Abstract
Patients with schizophrenia show impairments in motion processing, along with deficits in lower level processing primarily involving the magnocellular visual pathway. The present study investigates potential magnocellular contributions to impaired motion processing in schizophrenia using a combined neurophysiological and behavioral approach. As compared to prior motion studies in schizophrenia, thresholds were determined for both incoherent and coherent visual motion. In this study, velocity discrimination thresholds were measured for schizophrenia patients (n = 14) and age-matched normal control subjects (n = 16) using a staircase procedure. Early visual processing was evaluated using steady-state visual evoked potentials (ssVEP), with stimuli biased toward activation of either the magnocellular or parvocellular visual pathways through luminance contrast manipulation. Patients with schizophrenia showed poor velocity discrimination for both incoherent and coherent motion, with no significant group × task interaction. Further, when coherent motion performance was measured at individually determined incoherent motion thresholds, accuracy levels for patients were similar to controls, also indicating similarity of deficit for incoherent vs. coherent motion discrimination. Impairments in velocity discrimination correlated significantly with reduced amplitude of ssVEP elicited by magnocellular – but not parvocellular – selective stimuli. This study demonstrates that deficits in motion processing in schizophrenia are significantly related to reduced activation of the magnocellular visual system. Further, this study supports and extends prior reports of impaired motion processing in schizophrenia, and indicates significant bottom-up contributions to higher-order cognitive impairments.
Keywords: Schizophrenia, Motion, Magnocellular, Visual, NMDA
1. Introduction
Deficits in visual processing have been well documented in schizophrenia, including increased thresholds for visual stimulus detection (Butler et al., 2005; O'Donnell et al., 1996; Slaghuis, 1998), reduced neurophysiological response to isolated stimuli (Butler et al., 2001, 2005; Foxe et al., 2001; Kim et al., 2005), and deficits in motion processing (Braus et al., 2002; Chen et al., 2005, 2004, 2003, 1999; Li, 2002; Schwartz et al., 1999). The relationship between deficits is not well understood. The present study investigates the relationship between lower level visual processing deficits and motion discrimination abnormalities in schizophrenia.
The visual system, in general, is divided into two discrete pathways: a magnocellular pathway that is specialized for rapid analysis of low contrast, low spatial frequency information, and a parvocellular pathway that is specialized for analysis of fine detail and higher spatial frequency information. Magnocellular neurons project preferentially to dorsal visual stream (“where” system) structures, such as Middle Temporal area (area MT/V5), which process motion and location information, while parvocellular neurons project preferentially to ventral visual stream structures (“what” system), which process form and identity (Felleman and Van Essen, 1991; Orban et al., 2004; Ross et al., 2000). In schizophrenia, magnocellular processing may be particularly disturbed, with relative sparing of the parvocellular system (Butler et al., 2001, 2005; Keri et al., 2004; Kim et al., 2005; Schechter et al., 2003; Slaghuis, 2004).
Deficits in magnocellular function in schizophrenia are reflected in impaired generation of steady state visual evoked potentials (ssVEP) to magnocellular selective stimuli (Butler et al., 2003, 2001). Because the magnocellular system provides the primary input to dorsal stream (Chapman et al., 2004; Felleman and Van Essen, 1991; Thiele et al., 2001), deficits in magnocellular functioning would be predicted to give rise to deficits in motion processing. However, the relationship between deficits at these two levels of processing has not been explicitly examined. The present study therefore evaluates the degree to which disturbances in magnocellular dysfunction in schizophrenia may contribute to subsequent deficits in motion processing.
Motion processing, like other features of the visual pathway, is hierarchically organized with refinement of information extraction through successive stages of cortical processing. Magnocelluar neurons project from lateral geniculate nucleus (LGN) to primary visual (striate) cortex (V1) and subsequently to successive dorsal stream structures such as area MT (Felleman and Van Essen, 1991; Orban et al., 2004; Zeki, 1974). Motion sensitivity arises first in V1, where it depends upon reciprocal LGN/V1 interactions as well as local processing within each region (Heggelund and Hartveit, 1990; Humphrey et al., 1998; Humphrey and Weller, 1988; McKeefry et al., 1997). In V1, however, only a minority of cells show motion sensitivity, and each neuron is sensitive to motion in only a small part of the entire visual space. Detection of motion across space therefore depends upon convergent output from V1 to higher order regions, such as MT.
Neurons in MT receive convergent input from numerous motion sensitive V1 neurons, so that neurons with the same directional sensitivity but complementary visual fields impinge upon the same MT neurons. As a result, individual MT neurons show motion sensitivity across extensive regions of visual space (Felleman and Van Essen, 1991; McKeefry et al., 1997; Orban et al., 2004). Within MT, neurons show similar initial sensitivity to motion whether it occurs in random directions across the visual field (incoherent motion) or in organized flow across space (coherent motion) (Lam et al., 2000; McKeefry et al., 1997; Previc et al., 2000; Ulbert et al., 2001). However, neuronal outputs in MT are different to coherent vs. incoherent motion (Rees et al., 2000; Ulbert et al., 2001), suggesting that MT itself is responsible for decoding motion patterns across the visual field. Dissociations between incoherent and coherent motion discrimination thresholds would thus suggest impaired processing at the level of MT or other dorsal stream structures.
In the present study, motion processing thresholds for both incoherent and coherent motion stimuli were determined using a delayed discrimination task, in which subjects had to state whether two successive stimuli were of the same or different velocities. Tests of this type are specifically sensitive to deficits within dorsal stream structures such as MT (Bisley and Pasternak, 2000). Incoherent and coherent motion stimuli were presented in separate blocks. Relative deficits in incoherent and coherent motion were determined both by comparison of thresholds on each task, and by explicit testing of coherent motion discrimination performance at each subject's individually determined incoherent motion threshold. Our prediction was that motion processing would be similarly impaired in both tasks, as reflected in similarly elevated thresholds.
Because magnocellular neurons respond to lower levels of contrast than do parvocellular neurons, but show responses that saturate at higher contrast levels, ssVEP can be biased toward the magno- vs. parvocellular system by manipulation of background contrast levels (Butler et al., 2001, 2005). ssVEP to low contrast stimuli presented against a uniform background preferentially activate the magnocellular visual system. ssVEP presented using stimuli modulated around a high standing contrast level (“pedestal”) preferentially activate the parvocellular visual system. We have previously shown that deficits in magnocellular ssVEP generation in schizophrenia predict increased thresholds for detection of low contrast, low spatial frequency stimuli (Butler et al., 2005). The present study evaluates the degree to which magnocellular dysfunction, as reflected by impaired ssVEP generation, may contribute to impairments in motion sensitivity in schizophrenia as well.
2. Methods
2.1. Participants
Fourteen patients (13 males, 1 female) meeting DSM-IV criteria for schizophrenia and schizoaffective disorders at the Nathan Kline Institute for Psychiatric Research provided written informed consent after the procedures had been fully explained. Diagnoses were obtained by means of chart review, consultation with the treating psychiatrists, and the Structured Clinical Interview for DSM-IV (SCID). Patients were excluded if they had any neurological or ophthalmologic disorders that might affect performance. Eleven patients were taking atypical antipsychotics and three were receiving typical antipsychotic medications. The mean chlorpromazine-equivalent dose was 1334 mg/ day (range = 500−2500, SD = 600).
Sixteen comparison volunteers (11 males, 5 females), age matched to the patients provided written informed consent after the procedures had been fully explained. Comparison volunteers with a history of psychiatric, neurological, and/or ophthalmologic disorders were excluded.
The patient and comparison groups did not differ significantly in age (patients: 35.4 ± 11 years; controls: 30.9 ± 9.6 years; t = −1.2, df = 28, p = 0.2), although socioeconomic status, as measured with the four-factor Hollingshead scale (Hollingshead and Redlich, 1958), was significantly lower for the patients (22.8 ± 9.2) than for the comparison subjects (50.3 ± 11.8, t = 6.8, df = 26, p < 0.0001). Scores for general psychopathology on the Brief Psychiatric Rating scale (BPRS), negative symptoms and positive symptoms were 40.6 ± 9.3, 30.3 ± 10.3, and 10.0 ± 4.1, respectively.
All participants were screened for adequate binocular vision using a Snellen eye chart, and were included only if they showed better than 20/30 vision. Despite this screening, patients as a group had lower visual acuity than did the controls (t = 2.3, p = 0.03). ANCOVA analyses were, therefore, performed to evaluate potential contribution of between group acuity to observed between group differences.
2.2. Stimuli and procedure
The motion experiment was carried out in a dark room. Visual stimuli were displayed on an Iiyama Vision Master Pro 514 CRT monitor. The stimuli were generated using Presentation® (V0.6, Neurobehavioral Systems, http://nbs.neuro-bs.com).
For all tasks, the stimuli consisted of two separate fields of moving dots presented sequentially. Each field consisted of 100 dots, all of which moved at the same velocity within a field and in the same direction out of eight possible directions (up, down, left, right and oblique ×4 different directions). However, velocity of dots differed across fields. Fields (1000 ms each) were presented in pairs with a 500 ms interval separation between successive fields within a pair. Subjects were told to report whether velocity of dots in the second field was the same or different as velocity of dots in the first field. All participants responded vocally, and their responses (“same” or “different”) were recorded by the experimenter.
The experiment was carried out in 3 blocks: a coherence threshold task, an incoherence threshold task, and a percent accuracy block. In the coherence and incoherence threshold blocks, an up-down staircase method was used to determine threshold. In the percent accuracy task, accuracy in performing the coherent motion task was determined at each subject's individually determined incoherent motion threshold.
2.3. Coherence motion threshold task
For both the coherent and incoherent motion tasks, the dots in one field (either the first or second) moved at 10°/sec, and the dots in the other field moved at a speed that changed dynamically, according to a 3-down, 1-up staircase procedure. Speed of dots in the second field could be either faster, slower or the same as dots in the first field. Because of this, the difference in speed between the two fields could not exceed 100% since one of the two fields would be required to consist of stationary dots. In order to implement the 3-down, 1-up staircase, the difference in velocity was decreased by 20% following 3 correct answers, and increased by 20% following 1 incorrect answer. The number of same and different trials was balanced during the staircase. However, the staircase, which stopped after 10 reversals, adjusted only for incorrect responses to different trials. The 3-down/1-up rule produces a correct response rate equivalent to 79.4% accuracy. For each subject, the degree of velocity difference (Δv) at which the staircase converged, defined as the mean of the last 10 reversals, was recorded and used as that subject's coherent motion threshold.
2.4. Incoherence motion threshold task
For the incoherence threshold task, the same procedure was used as in the coherence threshold task, except that motion for each dot in a frame (out of the 8 possible directions) was randomly chosen for each dot in each frame. This resulted in each dot moving in a direction that was independent of all others. Nevertheless, all dots within a field moved at the same speed, albeit in random directions, out of the 8 possible directions described above. As in the coherent threshold task, a 3-down/1-up staircase was used, and the velocity level (Δv) for 79.4% correct performance was recorded.
2.5. Percent accuracy task
In the last block, accuracy in detecting differences in coherent motion between stimuli was tested for each subject at that subject's independently determined incoherent motion threshold. Stimuli were the same as used in the coherence motion threshold task, except that the level of Δv within a pair was fixed across trials at the individual subject's incoherent motion threshold. As in the prior blocks, stimulus velocity for stimuli could be the same or different within a pair. Same and different pairs were presented equiprobably. Fifty stimulus pairs were presented for each subject, and the number of correct responses was recorded.
2.6. Steady-state visual evoked potentials (ssVEP)
Steady-state visual potentials were recorded as previously described (Butler et al., 2005). Briefly, stimuli were biased toward activation of the magnocellular vs. parvocellular systems through the use of stimuli modulated around either a low luminance or high luminance contrast condition. The magnocellular system is selectively activated by low luminance contrast stimuli, but saturates once stimuli exceed 16% contrast. In contrast, the parvocellular system shows lower sensitivity to low (<16%) contrast stimuli, but progressive response with increasing contrast. Thus, use of low absolute luminances biases stimuli toward the magnocellular system, whereas modulation around a high standing level of contrast (48% pedestal) biases toward the parvocellular system. In order to evaluate early cortical contributions to motion sensitivity, correlational analyses were conducted between ssVEP to magnocellular-selective stimuli (16% contrast, no pedestal) vs. parvocellular-selective stimuli (16% contrast, pedestal condition). Data were collected using a VENUS stimulation system with isolated checks presented either in appearance–disappearance fashion (magnocellular condition), or around a standing level of luminance (parvocellular condition). Based upon shapes of the waveforms, plateau values for the magnocellular condition was defined as the average response to highest 3 levels of luminance contrast (8%, 16%, 32%). In the parvocellular condition, response to the highest levels (32%) was used as an index of maximal response (Butler et al., 2005). ssVEP analyses were performed on patients only, and were missing for 2 subjects. Mean time between motion and ssVEP testing was approximately 6 weeks.
2.7. Statistical analysis
Demographic comparisons were analyzed between groups by means of two-tailed t tests. Incoherent and coherent motion thresholds were analyzed using a repeated measures ANOVA with within group factor of task (incoherent vs. coherent) and between group factor of diagnostic cohort. Percent accuracy data were analyzed using between-group t-test. Correlational analyses were performed using a Spearman rank order (rs) correlation. For all analyses, a preset alpha level for significance of p < .05 was used. Data in text represent mean ± SD unless otherwise specified.
3. Results
3.1. Motion discrimination thresholds
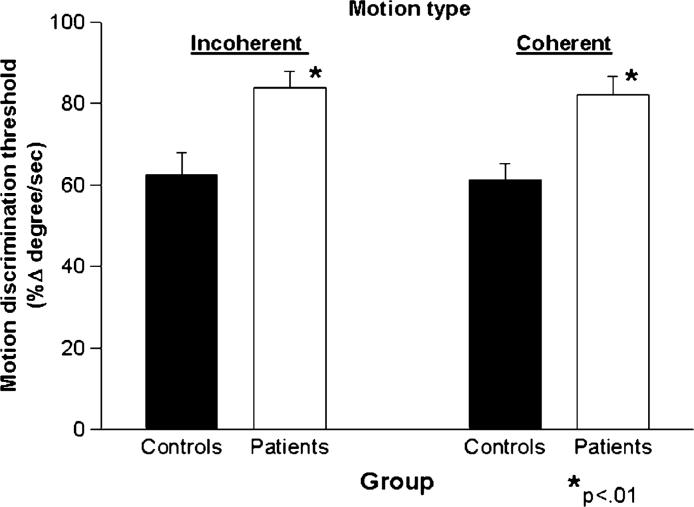
Patients and controls differed in both incoherent (t = −3.1, df = 28, p = 0.004) and coherent (t = −3.5, df = 28, p = 0.002) motion thresholds (Fig. 1). When both incoherent and coherent measures were entered into a repeated-measure ANOVA, there was a significant main effect of group ( F(1, 28) = 13.2, p = 0.001) but no significant group × task interaction ( F < 1.0), indicating equivalent level of performance decrement. Further, incoherent and coherent thresholds correlated significantly with each other for both patients and controls (rs = .60, p = .025) and controls (rs = .54, p = .03).
Fig. 1.
Bar chart showing mean (±sem) thresholds for detecting differences in velocity of coherent (left) and incoherent (right) motion stimuli for patients with schizophrenia (n = 14) vs. controls (n = 16).
When coherent motion performance was measured at individually determined incoherent motion thresholds, accuracy levels for patients (81.0 ± 8.3%) were similar to those of controls (78.3 ± 7.8%) with no significant between-group difference (t = 0.9, df = 28, p = 0.4), indicating that deficits in coherent motion discrimination were negated once correction was made for underlying deficits in incoherent motion discrimination.
The between-group difference in motion threshold remained significant even following covariation for potential between-group differences in visual acuity ( F(1, 27) = 8.2, p = 0.008) or between-group differences in SES ( F(1, 27) = 7.89, p = .01).
3.2. Correlation analysis
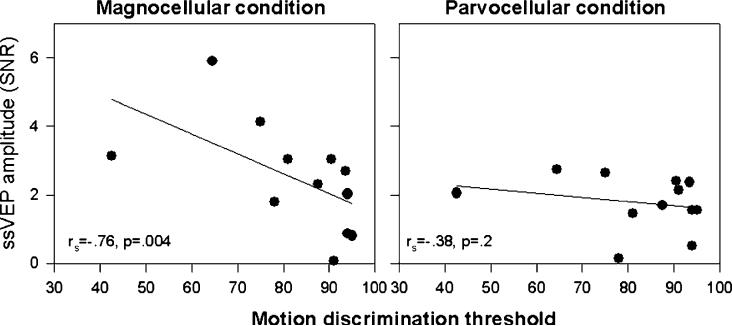
Correlational analyses evaluated the relationship between motion sensitivity and previously obtained ssVEP measures of magno- and parvocellular functioning (Butler et al., 2005). Because coherent and incoherent thresholds were not significantly different between from each other in either group, these were averaged prior to performing correlational analyses, and were compared vs. maximal magnocellular and parvocellular ssVEP amplitudes (Table 1) across subjects. In accordance with a priori predictions, the amplitude of the magnocellular ssVEP (rs = −.76, p = .004), but not the parvocellular (rs = −.38, NS), response function correlated with the motion discrimination threshold (Fig. 2).
Table 1.
Steady-state visual evoked potential (ssVEP) amplitudes to magnocellularly and parvocellularly biased stimuli at indicated levels of depth of modulation (DOM)
| DOM | Condition |
|
|---|---|---|
| Magnocellular | Parvocellular | |
| 0 | 0.48 ± 0.27 | 0.36 ± 0.21 |
| 1 | 0.52 ± 0.36 | 0.25 ± 0.15 |
| 2 | 0.62 ± 0.4 | 0.45 ± 0.32 |
| 4 | 1.13 ± 0.75 | 0.66 ± 0.36 |
| 8 | 2.40 ± 1.83 | 1.03 ± 0.64 |
| 16 | 2.53 ± 1.46 | 1.57 ± 1.12 |
| 32 | 2.52 ± 1.68 | 1.77 ± 0.80 |
| Peak | 2.49 ± 1.59 | 1.77 ± 0.80 |
In the magnocellular condition, the function plateaus between 8% and 32% DOM as previously reported (Butler et al., 2005), so that an average of these values was used to define the peak magnocellular response. In the parvocellular condition, the response does not saturate so the maximal response across DOM (32% DOM response) was used for correlational analyses (Fig. 2).
Fig. 2.
Correlation between average coherent/incoherent motion discrimination threshold and steady-state visual evoked potential (ssVEP) amplitude measured as signal-to-noise (SNR) ratio to magnocellularly (left) and parvocellularly (right) biased stimuli.
Additional correlational analyses investigated the relationship between motion discrimination thresholds and antipsychotic dose, expressed as CPZ equivalents. No significant correlations were observed for either coherent (r = .10, p = .7) or incoherent (r = −.2, p = .49) thresholds.
4. Conclusions
Although motion processing deficits have been well documented in schizophrenia (Braus et al., 2002; Brenner et al., 2003; Chen et al., 1999; Li, 2002; O'Donnell et al., 1996), underlying neurophysiological mechanisms remain to be determined. In this study, we investigated information processing using incoherent vs. coherent motion processing tasks, and related performance to measures of basic sensory processing.
The main findings of the present study are that patients with schizophrenia show elevated velocity discrimination thresholds, reflecting impaired motion processing. As compared to earlier studies, however, we demonstrate that these processes affect incoherent, as well as coherent motion processing, suggesting that decoding of coherent from incoherent motion, a process that takes place within dorsal stream regions such as MT (Lam et al., 2000; McKeefry et al., 1997; Previc et al., 2000; Rees et al., 2000; Ulbert et al., 2001), is relatively intact. In addition, motion-processing deficits were significantly related to deficits in functioning of the magnocellular visual system, which provides low-level input to the dorsal stream. Taken together, these findings suggest that motion-processing deficits in schizophrenia may be largely attributable to impaired bottom-up input to motion processing areas such as MT, rather than to local, intrinsic dysfunction within those regions.
On a neurophysiological level, global motion discrimination within the visual system is enabled by basic circuit-level mechanisms that permit subpopulations of neurons, beginning in primary visual cortex (V1) to develop differential sensitivity to stimuli moving in a specific direction. Motion-sensitive neurons take advantage of the timing differences provided by discrete subpopulations of neurons within LGN that respond either immediately (non-lagged) or following delay (lagged) to stimuli presented in a specific portion of space (Humphrey and Weller, 1988). On a molecular level, the delayed responses of lagged cells reflect the slower channel kinetics of N-methyl-d-aspartate (NMDA) receptors relative to other (non-NMDA) glutamate receptor types (Heggelund and Hartveit, 1990). Thus, deficits in NMDA transmission in schizophrenia, if present in schizophrenia (Abi-Saab et al., 1998; Coyle, 1996; Javitt and Zukin, 1991; Newcomer et al., 1999), would be predicted to lead to deficits in motion discrimination even at very low levels of the visual system (i.e. LGN/V1). Within V1, other NMDA-dependent processes, such as delayed GABAergic feedback, may also contribute to neuronal motion sensitivity (Rivadulla et al., 2001). Motion sensitivity deficits, as well as more general magnocellular dysfunction (Butler et al., 2005; Kwon et al., 1991, 1992), therefore may both be manifestations of proposed disturbances in glutamatergic and/or NMDA-dependent neurotransmission in schizophrenia.
Although functioning of lagged vs. non-lagged cells within V1 cannot be readily assessed in schizophrenia, we have recently observed that ssVEP responses are depressed to stimulus manipulations that elicit apparent motion, but not to those that elicit only apparent changes in form (Kim et al., 2005). Such findings, overall, are consistent as well with the concept that motion processing is impaired even at early stages of cortical analysis.
A limitation of the study is that all patients were receiving antipsychotic medication at the time of testing. Thus, a medication effect cannot be excluded. However, deficits in motion processing have been found in the past to be relatively insensitive to medication status (Chen et al., 1999). Moreover, in the present study, there was no significant correlation between antipsychotic type (typical vs. atypical) or dose and measures of either motion sensitivity or ssVEP amplitude.
In summary, motion-processing deficits have previously been reported in schizophrenia. This study demonstrates that the disease process affects sensitivity to incoherent motion equally to that of coherent motion. Further, deficits in motion processing are significantly predicted by neurophysiological dysfunction of the magnocellular, but not parvocellular, visual pathways, suggesting significant bottom-up contributions. Taken together, these findings suggest that deficits in dorsal stream processing may be driven in large part by failures in earlier magnocellular functioning, and supports hierarchical models of neurocognitive dysfunction in schizophrenia.
Acknowledgements
Supported in part by USPHS grants R01 MH49334 and K02 MH01439 and a Burroughs-Wellcome Translational Research Award to DCJ and R01 MH066374 to PDB.
References
- Abi-Saab WM, D'Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 1998;31(Suppl 2):104–109. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Pasternak T. The multiple roles of visual cortical areas MT/MST in remembering the direction of visual motion. Cereb. Cortex. 2000;10:1053–1065. doi: 10.1093/cercor/10.11.1053. [DOI] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch. Gen. Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Wilt MA, Lysaker PH, Koyfman A, O'Donnell BF. Psychometrically matched visual-processing tasks in schizophrenia spectrum disorders. J. Abnorm. Psychology. 2003;112:28–37. [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early stage visual processing in schizophrenia. Am. J. Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr. Res. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C, Hoag R, Giaschi D. The effect of disrupting the human magnocellular pathway on global motion perception. Vis. Res. 2004;44:2551–2557. doi: 10.1016/j.visres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch. Gen. Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr. Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol. Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr. Res. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv. Rev. Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. NeuroReport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Heggelund P, Hartveit E. Neurotransmitter receptors mediating excitatory input to cells in the cat lateral geniculate nucleus. I. Lagged cells. J. Neurophysiol. 1990;63:1347–1360. doi: 10.1152/jn.1990.63.6.1347. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FL. Social Class and Mental Illness. Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Weller RE. Functionally distinct groups of X-cells in the lateral geniculate nucleus of the cat. J. Comp. Neurol. 1988;268:429–447. doi: 10.1002/cne.902680311. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB, Feidler JC. Strobe rearing prevents the convergence of inputs with different response timings onto area 17 simple cells. J. Neurophysiol. 1998;80:3005–3020. doi: 10.1152/jn.1998.80.6.3005. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early stage visual processing in schizophrenia: harmonic analysis. Schizophr. Res. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Esguerra M, Sur M. NMDA and non-NMDA receptors mediate visual responses of neurons in the cat's lateral geniculate nucleus. J. Neurophysiol. 1991;66:414–428. doi: 10.1152/jn.1991.66.2.414. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Nelson SB, Toth LJ, Sur M. Effect of stimulus contrast and size on NMDA receptor activity in cat lateral geniculate nucleus. J. Neurophysiol. 1992;68:182–196. doi: 10.1152/jn.1992.68.1.182. [DOI] [PubMed] [Google Scholar]
- Lam K, Kaneoke Y, Gunji A, Yamasaki H, Matsumoto E, Naito T, Kakigi R. Magnetic response of human extrastriate cortex in the detection of coherent and incoherent motion. Neuroscience. 2000;97:1–10. doi: 10.1016/s0306-4522(00)00037-3. [DOI] [PubMed] [Google Scholar]
- Li CS. Impaired detection of visual motion in schizophrenia patients. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2002;26:929–934. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- McKeefry DJ, Watson JD, Frackowiak RS, Fong K, Zeki S. The activity in human areas V1/V2, V3, and V5 during the perception of coherent and incoherent motion. NeuroImage. 1997;5:1–12. doi: 10.1006/nimg.1996.0246. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Previc FH, Liotti M, Blakemore C, Beer J, Fox P. Functional imaging of brain areas involved in the processing of coherent and incoherent wide field-of-view visual motion. Exp. Brain Res. 2000;131:393–405. doi: 10.1007/s002219900298. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat. Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Rivadulla C, Sharma J, Sur M. Specific roles of NMDA and AMPA receptors in direction-selective and spatial phase-selective responses in visual cortex. J. Neurosci. 2001;21:1710–1719. doi: 10.1523/JNEUROSCI.21-05-01710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Badcock DR, Hayes A. Coherent global motion in the absence of coherent velocity signals. Curr. Biol. 2000;10:679–682. doi: 10.1016/s0960-9822(00)00524-8. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr. Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Maron BA, Evans WJ, Winstead DK. High velocity transient visual processing deficits diminish ability of patients with schizophrenia to recognize objects. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12:170–177. [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychology. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp. Brain Res. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- Thiele A, Dobkins KR, Albright TD. Neural correlates of chromatic motion perception. Neuron. 2001;32:351–358. doi: 10.1016/s0896-6273(01)00463-9. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Karmos G, Heit G, Halgren E. Early discrimination of coherent versus incoherent motion by multi-unit and synaptic activity in human putative MT+. Hum. Brain Mapp. 2001;13:226–238. doi: 10.1002/hbm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J. Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]