, and pAAV2.1-RHOK-EGFP plasmids were used. pAAV2.1-RHO-EGFP and pAAV2.1-RHOK-EGFP were obtained by exchanging the cytomegalovirus (CMV) promoter of pAAV2.1-CMV-EGFP with the RHO or RHO kinase (RHOK) promoter sequences. The RHO (−800 to +6; GenBank accession number U16824) and RHOK (−112 to +86 [58]) promoters were amplified from human genomic DNA. PCR was performed to insert the NheI and NotI sites at the 5′ and 3′ ends, respectively. For the RHO promoter, we used the following primers: NheI-RHOFor, 5′-AATTATGCTAGCAGATCTTCCCCACCTAGC-3′; and RHORev-NotI, 5′-ATTAATGCGGCCGCGGATGACTCTGGGTTCTG-3′. For RHOK we used the following primers: NheI-0.11KbRHOKFor, 5′-GCTAGCGGGCCCCAGAAGCCTGG-3′; and 0.11KbRHOKRev-NotI, 5′-GCGGCCGCCCCGGGGCTGACACAGC-3′. The PCR products then were digested with NheI and NotI and were cloned into pAAV2.1-CMV-EGFP after removing the CMV promoter. The chicken β-actin (CBA) promoter was amplified from the pCAGGS plasmid (a kind gift of M. Studer, TIGEM, Naples, Italy) by PCR to insert the NcoI and PstI sites at the 5′ and 3′ ends, respectively. The PCR product then was cloned into pAAV2.1-CMV-EGFP (with NcoI-PstI digestion) downstream of the CMV enhancer. To produce the pAAV2.1-CMV-, RHO-, RHOK-, and CBA-NLSEGFP-IRES-LUCIFERASE plasmids, we initially produced the pNLSEGFP-IRES-LUCIFERASE plasmid. The coding sequence for firefly luciferase (1.7 kb) was obtained from pZac2.1-CMV-LUCIFERASE plasmid (6) by cutting it with NheI-XbaI restriction enzymes and inserting it into XbaI-digested pIRES plasmid (Clonthech) to produce the pIRES-LUCIFERASE plasmid. The sequence of an expression cassette containing the coding sequence for the EGFP with a nuclear localization signal (NLS) (designated NLSEFGP) was amplified from pAAV2.1-CMV-EGFP plasmid with the following primers: forward, 5′-GCGGCCGCCATGCCTAAGAAGAAGAGAAAGGTGGAGGTGAGCAAGGGCGAGGAGCTG-3′; and reverse, 5′-TTAACTTGTACAGCTCGTCCATGCC-3′. The forward primer contains the NotI restriction site and the translational start site (ATG) followed by the nuclear localization sequence of the simian virus 40 (SV40) large T antigen (PKKKRKVE) (17). The PCR product was EcoRI digested from pCR2.1-TOPO plasmid (Invitrogen) and inserted in the pIRES-LUCIFERASE plasmid to produce the pNLSEGFP-IRES-LUCIFERASE plasmid. The NLSEGFP-IRES-LUCIFERASE sequence then was removed (with NotI-SalI) from pNLSEGFP-IRES-LUCIFERASE and was inserted into pAAV2.1-CMV-EGFP cut with NotI and BglII to produce pAAV2.1-CMV-NLSEGFP-IRES-LUCIFERASE. The pAAV2.1-RHO-NLSEGFP-IRES-LUCIFERASE, pAAV2.1-RHOK-NLSEGFP-IRES-LUCIFERASE, and pAAV2.1-CBA-NLSEGFP-IRES-LUCIFERASE plasmids were obtained by exchanging the CMV promoter of pAAV2.1-CMV-NLSEGFP-IRES-LUCIFERASE with the RHO, RHOK, and CBA promoter sequences obtained from pAAV2.1-RHO-EGFP, pAAV2.1-RHOK-EGFP, and pAAV2.1-CBA-EGFP plasmids, respectively, by NheI-NotI digestion. The SV40 intron present only in the pAAV2.1-CMV-NLSEGFP-IRES-LUCIFERASE and pAAV2.1-CBA-NLSEGFP-IRES-LUCIFERASE plasmids was eliminated by PstI and NotI digestion. The pAAV2.1-CMV-LacZ plasmid was previously described (6), while pAAV2.1-RHOK-LacZ was produced by exchanging the CMV promoter with RHOK (using NheI-NotI digestion). The AAV2/rh.64R1 capsid sequence was based on AAV isolate rh.64 (23) and was optimized for production by an R697W mutation in VP1 (L. H. Vanderberghe and J. M. Wilson, unpublished data).
AAV vector production and subretinal administration.
AAV vectors were produced by the TIGEM AAV Vector Core using pAAV2.1-CMV-, -CBA-EGFP, -RHO-EGFP, -RHOK-EGFP, pAAV2.1-CMV-, RHO-, RHOK-, CBA-NLSEGFP-IRES-LUCIFERASE, pAAV2.1-CMV, and RHOK-LacZ plasmids. Recombinant AAV2/5, AAV2/7, AAV2/8, AAV2/9, AAV2/rh.64R1, AAV2/rh.43, and AAV2/rh.29R viruses were produced by triple transfection of 293 cells followed by CsCl2 purification (6). For each viral preparation, physical titers (in genome copies [GC]/milliliter) were determined by both PCR quantification using TaqMan (Perkin-Elmer, Life and Analytical Sciences, Inc.) and dot blot analysis (20).
All procedures on animals were performed in accordance with institutional guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Four-week-old male C57BL/6 mice (Harlan) were used. Before vector administration, mice were anesthetized with an intraperitoneal injection of avertin (1.25% [wt/vol] 2,2,2-tribromoethanol and 2.5% [vol/vol] 2-methyl-2-butanol [Sigma-Aldrich]) at 2 ml/100 g of body weight (42). Subretinal vector administrations were performed as described previously (36).
Fundus photography.
Fundus photographs of mice were taken with a Topcon TRC-50IX retinal camera connected to a charge-coupled-device Nikon D1H digital camera (Topcon Medical System) after anesthetizing the animals, dilating their pupils, and fixing them on a stereotaxic table.
Histological analysis.
Mice were sacrificed, and their eyeballs were harvested and fixed overnight by immersion in 4% paraformaldehyde. The eyeballs were cut so that the lens and vitreous could be removed, leaving the eyecup. Mice eyecups were infiltrated with 30% sucrose for cryopreservation and were embedded in tissue-freezing medium (O.C.T. matrix; Kaltek). For each eye, 150 to 200 serial sections (10 μm thick) were cut along the horizontal meridian, and the sections were progressively distributed on 10 slides so that each slide contained 15 to 20 sections representative of the whole eye at different levels. The sections were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Vectashield; Vector Laboratories, Inc.), and retinal histology images were obtained with an Axiocam (Carl Zeiss) with ×20 magnification.
PNA immunolabeling on retinal sections and cone counting.
Sections were rinsed in phosphate-buffered saline (PBS) and incubated for 1 h in 10% fetal bovine serum (FBS) in PBT (0.1% Triton 100, 0.1% bovine serum albumin in PBS). The incubation with biotinylated peanut agglutinin (PNA) (400 μg/ml in PBT; Vector Laboratories) or anti-glutamine synthetase (GS6) (1:500 in PBT; Chemicon) was performed overnight at 4°C. The sections then were washed three times with PBS and incubated with Alexa Fluor 568-coupled streptavidin (1:250 in PBT; Molecular Probes) or Alexa Fluor 568-coupled anti-rabbit antibody (1:500 in PBT; Molecular Probes) for 1 h at room temperature (RT). After a final wash, sections were mounted with DAPI (Vectashield), and images were obtained with a confocal microscope (Leica DMIRE2 and Leica confocal software) with ×63 magnification. To quantify the transduced cones, we counted the number of EGFP-positive cones, defined by their typical morphology and by the colinearity of PNA-lectin labeling with EGFP expression, in a ×40 magnification area of at least three different sections for each eye (n = 3 eyes/group). The number of EGFP-positive cones was divided by the total number of cones present in the same ×40 magnification area to obtain the percentage of transduced cones. The percentage of transduced cones from each serotype then was averaged, and standard errors were calculated.
Luciferase and β-Gal assays.
Harvesting of retinas was performed as described previously (8). Luciferase activity and β-galactosidase (β-Gal) levels were measured in retina lysates using the luciferase reporter gene assay (Roche) and β-Gal enzyme-linked immunosorbent assay (Roche) according to the manufacturer's instructions.
Retinal stem cell culture and infection.
Retinal stem cells were isolated from adult rats and were cultured as described previously (46). Apoptosis in retinal degeneration involves cross talk between apoptosis-inducing factor and caspase-12 and is blocked by calpain inhibitors (46). Retinal neurospheres were allowed to differentiate with 1% FBS on a substrate of laminin (2.5 μg/ml) and poly-d-lysine (20 μg/ml) for 9 days. Cells then were infected with AAV2/5-CMV-EGFP, AAV2/7-CMV-EGFP, AAV2/8-CMV-EGFP, and AAV2/9-CMV-EGFP (1 × 105 GC of each vector/cell). Three days after infection, cells were fixed and incubated for 1 h with 10% FBS in PBS. The incubation with anti-RHO antibody 1D4 (1:400) was performed for 90 min at RT. After extensive washes with PBS, cells were incubated with Alexa Fluor 568 anti-mouse secondary antibody (1:1,000 in PBS; Molecular Probes) for 1 h at RT. After a final wash, slides were coverslipped with Vectashield and photographed using an Axioplan microscope (Zeiss) with ×63 magnification.
RESULTS AND DISCUSSION
Onset of transgene expression and tropism of novel AAV serotypes in the murine retina.
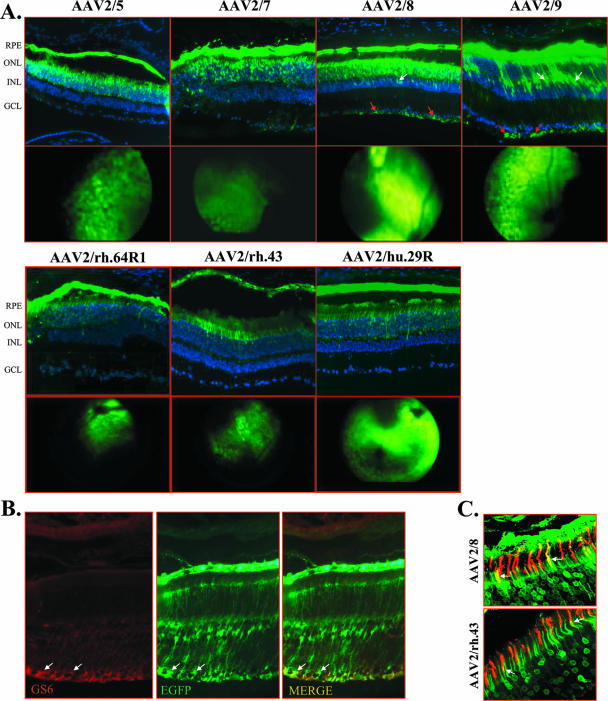
To evaluate the tropism of novel AAV serotypes following subretinal delivery to the adult murine retina and to determine if one is selective for photoreceptors, we generated AAV2/7 (25), AAV2/8 (25), AAV2/9 (23), AAV2/rh.43 (23), AAV2/rh.64R1 (23; Vanderberghe and Wilson, unpublished), AAV2/hu.29R (55), and AAV2/5 (11) vectors harboring the AAV2 vector genome, coding for EGFP, under the control of the ubiquitous CMV promoter. The novel serotypes were selected based on their ability to transduce various tissues (AAV2/7, AAV2/8, and AAV2/9 [25]) or the adult murine retina in a pilot experiment (L. H. Vandenberghe and J. M. Wilson, personal communication) and on their phylogenetic origins (23). Regarding the latter aspect, AAV2/7 belongs to clade D; AAV2/9 belongs to clade F; AAV2/8, AAV2/rh.43, and AAV2/rh.64R1 belong to clade E; and AAV2/hu.29R belongs to clade B (AAV2/5 is divergent from any other AAV isolated so far) (23). One eye of 4-week-old C57BL/6 mice (n = 4 animals/group) was administered 6.8 × 109 GC of AAV2/5, and the same dose of each of the novel AAV serotypes was separately administered in the controlateral eye. The onset of EGFP expression was assessed by indirect ophtalmoscopy and was evident at 5 days postinjection in the retinas that received AAV2/5, AAV2/7, and AAV2/8, at 7 days in those that received AAV2/rh.43, AAV2/rh.64R1, and AAV2/hu.29R, and at 11 days in those that received AAV2/9 (Fig. 1A, second and fourth rows). Four weeks after injection, retinal sections were analyzed by direct fluorescence evaluation to assess AAV vector tropism. As shown in the first and third rows of Fig. 1A, all vectors efficiently transduce photoreceptors and the RPE with distribution and expression levels apparently similar to those of AAV2/5. In addition, the retinas that received AAV2/8 and, in particular, AAV2/9 showed EGFP expression in Müller cells, as confirmed by colocalization with GS6, a known marker of Müller cells (18) (Fig. 1A, first row, and B). The same levels of EGFP expression and transduction patterns were observed in retinal sections 3 months after AAV administration. To assess the ability of AAV serotypes to transduce cones, we stained the cone outer segments with PNA-lectin, given their low density in the outer nuclear layer. Confocal immunofluorescence analysis revealed that cones, in addition to rods, were efficiently transduced by all AAV vectors tested (Fig. 1C). To quantify the number of cones transduced, we calculated the percentage of all individual cone sheaths expressing EGFP on ×40 magnification optical fields (n = 3 eyes/group). AAV2/8 (75% ± 6.7% cones) and AAV2/9 (75% ± 7.7% cones) transduced the highest number of cones, while AAV2/rh.64R1 (28% ± 5.1% cones), AAV2/rh.43 (23% ± 4.9% cones), and AAV2/rh.29R (33% ± 7.0% cones) transduced cones to levels similar to that of AAV2/5 (35% ± 6.7% cones). To assess potential detrimental effects to retinal function due to the subretinal injection procedure or to vector administration, electroretinographic analyses were performed. No significant changes in scotopic and photopic electroretinographic a- and b-wave amplitudes were observed between uninjected and AAV-injected eyes (data not shown). In addition, hematoxylin and eosin staining revealed no signs of inflammatory infiltration in the treated eyes (data not shown). We conclude that all novel AAV serotypes analyzed transduce various retinal cell types, including photoreceptors, and show early onset of transgene expression; none of the AAV serotypes tested transduces exclusively or predominantly photoreceptors.
FIG. 1.
Subretinal administration of novel AAV serotypes expressing EGFP in adult C57BL/6 mice. (A) The first and third rows depict fluorescence microscopy evaluations of EGFP expression 4 weeks after subretinal injection with AAV2/5-CMV-EGFP, AAV2/7-CMV-EGFP, AAV2/8-CMV-EGFP, AAV2/9-CMV-EGFP, AAV2/rh.64R1-CMV-EGFP, AAV2/rh.43-CMV-EGFP, and AAV2/rh.29R-CMV-EGFP. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. White arrows, Müller cell nuclei; red arrows, Müller cell endfoot membranes. The second and fourth rows depict in vivo imaging of EGFP fluorescence in mouse retinas 5 days after subretinal injections of AAV2/5-CMV-EGFP, AAV2/7-CMV-EGFP, and AAV2/8-CMV-EGFP, 11 days after injection of AAV2/9-CMV-EGFP, and 7 days after injection of AAV2/rh.64R1-CMV-EGFP, AAV2/rh.43-CMV-EGFP, and AAV2/rh.29R-CMV-EGFP. Fundus photographs show punctate EGFP fluorescence diffusing from the site of the subretinal injection to 30 to 40% of the treated retina. (B) Müller cell transduction by AAV2/9 vectors expressing EGFP from the CMV promoter. Staining with anti-GS6 antibody (GS6; red label) on retinal sections of animals injected with AAV2/9-CMV-EGFP is shown. Colocalization of EGFP expression and GS6 staining is indicated by the arrows (MERGE). Microscope magnification, ×20. (C) Cone transduction from novel AAV serotypes expressing EGFP from the CMV promoter. Staining with PNA-lectin (red label) on retinal sections of animals injected with AAV2/8-CMV-EGFP or AAV2/rh.43-CMV-EGFP is shown. Colocalization of EGFP expression and PNA staining is indicated by the arrows. Confocal microscope magnification, ×63.
Ubiquitous and photoreceptor-specific promoters in the retina.
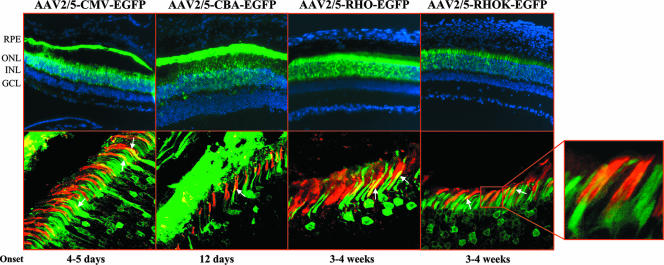
Various promoter elements have been tested so far and are routinely used to express exogenous genes in the retina following somatic gene transfer (5, 19). These include the photoreceptor-specific proximal portion of the human RHO promoter (22, 26) and the CMV (27) and CBA (48) promoters. In order to evaluate their activity side by side, we generated AAV2/5 vectors expressing EGFP from each of them. We additionally tested AAV2/5 vectors encoding EGFP from the proximal region of the human RHOK (−116 to +86 [58]) promoter, responsible for photoreceptor-restricted expression in transgenic animals (58). The AAV2/5 vectors with the various promoters were injected subretinally (6.8 × 109 GC of each vector/eye) into 4-week-old C57BL/6 mice (n = 4 animals/group). To assess the onset of EGFP expression, we performed ophthalmoscopic examinations every week after vector administration. The onset of EGFP expression appeared at 5 and 12 days after vector administration when the CMV and the CBA promoters, respectively, were used, while EGFP expression under the control of the RHO and the RHOK promoters was evident 3 to 4 weeks after vector delivery. The differences in the onset of gene expression between ubiquitous and photoreceptor-specific promoters may be due to the fact that EGFP expression in the RPE is more easily detectable by ophthalmoscopic examination than EGFP expression restricted to photoreceptors. One month after vector administration, the eyes were enucleated and histological analysis of EGFP expression was performed (Fig. 2, top row). Similar to what was reported in previous studies, EGFP expression from AAV2/5 vectors harboring the CMV or the CBA promoter is localized to both RPE and photoreceptors and, in some cells, to the inner nuclear layer, whereas the RHO and the RHOK promoters restrict transgene expression to photoreceptors. The fluorescence microscope analysis shows that the most robust expression levels among the promoters tested in photoreceptors seem to be provided by the RHO promoter element, while the lowest levels of expression seem to be provided by the RHOK promoter element (Fig. 2, top row). The same levels of EGFP expression and transduction patterns were observed in retinal sections 3 months after AAV administration. With a method similar to that used for the comparison between capsids, we analyzed cone transduction levels by the various promoters using PNA-lectin staining. Confocal microscopy analysis demonstrated that both ubiquitous and photoreceptor-specific promoters drive efficient EGFP expression in cone photoreceptors in the context of vectors with AAV5 capsids (Fig. 2, bottom row). This result suggests that AAV2/5 efficiently infects cones and that both the RHO and RHOK regulatory elements allow expression in both rods and cones, as previously shown (26, 40). Among the promoters tested (n = 3 eyes/group), the most efficient promoter for transgene expression in cones is the CBA promoter (58.4% ± 2.4% cones), followed by the RHOK (47% ± 6.7% cones), RHO (44.6% ± 3.8% cones), and CMV (36.1% ± 6.2% cones) promoters as assessed by counting the number of double-stained cones (PNA and EGFP)/microscopic field in the area of maximal transduction.
FIG. 2.
Subretinal injections of AAV2/5-EGFP vectors with various promoters into adult C57BL/6 mice. The images in the top row depict fluorescence microscopy evaluation of EGFP expression 4 weeks after subretinal injections of AAV2/5 encoding EGFP from the CMV, CBA, RHO, and RHOK promoters. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. The images in the bottom row show staining with PNA-lectin (red label) on retinal sections of animals injected with AAV2/5-CMV-EGFP, AAV2/5-CBA-EGFP, AAV2/5-RHO-EGFP, and AAV2/5-RHOK-EGFP. Colocalization of EGFP expression and PNA staining is indicated by the arrows. Confocal microscope magnification, ×63. The selected field shows the colocalization of cone sheathes (PNA, red label) and EGFP expression (green label) at increased magnification. The magnified portion depicts the onset of gene expression as assessed by indirect ophthalmoscopy.
Quantification of photoreceptor transduction with novel AAV serotypes and promoters.
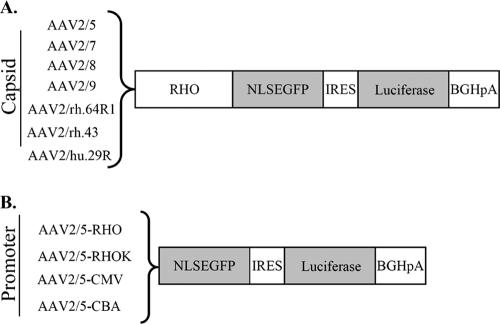
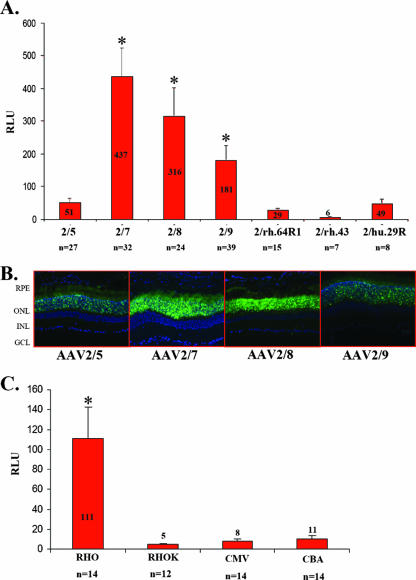
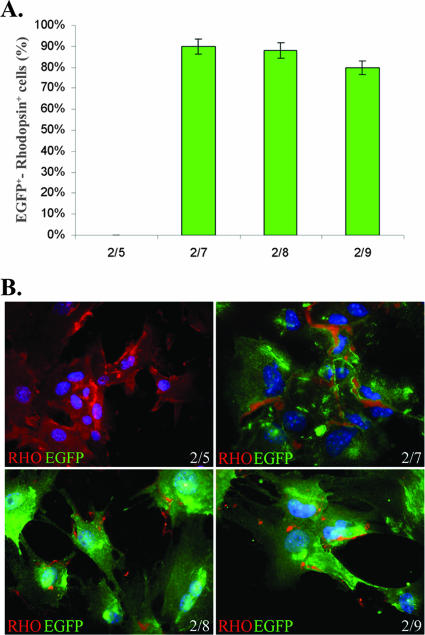
To measure transgene expression levels in photoreceptors following subretinal delivery of novel AAV serotypes and promoters, we generated an expression cassette containing the coding sequence for the EGFP with an NLS (designated NLSEFGP), followed by the internal ribosomal entry site sequence and the coding sequence for the firefly luciferase (Fig. 3). The NLSEGFP transgene produces lower, nonsaturating levels of green fluorescence than EGFP, and it is localized mainly but not exclusively to the nucleus. Therefore, NLSEGFP provides qualitative information on cellular targets, while luminometric measurements of luciferase levels provide quantitative information. To compare the impact of different capsids on photoreceptor-specific transduction levels, we cloned the RHO promoter element (which restricts expression to photoreceptors) upstream of the bicistronic construct; thus, the measure of luminescence due to luciferase expression provides an estimate of levels of photoreceptor-specific transduction. We subretinally coinjected adult C57BL/6 mice (4 weeks old) with 3 × 109 GC of each of the various vectors shown in Fig. 3A, together with 6.9 × 108 GC of an AAV2/5 vector encoding Escherichia coli β-Gal under the control of the CMV promoter (AAV2/5-CMV-LacZ). Relating luciferase levels to those of β-Gal allowed us to minimize the potential variability in the dose of the vector delivered following subretinal administration. After 1 month, eyes were harvested and the retinas were either processed for histological analysis or lysed to measure both luciferase activity and β-Gal levels. As shown in Fig. 4A, the ratio between luciferase and β-Gal was higher for the eyes treated with AAV2/7, AAV2/8, and AAV2/9 (437 ± 87, 316 ± 86, and 181 ± 44 relative light units [RLU], respectively) than for those treated with AAV2/5 (51 ± 12 RLU), while for serotypes AAV2/rh.64R1, AAV2/rh.43, and AAV2/hu.29R the values were similar to those for AAV2/5 or were lower (29 ± 4, 6 ± 1, and 49 ± 12 RLU, respectively). To rule out the possibility that the low values obtained with the AAV2/5 vector were due to competition with the same serotype used for normalization (although the doses of AAV2/5-CMV-LacZ administered were six times lower than those of the bicistronic vectors), we repeated the experiment using AAV2/1-CMV-LacZ as a normalizing vector. The results were comparable between the two sets of experiments (data not shown). We then analyzed the ability of the novel AAV serotypes to transduce in vitro-differentiated photoreceptors from retinal stem cells. Based on the in vivo quantitative analysis, we chose to compare transduction efficiencies of AAV2/5, AAV2/7, AAV2/8, and AAV2/9, which showed the highest levels of photoreceptor transduction. Retinal stem cells were isolated from the adult ciliary margin and were grown for 6 days as neurospheres. Neurospheres then were allowed to differentiate to photoreceptors (RHO-positive cells) for 9 days and were treated with the various AAV serotypes (1 × 105 GC/cell). Three days later the percentage of transduced photoreceptors was calculated by counting cells coexpressing EGFP and RHO (Fig. 5B). While AAV2/5 was not able to transduce in vitro-differentiated photoreceptors, we found that AAV2/7, AAV2/8, and AAV2/9 are the best serotypes for in vitro genetic modification of retinal stem cells, allowing expression of transgenes in more than 80% of RHO-positive cells (Fig. 5A).
FIG. 3.
Strategy to assess levels of transgene expression following AAV-mediated photoreceptor gene transfer. (A) To identify the AAV serotype that results in the highest number of transduced photoreceptors, a bicistronic reporter was expressed in photoreceptors using the photoreceptor-specific RHO promoter. The bicistronic reporter includes the sequence of NLSEGFP (see Results and Discussion for details) followed by that of luciferase. NLSEGFP allows the qualitative analysis of photoreceptor transduction by histology, while luciferase allows us to quantify the transduction levels by a luminometric assay. (B) To identify the promoter that results in the highest levels of expression in photoreceptors, we generated AAV2/5 vectors encoding the bicistronic reporter under the transcriptional control of various promoters. IRES, internal ribosomal entry site sequence; BGHpA, bovine growth hormone poly(A).
FIG. 4.
Levels of luciferase activity in the photoreceptors after subretinal injections of AAV. (A) Levels of luciferase activity 1 month after subretinal injections of AAV2/5, AAV2/7, AAV2/8, AAV2/9, AAV2/rh.64R1, AAV2/rh.43, and AAV2/rh.29R vectors encoding the bicistronic reporter from the RHO promoter. AAV2/5-CMV-LacZ was coinjected to normalize the variability due to subretinal administrations. The levels of luciferase activity were normalized for β-Gal expression levels. n, number of injected eyes. Differences from AAV2/5 expression levels that are statistically significant are marked with an asterisk; P ≤ 0.05. (B) Fluorescence microscopy evaluation of EGFP expression 4 weeks after subretinal injection of AAV2/5, AAV2/7, AAV2/8, or AAV2/9 vector encoding the bicistronic reporter. (C) Levels of luciferase activity 1 month after subretinal injection of AAV2/5 vectors encoding the bicistronic reporter gene from the CMV, CBA, RHO, or RHOK promoter and AAV2/1-CMV-LacZ. The levels of luciferase activity were normalized for β-Gal expression levels. n, number of injected eyes.
FIG. 5.
Transduction efficiency of in vitro-differentiated retinal stem cells by various AAV serotypes. In vitro-differentiated photoreceptors (RHO-positive cells) were infected with AAV2/5-CMV-EGFP, AAV2/7-CMV-EGFP, AAV2/8-CMV-EGFP, and AAV2/9-CMV-EGFP after 9 days of culture. EGFP expression in RHO-positive cells was evaluated 3 days later. (A) Statistical analysis of EGFP-positive (green bars) in vitro-differentiated photoreceptors (RHO positive). Values indicate the percentage of EGFP-positive cells compared to the total number of photoreceptor cells calculated by expression of RHO. The histogram shows the averages ± standard errors from results obtained from three infections/serotype. (B) Fluorescence microscopy evaluation of transduced EGFP expression (green) and endogenous RHO (red) in in vitro-differentiated retinal stem cells 3 days after infection with AAV2/5 (2/5), AAV2/7 (2/7), AAV2/8 (2/8), or AAV2/9 (2/9). Nuclei are stained with DAPI in blue. Microscope magnification, ×63.
To identify the most efficient promoter for expression in photoreceptors, retinas were transduced with vectors containing the AAV5 capsid and the bicistronic expression cassette under the control of the CMV, CBA, RHO, or RHOK promoter separately (Fig. 3B). The AAV2/5 vectors (1.9 × 109 GC/eye of each vector) were coinjected subretinally into adult C57BL/6 mice, with AAV2/1-CMV-LacZ being injected for normalization. Whole retinas (including the inner nuclear layer and sporadic RPE cells) were peeled 1 month after AAV administration and were analyzed for luciferase and β-Gal activities. The highest ratio between luciferase and β-Gal was obtained using the RHO promoter (111 ± 31.8 RLU), which had ratios that were 10- to 13-fold higher than those obtained using the ubiquitous CBA (11 ± 2.8 RLU) and CMV (8.1 ± 2.1 RLU) promoters and nearly 24-fold higher than that obtained using the RHOK promoter (4.6 ± 1.1 RLU). In the case of ubiquitous promoters, the values obtained are an overestimation of photoreceptor transduction, as luciferase expression is not restricted to photoreceptors in the samples analyzed, although the majority of the retinal cells transduced by AAV2/5-CMV-EGFP are photoreceptors (Fig. 2). In addition, the majority of RPE cells are carefully removed from the retinal samples when they are peeled from the eyecups. Therefore, nonphotoreceptor cells likely minimally influence the quantitative results. With a procedure similar to that used for capsid comparisons, this experiment was repeated using an AAV2/1-RHOK-LacZ vector for normalization to exclude the possibility that competition between CMV promoters could account for the low levels of luciferase measured when AAV2/1-CMV-LacZ was injected. The results were similar between the two sets of experiments (data not shown).
In conclusion, our data demonstrate that the AAV serotypes tested efficiently transduce photoreceptors and RPE following subretinal delivery. Interestingly, AAV2/9 also transduces Müller cells. AAV2/7, AAV2/8, and AAV2/9, as well as each of the promoters tested, determine high levels of gene expression in cone photoreceptors. We also show that AAV2/8 and AAV2/7 mediate six- to eightfold greater transgene expression levels in photoreceptors than AAV2/5, considered so far the most efficient vector for photoreceptor gene transfer. AAV2/9 also was more effective than AAV2/5 but was less effective than AAV2/7 and AAV2/8 at photoreceptor transduction. Interestingly, the ability of the AAV serotypes to transduce photoreceptors is independent of their homology in the capsid amino acid sequence. For instance, AAV8 and AAVrh.43 capsids are very closely related, with only 2% divergence in their VP1 capsid sequences (23). Nevertheless, the photoreceptor transduction efficiency in retinas injected with AAV2/8 was significantly higher than that in retinas injected with AAV2/rh.43. Conversely, AAV2/7, AAV2/8, and AAV2/9, which are the most efficient photoreceptor-transducing vectors tested in this study, belong to three different clades (AAV7, clade D; AAV8, clade E; and AAV9, clade F), suggesting that overall capsid sequence homologies are not predictive of AAV vector biological behavior and that few amino acid differences account for significantly different biological activities (23). Interestingly, AAV2/hu29 vectors show tropism similar to that of their closer homolog, AAV2/2 (3, 7), from which they differ in that AAV2/hu29 capsids lack the AAV2 heparin binding domain. Since the heparin binding domain contributes to antigen-presenting cell transduction and elicitation of cell-mediated immune responses to AAV2 capsids (55), it is possible that AAV2/hu29 capsid could avoid this. We also provide the first evidence that photoreceptors derived from retinal stem cells are amenable to genetic modification by AAV2/7, AAV2/8, and AAV2/9. The use of retinal stem cells for cell therapy of the retina is still very limited, primarily due to the high heterogeneity of retinal stem cells cultured in vitro. The possibility of genetically manipulating this cell population may be an important step towards the employment of stem cells for therapeutic purposes.
We believe that the remarkable results obtained with AAV-mediated gene transfer in RPE65-deficient small and large animal models (and hopefully soon in humans) may not necessarily apply to other retinal diseases. RPE65 animal models and patients show severe impairment of retinal function with a relatively preserved retinal structure, and the disease affects RPE cells that can be relatively easily transduced by viral vectors, thus representing an ideal setting for successful gene replacement (32). For the more common and therapeutically challenging photoreceptor diseases, vectors such as AAV2/7 and AAV2/8 may represent crucial tools to obtain therapeutic efficacy.
Acknowledgments
We thank Chiara Abrescia for generation of the plasmid pAAV2.1-CBA-EGFP, Graciana Diez Roux for critical reading of the manuscript, and the TIGEM AAV Vector and Microscopy and Imaging cores.
This work was supported by the Telethon grant TIGEM P21, the Milton & Steinbach Fund, the EC-FP6 projects LSHB-CT-2005-512146 DiMI and 018933 Clinigene, the National Institutes of Health grant 1R01EY015136-01, and the Italian Ministry of Agriculture grant D.M.589/7303/04.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Acland, G. M., G. D. Aguirre, J. Bennett, T. S. Aleman, A. V. Cideciyan, J. Bennicelli, N. S. Dejneka, S. E. Pearce-Kelling, A. M. Maguire, K. Palczewski, W. W. Hauswirth, and S. G. Jacobson. 2005. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 12:1072-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acland, G. M., G. D. Aguirre, J. Ray, Q. Zhang, T. S. Aleman, A. V. Cideciyan, S. E. Pearce-Kelling, V. Anand, Y. Zeng, A. M. Maguire, S. G. Jacobson, W. W. Hauswirth, and J. Bennett. 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28:92-95. [DOI] [PubMed] [Google Scholar]
- 3.Ali, R. R., M. B. Reichel, A. J. Thrasher, R. J. Levinsky, C. Kinnon, N. Kanuga, D. M. Hunt, and S. S. Bhattacharya. 1996. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum. Mol. Genet. 5:591-594. [DOI] [PubMed] [Google Scholar]
- 4.Ali, R. R., G. M. Sarra, C. Stephens, M. D. Alwis, J. W. Bainbridge, P. M. Munro, S. Fauser, M. B. Reichel, C. Kinnon, D. M. Hunt, S. S. Bhattacharya, and A. J. Thrasher. 2000. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 25:306-310. [DOI] [PubMed] [Google Scholar]
- 5.Allocca, M., A. Tessitore, G. Cotugno, and A. Auricchio. 2006. AAV-mediated gene transfer for retinal diseases. Expert Opin. Biol. Ther. 6:1279-1294. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio, A., M. Hildinger, E. O'Connor, G. P. Gao, and J. M. Wilson. 2001. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio, A., G. Kobinger, V. Anand, M. Hildinger, E. O'Connor, A. M. Maguire, J. M. Wilson, and J. Bennett. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10:3075-3081. [DOI] [PubMed] [Google Scholar]
- 8.Auricchio, A., V. Rivera, T. Clackson, E. O'Connor, A. Maguire, M. Tolentino, J. Bennett, and J. Wilson. 2002. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol. Ther. 6:238. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio, A., and F. Rolling. 2005. Adeno-associated viral vectors for retinal gene transfer and treatment of retinal diseases. Curr. Gene Ther. 5:339-348. [DOI] [PubMed] [Google Scholar]
- 10.Bennett, J. 2006. Commentary: an aye for eye gene therapy. Hum. Gene Ther. 17:177-179. [DOI] [PubMed] [Google Scholar]
- 11.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiorini, J. A., L. Yang, Y. Liu, B. Safer, and R. M. Kotin. 1997. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J. Virol. 71:6823-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke, G., E. Heon, and R. R. McInnes. 2000. Recent advances in the molecular basis of inherited photoreceptor degeneration. Clin. Genet. 57:313-329. [DOI] [PubMed] [Google Scholar]
- 14.Cremers, F. P., J. A. van den Hurk, and A. I. den Hollander. 2002. Molecular genetics of Leber congenital amaurosis. Hum. Mol. Genet. 11:1169-1176. [DOI] [PubMed] [Google Scholar]
- 15.Dejneka, N. S., and J. Bennett. 2001. Gene therapy and retinitis pigmentosa: advances and future challenges. Bioessays 23:662-668. [DOI] [PubMed] [Google Scholar]
- 16.Dejneka, N. S., E. M. Surace, T. S. Aleman, A. V. Cideciyan, A. Lyubarsky, A. Savchenko, T. M. Redmond, W. Tang, Z. Wei, T. S. Rex, E. Glover, A. M. Maguire, E. N. Pugh, Jr., S. G. Jacobson, and J. Bennett. 2004. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol. Ther. 9:182-188. [DOI] [PubMed] [Google Scholar]
- 17.Delhaye, S., V. van Pesch, and T. Michiels. 2004. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J. Virol. 78:4357-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derouiche, A., and T. Rauen. 1995. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res. 42:131-143. [DOI] [PubMed] [Google Scholar]
- 19.Dinculescu, A., L. Glushakova, S. H. Min, and W. W. Hauswirth. 2005. Adeno-associated virus-vectored gene therapy for retinal disease. Hum. Gene Ther. 16:649-663. [DOI] [PubMed] [Google Scholar]
- 20.Drittanti, L., C. Rivet, P. Manceau, O. Danos, and M. Vega. 2000. High throughput production, screening and analysis of adeno-associated viral vectors. Gene Ther. 7:924-929. [DOI] [PubMed] [Google Scholar]
- 21.Dryja, T. 2001. Retinitis pigmentosa and stationary night blindness, p. 5903-5933. In C. R. Scriver, A. L. Beaudet, W. Sly, and D. M. Valle (ed.), The metabolic and molecular bases of inherited disease, 8th ed.,vol. IV. McGraw-Hill, New York, NY. [Google Scholar]
- 22.Flannery, J. G., S. Zolotukhin, M. I. Vaquero, M. M. LaVail, N. Muzyczka, and W. W. Hauswirth. 1997. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl. Acad. Sci. USA 94: 6916-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao, G., L. H. Vandenberghe, M. R. Alvira, Y. Lu, R. Calcedo, X. Zhou, and J. M. Wilson. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao, G., L. H. Vandenberghe, and J. M. Wilson. 2005. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5:285-297. [DOI] [PubMed] [Google Scholar]
- 25.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glushakova, L. G., A. M. Timmers, T. M. Issa, N. G. Cortez, J. Pang, J. T. Teusner, and W. W. Hauswirth. 2006. Does recombinant adeno-associated virus-vectored proximal region of mouse rhodopsin promoter support only rod-type specific expression in vivo? Mol. Vis. 12:298-309. [PubMed] [Google Scholar]
- 27.Grant, C. A., S. Ponnazhagan, X. S. Wang, A. Srivastava, and T. Li. 1997. Evaluation of recombinant adeno-associated virus as a gene transfer vector for the retina. Curr. Eye Res. 16:949-956. [DOI] [PubMed] [Google Scholar]
- 28.Gregory-Evans, K., and R. G. Weleber. 2001. Retinitis pigmentosa and allied disorders in retina, p.362-460. In S. J. Ryan (ed.), Retina vol. 1, 3rd ed. Mosby, St. Louis, MO. [Google Scholar]
- 29.Hennig, A. K., J. M. Ogilvie, K. K. Ohlemiller, A. M. Timmers, W. W. Hauswirth, and M. S. Sands. 2004. AAV-mediated intravitreal gene therapy reduces lysosomal storage in the retinal pigmented epithelium and improves retinal function in adult MPS VII mice. Mol. Ther. 10:106-116. [DOI] [PubMed] [Google Scholar]
- 30.Hermonat, P. L., and N. Muzyczka. 1984. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA 81:6466-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho, T. T., A. M. Maguire, G. D. Aguirre, E. M. Surace, V. Anand, Y. Zeng, A. Salvetti, J. J. Hopwood, M. E. Haskins, and J. Bennett. 2002. Phenotypic rescue after adeno-associated virus-mediated delivery of 4-sulfatase to the retinal pigment epithelium of feline mucopolysaccharidosis VI. J. Gene Med. 4:613-621. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson, S. G., T. S. Aleman, A. V. Cideciyan, A. Sumaroka, S. B. Schwartz, E. A. Windsor, E. I. Traboulsi, E. Heon, S. J. Pittler, A. H. Milam, A. M. Maguire, K. Palczewski, E. M. Stone, and J. Bennett. 2005. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc. Natl. Acad. Sci. USA 102:6177-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Jacobson, S. G., S. L. Boye, T. S. Aleman, T. J. Conlon, C. J. Zeiss, A. J. Roman, A. V. Cideciyan, S. B. Schwartz, A. M. Komaromy, M. Doobrajh, A. Y. Cheung, A. Sumaroka, S. E. Pearce-Kelling, G. D. Aguirre, S. Kaushal, A. M. Maguire, T. R. Flotte, and W. W. Hauswirth. 2006. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum. Gene Ther. 17:845-858. [DOI] [PubMed] [Google Scholar]
- 33.Jomary, C., K. A. Vincent, J. Grist, M. J. Neal, and S. E. Jones. 1997. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 4:683-690. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan, J., J.-M. Rozet, I. Perrault, and A. Unnich. 2000. Leber congenital amaurosis, p. 5941-5943. In C. R. Scriver, A. L. Beaudet, W. Sly, and D. M. Valle (ed.), The metabolic and molecular bases of inherited disease, 8th ed.,vol. IV. McGraw-Hill, New York, NY. [Google Scholar]
- 35.Le Meur, G., K. Stieger, A. J. Smith, M. Weber, J. Y. Deschamps, D. Nivard, A. Mendes-Madeira, N. Provost, Y. Pereon, Y. Cherel, R. R. Ali, C. Hamel, P. Moullier, and F. Rolling. 2007. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 14:292-303. [DOI] [PubMed] [Google Scholar]
- 36.Liang, F. Q., V. Anand, A. Maguire, and J. Bennett. 2000. Intraocular delivery of recombinant virus. Methods Mol. Med. 47:125-139. [DOI] [PubMed] [Google Scholar]
- 37.Lotery, A. J., G. S. Yang, R. F. Mullins, S. R. Russell, M. Schmidt, E. M. Stone, J. D. Lindbloom, J. A. Chiorini, R. M. Kotin, and B. L. Davidson. 2003. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum. Gene Ther. 14:1663-1671. [DOI] [PubMed] [Google Scholar]
- 38.Min, S. H., L. L. Molday, M. W. Seeliger, A. Dinculescu, A. M. Timmers, A. Janssen, F. Tonagel, N. Tanimoto, B. H. Weber, R. S. Molday, and W. W. Hauswirth. 2005. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol. Ther. 12:644-651. [DOI] [PubMed] [Google Scholar]
- 39.Narfstrom, K., M. L. Katz, R. Bragadottir, M. Seeliger, A. Boulanger, T. M. Redmond, L. Caro, C. M. Lai, and P. E. Rakoczy. 2003. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Investig. Ophthalmol. Vis. Sci. 44:1663-1672. [DOI] [PubMed] [Google Scholar]
- 40.Palczewski, K., J. Buczylko, L. Lebioda, J. W. Crabb, and A. S. Polans. 1993. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J. Biol. Chem. 268:6004-6013. [PubMed] [Google Scholar]
- 41.Pang, J. J., B. Chang, A. Kumar, S. Nusinowitz, S. M. Noorwez, J. Li, A. Rani, T. C. Foster, V. A. Chiodo, T. Doyle, H. Li, R. Malhotra, J. T. Teusner, J. H. McDowell, S. H. Min, Q. Li, S. Kaushal, and W. W. Hauswirth. 2006. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol. Ther. 13:565-572. [DOI] [PubMed] [Google Scholar]
- 42.Papaioannou, V. E., and J. G. Fox. 1993. Efficacy of tribromoethanol anesthesia in mice. Lab. Anim. Sci. 43:189-192. [PubMed] [Google Scholar]
- 43.Pawlyk, B. S., A. J. Smith, P. K. Buch, M. Adamian, D. H. Hong, M. A. Sandberg, R. R. Ali, and T. Li. 2005. Gene replacement therapy rescues photoreceptor degeneration in a murine model of Leber congenital amaurosis lacking RPGRIP. Investig. Ophthalmol. Vis. Sci. 46:3039-3045. [DOI] [PubMed] [Google Scholar]
- 44.Rabinowitz, J. E., F. Rolling, C. Li, H. Conrath, W. Xiao, X. Xiao, and R. J. Samulski. 2002. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76:791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanges, D., A. Comitato, R. Tammaro, and V. Marigo. 2006. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. USA 103:17366-17371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarra, G. M., C. Stephens, M. de Alwis, J. W. Bainbridge, A. J. Smith, A. J. Thrasher, and R. R. Ali. 2001. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum. Mol. Genet. 10:2353-2361. [DOI] [PubMed] [Google Scholar]
- 48.Sawicki, J. A., R. J. Morris, B. Monks, K. Sakai, and J. Miyazaki. 1998. A composite CMV-IE enhancer/beta-actin promoter is ubiquitously expressed in mouse cutaneous epithelium. Exp. Cell Res. 244:367-369. [DOI] [PubMed] [Google Scholar]
- 49.Schlichtenbrede, F. C., L. Da Cruz, C. Stephens, A. J. Smith, A. Georgiadis, A. J. Thrasher, J. W. Bainbridge, M. W. Seeliger, and R. R. Ali. 2003. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J. Gene Med. 5:757-764. [DOI] [PubMed] [Google Scholar]
- 50.Smith, A. J., F. C. Schlichtenbrede, M. Tschernutter, J. W. Bainbridge, A. J. Thrasher, and R. R. Ali. 2003. AAV-mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol. Ther. 8:188-195. [DOI] [PubMed] [Google Scholar]
- 51.Surace, E. M., A. Auricchio, S. J. Reich, T. Rex, E. Glover, S. Pineles, W. Tang, E. O'Connor, A. Lyubarsky, A. Savchenko, E. N. Pugh, Jr., A. M. Maguire, J. M. Wilson, and J. Bennett. 2003. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J. Virol. 77:7957-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surace, E. M., L. Domenici, K. Cortese, G. Cotugno, U. Di Vicino, C. Venturi, A. Cellerino, V. Marigo, C. Tacchetti, A. Ballabio, and A. Auricchio. 2005. Amelioration of both functional and morphological abnormalities in the retina of a mouse model of ocular albinism following AAV-mediated gene transfer. Mol. Ther. 12:652-658. [DOI] [PubMed] [Google Scholar]
- 53.Tratschin, J. D., M. H. West, T. Sandbank, and B. J. Carter. 1984. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol. Cell. Biol. 4:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tropepe, V., B. L. Coles, B. J. Chiasson, D. J. Horsford, A. J. Elia, R. R. McInnes, and D. van der Kooy. 2000. Retinal stem cells in the adult mammalian eye. Science 287:2032-2036. [DOI] [PubMed] [Google Scholar]
- 55.Vandenberghe, L. H., L. Wang, S. Somanathan, Y. Zhi, J. Figueredo, R. Calcedo, J. Sanmiguel, R. A. Desai, C. S. Chen, J. Johnston, R. L. Grant, G. Gao, and J. M. Wilson. 2006. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 12:967-971. [DOI] [PubMed] [Google Scholar]
- 56.Weber, M., J. Rabinowitz, N. Provost, H. Conrath, S. Folliot, D. Briot, Y. Cherel, P. Chenuaud, J. Samulski, P. Moullier, and F. Rolling. 2003. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol. Ther. 7:774-781. [DOI] [PubMed] [Google Scholar]
- 57.Yang, G. S., M. Schmidt, Z. Yan, J. D. Lindbloom, T. C. Harding, B. A. Donahue, J. F. Engelhardt, R. Kotin, and B. L. Davidson. 2002. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J. Virol. 76:7651-7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young, J. E., T. Vogt, K. W. Gross, and S. C. Khani. 2003. A short, highly active photoreceptor-specific enhancer/promoter region upstream of the human rhodopsin kinase gene. Investig. Ophthalmol. Vis. Sci. 44:4076-4085. [DOI] [PubMed] [Google Scholar]
- 59.Zeng, Y., Y. Takada, S. Kjellstrom, K. Hiriyanna, A. Tanikawa, E. Wawrousek, N. Smaoui, R. Caruso, R. A. Bush, and P. A. Sieving. 2004. RS-1 gene delivery to an adult rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Investig. Ophthalmol. Vis. Sci. 45:3279-3285. [DOI] [PubMed] [Google Scholar]