Abstract
The pathogenesis of essential hypertension remains unknown, but thiazide diuretics are frequently recommended as first-line treatment. Recently, familial hyperkalemic hypertension (FHHt) was shown to result from activation of the thiazide-sensitive Na-Cl cotransporter (NCC) by mutations in WNK4, although the mechanism for this effect remains unknown. WNK kinases are unique members of the human kinome, intimately involved in maintaining electrolyte balance across cell membranes and epithelia. Previous work showed that WNK1, WNK4, and a kidney-specific isoform of WNK1 interact to regulate NCC activity, suggesting that WNK kinases form a signaling complex. Here, we report that WNK3, another member of the WNK kinase family expressed by distal tubule cells, interacts with WNK4 and WNK1 to regulate NCC in both human kidney cells and Xenopus oocytes, further supporting the WNK signaling complex hypothesis. We demonstrate that physiological regulation of NCC in oocytes results from antagonism between WNK3 and WNK4 and that FHHt-causing WNK4 mutations exert a dominant-negative effect on wild-type (WT) WNK4 to mimic a state of WNK3 excess. The results provide a mechanistic explanation for the divergent effects of WT and FHHt-mutant WNK4 on NCC activity, and for the dominant nature of FHHt in humans and genetically modified mice.
Introduction
Hypertension affects 50 million Americans, contributing importantly to myocardial infarction, kidney failure, and stroke. Its pathogenesis is multifactorial, but a large genetic component has long been recognized. Physiological studies suggested that renal processes play a central role in its pathogenesis, a suggestion that has been confirmed by the discovery that nearly all monogenic causes of human hypertension involve genes acting predominantly in the kidney (1). Treatment of hypertension remains largely empiric, but a consistent feature of the pharmacological approach during the past 50 years has been the central role of thiazide diuretics, drugs frequently recommended as first-line agents (2). These drugs were developed without an understanding of their mechanism of action, but it is now recognized that they are specific antagonists of an Na-Cl cotransporter (thiazide-sensitive Na-Cl cotransporter [NCC]; SLC12A3) that is expressed exclusively along a short segment of mammalian nephron, the distal convoluted tubule (DCT) (3). Recent discoveries are focusing new attention on the important role of the DCT and NCC in regulating sodium, chloride, and potassium excretion rates and in controlling human blood pressure (4, 5). These advances include the discovery that one form of monogenic human hypertension, familial hyperkalemic hypertension (FHHt; OMIM 145260; also called pseudohypoaldosteronism type II or Gordon syndrome), can be caused by activation of NCC within the DCT (6, 7).
FHHt can be caused by mutations in members of the WNK kinase family (8). The WNK kinases constitute a branch of the human kinome in which a catalytic lysine is located in subdomain I, rather than subdomain II, where it is located in other serine/threonine kinases (9); for this reason, these kinases were named WNK (with no lysine [K]). Among mammals, 4 members have been identified (10), all of which contain a short amino-terminal domain, a highly conserved serine/threonine kinase domain, and a long carboxyl terminus. Although the carboxyl termini of WNK kinases are not highly conserved among members, all include a putative autoinhibitory domain and at least 2 coiled-coil domains (11, 12). Functional effects of WNK kinases are still being elucidated, but the proteins are expressed in many organs and tissues, and deletion of WNK1 is lethal in utero (13), emphasizing that these proteins play essential roles in mammalian physiology and development.
Mutations of WNK1 and WNK4 cause FHHt. One of the cardinal manifestations of FHHt is increased sensitivity to thiazide diuretics (14), suggesting that WNK kinases regulate the NCC. WNK4 was shown to inhibit NCC activity by reducing NCC abundance at the plasma membrane of Xenopus oocytes and mammalian cells (15–19). WNK1 indirectly regulates NCC by inhibiting WNK4, providing support for the hypothesis that a predominant mechanism by which WNK kinases control blood pressure and electrolyte balance is via effects on NCC. Recently, this hypothesis was strongly confirmed in experimental animals made transgenic for wild-type and FHHt-mutant WNK4 (6) and in animals with FHHt-mutant WNK4 knocked in (7). Expression of the wild-type WNK4 transgene reduced blood pressure and serum potassium concentration; expression of the FHHt mutant WNK4 transgene (differing by only a single amino acid residue, Q562E, or D561A) elevated arterial pressure and serum potassium concentration. The mechanism responsible for this switch of WNK4 effects on NCC from inhibitory to stimulatory remains undetermined, but the results emphasize that the WNK4-induced FHHt phenotype results predominantly from activation of NCC in the DCT.
WNK3 is a third member of the WNK kinase family that is expressed by cells of the DCT and modulates NCC activity (11, 20). In contrast to effects of other WNK kinases on transport proteins, most of which are inhibitory, the WNK3 effect on NCC is stimulatory (20). We have reported previously that WNK1, WNK4, and KS-WNK1, a kidney-specific splice form of WNK1, form protein complexes and interact functionally (15, 21–23). WNK1 can inhibit the effects of WNK4 on NCC, and KS-WNK1 can inhibit the effects of WNK1 on WNK4 and, indirectly, NCC. These results suggest that WNK kinases do not act alone, but instead constitute a signaling complex in the aldosterone-sensitive distal nephron (ASDN). Here, we show that WNK3 is part of this signaling complex and that interactions between WNK3 and WNK4 enhance regulatory gain, permitting robust regulation of NCC activity, based on physiological need. We also show that FHHt-mutant WNK4 is a dominant-negative regulator of wild-type WNK4 effects on WNK3. These results provide a model to explain the surprising ability of a single amino acid substitution within WNK4 to convert its effects on NCC from inhibitory to stimulatory.
Results
WNK3 stimulates NCC activity via its carboxyterminal domain.
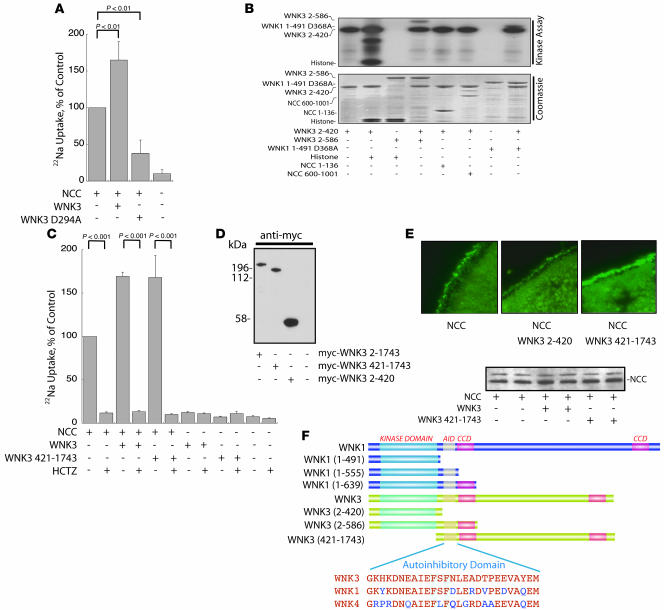
Kinase-active WNK3 has been reported to stimulate NCC activity, whereas kinase-dead WNK3 has been reported to inhibit it (20). We confirmed these results by expressing full-length and kinase-dead WNK3 with NCC in Xenopus oocytes and measuring sodium uptake. The results (Figure 1A) suggest that the kinase activity of WNK3 is essential for its effects on NCC and raise the possibility that WNK3 might phosphorylate NCC directly. We tested whether WNK3 exhibits kinase activity in vitro. WNK3 2–420 phosphorylated itself (autophosphorylation) and the substrate protein, histone (Figure 1B). We could not, however, detect any phosphorylation of either the amino-terminal or the carboxyterminal cytoplasmic domains of NCC by WNK3 2–420 (Figure 1B).
Figure 1. WNK3 stimulates but does not phosphorylate NCC.
(A) Full-length WNK3-stimulated Na uptake by Xenopus oocytes (expressed as percentage of Na uptake by oocytes injected with NCC alone), whereas kinase-dead WNK3 D294A downregulated Na uptake, compared with NCC alone. n = 4. (B) GST-WNK3 2–420 phosphorylated itself and the substrate histone, whereas GST-WNK3 2–586 was inactive. WNK3 2–420 also phosphorylated the kinase-inactive GST-WNK3 2–586 and GST-WNK1 1–491 D368A. WNK3 did not phosphorylate NCC, within either the N terminus (GST-NCC 1–136) or the C terminus (GST-NCC 600-1,001). Top: kinase assay; bottom: Coomassie-stained gel. Results are representative of experiments performed in triplicate. (C) The C terminus of WNK3 (WNK3 421–1,743) increased NCC activity in a manner similar to full-length WNK3. The NCC stimulation was inhibited by hydrochlorothiazide (HCTZ). Neither WNK3 alone nor WNK3 421–1,743 alone stimulated Na uptake in the absence of NCC. WNK3 2–420 had no effect on NCC activity (data not shown); n = 5. (D) WNK3 constructs were all expressed at the protein level (Western blot of Xenopus oocyte lysate). (E) WNK3 421–1,743, but not the kinase domain, WNK3 2–420, increased the abundance of NCC at the plasma membrane of oocytes, as detected by immunocytochemistry (results are representative of experiments performed in triplicate). Original magnification, ×400. Western blot of oocyte lysate showed no effect on total NCC. (F) Comparison of the domain structure of WNK1 and constructs employed in the present experiments. AID, autoinhibitory domain; CCD, coiled-coil domain. Sequences of autoinhibitory domains of WNK kinases are compared. Key phenylalanines shown to be essential for autoinhibition of WNK1 (24) are highlighted in blue.
WNK1 contains an autoinhibitory domain, just C-terminal to its kinase domain (24), which inhibits its activity. Thus, the construct WNK1 1–555 is relatively kinase inactive, in contrast to the shorter construct WNK1 1–491 (21, 24). A longer construct, WNK1 1–639, containing additional amino acid residues, including a coiled-coil domain, however, does exhibit kinase activity. This indicates that the WNK1 region between 555 and 639 represses the autoinhibitory domain (24). To test whether WNK3 contains a similar domain structure, we expressed the longer construct glutathione S transferase–WNK3 (GST-WNK3) 2–586, which contains both the putative autoinhibitory domain and the adjacent and coiled-coil domain (see schematic, Figure 1F). This construct did not demonstrate any detectable kinase activity (Figure 1B), indicating that the WNK3 coiled-coil domain does not repress the autoinhibitory domain. WNK3 2–420 also phosphorylated a kinase-dead WNK1 (WNK1 1–491 D368A) and the kinase-inactive WNK3 2–586, indicating that WNK3 is capable of phosphorylating other members of the WNK family and cross-phosphorylating WNK3 (Figure 1B).
Because the actions of WNK3 on NCC appeared to be dependent functionally on WNK3 kinase activity (Figure 1A and ref. 20), we used a deletion strategy to test whether the kinase domain of WNK3 can stimulate NCC. Surprisingly, the WNK3 kinase domain (WNK3 2–420), a construct that exhibits full kinase activity in vitro (Figure 1B), had no effect on NCC activity (uptake: 102% ± 15% of NCC alone; P = NS). In contrast, the carboxyl terminus, WNK3 421–1,743, a construct devoid of kinase activity, had the same stimulatory effect on NCC as full-length WNK3 (Figure 1C). The ability of WNK3 to stimulate NCC was fully inhibited by hydrochlorothiazide (HCTZ), indicating that it results from increased NCC activity. In addition, neither WNK3 nor the amino or carboxyl terminal WNK3 constructs stimulated Na uptake in the absence of NCC, further confirming that the observed stimulation is dependent entirely on NCC activation. All of the WNK3 constructs were expressed at the protein level, indicating that differential WNK3 protein expression does not explain these results (Figure 1D).
We confirmed that the effects on NCC activity were associated with parallel changes in NCC surface abundance (20), as documented by immunofluorescence of fixed oocytes (Figure 1E). There was no detectable effect of WNK3 on total abundance (Figure 1E).
WNK3 and WNK1 phosphorylate WNK4.
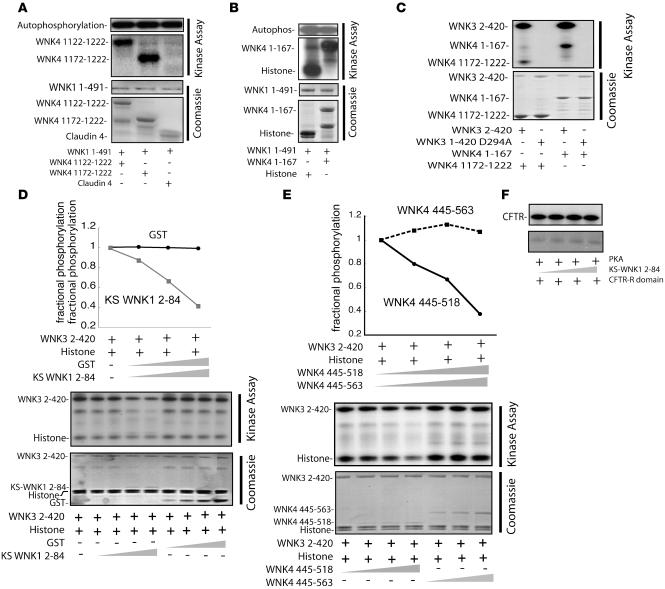
In view of the evidence that the effects of WNK3 on NCC are separable from the activity of WNK3 as a kinase, we were interested in seeking the molecular basis of WNK kinase interactions. Previously, we showed that WNK1 and WNK4 interact to regulate NCC activity (15, 21, 22), and Cobb and colleagues showed that WNK1 can phosphorylate WNK4 within the kinase domain (25). Here, we tested whether WNK1 could phosphorylate WNK4 outside of the kinase domain. Figure 2, A and B, shows that WNK1 is capable of phosphorylating both the carboxy- (WNK4 1,172–1,222) and amino-terminal domains (WNK4 1–167). Interestingly, WNK1 was not capable of phosphorylating claudin 4 (Figure 2A). We then tested whether WNK3 exhibits similar activity. Figure 2C shows that WNK3 2–420 phosphorylated both the amino- and carboxyl termini of WNK4 in vitro. Thus, both WNK1 and WNK3 are capable of phosphorylating WNK4 in vitro.
Figure 2. WNK kinase and inhibitory activity.
(A) WNK1 1–491 phosphorylated the WNK4 carboxyl terminus, but not claudin 4. (B) WNK1 1–491 phosphorylated histone and the amino-terminal domain of WNK4 (WNK4 1–167). (C) WNK3 2–420, but not WNK3 2–420 D294A, phosphorylated both the amino and carboxyl termini of WNK4. Results are representative of 5 identical experiments. (D) KS-WNK1 2–84 inhibited WNK3 kinase activity in a dose-dependent manner, whereas GST alone had no effect. Results are representative of experiments performed in triplicate. (E) GST-WNK4 445–518 inhibited WNK3 phosphorylation of itself and of histone in a dose-dependent manner. WNK4 445-563, which extends beyond the autoinhibitory domain, had no effect. Results are representative of experiments performed in triplicate. (F) KS-WNK1 2–84 did not inhibit cAMP-activated PKA activity, as detected by phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) R domain.
KS-WNK1 and WNK4 inhibit WNK3 kinase activity.
We reported previously that KS-WNK1, a kidney-specific kinase-deficient isoform of WNK1, exerts a dominant-negative effect on WNK1 (23). KS-WNK1 also inhibits WNK1 effects on the renal outer medullary K channel (ROMK; Kir 1.1) (26, 27), suggesting that this is a general action of KS-WNK1. We also reported that the autoinhibitory domain of WNK4 suppresses WNK1 kinase activity in vitro (21), providing evidence that WNK kinases form a regulatory complex. Here, we tested whether KS-WNK1 and WNK4 could inhibit WNK3 kinase activity, as they do for WNK1. The results (Figure 2D) showed that the unique KS-WNK1 amino-terminal domain (KS-WNK1 2–84) inhibited WNK3 kinase activity in a dose-dependent manner. We (21) and others (25) have shown previously that the WNK4 autoinhibitory domain suppresses the kinase activity of WNK1. Here, we show that the WNK3 kinase activity can also be suppressed by the WNK4 autoinhibitory domain (WNK4 445–518) (Figure 2E). A longer WNK4 that does not possess autoinhibitory activity did not inhibit WNK3 kinase activity (Figure 2E). These inhibitor effects are specific for WNK3, because KS-WNK1 2–84 does not inhibit cAMP-dependent PKA activity (Figure 2F). The results provide further evidence that WNK kinase functional domains regulate not only themselves but other members of the WNK kinase family as a signaling complex.
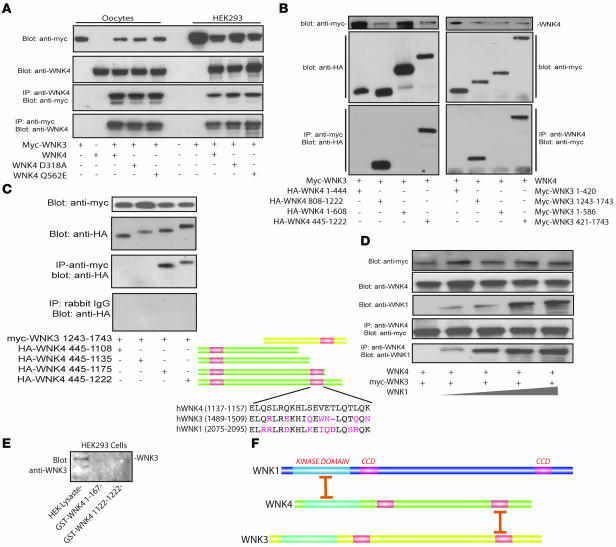
WNK3 associates with WNK4 in a protein complex.
WNK1 has been reported to form homotetramers (25). We reported previously that WNK1 and WNK4 form protein complexes when expressed in oocytes (22), suggesting that heteromeric complexes may also occur. WNK1, WNK4, and WNK3 are all expressed by DCT cells (20, 28). Here, we tested whether WNK3 and WNK4 associate in a protein complex. Figure 3A shows that anti-myc precipitated WNK4 and that anti-WNK4 can precipitate myc-WNK3 when the proteins are expressed in Xenopus oocytes or in a mammalian cell line (HEK293t). A mutant WNK4 that causes FHHt (WNK4 Q562E) retains its ability to associate with WNK3, as does kinase-dead WNK4 D318A. Surprisingly, WNK3 and WNK4 interact via their carboxyl termini (Figure 3B), whereas WNK1 and WNK4 interact via their amino-terminal domains (22).
Figure 3. WNK3 associates with WNK4 and WNK1.
(A) WNK3 associated with WNK4, FHHt mutant WNK4 (WNK4 Q562E), and kinase-dead WNK4 (D318A) in Xenopus oocytes and HEK293t cells. (B) WNK3 and WNK4 associate within their C termini. Left: myc-WNK3 expressed with HA-tagged fragments of WNK4. Anti-myc antibody precipitated only WNK4 fragments that included the carboxyl terminal domain (WNK4 445–1,222 and 808–1,222). Right: WNK4 precipitated only WNK3 fragments that contain the carboxyl terminal domain (WNK3 421–1,743 and 1,243–1,743). (C) Identification of the WNK4 region involved in interaction with WNK3. Progressive truncation of the WNK4 carboxyl terminus identified a region between residues 1,135 and 1,175 as essential for interaction. This region encompasses the second WNK4 coiled-coil domain, as shown schematically. The coiled-coil domains of WNK1, -3, and -4 are compared. (D) WNK1, WNK3, and WNK4 formed protein complexes in Xenopus oocytes. Myc-WNK3 and WNK4 were expressed with increasing amounts of WNK1 cRNA. The WNK3/WNK4 expression ratio was 1:1. The WNK1/WNK3 and WNK1/WNK4 ratios were 0.25:1 to 2:1. Lysates were precipitated using an anti-WNK4 antibody and detected using anti-myc and anti-WNK1. Increasing expression of WNK1 did not dissociate the complex. (E) GST-WNK4 1,122–1,222, but not GST-WNK4 1–167, pulls down endogenous WNK3 from HEK293 cells. Endogenous WNK3 is present in cell lysate. Results are representative of experiments performed in triplicate. (F) Schematic comparing sites of association between WNK4 with WNK3 (these studies) and WNK4 with WNK1 (22).
Coiled-coil domains are protein-protein interaction motifs. We tested whether WNK4 coiled-coil domains are involved in the association with WNK3. Figure 3C shows that deleting amino acid residues between 1,136 and 1,174 (containing the second coiled-coil domain) abrogates the ability of WNK4 to interact with WNK3. These results indicate that the WNK4 amino acid residues that interact with WNK3 are within, or immediately adjacent to, the second coiled-coil domain (Figure 3C). The results of transfection experiments may not mimic protein interactions that occur in situ. We therefore tested whether GST-WNK4 could pull down endogenous WNK3 from HEK293 cells. Figure 3D shows the carboxyl terminus of WNK4 pulled down endogenous WNK3 from HEK cells; in contrast, the amino terminus did not.
WNK1 forms a protein complex with WNK4 (22). We tested whether WNK1 might compete with WNK3 for WNK4 interaction, but expressing WNK1 did not decrease the ability of WNK4 to immunoprecipitate WNK3 (Figure 3E). Figure 3F shows schematically that WNK4 interacts with both WNK1 and WNK3, but via different domains.
WNK3 and WNK4 compete to regulate NCC.
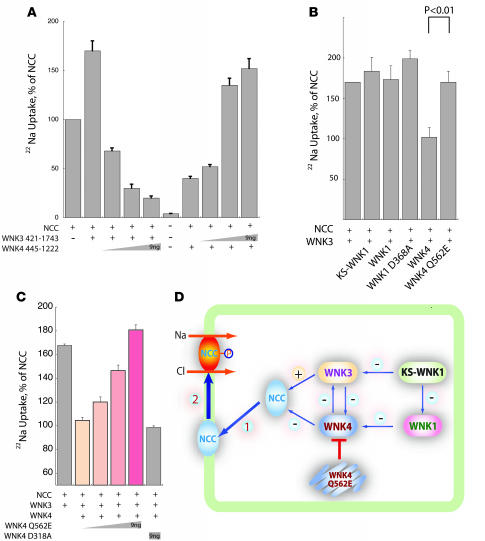
In view of the fact that WNK3 and WNK4 form protein complexes, and because they exert opposite effects on NCC trafficking and activity, we tested whether WNK kinases interact functionally, with respect to NCC. We confirmed that the carboxyl terminus of WNK3 (WNK3 421–1,743) stimulates NCC activity, as shown above. We also confirmed our previous report (22) that the carboxyl terminus of WNK4 (WNK4 445–1,222) inhibits NCC activity when coexpressed in Xenopus oocytes. Interestingly, WNK4 445–1,222 inhibited WNK3’s stimulatory effect on NCC in a dose-dependent manner (Figure 4A). Similarly, WNK3 421–1,743 inhibited WNK4’s effect on NCC in a dose-dependent manner (Figure 4A). Note that the range of NCC activity is greater when WNK3 and WNK4 are expressed together than when either WNK3 or WNK4 is expressed alone.
Figure 4. WNK3 and WNK4 compete to regulate NCC.
(A) Increasing WNK4 445–1,222 cRNA reverses WNK3 421–1,743–mediated NCC stimulation, whereas WNK3 overcame WNK4-mediated NCC inhibition. Note that the amplitude of NCC activity, when regulated by both WNK3 and WNK4, was considerably greater than when regulated by either WNK3 or WNK4 alone. Injected amounts were: 3 ng NCC, 0–9 ng WNK3, 0–9 ng WNK4; n = 6. (B) WNK4, but not FHHt mutant WNK4 (WNK4 Q562E), attenuated stimulation of NCC activity by WNK3. KS-WNK1, full-length WNK1, and kinase-dead WNK1 had no effect on WNK3-mediated NCC stimulation. n = 4. (C) FHHt-mutant WNK4 Q562E, but not kinase-dead WNK4 D318A, inhibited wild-type WNK4 inhibition of WNK3 effects on NCC. Amounts of injected WNK4 are shown. n = 3. (D) The current results show that NCC abundance at the plasma membrane (step 1) is determined by WNK3 and WNK4 directly and by interaction between WNK3 and WNK4. FHHt-mutant WNK4 Q562E acts as a dominant-negative regulator of WNK4 actions on WNK3. WNK1 also interacts with WNK4 to regulate NCC and in turn is regulated by KS-WNK1. KS-WNK1 also inhibits WNK3 kinase activity, although it is not clear whether this affects WNK3 actions on NCC. As noted in the text, NCC is also activated by phosphorylation by unknown kinases (step 2). WNK kinases may be involved in this process. +, positive regulation; –, negative regulation.
WNK4 mutations that cause FHHt exhibit some loss of their ability to inhibit NCC activity in oocytes (15–17, 29). Yet a simple loss of WNK4 action on NCC cannot explain the FHHt phenotype. Animals transgenic for FHHt-mutant WNK4 exhibit increased NCC activity, despite the presence of 2 wild-type WNK4 alleles (6); this result is not compatible with simple loss of function with respect to NCC. In view of the data indicating that WNK4 also regulates NCC activity through effects on WNK3, we tested the hypothesis that FHHt-causing WNK4 mutations affect the interaction between WNK4 and WNK3. Figure 4B shows that, unlike WNK4, KS-WNK1, WNK1, and kinase-dead WNK1 (WNK1 D368A) did not affect WNK3-mediated NCC stimulation. In contrast to wild-type WNK4, however, the FHHt-causing WNK4 Q562E completely lacked the ability to suppress WNK3 activation of NCC.
As noted, FHHt is inherited in an autosomal dominant manner; this is not compatible with a simple loss of WNK4 function. We therefore tested the hypothesis that FHHt-mutant WNK4 inhibits wild-type WNK4 actions on WNK3. Figure 4C shows that FHHt-mutant WNK4 Q562E inhibited WNK4-mediated suppression of WNK3 effects on NCC in a dose-dependent manner; these results indicate that FHHt-mutant WNK4 is a dominant-negative inhibitor of wild-type WNK4.
Discussion
WNK kinases constitute a unique branch of the human kinome that modulates electrolyte transport across plasma membranes and epithelia (12, 30). WNK kinases also affect cell growth and apoptosis (31, 32) and are essential for normal embryonic development (13). Despite their widespread expression, human mutations in 2 WNK kinases, WNK1 and WNK4, affect predominantly renal electrolyte transport, causing the monogenic disease FHHt (8). For this reason, efforts have been directed toward identifying effects of WNK kinases on ion transport pathways, especially ion transport pathways expressed in renal cells, using in vitro approaches (reviewed in refs. 12, 30, 33, 34) and genetic and physiological manipulation (6, 13). WNK4 inhibits NCC activity in Xenopus oocytes (15, 17, 35), mammalian cells (18, 19), and mammalian kidney tubules (6). WNK kinases, however, also interact to regulate each other. We showed previously that WNK1 inhibits WNK4 actions on NCC (15, 21, 22), suggesting that WNK kinases form a negative-regulatory signaling cascade (36). We later showed that the effects of WNK1 on WNK4 are kinase activity–dependent and that WNK1 and WNK4 can form protein complexes (21, 22). We also showed that KS-WNK1, a kidney-specific WNK1 isoform, inhibits the kinase activity of WNK1 (23), thereby acting as a dominant-negative WNK1 regulator. These results suggest that WNK kinases not only modulate transport pathways directly, but also constitute a signaling complex. Here, we report that WNK3, another WNK kinase expressed by cells of the ASDN (20, 37), is an important constituent of this regulatory complex, modulating the effects of WNK1 and WNK4 on NCC activity. The results provide novel insights into the mechanisms by which Na, K, and Cl excretion rates are regulated, both physiologically and in the setting of human disease.
The present results confirm work showing that WNK3 stimulates NCC activity when coexpressed in Xenopus oocytes (20). We also confirm that this effect is mediated, at least in part, by increased NCC abundance at the plasma membrane (Figure 1), although it is not yet clear whether this reflects enhanced NCC trafficking to the plasma membrane or retarded removal. We also confirm that a kinase-defective WNK3 mutant has effects on NCC activity that are opposite to those of the wild-type kinase (20). NCC is regulated by phosphorylation of 3 amino acid residues (T53, T58, S71) within its amino-terminal cytoplasmic domain (38). Based on the activity of WNK3 as a kinase (Figure 1 and ref. 30), and the contrasting effects of kinase-active and kinase-dead WNK3, it is tempting to speculate that the stimulatory effects of WNK3 on NCC activity result from phosphorylation of the transport protein. It is surprising, therefore, that the carboxyl terminus of WNK3 is capable of stimulating NCC. This does not exclude a role for WNK3 kinase activity or NCC phosphorylation in the effects of WNK3, but other observations suggest that WNK3 has effects that are mechanistically distinct. NCC activation in response to hypotonic stimuli involves amino-terminal phosphorylation (38); such stimulation does not correspond to changes in NCC abundance at the plasma membrane, suggesting that it involves activation of proteins already present (38). In contrast, the effects of WNK3 on NCC activity (Figure 1 and ref. 20) do correlate with changes in NCC abundance at the plasma membrane. Coupled with the observation that WNK3 does not directly phosphorylate NCC in vitro (Figure 1B), this suggests that NCC can be activated via 2 mechanisms: (a) increased abundance at the plasma membrane (presumably via protein trafficking; step 1 in Figure 4D); and (b) allosteric activation of existing NCC proteins (presumably via phosphorylation; step 2 in Figure 4D).
A puzzling aspect of some recent work on WNK kinases is the role played by phosphorylation events in transducing WNK-dependent signaling, inasmuch as effects of WNK kinases have been variably reported to be dependent or independent of kinase activity (15, 16, 26, 27, 39). When point mutations in WNK1, WNK3, and WNK4 that disrupt kinase activity (WNK1 D368A, WNK3 D294A, and WNK4 D318A) abrogate functional effects of these proteins, it has often been inferred that WNK actions are kinase activity–dependent. While it is clear that these point mutations generate proteins that are devoid of kinase activity in vitro (9, 20, 40), work using truncated WNK constructs has sometimes led to different conclusions. To date, evidence suggests that WNK kinases do not phosphorylate NCC, ROMK, or Na-K-2Cl cotransporter (NKCC1) directly in vitro, even though they may affect the phosphorylation status in cells. The ability of WNK3 to stimulate NCC and the ability of WNK4 to inhibit NCC do not appear to depend on WNK kinase activity, as the carboxyl terminus of each kinase, without the associated kinase domain, generates effects similar to those of the full-length protein. This suggests that one mechanism by which WNKs regulate NCC is via their effects as scaffolds or adaptor proteins that regulate protein trafficking. On the other hand, WNK3 has been shown to enhance phosphorylation of amino-terminal residues that are conserved among the cation chloride cotransporters (20). As noted above, this raises the possibility that WNK kinases also regulate NCC activity by phosphorylation events; this hypothesis will require further investigation.
Cobb and colleagues observed that WNK1 can form tetrameric complexes in kidney cells (9, 25). The present results, showing that WNK3 and WNK4 form protein complexes in mammalian kidney cells and Xenopus oocytes (Figure 3A), suggest that the formation of WNK kinase complexes containing multiple, but perhaps varying, ratios of individual WNK kinases, may explain the variable and sometimes discrepant effects of WNKs observed in experimental work. For example, the current work indicates that WNK3 and WNK4 associate in protein complexes and inhibit each other’s effects on NCC activity, as shown schematically in Figure 4D. Both WNK3 and WNK4 are expressed with NCC by cells of the distal tubule, where WNK4 abundance is increased by dietary potassium loading (20, 28). An increased WNK4 abundance should increase the molar ratio of WNK4 to WNK3, thereby inhibiting NCC activity both directly (via WNK4 effects on NCC) and indirectly (via WNK4 effects on WNK3; see Figure 4D).
An important consequence of the present results is that the formation of WNK kinase complexes increases the regulatory signal gain with respect to NCC. Results presented in Figure 4A indicate that the coexpression of WNK3 with WNK4 significantly increases the range of NCC activity, from negligible levels (when WNK4 greatly exceeds WNK3) to highly stimulated levels (when WNK3 greatly exceeds WNK4). This range is considerably higher than when NCC is expressed with either WNK3 or WNK4 alone. According to this model, the WNK kinase complex acts as a rheostat-controlled amplifier, able to achieve graded regulation of NCC activity according to physiological need.
The proposed WNK kinase signaling complex also suggests a resolution to a persistent paradox concerning WNK kinases and the molecular pathogenesis of FHHt. Several, though not all (19), groups have reported that FHHt-causing WNK4 mutant proteins completely (16–18) or partially (15) lose their ability to suppress NCC activity. Yet FHHt is inherited as an autosomal dominant disease of increased NCC activity (12) and is reproduced phenotypically when mice express WNK4 Q562E on a wild-type background (6). Simple loss of WNK4 function with respect to NCC does not explain these results. In contrast, the observation that WNK4 Q562E loses it ability to inhibit WNK3 (but still associates with WNK3 in a protein complex) suggests a possible resolution. Figure 4C shows that FHHt-mutant WNK4 is a dominant-negative WNK4 inhibitor. In a patient or animal in which FHHt-mutant WNK4 is present, the mutant protein would inhibit wild-type WNK4 activity, leaving WNK3 unopposed to stimulate NCC. In contrast, in the setting of increased wild-type WNK4 protein abundance (as seen in animals transgenic for wild-type WNK4), the enhanced WNK3-inhibiting effects of WNK4 would contribute to the Gitelman-like phenotype (6). Thus, our results provide a novel model to explain the opposite effects of wild-type and mutant WNK4 on NCC activity. Further studies will be required to test this model in more physiological model systems.
WNK4 has also been postulated to act as a molecular switch (41, 42), because it can inhibit NCC activity in its native state but loses this ability in human disease. It was postulated that an unidentified ligand mimics the switching activity that occurs when FHHt mutations are introduced (41, 42). A similar hypothesis has been proposed to explain the physiological role of WNK3. In this case, the switch in functional activity occurs when the kinase-active WNK3 is mutated to make it kinase inactive. This too has been suggested to mimic the effects of physiological perturbations, perhaps achieved by interaction with unidentified ligands (20). According to the WNK kinase complex model described here, WNK3 can bind to and inhibit the effects of WNK4, and WNK4 can bind to and inhibit the effects of WNK3. Thus, WNK4 may be a physiologically relevant ligand for WNK3 that switches its net effect from stimulation to inhibition; conversely, WNK3 may be a physiologically relevant ligand for WNK4, which switches its net effect from inhibition to stimulation. This model requires further direct testing but provides a plausible link between physiological processes and disease-related events.
Methods
DNA constructs.
Mouse WNK4, rat WNK1, and human WNK3 were generated previously (15, 21–23). Other constructs were generated by PCR, such as GST-claudin and GST-CFTR R-domain or by site-directed mutagenesis using the QuickChange kit (Stratagene). All sequences were confirmed by sequencing (Vollum Institute, Oregon Health & Science University).
Expression and purification of GST fusion proteins.
An overnight bacterial culture of E. coli strain BL21(DE3) transformed with the appropriate plasmid was diluted and grown at 37°C for 3 hours. Protein expression was induced by adding isopropyl-1-thio- d-galactopyranoside to a final concentration of 0.1–0.4 mM. The culture grew at 28°C for another 3–6 hours, and cells were harvested by centrifugation. The protocol for purification was modified from the manufacturer’s instructions for glutathione–Sepharose-4B beads and has been used by us extensively (22). Fractions were pooled and concentrated in a column with a molecular mass cutoff of 30 kDa and stored in aliquots containing 10% glycerol at –20°C until used.
GST pull-down assay.
Fifteen nanograms of GST or expressed GST-WNK4 N- and C-terminal constructs were immobilized on 20 μl of glutathione-agarose beads in 0.5 ml of PBS containing 0.1 mg/ml BSA. After washing 3 times with PBS, 50 mg of HEK293 cell lysate were incubated with the beads for 1 hour at 4°C. Beads were pelleted and washed 3 times with 50 mM Tris (pH 7.4), 0.3 M NaCl, and 0.1% Triton X-100. Precipitated proteins were separated by SDS-PAGE and Western blotted using rabbit anti-human WNK3 (a kind gift of Peter Jordan, Centro de Genética Humana, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon, Portugal; ref. 32)
In vitro phosphorylation assays.
Kinase reactions were carried out in 30 μl of 10 mM HEPES (pH 8.0), 10 mM MgCl2, 1 mM benzamidine, 1 mM dithiothreitol, and 50 μM cold ATP, with 0.22 mCi/ml [32P]ATP containing the active kinase and a substrate at 30°C for 30 minutes. Reactions were stopped by adding SDS sample buffer, followed by boiling for 2 minutes. Reactants (30 μl) were separated by SDS-PAGE on a 4%–12% Bis-Tris gradient gel. Phosphorylated products were visualized by autoradiography. The cAMP-dependent PKA kinase assay was performed according to the manufacturer’s protocol (New England Biolabs Inc.). Quantification of results was as described previously (23).
Immunofluorescence labeling of NCC expressed on oocyte surface.
Four days after injection, oocytes were fixed with 3% paraformaldehyde in PBS for 4 hours at 4°C. Six-micrometer-thick cryosections were placed on chrome-alum gelatin–coated glass slides, blocked with PBS buffer containing 0.1% BSA, and incubated with anti-mouse NCC, diluted in PBS buffer containing 0.1% BSA and 0.05% Tween-20 (1:100) at 4°C overnight. Sections were rinsed with PBS 4 times, incubated with FITC-conjugated goat anti-rabbit antibody IgG H+L (1:200; Zymed Laboratories Inc.) for 1 hour at 22°C, and rinsed with PBS buffer 4 times. The slides were then viewed on a Zeiss Axiophot phase-contrast microscope.
22Na uptake.
22Na uptake experiments were performed as described previously (15), with some modifications. Three days after injection of cRNA, oocytes were incubated in chloride-free medium for 3–4 hours at 18°C, before Na uptake was measured. DNA template (NCC, WNK1, KS-WNK1, WNK3, and WNK4) was linearized downstream from the 3′ untranslated region, using appropriate restriction enzymes, and transcribed using T7 RNA polymerase (mMESSAGE mMACHINE; Ambion). Sorted Xenopus oocytes were injected with 50 nl of water each, with 3–5 ng each of NCC, WNK3, WNK4, or various truncated constructs. Unless otherwise noted, the amount of NCC cRNA injected was 3 ng, and the WNK3 cRNA injected was 0.5–1 ng. For each experimental condition, 10–20 oocytes were injected. n, given in the figure legends, represents the number of independent experiments. All animal experiments were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Immunoblotting.
Immunoblots of oocytes were performed as described by our group previously (22, 23)
Immunoprecipitation.
Oocytes were prepared as described above for Western blotting. Primary antibody (2 μl) was added to oocyte homogenate (10 oocytes in 600 μl) and incubated at room temperature for 1 hour (or at 4°C overnight). Thirty microliters of protein A–sepharose (Amersham Biosciences) were added and incubated at 4°C overnight. The reaction mixture was then centrifuged, and the beads were washed 5 times in homogenizing buffer. Immunoprecipitated protein was eluted from the protein A–sepharose by heating the mixture to 100°C for 10 minutes with sample buffer containing 2.5% SDS and 2.5% β-mercaptoethanol. Immunoprecipitates were analyzed by SDS-PAGE and blotted, as described above.
Transient transfections and immunoprecipitation.
HEK293T cells (ATCC) were cultured at 37°C with 5% CO2 in Vitacell MEM supplemented with 2 mM streptomycin/penicillin and 10% FCS. Transient transfections were performed using SuperFect Transfection Reagent (QIAGEN) in accordance with the manufacturer’s instructions. After transfection, cells were incubated for 48–72 hours. Cells were treated with 100 mM NaCl for 10 minutes before harvest. Cells were then washed in cold PBS and lysed at 4°C in buffer (10 mM Tris HCl, pH 8.0, 2.5 mM MgCl2, 5 mM EGTA, pH 8.0, 0.5% Triton X-100, 1 mM Na3VO4, 50 mM NaF, and 100 μl protease inhibitor cocktail set III [Calbiochem] per 10 ml of buffer). Lysates were cleared by centrifugation, and the supernatant was used for immunoprecipitation. The protein concentrations were determined by the Bradford method (Bio-Rad). For each immunoprecipitation, extracts were incubated with 0.5 μg of mouse monoclonal anti-HA or anti-Myc (12CA5, Roche Diagnostics Corp.; or Sigma-Aldrich) or 0.5 μg of purified rabbit polyclonal anti-WNK1/WNK4 for 2 hours or overnight at 4°C. The immunocomplex was precipitated by incubating with 40 μl of 50% protein A–sepharose slurry (Amersham Biosciences) for 1 hour at 4°C. Immunoprecipitates were washed 3 times with PBS. Bound protein was eluted by boiling for 10 minutes in 2× SDS sample buffer. Immunoprecipitates were analyzed by SDS-PAGE and blotted as described above.
Statistics.
Group comparisons were by unpaired 2-tailed Student’s t test. When multiple comparisons were made, the Bonferroni correction was employed. A P value less than 0.05 was considered significant.
Acknowledgments
The authors thank Arohan Subramanya and James McCormick for helpful discussions and provision of constructs. The authors also acknowledge Sara Humphreys for technical assistance and Peter Jordan for the anti-WNK3 antibody. This work was supported by a grant from the NIH (DK51496).
Footnotes
Nonstandard abbreviations used: DCT, distal convoluted tubule; FHHt, familial hyperkalemic hypertension; GST, glutathione S transferase; NCC, thiazide-sensitive Na-Cl cotransporter; WNK, with no lysine (K).
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:3403–3411 (2007). doi:10.1172/JCI32033
See the related Commentary beginning on page 3179.
References
- 1.Lifton R.P., Gharavi A.G., Geller D.S. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian A.V., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 4.Reilly R.F., Ellison D.H. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 5.Meneton P., Loffing J., Warnock D.G. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am. J. Physiol. Renal Physiol. 2004;287:F593–F601. doi: 10.1152/ajprenal.00454.2003. [DOI] [PubMed] [Google Scholar]
- 6.Lalioti M.D., et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat. Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 7.Yang S.S., et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Wilson F.H., et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 9.Xu B., et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 10.Verissimo F., Jordan P. Sep 6. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- 11.Holden S., Cox J., Raymond F.L. Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3). Gene. 2004;335:109–119. doi: 10.1016/j.gene.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am. J. Physiol. Renal Physiol. 2005;288:F245–F252. doi: 10.1152/ajprenal.00311.2004. [DOI] [PubMed] [Google Scholar]
- 13.Zambrowicz B.P., et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayan H., et al. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J. Clin. Endocrinol. Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 15.Yang C.L., Angell J., Mitchell R., Ellison D.H. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Invest. 2003;111:1039–1045. doi: 10.1172/JCI200317443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson F.H., et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl. Acad. Sci. U. S. A. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golbang A.P., et al. A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension. 2005;46:295–300. doi: 10.1161/01.HYP.0000174326.96918.d6. [DOI] [PubMed] [Google Scholar]
- 18.Cai H., et al. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 19.Yang S.S., et al. Regulation of apical localization of the thiazide-sensitive NaCl cotransporter by WNK4 in polarized epithelial cells. Biochem. Biophys. Res. Commun. 2005;330:410–414. doi: 10.1016/j.bbrc.2005.02.172. [DOI] [PubMed] [Google Scholar]
- 20.Rinehart J., et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Yang C.L., Ellison D.H. Comparison of WNK4 and WNK1 kinase and inhibiting activities. Biochem. Biophys. Res. Commun. 2004;317:939–944. doi: 10.1016/j.bbrc.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 22.Yang C.L., Zhu X., Wang Z., Subramanya A.R., Ellison D.H. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J. Clin. Invest. 2005;115:1379–1387. doi: 10.1172/JCI200522452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanya A.R., Yang C.L., Zhu X., Ellison D.H. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am. J. Physiol. Renal Physiol. 2006;290:F619–F624. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- 24.Xu B.E., et al. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J. Biol. Chem. 2002;277:48456–48462. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- 25.Lenertz L.Y., et al. Properties of WNK1 and implications for other family members. J. Biol. Chem. 2005;280:26653–26658. doi: 10.1074/jbc.M502598200. [DOI] [PubMed] [Google Scholar]
- 26.Wade J.B., et al. WNK1 kinase isoform switch regulates renal potassium excretion. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8558–8563. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazrak A., Liu Z., Huang C.L. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Reilly M., et al. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J. Am. Soc. Nephrol. 2006;17:2402–2413. doi: 10.1681/ASN.2005111197. [DOI] [PubMed] [Google Scholar]
- 29.Cai H., et al. The surface expression of sodium chloride cotransporter (NCC) is down-regulated by WNK4 in mammalian cells. J. Am. Soc. Nephrol. 2004;15:541A. [Google Scholar]
- 30.Kahle K.T., et al. WNK protein kinases modulate cellular Cl- flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda). 2006;21:326–335. doi: 10.1152/physiol.00015.2006. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z.Y., et al. Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3-L1 cells. J. Biol. Chem. 2005;280:21622–21628. doi: 10.1074/jbc.M414464200. [DOI] [PubMed] [Google Scholar]
- 32.Verissimo F., Silva E., Morris J.D., Pepperkok R., Jordan P. Protein kinase WNK3 increases cell survival in a caspase-3-dependent pathway. Oncogene. 2006;25:4172–4182. doi: 10.1038/sj.onc.1209449. [DOI] [PubMed] [Google Scholar]
- 33.Kahle K.T., et al. WNK kinases: molecular regulators of integrated epithelial ion transport. Curr. Opin. Nephrol. Hypertens. 2004;13:557–562. doi: 10.1097/00041552-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Xie J., Craig L., Cobb M.H., Huang C.L. Role of with-no-lysine [K] kinases in the pathogenesis of Gordon’s syndrome. Pediatr. Nephrol. 2006;21:1231–1236. doi: 10.1007/s00467-006-0106-6. [DOI] [PubMed] [Google Scholar]
- 35.Wilson F.H., et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl. Acad. Sci. U. S. A. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossier B.C. Negative regulators of sodium transport in the kidney: key factors in understanding salt-sensitive hypertension? J. Clin. Invest. 2003;111:947–950. doi: 10.1172/JCI200318232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng Q., et al. WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkalaemia, regulates the K+ channel ROMK1 (Kir1.1). J. Physiol. 2006;571:275–286. doi: 10.1113/jphysiol.2005.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacheco-Alvarez D., et al. The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J. Biol. Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 39.Cope G., et al. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J. Am. Soc. Nephrol. . 2006;17:1867–1874. doi: 10.1681/ASN.2005111224. [DOI] [PubMed] [Google Scholar]
- 40.Vitari A.C., Deak M., Morrice N.A., Alessi D.R. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahle K.T., et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat. Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 42.Bindels R.J. A molecular switch controlling renal sodium and potassium excretion. Nat. Genet. 2003;35:302–303. doi: 10.1038/ng1203-302. [DOI] [PubMed] [Google Scholar]