Abstract
Three neutralizing monoclonal antibodies (MAbs), 2G12, 2F5, and 4E10, with activity in vitro and in vivo were administered in an open-label, nonrandomized, proof-of-concept study to attempt to prevent viral rebound after interruption of antiretroviral therapy (ART). Ten human immunodeficiency virus type 1-infected individuals identified and treated with ART during acute and early infection were enrolled. The first six patients were administered 1.0 g of each of the three MAbs per infusion. The remaining four patients received 2G12 at 1.0 g/infusion and 2.0 g/infusion of 2F5 and 4E10. The MAbs were well tolerated. Grade I post-partial thromboplastin time prolongations were noted. Viral rebound was observed in 8/10 subjects (28 to 73 days post-ART interruption), and 2/10 subjects remained aviremic over the course of the study. In seven of eight subjects with viral rebound, clear resistance to 2G12 emerged, whereas reductions in the susceptibilities of plasma-derived recombinant viruses to 2F5 and 4E10 were neither sustained nor consistently measured. Viral rebound was associated with a preferential depletion of CD4+ T cells within the gastrointestinal tract. Though safe, the use of MAbs generally delayed, but did not prevent, virologic rebound. Consideration should be given to further pilot studies with alternative combinations of MAbs and perhaps additional novel treatment modalities.
Highly active antiretroviral therapy has made a significant impact on the natural history of human immunodeficiency virus type 1 (HIV-1) infection. A nearly uniformly fatal infection has been transformed into one that is treatable and chronic when managed properly, particularly in resource-abundant settings (42). Treatment regimens have evolved, with less toxicity in the short and long term, as has compactness, accompanying improved ease of administration. At the same time, new agents directed against established and new targets, both viral and cellular, are on the therapeutic horizon.
Despite this apparent success over the past decade, we are still left with a treatment paradigm of lifelong antiviral therapy for the majority of HIV-1-infected individuals. Concerns regarding current therapies include high cost (5), emergence of drug resistance in the face of less than perfect adherence (4), cardiovascular complications due to hyperlipidemia (12, 13), metabolic complications due to hyperglycemia and insulin resistance (9, 17, 31), the possibility of renal disease with chronic use of nucleotide reverse transcriptase inhibitors (44), and perhaps persistent low-level viral replication during therapy (20, 56). For all of these reasons, we and others have attempted to pilot alternative treatment paradigms—antiviral therapy for finite periods of time with therapeutic vaccination, followed by treatment termination or structured treatment interruptions (32, 33, 40). Such efforts have not resulted in sustained control of viral replication in vivo in most of the participants in these studies; nevertheless, despite “failure,” lessons have been learned. One striking observation in our treatment interruption studies in patients treated during acute and early infection was that after viral rebound, a spontaneous reduction in HIV-1 RNA levels in plasma was observed, 1.7 log10 units on average (range, 0.3 to 3.1) (33). This is comparable to what is seen with potent antiviral agents and we believe is likely due to an anamnestic immune response in an already primed patient with an intact, inducible immune system.
Given these findings, we hypothesized that if we could complement the autologous cellular immune response induced by virologic rebound with a neutralizing serologic response obtained with infusions of potent neutralizing monoclonal antibodies (MAbs), perhaps sustained virological remission could be achieved. Three such MAbs were made available for clinical use: 2G12, which binds to a carbohydrate moiety on the silent face of gp120 (54), and 4E10 and 2F5, both of which bind to the membrane-proximal ectodomain of gp41 (45, 52). At the time of the design of our study, these antibodies had been shown to prevent viral infection in the simian immunodeficiency virus/macaque model following oral (18, 24, 34), intravenous (11), or intravaginal (35) challenge. In addition, these MAbs were shown to reduce peak viremia postinfection and to exhibit antiviral activity in established infection (2, 51). Subsequently, Trkola et al. have reported on a passive-immunization experiment using a combination of 2G12, 2F5, and 4E10 in acutely and chronically HIV-1-infected humans and demonstrated that viral rebound was delayed in a subset of patients (53).
We selected a cohort of 10 individuals who were treated with combination antiretroviral therapy (ART) during acute and early infection and in whom undetectable viral loads were measured for at least 6 months prior to study entry. Patient selection was based on the susceptibility of the baseline virus at the time of diagnosis, prior to ART initiation, to each of the three antibodies. The aims of this proof-of-concept trial were as follows: (i) to prevent or dramatically alter the dynamics of plasma HIV-1 RNA rebound after discontinuation of ART by the use of a combination of MAb infusions, (ii) to establish the safety profile of the MAb infusions, and (iii) To compare immunological and virological events in the peripheral blood prior to and after viral rebound with those in tissue, specifically the lower gastrointestinal (GI) tract, a site identified in recent studies as critical to the pathogenesis of HIV-1 infection (6, 22, 36).
(A portion of these data were presented in abstract form at the 13th Conference on Retroviruses and Opportunistic Infections, February 2006 [Denver, CO]), and the 2nd International Workshop for Acute and Early HIV-1 Infection, May 2004 [Bethesda, MD].)
MATERIALS AND METHODS
Study subjects.
Patients were recruited from the Aaron Diamond AIDS Research Center Primary Infection Program, a site of the Acute Infection and Early Disease Research Program (AIEDRP). Entry and infection duration criteria, all laboratory based, have been previously described (36). All patients were treated during acute or early infection with combination antiviral therapy, including nucleoside reverse transcriptase inhibitors (NRTI) and either non-NRTI or protease inhibitor (Table 1) for at least 15 months and had HIV-1 RNA levels below detection in the 6 months preceding screening. Stored pretreatment patient plasma was tested for susceptibility to the three MAbs using a recombinant assay as previously described (43). Susceptibility in this assay was scored as an inhibitory concentration at the 50% level (IC50) below 50 μg/ml. The entry criteria allowed patients to be entered if their baseline virus was susceptible to 2G12 and to at least one of the remaining two antibodies; however, all 10 subjects harbored viruses deemed susceptible to all three MAbs (Table 1). The treatment protocol and amendments were reviewed by the Rockefeller University Hospital Institutional Review Board, and participants gave informed consent by signature. All clinical investigation was conducted according to the principles expressed in the Helsinki Declaration.
TABLE 1.
Baseline patient characteristics
| Subject | Days from symptoms to treatment | Treatment duration (mo) | Baseline HIV-1 RNA (log10 copies) | CD4+ T-cell count (cells/mm3)
|
IC50 to MAb (μg/ml):
|
|||
|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment interruption | 2G12 | 2F5 | 4E10 | ||||
| 700 | 20 | 60 | 6.4 | 703 | 964 | 25.7 | 4.6 | 1.2 |
| 701 | 30 | 20 | 5.4 | 252 | 551 | 36.1 | 5.7 | 2.0 |
| 702 | 30 | 19 | 5.7 | 509 | 546 | 23.2 | 8.0 | 9.6 |
| 703 | 120a | 15 | 4.1 | 498 | 1061 | 1.5 | 2.0 | 3.8 |
| 704 | 30 | 23 | 7.1 | 175 | 471 | 34.6 | 18.9 | 14.6 |
| 705 | 90 | 51 | 5.6 | 394 | 801 | 0.9 | 1.4 | 14.7 |
| 706 | 30 | 23 | 5.2 | 588 | 880 | 1.3 | 1.0 | 1.5 |
| 707 | 25 | 26 | 5.5 | 449 | 678 | 0.7 | 18.6 | 20.1 |
| 708 | 9 | 27 | 7.3 | 258 | 873 | 7.2 | 1.8 | 1.9 |
| 709 | 30 | 27 | 5.1 | 575 | 1106 | 6.1 | 1.3 | 1.4 |
| Mean ± SD | 33 ± 23 | 29 ± 15 | 5.7 ± 1.0 | 440 ± 170 | 793 ± 223 | 13.7 ± 22.6 | 6.3 ± 6.9 | 7.1 ± 7.1 |
Asymptomatic acute seroconversion; the duration of infection was based on a nonreactive detuned enzyme-linked immunosorbent assay at presentation.
Antibodies.
MAbs 2G12, 2F5, and 4E10 were produced by recombinant expression in Chinese hamster ovary (CHO) cells as immunoglobulin G1(κ) [IgG1(κ)]. The generation, production, and characterization of the MAbs were described previously (7, 27, 28).
Antibodies in concentrations of 8 to 15 mg/ml were provided in 1.0- and 2.0-g doses in 10% maltose solution at pH 4.5 by Polymun Scientific (Vienna, Austria). They were shipped and stored at 4°C for subsequent use. 2G12, 4E10, and 2F5 were routinely infused intravenously over 20 min in order and were followed by a 50-ml normal saline infusion over 20 min. The initial six subjects received 1.0 g of each antibody at each infusion. The remaining four patients received 2.0 g of 2F5 and 4E10 and the same dose of 2G12 for reasons explained below.
Study procedures.
The subjects were screened and, if eligible, entered the 28-week study, which included 4 weeks of lead-in and 24 weeks of infusions, treatment interruption, and postinfusion follow-up. Infusions were given weekly for 3 weeks while ART was continued. At the fourth visit, ART was discontinued and antibody infusions were continued weekly for an additional 12 weeks. A total of 16 infusions were planned. Infusions were stopped if patient plasma HIV-1 RNA levels were above 10,000 copies/ml on two occasions at least 2 weeks apart. Patients were advised to resume antiviral therapy for HIV-1 RNA levels above 55,000 copies/ml or if CD4 cell counts fell below 350 cells/mm3 or 50% of the count from the time of treatment interruption. Once infusions were complete, the subjects were followed at 2- to 4-week intervals as indicated for 12 weeks (day 168). Subsequent visits occurred every 12 weeks as part of the ongoing AIEDRP-sponsored cohort study. Safety monitoring included weekly medical history and physical examination, hematology, chemistry, urinalysis, and serum complement levels. Longitudinal HIV-1 RNA determinations were performed with the Roche Ultrasensitive Amplicor COBAS assay with a lower limit of detection of 50 copies/ml plasma. Pre- and postinfusion plasma was collected and stored at −80°C to measure antibody levels. Safety laboratory values during treatment and follow-up were examined according to the Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, toxicity guidelines for adults (16).
Antibody level determinations.
The plasma concentrations of MAbs 2G12, 4E10, and 2F5 were determined by specific double-sandwich enzyme-linked immunosorbent assays using gp160 (MAb 2G12) or peptide KKWNWFDITNWGGG (MAb 4E10) or GGGLELDKWASL (MAb 2F5) as the capture antigen. The measurement of 2G12 made use of anti-idiotypic antibody conjugated with biotin, whereas goat anti-human IgG conjugated with horseradish peroxidase was chosen to detect 4E10 and 2F5. Each sample was analyzed at eight different dilutions. The limit of detection was 3 ng/ml for each MAb. The methods were described in detail in previous publications (1, 2, 28).
Determination of virus neutralization by MAbs 2F5, 4E10, and 2G12.
A previously described recombinant-virus assay was used to measure virus-antibody neutralization (43). In brief, RNA derived from HIV-1-positive plasma was amplified by reverse transcription-PCR and incorporated into an expression vector (pCXAS) by conventional cloning methods. Recombinant HIV-1 stocks expressing patient virus envelope proteins were prepared by cotransfecting HEK293 cells with a replication-defective, luciferase expression cassette-containing HIV-1 genomic viral vector and an appropriate envelope expression vector. Pseudotyped recombinant viruses were harvested 48 h posttransfection and incubated for 1 h at 37°C with serial fourfold dilutions of the three MAbs and plasma controls. U87 cells that expressed CD4, chemokine receptor 5 (CCR5), and CXCR4 were inoculated with virus-antibody dilutions. Luciferase activity determined 72 h postinoculation was used as the indicator of infectivity. Neutralizing activity was displayed as the percent inhibition of luciferase production at each antibody concentration compared to that of an antibody-negative control. The IC50 was defined as the concentration of MAb required to inhibit virus infectivity by 50%. For the purposes of this study, viruses were classified as susceptible to neutralization if the IC50 for that antibody was ≤50 μg/ml.
Measurement of HIV-specific neutralizing-antibody responses.
HIV-1-specific neutralizing-antibody responses were measured by a recombinant-virus assay as described previously (43, 46), using recombinant viruses containing a firefly luciferase indicator gene. Virus infectivity was determined by measuring the amount of luciferase activity expressed in infected cells compared with an antibody-negative control after a single cycle of replication. Neutralizing-antibody titers were calculated as the reciprocal of the IC50. In addition to virus isolated from the study subjects, we also measured heterologous neutralization against the HIV-1 strains NL4-3 (GenBank accession no. AY669735) and JR-CSF (GenBank accession no. AY669726), as well as a virus pseudotyped with a non-HIV envelope (amphotropic murine leukemia virus) as a specificity control.
Sequencing of the HIV-1 env gene.
HIV-1 genomic RNA was isolated from patient plasma by using oligo(dT) magnetic beads, and first-strand cDNA was synthesized in a standard reverse transcription reaction using oligo(dT) primers. The entire envelope (gp160) was PCR amplified by using forward and reverse primers located immediately upstream and downstream of the env initiation and termination codons, respectively. The forward and reverse primers contained unique recognition sites for PinAI and MluI. env PCR products were digested by using PinAI and MluI and ligated into the pCXAS expression vector, which uses the cytomegalovirus immediate-early promoter enhancer to drive expression of the env insert in transfected cells. The ligation products were introduced into competent Escherichia coli cells (Invitrogen) by transformation, and DNA was purified from the bacterial culture. An aliquot of each transformation was spread onto agar plates, and colony counts were used to estimate the number of env sequences represented in each library (500 to 5,000 colonies). The DNA was diluted, retransformed, and replated to allow easy picking of individual colonies (usually 100 to 200 per plate). Sequencing analysis was performed by using a thermocycling method with fluorescent-dye-labeled dideoxynucleotide chain terminator chemistry (Applied Biosystems). Sequencing reaction products were resolved by using a 96 parallel capillary gel electrophoresis system (Applied Biosystems 3700). Multiple-sequence alignment was performed with a Clustal W algorithm, employing DNA Star (Megalign) software.
Examination of HIV-1 peptide-specific cellular immune responses.
Pooled, overlapping 15-mer clade B peptides representing GAG, VPR, and NEF proteins were obtained from the NIH AIDS repository. Aliquots of 0.5 × 106 to 2 × 106 cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and resuspended in culture medium. The PBMCs were incubated alone and in the presence of SEB and HIV-1 peptides as previously described (33). After a 6-h stimulation, the cells were harvested and stained with anti-CD3 Pacific Blue (BD Pharmingen; clone UCHT-1), anti-CD4 Alexa Fluor 700 (BD Pharmingen; clone RPA-T4), and anti-CD8 allophycocyanin-Cy7 (BD Pharmingen; clone SK1) antibodies. After surface staining, the cells were permeabilized using Cytofix-Cytoperm solution (BD Biosciences) according to the manufacturer's instructions. Intracellular staining was performed using anti-interleukin-2 fluorescein isothiocyanate (BD Fastimmune; clone 5344.111) and anti-gamma interferon (IFN-γ) phycoerythrin (BD Fastimmune; clone 25723.11). The cells were acquired using a BD LSRII flow cytometer (BD Biosciences, La Jolla, CA), and data were analyzed using BD FACS DIVA software.
GI-biopsy sample acquisition.
Peripheral blood and recto-sigmoid colonic mucosal tissue were collected from the study patients sequentially, prior to ART discontinuation, 2 to 6 weeks after ART discontinuation, and 8 to 12 weeks after ART discontinuation. Informed consent was obtained from all patients for each biopsy performed, and the study was approved by the Institutional Review Board of the Rockefeller University Hospital. All clinical investigation was conducted according to the principles expressed in the Helsinki Declaration.
Endoscopic-biopsy specimens were obtained from macroscopically normal colonic mucosa and were processed as described previously (36). Briefly, the biopsy specimens were taken using large-cup endoscopic-biopsy forceps (Microvasive Radial Jaw; outside diameter, 3.3 mm; Boston Scientific, Boston, MA) and placed immediately in tissue culture medium (RPMI 1640; Mediatech, Herndon, VA). Phlebotomy was undertaken immediately prior to endoscopy. Immediately after acquisition, mucosal mononuclear cells (MMCs) were enzymatically isolated from the mucosal biopsy specimens using a 30-min incubation in collagenase type II (clostridiopeptidase A; Sigma-Aldrich, St. Louis, MO), followed by mechanical separation through a blunt-ended 16-gauge needle. The digested cell suspension was strained through a 70-μm disposable plastic strainer. Immediately after isolation, the cells were washed with phosphate-buffered saline and resuspended in phosphate-buffered saline containing antibodies for flow cytometry. The PBMCs were prepared by centrifugation on a Ficoll-Hypaque density gradient (Mediatech) and stained for flow cytometry immediately after isolation.
Flow cytometry.
Cell surface expression of lymphocyte antigens was identified by MAb staining of freshly isolated MMCs and PBMCs, followed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems [BDIS], Mountain View, CA) with analysis using CellQuest software (BDIS, Mountain View, CA). The MAbs used in this study included anti-human CD3-fluorescein isothiocyanate (clone UCHT1; BDIS, Mountain View, CA), anti-human CD4-allophycocyanin (clone RPA T4; Pharmingen, San Diego, CA), and anti-human CD8 phycoerythrin (clone RPA T8; Pharmingen, San Diego, CA). During flow cytometry, lymphocytes, initially identified by their forward and side scatter characteristics, were subjected to phenotypic analysis. Dead cells were excluded from analysis using 7-aminoactinomycin D (Calbiochem, La Jolla, CA). To determine the percentages of CD4+ and CD8+ cells in the T-cell population, gated lymphocytes were initially examined for the expression of CD3. The CD3+ lymphocytes were then analyzed for expression of CD4 and CD8 receptors.
Statistical analysis.
Baseline characteristics, as well as clinical and laboratory adverse experiences, were recorded and analyzed descriptively. Additional statistical analyses included paired and unpaired Student t tests, the Mann-Whitney test, and simple regressions and were performed with StatView (SAS Institute, Carey, NC).
RESULTS
Study subjects.
Ten subjects (nine Caucasian; one Asian) were deemed eligible for the study. All were men who had sex with men and were treated for anywhere from 15 to 60 months (Table 1) with protease inhibitor-based (n = 2) or non-NRTI-based (n = 8) therapy. The estimated intervals from infection to treatment initiation ranged from 23 to 120 (mean, 54) days. Baseline virologic and immunologic data reflected the duration of infection (Table 1). All pretreatment viruses were susceptible to all three MAbs, as defined above, with mean IC50s lowest for 2F5 and highest for 2G12 (Table 1). Suppression of viral replication during ART was accompanied by CD4+ T-cell increases of 350 cells/mm3 on average (range, 37 to 563 cells/mm3).
Safety and tolerability of antibody infusions.
A total of 149 infusions were performed. There were no serious adverse events recorded. Fifty-six adverse events occurred, all mild and transient, and were possibly related to the MAb infusions. These included body aches (9 events), fatigue (1 event), flushed sensation (1 event), joint soreness (10 events), limb pain (1 event), low-back ache (5 events), myalgia (2 events), redness at infusion site (1 event), tachycardia without change in blood pressure (14 episodes in one subject, likely not related to MAb infusion), tiredness (11 events), and “floaters in visual field” (1 event). There was no difference in the occurrence or frequency of adverse effects between patients who received 1 g of 2F5/4E10 and those who received 2 g of the two antibodies.
There were no laboratory adverse events reported, other than post-partial thromboplastin time (PTT) prolongations as described below. Complement levels remained constant pre- and postinfusion and within normal limits in all 10 subjects. Following the report by Haynes et al. (23) describing in vitro anticardiolipin antibody activity of 2F5 and 4E10, 12 pre-partial thromboplastin time and PTT determinations were performed in four subjects. All patients had normal coagulation profiles before study entry. Of the 12 determinations preinfusion, 9/12 were within normal limits. Of the three outside the normal range, all were grade 1 (1.1 to 1.25 times the upper limit of normal) (16). Postinfusion PTT levels were drawn 20 min after antibody infusions were completed at a site on the opposite arm from the infusion site and were prolonged (grade 1) in six of nine cases that were normal preinfusion. Three that were prolonged preinfusion were similarly prolonged postinfusion and remained prolonged at the grade 1 level. A mixing study was subsequently performed on one postinfusion sample from one patient to ascertain whether the PTT prolongation was due to the presence of an inhibitor. The patient PTT premix was 56.9 s and with mixing was 53.0 s. After a 1-hour incubation, the patient PTT was 53.1 s and the 50/50 mix was 49.4 s. These results—partial correction with mixing—are consistent with the presence of an inhibitor. The dilute Russel's Viper Venom time was only slightly prolonged (1.38; normal, less than 1.2) and consistent with the presence of a low-level inhibitor. The PTT prolongations were transient, that is, they returned to the normal range within a week of infusion on six of nine occasions and returned to the normal range once infusions were completed. Of note, there were no thrombotic complications associated with MAb therapy.
Clinical, virologic, and immunologic profiles after treatment interruption.
Eight of 10 subjects had rebound plasma viremia during the course of the 28-week study (Fig. 1 and Table 2). All subjects who experienced rebound were asymptomatic, and no HIV-1-related conditions were documented after 24 months of follow-up. Three subjects terminated MAb infusions prematurely based on virologic criteria. The remaining five subjects had virologic rebound to low levels and did not meet stopping criteria. No patients reinitiated ART during the 24-week study. Virologic rebound (that is, going from levels that were undetectable to levels that could be detected using the Roche Amplicor Ultrasensitive assay) occurred 72 days after treatment interruption on average (range, 28 to >168 days). This was substantially prolonged from the 27-day interval previously published (33). Once rebound was detected, the subsequent peak viremia postrebound (mean, 4.3 log10 copies/ml) occurred at day 24 on average and spontaneously fell approximately 1.0 log10 copy/ml (range, 0.4 to 2.2). Once they rebounded, the initial plasma doubling times ranged from 1.5 to 3.9 days (2.3 days on average) (Fig. 2), a value not substantially different from that observed in our earlier treatment interruption studies (33).
FIG. 1.
Virologic and immunologic profiles of the 10 study subjects. The viral loads (purple circles) and CD4+ T-cell counts (blue squares), both represented on the y axis, are plotted over time (x axis) for each study subject. The yellow shaded areas represent the periods during which patients received MAb infusions, and the orange lines depict the day of ART discontinuation. Three subjects with early viral rebound requiring MAb discontinuation are shown in the top row, four subjects with intermediate viral rebound are shown in the middle row, and the three subjects with prolonged control of plasma viremia in the absence of ART are shown in the bottom row.
TABLE 2.
Characteristics of virologic rebound
| Subject | Time to rebounda (days) | Peak viremia (log copies) | Change in viral load post-peak viremia (log copies) | Initial plasma viremia doubling time (days) |
|---|---|---|---|---|
| 700 | 49 | 5.1 | 0.6 | 1.7 |
| 701 | 42 | 3.6 | 1.8 | 2.7 |
| 702 | 42 | 5.4 | 1.1 | 1.7 |
| 703 | 49 | 3.9 | 2.2 | 1.9 |
| 704 | 35 | 4.9 | 0.7 | 1.5 |
| 705 | 168b | |||
| 706 | 168b | |||
| 707 | 63 | 3.7 | 0.4 | 2.2 |
| 708 | 28 | 4.3 | 0.8 | 3.9 |
| 709 | 73 | 3.7 | NDc | 2.7 |
| Mean | 72 ± 53 | 4.3 ± 0.7 | 1.0 ± 0.4 | 2.3 ± 0.8 |
Reflects the time when plasma viremia was first detected.
Patient did not rebound during the course of observation (24 weeks).
ND, not determined.
FIG. 2.
Initial doubling times of rebounding plasma virus. Shown are the plasma viral loads (y axis) before and after virologic rebound (x axis) in eight study subjects. Linear regression analysis assuming first-order kinetics was used to calculate the initial doubling times of virus rebound in plasma, as previously published (33). T2, initial doubling time.
After 24 months of follow-up, 5 of 10 subjects had either restarted antiviral therapy or had met criteria for restarting therapy based on either CD4+ T-cell counts below 350 cells/mm3 (n = 4) or plasma viremia above 100,000 copies/ml (n = 1). Four subjects remained off therapy, with one subject remaining aviremic and two with HIV-1 RNA levels below 1,000 copies/ml. One individual remained asymptomatic, with CD4+ T-cell counts in the mid-400 cells/mm3 range and HIV-1 RNA levels of approximately 60,000 copies/ml. One subject was lost to follow- up.
Pharmacokinetics.
Peak plasma concentrations of each of the three antibodies were measured 0.5 hour postinfusion, and trough plasma concentrations were determined preinfusion. The first six subjects (subjects 700 to 705) received 1.0 g of each of the three antibodies. On day zero, the peak plasma levels of the three MAbs were 701.3 ± 45.7 μg/ml for 2G12, 448.2 ± 50.8 μg/ml for 2F5, and 378.6 ± 45.0 μg/ml for 4E10 (P < 0.01 for 2G12 levels versus 2F5 levels and P < 0.01 for 2G12 levels versus 4E10 levels). Similarly, the mean trough concentrations of 2F5 (47.8 ± 26.9 μg/ml) and 4E10 (71.5 ± 20.1 μg/ml) were significantly lower than the mean trough concentration of 2G12 (368.0 ± 70.4 μg/ml) at baseline (day zero; P < 0.01 and P < 0.01, respectively).
Three of the initial six study participants received all 16 infusions (subjects 701, 703, and 705), whereas for three patients (subjects 700, 702, and 704), infusions were discontinued at weeks 10, 9, and 10, respectively. Interindividual differences in the trough or peak plasma concentration of each of the three antibodies did not correlate with the clinical outcome. In these six subjects, it was evident that 2G12 trough levels increased over time, whereas 4E10 and 2F5 levels did not (Fig. 3A). Based on these data, we attempted to augment the accumulation of 2F5 and 4E10 by increasing the dose of both to 2.0 g/infusion in the next four subjects.
FIG. 3.
Plasma concentration of each of the three MAbs. The concentration of each of the three MAbs—2G12 (green), 2F5 (purple), and 4E10 (orange)—were measured 0.5 hour postinfusion (peak levels) and 7 days later, prior to the next infusion (trough levels). (A) The first six subjects received 1 g/infusion of each of the three MAbs. (B) In the last four subjects, the doses of 2F5 and 4E10 were increased to 2 g/infusion as described in the text. MAb levels (μg/ml) are represented on the y axis and are plotted over time in days (shown on the x axis).
In this group, the mean peak levels of 2G12 (739.6 ± 102.1 μg/ml), 2F5 (886.5 ± 108.4 μg/ml), and 4E10 (882.1 ± 178.2 μg/ml) were comparable on day zero in the last four subjects. However, mean trough levels of 2G12 (336.6 ± 44.1 μg/ml) were significantly higher than levels of 2F5 (93.6 ± 62.4 μg/ml; P = 0.001) and 4E10 (144.4 ± 71.9 μg/ml; P < 0.01). Three out of four subjects completed the study, and one (subject 708) discontinued antibody infusions at week 11 due to virologic stopping criteria. Interindividual differences in antibody levels did not account for the clinical outcome as in the previous group. We did not observe a significant accumulation of either 2F5 or 4E10, as reflected by plasma trough levels, despite dose escalation to 2.0 g per infusion (Fig. 3B).
Based on the above data, elimination half-lives (T1/2β) of each of the three antibodies were determined as described previously (25) by fitting to a two-compartment first-order elimination model (Table 3). The mean T1/2βs of 2G12, 2F5, and 4E10 were 19.9 ± 4.3 days, 5.8 ± 1.4 days, and 7.8 ± 1.5 days, respectively, similar to previously published data (25).
TABLE 3.
Plasma half-lives of the three MAbsa
| Subject |
T1/2 α (days)
|
T1/2 β (days)
|
||||
|---|---|---|---|---|---|---|
| 2G12 | 4E10 | 2F5 | 2G12 | 4E10 | 2F5 | |
| 700 | 0.55 | 0.66 | 0.52 | 14.71 | 5.89 | 3.64 |
| 701 | 0.59 | 0.67 | 0.62 | 26.86 | 11.41 | 8.52 |
| 702 | 0.73 | 0.68 | 0.65 | 19.02 | 7.13 | 6.68 |
| 703 | 0.71 | 0.58 | 0.65 | 20.64 | 7.04 | 7.45 |
| 704 | 0.73 | 0.71 | 0.67 | 19.11 | 7.77 | 4.80 |
| 705 | 0.61 | 0.54 | 0.56 | 26.13 | 6.68 | 5.12 |
| 706 | 0.65 | 0.45 | 0.47 | 17.97 | 8.32 | 5.75 |
| 707 | 0.71 | 0.58 | 0.59 | 19.40 | 8.64 | 5.26 |
| 708 | 0.64 | 0.53 | 0.67 | 13.25 | 7.62 | 5.45 |
| 709 | 0.73 | 0.79 | 0.71 | 21.83 | 7.28 | 5.24 |
| Mean | 0.67 | 0.62 | 0.61 | 19.89 | 7.78 | 5.79 |
| SD | 0.07 | 0.10 | 0.08 | 4.32 | 1.50 | 1.40 |
T1/2 α, first-phase elimination half-life.
Susceptibilities of the rebounding virus to MAbs.
A recombinant viral assay (43) was used to test the susceptibilities of baseline and rebounding virus. A delay in viral rebound did not correlate with plasma neutralization titers of baseline virus (Spearman's rho, −0.1; P = 0.79). Assigning the two patients who did not rebound during the 24-week infusion/ART interruption/observation phase of the study a value of 168 days, the time to rebound was negatively and significantly correlated with baseline susceptibility to 2G12 (Spearman's rho, −0.7; P = 0.03). There was no relationship between the time to rebound and baseline susceptibility to 2F5 or 4E10. In seven of eight subjects, a decrease in susceptibility to 2G12 accompanied virologic rebound, 2.8-fold on average, whereas reductions in susceptibility to 2F5 and 4E10 were neither sustained nor consistently measured (Fig. 4). Full-length envelope sequencing was performed to characterize the rebounding virus populations. In six of the seven patients who developed 2G12 resistance, N-linked glycosylation site mutations (49) were found at baseline or posttreatment (Fig. 5). In the one patient with rebound viremia in whom 2G12 resistance was not detected, no such mutations were noted. There were no amino acid substitutions detected in the gp41 binding domains for 2F5 or 4E10, though a deletion of lysine in ELDWKA, the 2F5 binding domain, was detected in patient 704 (Fig. 4). This deletion, first appearing at day 56 and maintained to day 140, was not temporally associated with the emergence of measured changes in 2F5 susceptibility (11.2 μg/ml at baseline; 14.8 μg/ml at day 56; 17.6 μg/ml at day 140), although this individual did exhibit a brief though unsustained 2.5-fold reduction (27.5 μg/ml) in susceptibility to 2F5 at day 94 (24 days after infusions were discontinued).
FIG. 4.
Neutralization profiles of patient-derived virus at baseline and after rebound. Using a recombinant-virus assay (43), the susceptibilities of baseline and rebounding virus to the three MAbs (measured in μg/ml) (shown on the y axis) were followed over time in days (x axis) in eight study subjects with rebound viremia. The range of susceptibility is shown by the arrows; values above 50 μg/ml were considered resistant and for the sake of illustration were plotted as 60 μg/ml.
FIG. 5.
HIV-1 Env amino acid sequences in rebounding plasma HIV-1. Full-length envelope sequencing was performed on plasma-derived HIV-1 prior to and after rebound. Sequences from each patient were aligned with the reference strain HXB2, and changes at N-linked glycosylation sites known to result in reduced susceptibility to 2G12 (49) are highlighted in red.
Neutralization of patient virus by patient plasma.
In order to examine the overall neutralization capacities of patient plasmas, the dilutions (n-fold) that neutralized 50% of recombinant viruses constructed from patient plasma-derived envelopes at multiple time points were determined (see the supplemental material). In general, we observed that patient plasma neutralized contemporaneous recombinant virus less well than recombinant viruses constructed from circulating plasma virus from earlier time points. Furthermore, viruses became less neutralizable by plasma over time, consistent with rapid escape from the effects of the combination of MAbs and/or from an autologous antibody response.
Despite continued infusions of MAbs, we did not observe sustained changes in the susceptibilities of rebounding viruses to either 2F5 or 4E10, despite trough levels in excess of the neutralization IC50. To attempt to understand the relative effects of 2F5 and 4E10, we analyzed the neutralization capacity of plasma after the emergence of resistance to 2G12 but prior to antibody discontinuation. This was compared with the plasma neutralization capacity after antibody infusions were discontinued (Table 4). In five of eight patients, there was no decline in plasma neutralization capacity after discontinuation of 4E10 and 2F5 infusions. Of the three patients in whom a decline in plasma neutralization capacity was observed, there was a modest (0.5- and 0.4-log10 unit) increase in the plasma viral load in two of the subjects after antibody infusions were discontinued (subjects 701 and 704, respectively), consistent with some in vivo neutralization effects of the two MAbs.
TABLE 4.
Plasma neutralization of autologous viruses
| Patient | Plasma neutralization capacity
|
Δ Plasma HIV-1 RNA post-MAb DC (log10 copies/ml) | Plasma trough level/IC50 ratio (day of MAb DC)
|
|||
|---|---|---|---|---|---|---|
| Initial viral rebound | First 2G12 resistance | 2 wk post-MAb DCa | 2F5 | 4E10 | ||
| 700 | 1:295 | 1:627 | 1:401 | −0.1 | 28.2/3.9 = 7.2 | 63/1.7 = 37.1 |
| 701 | 1:167 | 1:167 | 1:248 | +0.5 | 70.0/3.2 = 21.9 | 173.2/1.8 = 96.2 |
| 702 | 1:184 | 1:212 | 1:73 | −0.5 | 61/3.4 = 17.9 | 100.4/3.6 = 27.9 |
| 703 | 1:95 | 1:95 | 1:177 | +0.1 | 29.5/8.3 = 3.6 | 103.3/10.6 = 9.8 |
| 704 | 1:166 | 1:140 | 1:76 | +0.4 | 31.2/9.5 = 3.3 | 76.4/3.4 = 22.5 |
| 707 | 1:90 | 1:90 | 1:82 | +0.7 | 64.4/12.6 = 5.1 | 173.3/10.5 = 16.5 |
| 708 | 1:345 | 1:200 | 1:222 | −0.6 | 95.1/2.6 = 36.6 | 235.5/3.1 = 75.9 |
| 709 | 1:166 | NAb | 1:302c | +0.6 | 133.2/3.6 = 37 | 224.3/4.6 = 48.8 |
DC, discontinuation.
NA, not applicable.
One week post-MAb DC, the patient was lost to follow-up.
Cellular immune responses during MAb infusions and viral rebound.
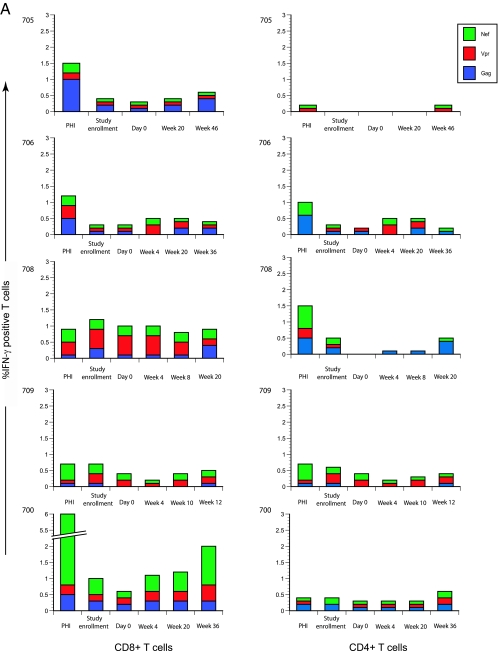
To determine the role of cellular immune responses in the study subjects, we measured intracellular IFN-γ secretion in CD4+ and CD8+ T cells when pulsed with pooled, overlapping, 15-mer HIV-1 clade B peptides constituting GAG, NEF, and VPR proteins. Five representative subjects were chosen for study. They included the two patients with persistent plasma HIV-1 RNA levels below the detection level (subjects 705 and 706), one patient with early viral rebound requiring premature antibody infusion discontinuation (subject 700), and a genetically unrelated transmission pair with near-identical viruses at baseline (subjects 708 and 709). Within the transmission pair, subject 708 experienced early virologic rebound, 28 days after ART discontinuation, whereas subject 709 demonstrated late rebound 73 days after ART discontinuation. Thus, we had the rare opportunity to examine cellular immune responses in a genetically unrelated transmission pair with nearly identical viruses at baseline and somewhat disparate outcomes. The time points chosen for these experiments were as follows: (i) acute and early HIV-1 infection prior to ART initiation, (ii) at the time of MAb study enrollment, (iii) after three infusions of MAb prior to ART discontinuation, (iv) after ART discontinuation, (v) at the time of initial viral rebound, and (vi) at the end of the MAb study.
In general, the magnitudes of the cellular immune responses were related to the rebounding plasma viral load, and the breadth of these responses remained relatively unchanged over time (Fig. 6A). For example, in subject 700, a strong response directed against NEF was observed during acute HIV-1 infection. This NEF-predominant response waned during the aviremic period and reappeared after discontinuation of ART, during viral rebound. Of note, the breadth of the CD8+ T-cell responses in this patient or others did not change over time.
FIG. 6.
Cellular immune responses at acute and early HIV-1 infection and during the course of study. (A) Five representative subjects were chosen, and CD4+ and CD8+ T-cell responses to pooled peptides representing GAG, VPR, and NEF from HIV-1 clade B were measured. The percentages of IFN-γ-producing cells (y axis) are plotted at sequential time points (x axis). (B) In subject 706, CD4+ and CD8+ T-cell responses were compared between the peripheral blood and GI tract (x axis), and the percentages of cytokine-producing cells (depicted on the y axis) were examined.
We observed a broad CD8+ T-cell response in subject 708, who experienced early viral rebound, whereas in subject 709, low-level responses were observed during acute and early HIV-1 infection and after viral rebound.
In the two patients with persistent plasma HIV-1 RNA levels below the detection level (subjects 705 and 706), broad HIV-1-specific CD8+ T-cell responses were observed in the peripheral blood of one, 705, but not that of 706. Subject 706 consented to GI biopsy, facilitating quantitation of IFN-γ-secreting CD4+ and CD8+ T cells pulsed with HIV-1 peptides, as described above. While responses in the peripheral blood were detected at low levels, we observed a strong constitutive, as well as antigen-specific, CD4+ T-cell-dominated response in the MMC population (Fig. 6B).
We were concerned that perhaps delivery of MAbs to tissue compartments could affect treatment response, in that inadequate MAb levels at these sites could be correlated with viral outcome. We therefore attempted to determine MAb levels in tissues by using immunohistochemistry, but we were unable to do so because of nonspecific tissue binding of detection antibodies.
Examination of GI tract-associated lymphoid tissue.
Recent studies have demonstrated an important role played by mucosal sites, such as the GI tract, in establishing HIV-1 infection in a new host (22, 36). Along these lines, we thought that in patients discontinuing ART after prolonged periods, the reignition of rapid rounds of viral replication could similarly emerge in the GI mucosa, where higher levels of activated CD4+ T cells expressing CCR5 reside. We performed serial biopsies of the rectosigmoid colon to (i) examine and compare CD4+ T-cell subsets in tissue with peripheral blood and (ii) determine the role of tissue compartments during virologic rebound.
Seven of 10 patients consented to sequential flexible sigmoidoscopy with multiple biopsies. The percentages of CD4+ T cells were measured using flow cytometry in the peripheral blood and GI tract prior to and after viral rebound (Table 5). In the two subjects who remained aviremic, GI mucosal CD4+ T-cell percentages did not decline after discontinuation of ART. In contrast, in the five patients examined for whom viral rebound followed ART discontinuation, there was a rapid, preferential, and significant decline in the percentages of GI CD4+ T cells. In fact, in subject 700, the CD4+ T-cell percentage in the GI tract declined from 66% at baseline to 30% within 10 weeks of ART discontinuation. In contrast, peripheral-blood CD4+ T cells declined from 61% to 52% over the same period. A similar pattern was seen in every patient studied in whom viral rebound occurred.
TABLE 5.
T-cell subsets in the peripheral blood and the GI tract
| Subject no. | % CD4+ T cells
|
% CD8+ T cells
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBMCs at biopsy no. (wk)
|
MMCs at biopsy no. (wk)
|
PBMCs at biopsy no. (wk)
|
MMCs at biopsy no. (wk)
|
|||||||||
| 1 (−3 to 0) | 2 (5 to 7) | 3 (12 to 14) | 1 (−3 to 0) | 2 (5 to 7) | 3 (12 to 14) | 1 (−3 to 0) | 2 (5 to 7) | 3 (12 to 14) | 1 (−3 to 0) | 2 (5 to 7) | 3 (12 to 14) | |
| 700 | 60.7 | 62.1 | 52.2 | 65.8 | 65.8 | 29.7 | 34.8 | 34.6 | 43.2 | 31.3 | 32.8 | 68.3 |
| 701 | 60.6 | 59.7 | 54.5 | 43.5 | 44.2 | 32.3 | 33.5 | 34.2 | 39.5 | 48.3 | 50.9 | 63.7 |
| 703 | 53.6 | 52.3 | 49.0 | 41.7 | 33.9 | 32.8 | 33.9 | 33.7 | 41.9 | 47.5 | 56.7 | 55.9 |
| 704 | 40.9 | 41.8 | 34.8 | 44.7 | 45.7 | 21.4 | 56.6 | 54.8 | 61.8 | 49.8 | 46.0 | 71.6 |
| 705 | 64.0 | 62.9 | 63.9 | 41.9 | 44.6 | 58.9 | 34.8 | 33.0 | 35.3 | 50.0 | 46.3 | 39.4 |
| 706 | 57.2 | 61.5 | 63.4 | 44.5 | 41.4 | 45.6 | 39.5 | 37.0 | 33.9 | 52.7 | 50.8 | 50.5 |
| 707 | 63.2 | 64.4 | 56.9 | 39.4 | 36.3 | 28.3 | 36.7 | 34.2 | 40.2 | 55.7 | 59.7 | 68.9 |
In summary, it appears that, akin to primary HIV-1 infection, ART interruption resulted in an early and preferential depletion of CD4+ T cells at mucosal sites in patients experiencing viral rebound. Interestingly, this was not seen in those who remained aviremic.
DISCUSSION
There is much interest in the antiviral activities of MAbs as therapeutics, particularly in the postexposure prophylaxis setting, as well as in the area of vaccine development, as the identification of broadly neutralizing antibodies may allow the design of immunogens capable of stimulating this potential protective response. In the context of simian immunodeficiency virus infection, MAbs have been used for pre- and postexposure prophylaxis with success (34, 35, 50). Akin to previous therapeutic vaccine studies, we performed this trial to provide humoral immunity passively while anticipating the emergence of a host-derived anamnestic cellular immune response to control viral replication in vivo following treatment interruption.
Given that this was a proof-of-concept study, we carefully selected patients who were treated during acute and early HIV-1 infection. The advantages of choosing this population include the likelihood that prolonged ART, initiated early, could preserve autologous cellular immune responses (41, 47) and that the pretreatment viral population was relatively homogeneous (57), resulting in a low likelihood that susceptibility to the MAbs would be compromised by the presence of complex mixtures of viral quasispecies. The three neutralizing antibodies, 2G12, 2F5, and 4E10, were chosen solely on the basis of availability. Data from our laboratory (38) and additional studies by others (3, 8) demonstrated that 2F5 and 4E10 had broad and potent neutralizing activities against a panel of nearly 100 primary isolates. However, the neutralization capacity of 2G12 was variable and limiting, with only a third of the primary viral isolates tested showing in vitro susceptibility (3, 38). Notably, the neutralization activities of all MAbs tested (including 2F5 and 4E10) was greater in pseudovirus-based assays than in assays that were PBMC based (3). For the purposes of this study, we selected patients in whom the baseline virus was neutralized by all three antibodies in vitro, defined as an IC50 at or below 50 μg/ml. Finally, the trial design provided for MAb infusions overlapping with ART for 3 weeks to allow the accumulation of the three IgG1 antibodies in extravascular spaces prior to withdrawal of ART.
Though the results fell far short of our goal of sustained control of viremia after ART discontinuation in at least six subjects, we believe that our findings could support further studies along this line.
First and foremost, MAb infusions were very well tolerated, with essentially no toxicity. We had the opportunity to test coagulation parameters in four subjects as described above. In all four subjects, transient, reversible, grade 1 PTT prolongations were noted postinfusion. Mixing studies performed in one patient suggested the presence of a low-level lupus anticoagulant-like inhibitor. Notably, no thrombotic events were noted. Detailed in vitro analyses of 2F5 and 4E10 have been performed and suggest that 4E10 and not 2F5 exhibits anti-cardiolipin-like activity (55).
Viral rebound, though not prevented, was significantly delayed in the majority (8/10) of our study subjects compared to historic controls. Importantly, baseline susceptibility to 2G12 was inversely associated with the time to viral rebound and escape from 2G12 was clearly associated with viral rebound during antibody infusions. Examination of the rebounding virus population revealed that of the eight patients, decreased susceptibility to 2G12 developed in seven. In the only patient (subject 709) for whom 2G12 resistance was not observed, low-level viral rebound occurred on study day 73 and MAb infusions were stopped on day 84 according to the study protocol; at that point, the patient was essentially lost to follow-up. It is conceivable that with time a more resistant viral population may have emerged. Taken together, our results confirm that the activity of 2G12 was dominant among the MAbs and substantially delayed rebound, concordant with the findings of Trkola et al. (53). We remain unsure, however, of the degree to which the neutralizing activities of 2F5 and 4E10 contributed to our in vivo observations.
Though susceptibilities to 2F5 and 4E10 were demonstrated in the recombinant assay and the antibody levels at trough were in excess of the IC50, antiviral activity was not clearly demonstrated for the following reasons: (i) changes in susceptibility (resistance) to 2F5 and 4E10 were not consistently measured despite ongoing viral replication in the presence of MAb infusions, (ii) no consistent increase in levels of plasma HIV-1 RNA were observed in patients when the antibody infusions were stopped, and (iii) despite continued infusions of 2F5 and 4E10 in the face of 2G12 resistance, initial doubling times of plasma viremia were essentially identical to that seen in ART-treated subjects whose therapy was interrupted without adjunctive therapies (33). Furthermore, unlike 2G12, both 2F5 and 4E10 failed to accumulate with repeated infusions for reasons that remain unclear. Of note, in a phase Ib study of 2F5 and 2G12 infusions in seven chronically infected subjects, 2F5 clearly exerted antiviral activity in vivo in three of five subjects with 2F5-susceptible, 2G12-resistant HIV-1 infections, though the duration of antiviral activity was generally brief (51). In two patients in whom a response was followed by a rebound, a shift in 2F5 susceptibility (IC90) was demonstrated using a PBMC-based viral-neutralization assay; however, no genotype data were generated. We believe it is critical to better understand the relative contributions of both 2F5 and 4E10 in vivo, as these MAbs have generated considerable attention in the area of vaccine development and in settings where passive immunization as prophylaxis may be desirable. Currently, we are performing studies to address this critical issue.
Although we cannot make generalizable conclusions from the prolonged control of viral replication in 2 of 10 study participants, prolonged viral suppression to undetectable levels in the absence of antiretroviral treatment is rare. In previous studies conducted by us and others, viral rebound following ART interruption has been more or less universal (14, 21, 33), with only anecdotal reports of control of viremia to levels below 500 copies in the absence of therapy (29, 40). That 3 of 10 individuals appear to be controllers over the longer term provides a glimmer of optimism.
Our results are consistent with the findings of Trkola et al. (53), although there are some differences that merit attention. Specifically, Trkola et al. examined eight patients treated during chronic HIV-1 infection and six patients treated during acute infection. One gram of MAbs 2G12 and 4E10 and 1.3 g of 2F5 were infused into the patients on a weekly basis. Two of the eight patients with chronic HIV-1 infection showed delay in viral rebound, whereas all six patients treated during acute HIV infection showed viral-rebound delay similar to that noted in the present study. This observation underscores a potential limitation of passive-immunization strategies and highlights the fact that MAb neutralization potential may be significantly curtailed by viral-quasispecies diversity generated during chronic HIV-1 infection. In both the present study and the one by Trkola et al., viral rebound correlated with susceptibility to 2G12, although two different neutralization assays were used. While Trkola et al. used a PBMC-based assay to determine IC90 neutralization titers, the present study employed a pseudovirus-based assay and measured IC50 levels.
In order to understand the mechanisms behind persistent viral control in subjects 705 and 706, we performed detailed virological, genetic, and immunological studies. In both subjects 705 and 706, the baseline virus was highly susceptible to 2G12, with IC50s of ∼1 μg/ml (Table 1). In addition, patient 705 had high plasma neutralization capacity at baseline (∼1/1,000). This appeared to decrease after MAb infusions were stopped suggesting that MAbs contributed to the autologous plasma neutralization capacity (1/1,039 on the day of the last MAb infusion to 1/639 2 weeks following MAb discontinuation).
Genetic studies performed on the 10 study subjects were not relevant to the clinical-outcome data (see the supplemental material). Both subjects 705 and 706 did not have any known HLA class I alleles associated with good prognosis or slow clinical progression (10, 19) or a 32-bp deletion in the open reading frame of the CCR5 gene (Δ32-CCR5) that induces a frameshift and loss of HIV-1 coreceptor activity (15, 30, 48). Actually, patient 706 was homozygous for the HLA class I allele, A*0301. Patient 702, with high-level virologic rebound, harbored one copy of the Δ32-CCR5 allele but was also homozygous at the HLA class I loci B*3801 and C*1203.
To characterize cellular immune responses in both the peripheral blood and the GI-tract-associated lymphoid tissue, we measured the percentages of CD4+ and CD8+ T cells secreting IFN-γ in response to HIV-1 overlapping 15-mer peptides representing clade B GAG, VPR, and NEF. Though it is now clear that these responses may not correlate with levels of plasma viremia, this assay was considered the standard in the field at the time these studies were performed. In subject 705, broad CD8+ T-cell responses were observed (Fig. 6). In subject 706, we detected lower-level, narrower CD8+ T-cell responses in the peripheral blood. However, on detailed examination of the MMCs isolated from the GI tract, we observed a significant constitutive and antigen-specific, CD4-dominated response. This finding is unusual and in our experience has been observed in only 1 of 5 acutely and none of 10 chronically HIV-1-infected subjects similarly studied. Clearly, the significance of this finding requires further study.
It is interesting that patients 708 and 709 were infected with the same virus, with the same susceptibilities to the three MAbs, yet patient 708 rebounded earlier than all other subjects whereas patient 709 did not rebound until day 73. We expected to find that cellular immune responses would explain this difference in these genetically unrelated hosts. Patient 708 had low-level, broad responses against all three antigens (GAG, VPR, and NEF) tested (Fig. 6), whereas patient 709 had low-level cellular responses. We can only conclude that the differences between the outcomes in these individuals could not be detected with the use of this assay. Furthermore, we did not observe a correlation between GAG-predominant, narrow cellular immune responses and a favorable viral outcome, as has been recently demonstrated at the population level in a heterogeneous cohort of chronically HIV-1-infected individuals (26). Also of interest was the fact that measured responses to HIV-1 antigens pre-ART and posttreatment interruption remained fixed. That is, a patient who had a NEF-dominated response at baseline showed a similar response (with variable magnitude) post-ART discontinuation. These findings are consistent with the concept that the autologous cellular immune responses may indeed be fixed due to genetic determinants, and though the early use of ART may preserve these responses, it is unlikely that such responses will be substantially altered qualitatively.
Though we cannot pinpoint the exact mechanisms of persistent aviremia in subjects 705 and 706, our findings suggest that a convergence of multiple factors as opposed to one unifying observation (high susceptibility to 2G12, potent autologous plasma neutralization activity, and broad cellular immune response in the peripheral blood or in the GI tract) resulted in a favorable outcome. We urge caution in the overinterpretation of these data. However, we believe that the success of these two patients, as well as a third patient who exhibited modest rebound viremia that has been controlled over the long term, does raise the possibility that in the right clinical setting, with the use of a combination of potent MAbs to which the patient's virus is highly susceptible (i.e., an IC50 below 1.0 μg/ml), prolonged viral suppression in the absence of ART may be feasible.
We sampled the GI tract, the largest reservoir of immune cells in the body (39) and a site that has been demonstrated to play a critical role in the pathogenesis of acute (22, 36, 37) and chronic (6) HIV-1 infection. In the two patients with persistent control of viremia, serial biopsies did not demonstrate a decline in GI CD4+ T-cell percentages. In contrast, in five subjects with viral rebound who consented to sequential examinations of the GI tract, an early and preferential decline of CD4+ T cells was observed in the GI tract prior to such changes in the peripheral blood. Although at this point we cannot state whether rebounding virus emerges from a tissue reservoir or the tissue is seeded from elsewhere, these findings strengthen the argument that activated CD4+ T cells resident in the GI tract are critical targets as rapid rounds of viral replication become reignited after ART is withdrawn.
In summary, this study provides a number of important conclusions. First, we have confirmed the efficacy of 2G12 as a neutralizing MAb that has potential in future trials of passive immunization, though its utility may be limited by a lack of broad efficacy against patient-derived viruses. Secondly, this study raises the possibility of discordance between the strong and broad in vitro neutralization profiles of MAbs like 2F5 and 4E10 to the membrane-proximal ectodomain of gp41 and their apparent in vivo efficacy. Given the interest in theses MAbs as models for vaccine-induced humoral responses, we believe it is critical that we better understand the relative contributions of these MAbs to the virologic effects we have observed, and indeed, these studies are in progress. Thirdly, though we fell far short of our goal of long-term control of viremia in at least 6 of 10 subjects, long-term suppression of viremia was observed in 3/10 participants, raising the possibility that with more careful selection of MAbs and trial subjects, the results of such pilot trials could be improved and could pave the way for larger phase II and III trials. Finally, the present study demonstrates that viral rebound in the absence of therapy is associated with a rapid decline in mucosal CD4+ T cells, establishing the importance of the activated tissue effector memory CD4+ T-cell population in establishing and perhaps fueling virologic rebound. We suggest that protection of these cells using innovative approaches, such as CCR5 antagonists, in addition to immune-based adjuncts may alter the balance between innate, cellular, and humoral responses on the one side and the reigniting of rapid rounds of viral replication on the other, so that our goal of long-term control of HIV-1 replication in the absence of therapy may be realized.
Supplementary Material
Acknowledgments
This work was supported in part by the AIEDRP (AI-41534) and a Clinical and Translational Science Award from the National Institutes of Health (UL1-RR024143). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
G.S., B.V., and H.K. are affiliated with Polymun Scientific, a private company involved in the development of human MAbs. T.W., C.P., and J.G. are employees of Monogram Biosciences, Inc., the property company of the Phenosense Entry/Neutralization Assay used in this study.
All three MAbs used in this trial (2F5, 2G12, and 4E10) were provided by Polymun Scientific.
Footnotes
Published ahead of print on 8 August 2007.
Supplemental material for this article may be found at http://jvi.asm.org.
REFERENCES
- 1.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, U. Koller, R. Jilch, C. G. Ammann, M. Pruenster, H. Stoiber, and H. W. Katinger. 2004. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J. Antimicrob. Chemother. 54:915-920. [DOI] [PubMed] [Google Scholar]
- 2.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, N. L. Michael, N. Vetter, and H. W. Katinger. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16:227-233. [DOI] [PubMed] [Google Scholar]
- 3.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden, D., A. Hurley, L. Zhang, Y. Cao, Y. Guo, E. Jones, J. Tsay, J. Ip, C. Farthing, K. Limoli, N. Parkin, and M. Markowitz. 1999. HIV-1 drug resistance in newly infected individuals. JAMA 282:1135-1141. [DOI] [PubMed] [Google Scholar]
- 5.Bozzette, S. A., G. Joyce, D. F. McCaffrey, A. A. Leibowitz, S. C. Morton, S. H. Berry, A. Rastegar, D. Timberlake, M. F. Shapiro, D. P. Goldman, and the H I V Cost and Services Utilization Study Consortium. 2001. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N. Engl. J. Med. 344:817-823. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins: electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. [DOI] [PubMed] [Google Scholar]
- 8.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 9.Carr, A., K. Samaras, S. Burton, M. Law, J. Freund, D. J. Chisholm, and D. A. Cooper. 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51-F58. [DOI] [PubMed] [Google Scholar]
- 10.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535-551. [DOI] [PubMed] [Google Scholar]
- 11.Conley, A. J., J. A. Kessler II, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark III, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Data Collection on Adverse Events of Anti-HIV Drugs Study Group. 2003. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 349:1993-2003. [DOI] [PubMed] [Google Scholar]
- 13.Data Collection on Adverse Events of Anti-HIV Drugs Study Group. 2007. Class of antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med. 356:1723-1735. [DOI] [PubMed] [Google Scholar]
- 14.Davey, R. T., Jr., N. Bhat, C. Yoder, T.-W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, Hemophilia Growth and Development Study, Multicenter Aids Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, Alive Study, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 16.Division of AIDS. 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD.
- 17.Dube, M. P., D. L. Johnson, J. S. Currier, and J. M. Leedom. 1997. Protease inhibitor-associated hyperglycaemia. Lancet 350:713-714. [DOI] [PubMed] [Google Scholar]
- 18.Ferrantelli, F., R. Hofmann-Lehmann, R. A. Rasmussen, T. Wang, W. Xu, P. L. Li, D. C. Montefiori, L. A. Cavacini, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2003. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17:301-309. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Villanueva, P. O., E. J. Yunis, J. C. Delgado, E. Vittinghoff, S. Buchbinder, J. Y. Leung, A. M. Uglialoro, O. P. Clavijo, E. S. Rosenberg, S. A. Kalams, J. D. Braun, S. L. Boswell, B. D. Walker, and A. E. Goldfeld. 2001. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl. Acad. Sci. USA 98:5140-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, F., M. Plana, C. Vidal, A. Cruceta, W. A. O'Brien, G. Pantaleo, T. Pumarola, T. Gallart, J. M. Miro, and J. M. Gatell. 1999. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS 13:F79-F86. [DOI] [PubMed] [Google Scholar]
- 22.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes, B. F., J. Fleming, E. W. St Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T. C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joos, B., A. Trkola, H. Kuster, L. Aceto, M. Fischer, G. Stiegler, C. Armbruster, B. Vcelar, H. Katinger, and H. F. Gunthard. 2006. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob Agents Chemother. 50:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 27.Kunert, R., W. Steinfellner, M. Purtscher, A. Assadian, and H. Katinger. 2000. Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3/IgG1 subclass switch in CHO cells. Biotechnol. Bioeng. 67:97-103. [DOI] [PubMed] [Google Scholar]
- 28.Kunert, R., S. Wolbank, G. Stiegler, R. Weik, and H. Katinger. 2004. Characterization of molecular features, antigen-binding, and in vitro properties of IgG and IgM variants of 4E10, an anti-HIV type 1 neutralizing monoclonal antibody. AIDS Res. Hum. Retrovir. 20:755-762. [DOI] [PubMed] [Google Scholar]
- 29.Lisziewicz, J., E. Rosenberg, J. Lieberman, H. Jessen, L. Lopalco, R. Siliciano, B. Walker, and F. Lori. 1999. Control of HIV despite the discontinuation of antiretroviral therapy. N. Engl. J. Med. 340:1683. [DOI] [PubMed] [Google Scholar]
- 30.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 31.Lo, J. C., K. Mulligan, V. W. Tai, H. Algren, and M. Schambelan. 1998. “Buffalo hump” in men with HIV-1 infection. Lancet 351:867-870. [DOI] [PubMed] [Google Scholar]
- 32.Lori, F., and J. Lisziewicz. 2001. Structured treatment interruptions for the management of HIV infection. JAMA 286:2981-2987. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz, M., X. Jin, A. Hurley, V. Simon, B. Ramratnam, M. Louie, G. R. Deschenes, M. Ramanathan, Jr., S. Barsoum, J. Vanderhoeven, T. He, C. Chung, J. Murray, A. S. Perelson, L. Zhang, and D. D. Ho. 2002. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J. Infect. Dis. 186:634-643. [DOI] [PubMed] [Google Scholar]
- 34.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 36.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehandru, S., K. Tenner-Racz, P. Racz, and M. Markowitz. 2005. The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. J. Allergy Clin. Immunol. 116:419-422. [DOI] [PubMed] [Google Scholar]
- 38.Mehandru, S., T. Wrin, J. Galovich, G. Stiegler, B. Vcelar, A. Hurley, C. Hogan, S. Vasan, H. Katinger, C. J. Petropoulos, and M. Markowitz. 2004. Neutralization profiles of newly transmitted human immunodeficiency virus type 1 by monoclonal antibodies 2G12, 2F5, and 4E10. J. Virol. 78:14039-14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowat, A. M., and J. L. Viney. 1997. The anatomical basis of intestinal immunity. Immunol. Rev. 156:145-166. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz, G. M., D. F. Nixon, A. Trkola, J. Binley, X. Jin, S. Bonhoeffer, P. J. Kuebler, S. M. Donahoe, M. A. Demoitie, W. M. Kakimoto, T. Ketas, B. Clas, J. J. Heymann, L. Zhang, Y. Cao, A. Hurley, J. P. Moore, D. D. Ho, and M. Markowitz. 1999. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J. Clin. Investig. 104:R13-R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and the HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 43.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyriere, H., J. Reynes, I. Rouanet, N. Daniel, C. M. de Boever, J. M. Mauboussin, H. Leray, L. Moachon, D. Vincent, and D. Salmon-Ceron. 2004. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J. Acquir. Immune Defic. Syndr. 35:269-273. [DOI] [PubMed] [Google Scholar]
- 45.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 46.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 48.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 49.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 51.Stiegler, G., C. Armbruster, B. Vcelar, H. Stoiber, R. Kunert, N. L. Michael, L. L. Jagodzinski, C. Ammann, W. Jager, J. Jacobson, N. Vetter, and H. Katinger. 2002. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS 16:2019-2025. [DOI] [PubMed] [Google Scholar]
- 52.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 53.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 54.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vcelar, B. A., G. M. Stiegler, H. M. Wolf, W. Muntean, B. Leschnik, S. Mehandru, M. Markowitz, C. Armbruster, R. Kunert, M. M. Eibl, and H. Katinger. Reassessment of autoreactivity of the broadly neutralizing HIV antibodies 4E10 and 2F5 and retrospective analysis of clinical safety data. AIDS, in press. [DOI] [PubMed]
- 56.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, D. D. Ho, Y. Guo, M. Duran, A. Hurley, J. Tsay, Y.-C. Huang, and C.-C. Wang. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.