Abstract
Nanodisks are nanoscale, disk-shaped phospholipid bilayers whose edge is stabilized by association of apolipoprotein molecules. Self assembled ND particles enriched with all-trans-retinoic acid (ATRA) [phospholipid:ATRA molar ratio = 5.5:1] were generated wherein all reaction components were solubilized. ATRA-ND migrated as a single band (Stokes’ diameter ∼20 nm) on native gradient polyacrylamide gel electrophoresis. ATRA, phospholipid and apolipoprotein co-eluted from a Sepharose 6B gel filtration column, consistent with stable integration of ATRA into the ND particle milieu. Spectroscopic analysis of ATRA-ND in buffer yielded an absorbance spectrum characteristic of ATRA. ATRA-ND mediated time-dependent inhibition of cultured HepG2 cell growth more effectively than free ATRA. The nanoscale size of the formulation particles and the stable integration of biologically active ATRA suggest ND represent a potentially useful vehicle for solubilization and in vivo delivery of ATRA.
Keywords: all-trans-retinoic acid, apolipoprotein, nanodisk, phospholipid, cell culture, drug delivery
Introduction
All-trans-retinoic acid (ATRA) is a naturally occurring vitamin A derivative that functions as a regulator of gene transcription (Umosono et al., 1988; Gianni et al., 2000; Duprez et al., 2003). ATRA interacts with members of the hormone receptor superfamily including the retinoic acid receptor (RAR) and the retinoid X receptor (RXR) (Sun and Lotan, 2002). ATRA binding to these transcription factors modulates their interaction with “retinoid response elements” on a wide range of genes that function in cell proliferation, differentiation and apoptosis. When ligand-activated, RAR-RXR heterodimers dissociate from co-repressor proteins they recruit co-activators that lead to chromatin decondensation and activation of gene transcription (Freedman, 1999; Sun and Lotan, 2002). Subsequently, pathways controlling growth, differentiation and cell death are activated.
Recognized as a potent pharmacological agent, ATRA has been used to treat various forms of cancer including leukemia, breast cancer and liver cancer (Freemantle et al., 2003; Arce et al., 2005). In the case of hepatomas, retinoid therapy has successfully reduced both primary and secondary malignancies. This effect may be attributed to a defect in retinoic acid metabolism in patients with hepatocarcinoma, many of whom have significantly lower levels of circulating retinol (Arce et al., 2005). At the same time, other cases may be related to RAR activation following integration of hepatitis B virus (Benbrook et al., 1988).
While beneficial as an anti-cancer agent, the use of ATRA is not without complications. ATRA is a water insoluble, toxic agent with limited bioavailability (Freemantle et al., 2003). Pharmacological levels can cause retinoic acid syndrome and neurotoxicity, particularly in children (Takitani et al., 2006). In addition, drug resistance has been reported in cases of sustained ATRA treatment requiring the use of additional cytotoxic chemotherapy (Freemantle et al., 2003). Although liposomal formulations were developed a number of years ago in an effort to address these issues, they have not progressed past the clinical trial stage (Estey et al., 2005). Despite this, the potential benefits of associating ATRA with a lipid-based carrier are many. Not only do lipid-drug formulations address solubility issues, they also decrease toxicity and potentially avoid triggering ATRA resistance, thereby minimizing the need for additional chemotherapy (Freemantle et al., 2003).
In the present study we show that significant quantities of ATRA can be stably incorporated into novel, nanometer scale, apolipoprotein stabilized phospholipid bilayer disk complexes, termed nanodisks (ND). ND associated ATRA is stably associated, fully soluble and retains potent biological activity, suggesting its potential for use as a delivery vehicle for this compound.
Materials and Methods
Materials
ATRA was obtained from Sigma Chemical Co. Dimyristoylphosphatidylcholine (DMPC) and dimyristoylphosphatidylglycerol (DMPG) were obtained from Avanti Polar Lipids Inc. Recombinant apolipoprotein E3 N-terminal domain (apoE3-NT) was expressed in E. coli and isolated as described previously (Fisher et al., 1997). HepG2 cells were purchased from ATTC.
ATRA-ND
Ten mg of a DMPC / DMPG mixture (7:3 weight ratio) were dissolved in chloroform / methanol (3:1 v/v) and dried under a stream of N2 gas, forming a thin film on the vessel wall. Residual organic solvent was removed under vacuum. The prepared lipids were then dispersed in 1 ml phosphate buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.0) by vortexing and intermittent heat (37 °C). Subsequently, 0.8 mg ATRA was added from a 30 mg/ml stock solution in dimethylsulfoxide (DMSO). Following this, 4 mg apoE3-NT (3–4 mg/ml in PBS) was added and the solution (2.0 ml final volume) subjected to bath sonication under a N2 atmosphere, with the sample temperature maintained between 22° C and 25 °C. After 1–4 h the turbid mixture became clear, indicating apolipoprotein / phospholipid complexes (i.e. ND) had formed. The solution was then dialyzed overnight to remove DMSO, followed by 0.22 μm filter sterilization.
Analytical procedures
Protein concentrations were determined by the bicinchoninic acid assay (Pierce Chemical Co.) with bovine serum albumin as standard. Choline containing phospholipids were quantified by enzyme based colorimetric assay (Nie et al., 1993). Nondenaturing polyacrylamide gel electrophoresis (PAGE) was performed on 4–20% acrylamide slab gels. Samples were electrophoresed at a constant 150 V for 20 h and stained with Coomassie Blue.
UV/Vis absorbance Spectroscopy
Absorbance spectroscopy was performed on a Perkin-Elmer Lambda 20 spectrometer at 20 °C. ATRA levels were determined using an extinction coefficient at 341 nm = 45,300 M−1 cm−1 in ethanol (Barua and Furr, 1998). Samples were scanned from 250 – 450 nm. Spectra of ND samples in aqueous media were obtained in PBS. Control spectra of “empty” ND that lack ATRA confirmed that other ND components do not interfere with or contribute to the spectral absorbance of ATRA between 300 and 450 nm.
Gel filtration
A sterile filtered sample (2 ml) of ATRA-ND was applied to a 75 ml bed volume Sepharose 6B (GE Biosciences) gel filtration column equilibrated in PBS. Following application of the sample, the column was eluted with collection of two ml fractions. Subsequently, each fraction was analyzed for protein, phospholipid and ATRA content as described above. In control experiments, empty ND gave rise to the same elution profile as ATRA-ND.
Cell growth inhibition assays
HepG2 human hepatoma cells were maintained in minimal essential media supplemented with 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 10% fetal bovine serum (FBS). Prior to experiments, 4 x 104 cells per well were seeded into 24 well culture plates with 2 ml complete media. After 24 h, the media was replaced with 4% FBS complete media. At 48 h, cells were washed, and provided fresh 4% FBS complete media in addition to specified concentrations of free ATRA (in ethanol) or ATRA-ND. 72 h and 120 h after administering the ATRA, cell viability was determined using the MTT assay (Mosmann, 1983). Values expressed are the mean ± S.D. (n = 3) percent cell viability relative to control.
Results and Discussion
ND are nanoscale (8 – 20 nm diameter) noncovalent assemblies organized as a disk-shaped phospholipid bilayer that is circumscribed by two or more amphipathic apolipoprotein molecules (Narayanaswami and Ryan, 2000). The bilayer portion of ND provides an environment capable of solubilizing and sequestering hydrophobic molecules and, as such, they can serve as bioactive agent delivery vehicles. Previous studies have shown that ND enriched with the water insoluble polyene antiobiotic, amphotericin B (Hargreaves et al., 2006) possess potent biological activity vitro and in vivo (Oda et al., 2006; Nelson et al., 2006). To further explore the utility of ND as a drug delivery vehicle, experiments were designed to incorporate the bioactive lipid ATRA into ND. The formulation strategy takes advantage of the unique ability of amphipathic apolipoproteins to transform specific phospholipid vesicle substrates into ND (Weers et al., 2001). By introducing ATRA into the reaction mix, it was hypothesized that a ternary ND particle, comprised of phospholipid, apoE3-NT and ATRA, would be generated. A general scheme describing ATRA-ND formation and particle structure is depicted in Figure 1.
Figure 1. ATRA-ND formulation scheme and structure model.
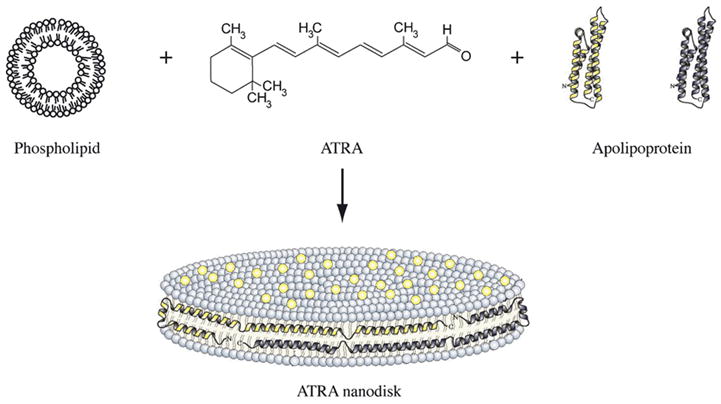
Phospholipid vesicles, ATRA and apoE3-NT (as a lipid-free 4-helix bundle; Wilson et al., 1991) were combined to form ATRA-ND. Lipid interaction converts apo E3-NT from a soluble 4-helix bundle into an open, extended lipid bound conformation (Narayanaswami et al., 2004). ATRA ND are comprised of a disk-shaped phospholipid bilayer in which ATRA molecules (yellow dots) are integrated. The edge of the ND bilayer is stabilized by the apolipoprotein “scaffold”. Component images are not to scale.
Formulation and characterization of ATRA-ND
When DMPC, DMPG, and ATRA (phospholipids:ATRA molar ratio = 5.5:1) were incubated with recombinant apolipoprotein at 24 °C with bath sonication, the sample changed from an opaque, turbid suspension to a clarified solution. No precipitate appeared upon centrifugation of the product solution indicating all of the reaction components had been solubilized. When the relative amount of ATRA in the starting mixture was increased, however, solution clarification was not complete and a precipitate appeared upon centrifugation. Dialysis of solubilized reaction product for 48 h resulted in loss of <15% of original ATRA. Thus, the solubilization efficiency of ATRA (the amount of ATRA in solution following centrifugation / ATRA added to the incubation x 100) ≥ 85 %. When considered in light of earlier studies with amphotericin B (Hargreaves et al., 2006; Oda et al., 2006), it is evident that ATRA has been stably incorporated into the ND particle matrix. Consistent with this interpretation, UV/Vis absorbance spectra of ATRA-ND displayed characteristic spectral features of ATRA including a single absorbance maximum at 341 nm (Figure 2), as reported by others (Szuts and Harosi, 1991). Furthermore, native gradient PAGE revealed a population of particles with a diameter of ∼20 nm (Figure 3). In other studies (Yamamoto and Ryan, 2007) it has been shown that lipid-free apoE3-NT migrates much further into the gel and is well separated from ND samples. In studies of ATRA-ND stability, no changes in electrophoretic properties were noted over the course of two week following storage of ATRA-ND at 4 °C in the dark. gel filtration chromatography of ATRA-ND, apolipoprotein, phospholipid and ATRA co-eluted, indicating stable association of ATRA with the ND particle (Figure 4). Given the stability of ATRA association, the relatively high amount of ATRA incorporated and the potent biological activity of this compound, studies were performed to evaluate the cell growth inhibition properties of ATRA-ND.
Figure 2. UV/visible absorbance spectra of ATRA.
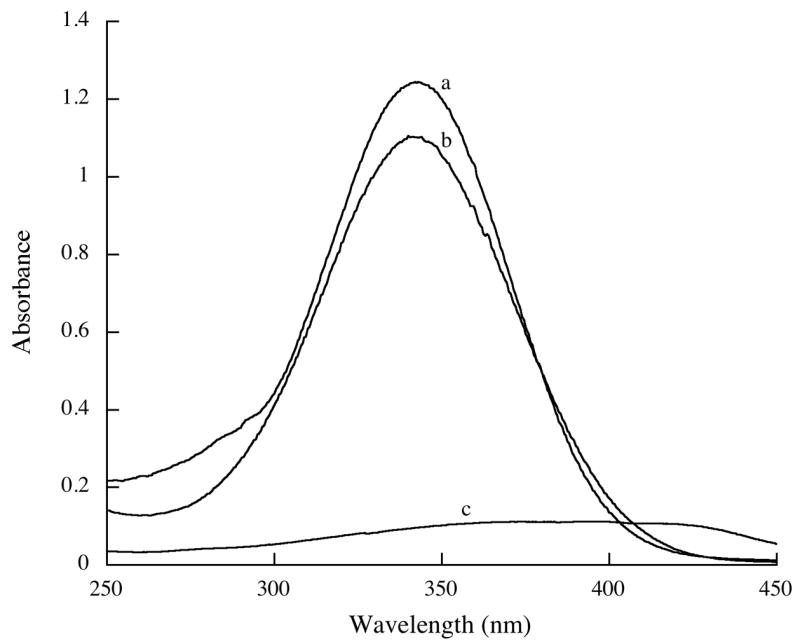
Scans were recorded from 250 – 450 nm. Samples included free ATRA (5 μg/ml ) in a) ethanol c) PBS and b) ATRA-ND in PBS.
Figure 3. Native PAGE of ND.
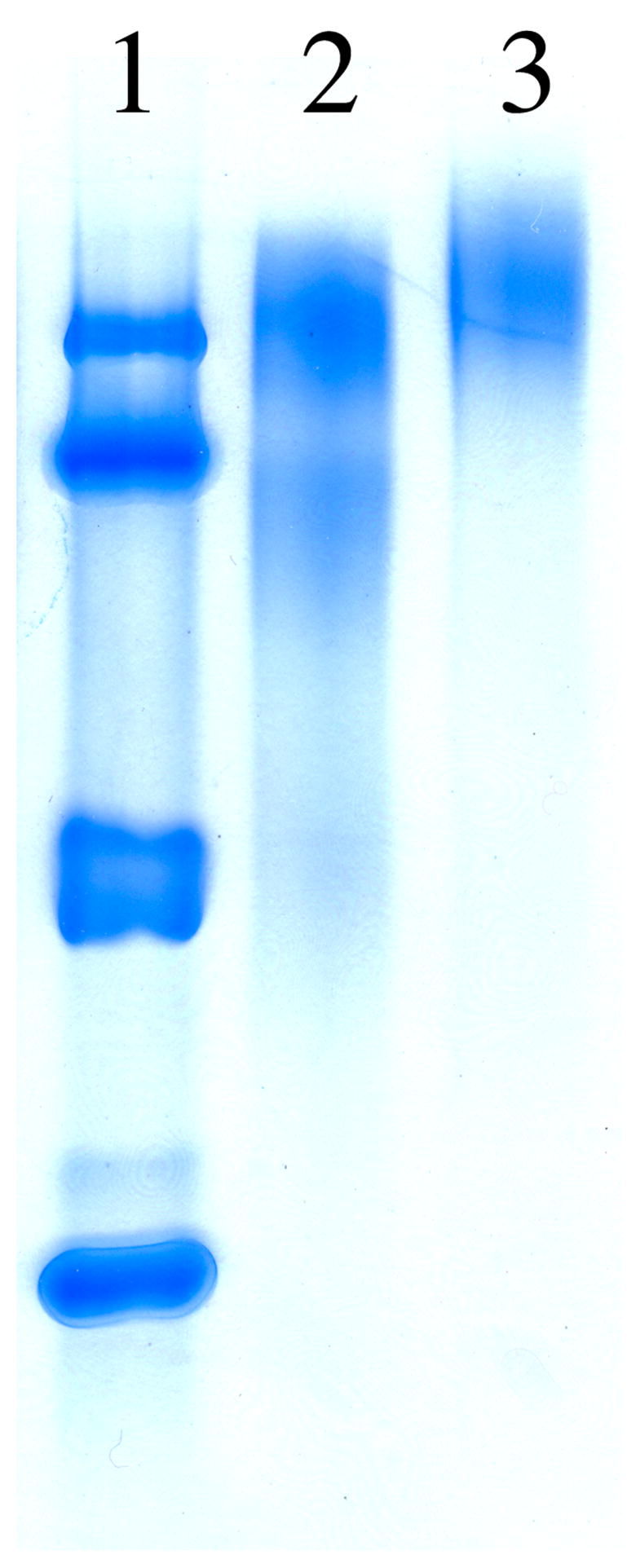
Lane 1) protein standards with known Stokes’ diameters [from top to bottom: thyroglobulin (17 nm), horse ferritin (12.2 nm), catalase (9.2 nm) and lactate dehydrogenase (8.2 nm)]; Lane 2) empty ND; Lane 3) ATRA-ND.
Figure 4. filtration chromatography of ATRA-ND.
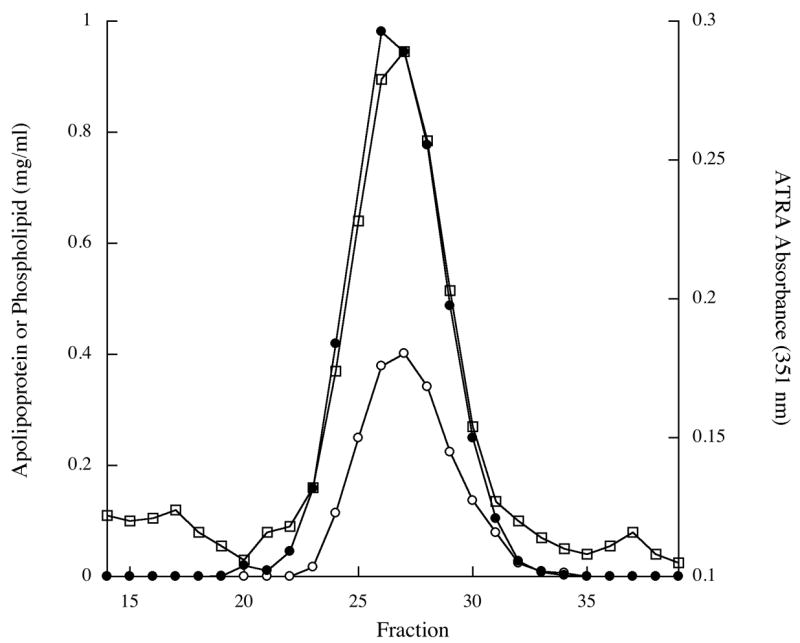
ATRA-ND were applied to a Sepharose 6B column equilibrated in PBS and eluted with collection of 2 ml fractions. The phospholipid (filled circles), protein (open circles) and ATRA (open squares) content in each fraction were determined as described in Material and Methods.
Cell viability assays
Cultured HepG2 human hepatoma cells were used in cell viability assays. Cells were exposed to culture media supplemented with buffer (control), free ATRA (dissolved in ethanol) or ATRA-ND (in PBS) and cell viability determined after 3 or 5 days (Figure 5). At both time points, ATRA-ND inhibited hepatoma cell growth more effectively than free ATRA. After 3 days cell viability was 43 % when treated with ATRA-ND compared to 77 % for cells treated with free ATRA. Cell viability after 5 days decreased to 37 % in the case of ATRA-ND and was 81 % in the case of free ATRA. The data suggest incorporation of ATRA into ND enhances its ability to inhibit HepG2 cell growth. At present it is not known if this effect may be attributed to facilitated entry of ND associated ATRA into the cells or whether other factors, such as increased ATRA solubility when presented as ND, improves its effectiveness. Given the possibility that lower doses of ATRA may be used to achieve the same pharmacological effect, further investigation of the in vivo efficacy of ATRA-ND is warranted.
Figure 5. Effect of ATRA on viability of cultured hepatoma cells.
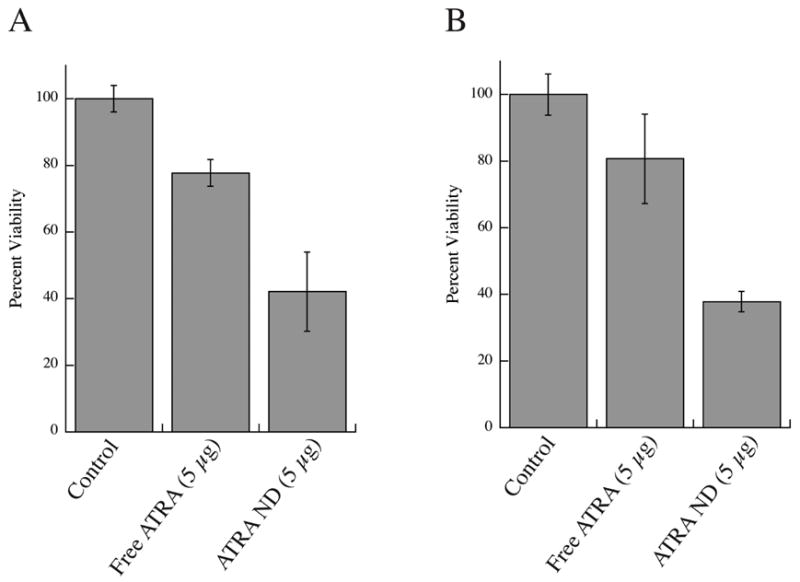
HepG2 cells were treated with buffer (control), free ATRA (5 μg/ml from ethanol stock solution) or ATRA-ND in PBS (5 μg ATRA/ml). After 72 h (panel A) and 120 h (panel B), cell viability was measured using the MTT assay. Values reported are the mean ± S.D. of 3 determinations.
The results presented indicate that incorporating ATRA into ND overcomes the poor water solubility of this bioactive lipid. The present results extend previous studies that showed amphotericin B-ND have efficacy in mice infected with the pathogenic fungus, Candida albicans (Oda et al., 2006) or the protozoal parasite Leishmania major (Nelson et al., 2006). Thus, it is conceivable that ND may provide a broad platform for hydrophobic bioactive agent delivery. Further studies will be required to evaluate ATRA-ND as an in vivo delivery vehicle for this potent inducer of cell differentiation and apoptosis. Since ND particles containing amphotericn B have been generated using apoliprotein A-I (Oda et al., 2006) or a synthetic amphipathic peptide (Tufteland et al., 2007), it is anticipated that ATRA-ND can be generated that contain one of several potential alternate scaffold proteins / peptides.
The observation that the spectrophotometric properties of ATRA are unaffected by association with ND indicates that the molecule has not been altered during formulation or as a result of its association with ND. Interestingly, as seen previously with liposomal formulations in both animal studies and human clinical trials (Mehta et al., 1994; Estey et al., 2005), ATRA bioavailability was enhanced by incorporation into ND compared to free ATRA. With oral ATRA treatment, some patients develop resistance to ATRA with serum levels decreasing as a function of time, apparently due to clearance of the drug through the liver (Mehta et al., 1994). On the other hand, liposomal ATRA delivered intravenously avoids this pathway, minimizing the need for increased doses.
Cancers associated with defects in the retinoic acid pathway respond well to ATRA therapy. These include hepatocellular carcinoma (HCC) and acute myeloid leukemias (Sun and Lotan, 2002). Clinical studies have shown a significant decrease in the occurrence of both primary and secondary malignancies in patients with HCC when treated with ATRA, encouraging further development of drug delivery methods (Arce et al., 2005). Also, it has been reported that hepatoma cells infected with the hepatitis B virus are more responsive to ATRA than hepatoma cells with no known viral infection (Arce et al., 2005). Our results confirmed that virus-free HepG2 cells are relatively resistant to treatment with free ATRA. However, incubation with ND at the same ATRA dose elicited more dramatic effects. The enhanced acivity of ND associated ATRA may be related to the mode of entry of ND into the cell. Interestingly, a primary component of the present ND formulation is apolipoprotein E3, a well-known ligand of the low-density lipoprotein receptor (Weisgraber, 1994). Given the fact that this receptor is upregulated in HepG2 cells as well as leukemia cells (Izem et al., 1998, Tatidis et al., 2002), it is conceivable that ATRA-ND gain entry into the cell via receptor-mediated endocytosis of ND particles.
Acknowledgments
The authors than Mariko Ishiyama for technical assistance. This work was supported by grants from the National Institutes of Health (HL-64159 and AI-65241).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publicationAs a service to our customers we are providing this early version of the manuscriptThe manuscript will undergo copyediting typesetting and review of the resulting proof before it is published in its final citable formPlease note that during the production process errors may be discovered which could affect the content and all legal disclaimers that apply to the journal pertain
References
- Arce F, Gätjens-Boniche O, Vargas E, Valverde B, Dìaz C. Apoptiotic events induced by naturally occurring retinoids ATRA and 13-cis retinoic acid on human hepatoma cell lines Hep3B and HepG2. Cancer Lett. 2005;229:271–281. doi: 10.1016/j.canlet.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Barua AB, Furr HC. Properties of retinoids. Structure, handling, and preparation. Mol Biotechnol. 1998;10:167–182. doi: 10.1007/BF02760863. [DOI] [PubMed] [Google Scholar]
- Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatoceullular carcinoma. Nature. 1988;333:669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Duprez E, Wagner K, Koch H, Tenen DG. C/EBPβ: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. EMBO J. 2003;22:5806–5816. doi: 10.1093/emboj/cdg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estey EH, Koller C, Tsimberidou AM, O’Brian S, Beran M, Cortes J, Tirado-Gomez M, Lopez-Berestein G, Katarjian H. Potential curability of newly diagnosed acute promyelocytic leukemia without use of chemotherapy: the example of lipsomal all-trans retinoic acid. Blood. 2005;105:1366–1367. doi: 10.1182/blood-2004-09-3437. [DOI] [PubMed] [Google Scholar]
- Fisher CA, Wang J, Francis GA, Sykes BD, Kay CM, Ryan RO. Bacterial overexpression, isotope enrichment, and NMR analysis of the N-terminal domain of human apolipoprotein E. Biochem Cell Biol. 1997;75:45–53. [PubMed] [Google Scholar]
- Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- Freedman LP. Increasing the complexity of coactivation in nuclear receptor signaling. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Gianni M, Ponzanelli I, Mologni L, Reichert U, Rambaldi A, Terao M, Garattini E. Retinoid-dependent growth inhibition, differentiation, and apoptosis in acute promyelocytic leukemia cells. Expression and activation of caspases. Cell Death Differ. 2000;7:447–460. doi: 10.1038/sj.cdd.4400673. [DOI] [PubMed] [Google Scholar]
- Hargreaves PL, Nguyen TS, Ryan RO. Spectroscopic studies of amphotericin B solubilized in nanoscale bilayer membranes. Biochim Biophys Acta. 2006;1758:38–44. doi: 10.1016/j.bbamem.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Izem L, Rassart E, Kamate L, Fasltrault L, Rhainds D, Brissette L. Effect of reduced low-density lipoprotein receptor level on HepG2 cell cholesterol metabolism. Biochem J. 1998;329:81–89. doi: 10.1042/bj3290081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K, Sadeghi T, McQueen T, Lopez-Berestein G. Liposome encapsulation circumvents the hepatic clearance mechanisms of all-trans retinoic acid. Leuk Res. 1994;18:587–596. doi: 10.1016/0145-2126(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Ryan RO. The molecular basis of exchangeable apolipoprotein function. Biochim Biophys Acta. 2000;1483:15–36. doi: 10.1016/s1388-1981(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Maiorano JN, Dhanasekaran P, Ryan RO, Phillips MC, Lund-Katz S, Davidson WS. Helix Orientation of the Functional Domains in Apolipoprotein E in Discoidal High Density Lipoprotein Particles. J Biol Chem. 2004;279:14273–14279. doi: 10.1074/jbc.M313318200. [DOI] [PubMed] [Google Scholar]
- Nelson KG, Bishop JV, Ryan RO, Titus R. Nanodisk-associated amphotericin B clears Leishmania major cutaneous infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2006;50:1238–1244. doi: 10.1128/AAC.50.4.1238-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, He JL, Hsia SL. A micro enzymic method for determination of choline-containing phospholipids in serum and high density lipoproteins. Lipids. 1993;28:949–951. doi: 10.1007/BF02537506. [DOI] [PubMed] [Google Scholar]
- Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Anwerpen R, Ryan RO. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J Lipid Res. 2006;47:260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun S, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- Szuts EZ, Harosi FI. Solubility of retinoids in water. Arch Biochem Biophys. 1991;287:297–304. doi: 10.1016/0003-9861(91)90482-x. [DOI] [PubMed] [Google Scholar]
- Tatidis L, Masquelier M, Vitols S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem Pharmacol. 2002;63:2169–2180. doi: 10.1016/s0006-2952(02)01018-3. [DOI] [PubMed] [Google Scholar]
- Takitani K, Koh M, Inoue A, Kawakami C, Kuno T, Tamai H. Pharmacokinetics of all-trans retinoic acid in adults and children with acute promyelocytic leukemia. Am J Hematol. 2006;81:720–721. doi: 10.1002/ajh.20717. [DOI] [PubMed] [Google Scholar]
- Tufteland M, Pesavento JB, Bermingham RL, Hoeprich PD, Jr, Ryan RO. Peptide stabilized amphotericin B nanodisks. Peptides. 2007;28 doi: 10.1016/j.peptides.2007.01.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K, Gigeure V, Glass CK, Rosenfeld MG, Evans RM. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988;336:262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–3735. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]


