Abstract
Since the original description of T cell anergy in CD4 clones from mice and humans, a number of different unresponsive states have been described, both in vivo and in vitro, that have been called anergic. While initial attempts were made to understand the similarities between the different models, it has now become clear from biochemical experiments that many of them have different molecular mechanisms underlying their unresponsiveness. In this review we will detail our own work on the in vivo model referred to as adaptive tolerance and then attempt to compare this biochemical state to the multitude of other states that have been described in the literature.
INTRODUCTION
Anergy is a hyporesponsive state that a lymphocyte can assume following an encounter with antigen. This state is to be distinguished from programmed cell death by a half life of greater than several days and from immunoregulation and suppression by the intrinsic nature of the biochemical alteration. T cell clonal anergy was first described as an in vitro model of tolerance for T cell clones from both humans and mice [1,2]. Presentation of antigen under suboptimal activation conditions led to a hyporesponsive state in which IL-2 production and T cell proliferation were subsequently impaired following rechallenge of the T cells with optimum stimulation conditions. Anergy was induced in several different ways. Initially, it was achieved by T-cell receptor (TCR) occupancy with agonist peptide/MHC complexes in the absence of APC costimulation [3,4]. Then it was induced by TCR occupancy with partial agonist peptides in the presence of APC costimulation [5]. Other methods included TCR cross-linking with anti-CD3 on a plate [6], treatment of the T cells with ionomycin followed by high density culture [7,8], and T cell self-presentation of agonist peptides by human clones that express MHC class II molecules [1]. Freshly explanted T cells were also anergized by the blocking of costimulation with CTLA4-Ig during stimulation with soluble anti-CD3 and splenic APC [9]. In vivo studies soon followed in which a variety of manipulations were shown to produce an anergic-like state. The first was through injection of superantigens in which a fraction of the cohort of Vβ-stimulated cells becomes anergized (instead of being deleted) after the initial expansion and contraction phases of the response [10-12]. Subsequent antigen-specific models used TCR transgenic T cells stimulated once or twice with soluble peptides i.v. [13] or stimulated once after transfer of the T cells into syngeneic hosts to decrease their frequency [14]. In another variation, the TCR transgenic T cells were transferred into intact, irradiated, or lymphopenic (Rago/o or CD3εo/o) hosts expressing the antigen [15-18]. Finally, these transgenic mice have been used in an oral tolerance model when the antigen is fed at high doses [19] and in a transfusion-induced tolerance model prior to an allogeneic cardiac transplant [20].
Initially it was thought that these various forms of anergy induction produced the same biological state because all of them were characterized by a failure to produce IL-2 and sustain proliferation following T cell activation [21]. However, as each of these models has been explored in more detail, it has become clear that many of them are quite unique and probably represent different biological states of unresponsiveness. In our laboratory we have compared T cell clonal anergy with the in vivo double transgenic model called adaptive tolerance, and found them to be quite different [22]. Biologically, clonal anergy represents a growth arrest state in which the production of only selective cytokines (IL-2 and IL-3) is significantly impaired in response to TCR stimulation. It does not require antigen to maintain the state and it can be reversed by stimulation with IL-2 [23]. In contrast, adaptive tolerance results in the down-regulation of all TCR-induced cytokine production, requires antigen persistence to maintain the state, and is not reversed by the addition of IL-2 [18,21,24]. A recent biochemical comparison showed that adaptive tolerance entails a block in TCR stimulation at the level of Zap-70’s phosphorylation of LAT, while clonal anergy was confirmed to be a block further downstream in the Ras-MAP kinase pathway [22]. Thus, the possibility exists that each of the models described above represents a different biologic state with its own biochemical negative feedback regulation preventing T cell activation. In this brief review, we have decided to summarize our recent published and unpublished biochemical experiments on the adaptive tolerance model of anergy and then to compare this type of anergy with what is known about all the other models currently described in the literature.
THE MODEL OF ADAPTIVE TOLERANCE
EARLY BIOCHEMICAL EVENTS IN TCR SIGNAL TRANSDUCTION
TCR signaling begins with allosteric changes in the receptor following its cross-linking by pMHC complexes presented by the APC [25]. This leads to phosphorylation of the TCRζ-chain by the Src family kinases Lck and Fyn [26]. This phosphorylation creates a docking site for Zap70 recruitment to the TCR and its subsequent activation by Lck and autophosphorylation [27]. Activated Zap-70 in turn phosphorylates the membrane-associated Linker of Activated T cells (LAT), which then serves as a membrane docking site for phospholipase C-γ1 (PLC-γ1) and Grb2/mSOS [28,29]. The latter acts as a guanine nucleotide exchange factor to convert Ras-GDP to Ras-GTP at the plasma membrane, leading to activation of Raf1 and the Erk/MAP kinase pathway, as well as PI3Kinase [30]. The former becomes activated by Zap-70 phosphorylation and cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) in the membrane into the two second messengers inositol triphosphate (IP3) and diacylglycerol (DAG) [31]. IP3 interacts with receptors on the endoplasmic reticulum (ER) to release calcium from intracellular stores, which in turn opens CRAC channels in the plasma membrane, allowing extracellular calcium to enter the cell [32]. These increases in intracellular calcium activate calmodulin, which binds to the phosphatase calcineurin and activates it to dephosphorylate the transcription factor NF-AT [33]. This allows NF-ATs to move into the nucleus and participate in gene activation [34]. In the other arm of the response, DAG recruits protein kinase C theta (PKC-θ) to the plasma membrane [35] where it interacts with CARMA1 [36] to eventually activate the NF-κB pathway via degradation of the inhibitor of NF-κB (IκB) [37]. DAG also synergizes with the increased calcium to activate Ras-GRP1, another activator of the Erk/MAP kinase pathway [30]. This pathway augments the production and activation of the transcription factor AP-1, which synergizes with NF-AT and NF-κB to initiate transcription of new genes such as IL-2 [38]. Costimulation through the CD28 molecule plays a key role in amplifying the signaling of the NF-κB pathway through activation of PI3Kinase and AKT [39].
THE MODEL
In search of a simplified in vivo model for peripheral tolerance to a persistent neo-self antigen, we isolated CD4+ T cells from a pigeon cytochrome c (PCC)/I-Ek-specific, TCR-transgenic mouse (5C.C7I-Vβ3+Vα11+) crossed onto a Rag2-deficient background. This naïve monoclonal population (>90% CD4+) was then transferred into a second transgenic mouse expressing a membrane-targeted version of PCC (mPCC) expressed under the control of an MHC class I promoter and an Ig enhancer [18]. In order to obtain enough T cells for a traditional biochemical analysis of signal transduction events, the recipient mPCC transgenic was crossed onto a CD3εo/o background. In this lymphopenic environment, the naïve T cells undergo a 100 fold expansion over a 4 day period. The subsequent contraction phase is only about 2 fold and the remaining cells enter a hyporesponsive state in which all cytokine production following TCR signaling is decreased about 10 fold and proliferation to antigen in vivo is greatly reduced; turnover in BrdUrd-labeling studies revealed about 5% new cell cycling per day [24]. This state of adaptive tolerance is stably entrenched by 2-3 weeks and persists for at least 4 or 5 months. For biochemical studies of T cell activation, the cells were purified 1-2 months after transfer from lymph nodes and spleen of the recipient mice by negative selection using magnetic beads coupled with antibodies specific for mouse IgG, CD45(B220), CD11b, and MHC class II [22]. The analyzed population was generally >90% CD4+ Vβ3+ T cells. The cells were stimulated for 2 to 30 min with biotinylated anti-TCRβ and anti-CD4 cross-linked with Streptavidin. The reaction was stopped by cell lysis with a buffer containing 1% NP40 and total lysates or specific immunoprecipitates were analyzed by SDS PAGE and Western blotting. Control T cells were either naïve cells or these cells pre-activated with PCC and APC followed by expansion in IL-2 and resting for approximately 2 weeks.
T CELL RECEPTOR SIGNALING
TCR and CD4 Levels
Following initial T cell activation with PCC and I-Ek APC, the TCR is down-regulated within a few hours of stimulation, but then returns to levels found on naïve T cells by around 24 hours. T cells that have entered the adaptive tolerant state show the same mean fluorescence intensity of TCR staining as naïve cells, although the distribution of the population is broader. Preactivated T cells show a similar pattern. In contrast to TCR, CD4 levels are significantly increased (1.4 fold) on the tolerant T cells [22]. Preactivated T cells showed a similar increase, suggesting it is a property of antigen-activated CD4+ T cells [unpublished data].
Src Family Kinases
The initiating tyrosine kinases following TCR and CD4 cross-linking are thought to be Lck and Fyn. The amount of Lck in the tolerant T cells is the same as that in naïve T cells. Its activation following TCR cross-linking is also the same as measured in total cell lysates by the amount of Lck tyrosine phosphorylation induced at amino acid position 394. Immunoprecipitation of Lck and performance of an in vitro kinase assay showed a slight decrease (35%) in activity on the exogenous substrate myelin basic protein as well as by autophosphorylation [22]. Since CD4 levels are increased on the tolerant cells, there is also more associated Lck available for kinase activity at the cell surface. Thus, it appears that this initiating kinase is not rate limiting for TCR/CD4 signaling of the tolerant cells.
In contrast, the amount of Fyn in resting tolerant T cells is significantly elevated over that in naïve T cells [22]. This increase ranged from 1.75 to 10.6 fold (geometric mean of 3.75) and was not further augmented by TCR and CD4 cross-linking. Immunoprecipitation followed by an in vitro kinase assay showed enhanced activity of Fyn on itself and the exogenous substrate enolase. This activity did not change with T cell stimulation. Most of the enhancement could be accounted for by the increase in the amount of Fyn in the cells. The possibility that the augmentation in Fyn is an instrumental part of maintaining the adaptive tolerant state was tested by crossing the Fyno/o onto the B10.A TCR-5C.C7 Rag2o/o background. T cells from this animal still became anergic following adoptive transfer, suggesting that Fyn was not required for the induction or maintenance of adaptive tolerance [unpublished data].
Tyrosine Phosphorylation
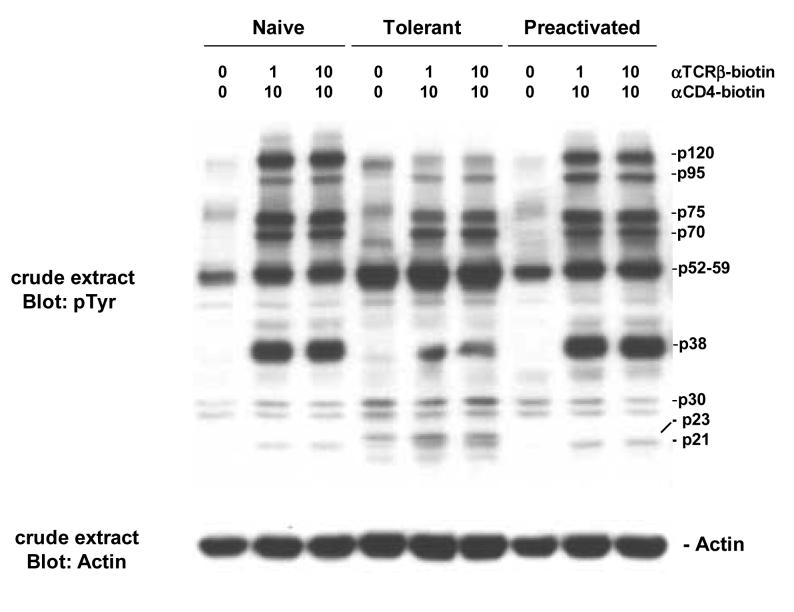
Within one minute after TCR and CD4 cross-linking, a number of proteins become phosphorylated on tyrosine residues as seen by Western blotting of whole cell lysates with a generic anti-phospho-tyrosine antibody. Adaptively tolerant T cells show a number of differences in this pattern compared to both naïve and pre-activated T cells (Figure 1). The induced phosphorylations of proteins of 38kD (LAT?) and 120kD (cCbl?) are greatly curtailed in the tolerant cells. Phosphorylations of p95 and p75 proteins were slightly reduced. In contrast, phosphorylation of a p23 protein (TCRζ?) was enhanced. There was also a constitutive enhancement in the amounts of two phospho-proteins of 30 and 52-59kD that were not induced very much by T cell activation. The latter most likely includes Fyn as discussed above.
Figure 1.
TCR induction of protein tyrosine phosphorylation.
Purified naïve, adaptively tolerant, or preactivated T cells were stimulated for 1 min with varying concentrations of anti-TCRβ-biotin mAb (0, 1, or 10μg/ml) and anti-CD4-biotin mAb at either 0 or 10 μg/ml, followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and run on SDS-PAGE. Western blots were performed and probed with an anti-phosphotyrosine mAb (anti-pTyr) and then reprobed with an anti-actin mAb.
TCR-Zeta Chain Phosphorylation
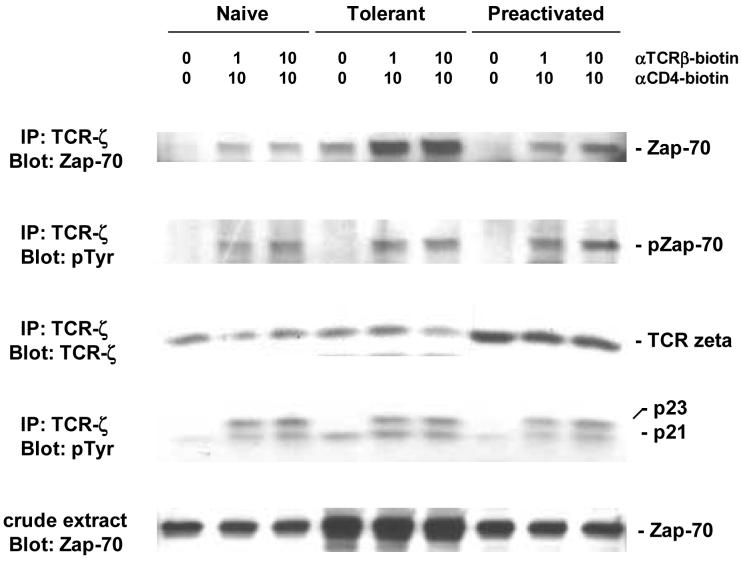
The critical substrates for Src family kinases in initiating TCR signal transduction are the ITAM motifs in the cytoplasmic tails of the CD3γ, CD3δ, CD3ε, and CD3ζ chains of the receptor complex. The epsilon chain has one pair of these motifs, while the zeta chain has three pairs. Phosphorylated zeta chain protein migrates as two forms on PAGE: a 21kD form representing phosphorylation of 4 tyrosines in these motifs, and a 23kD form representing full phosphorylation of 6 tyrosines [40]. The amount of zeta chain immunoprecipitated from tolerant T cells was equivalent to that from naïve T cells and did not change following activation (Figure 2 - row 3). The increase shown in pre-activated cells was not reproducible. Blotting with the anti-phospho-tyrosine mAb shows the presence of p21 in unstimulated tolerant cells (Figure 2 - row 4). This is observed to a much less degree in naïve and pre-activated T cells. Nonetheless, cross-linking of TCR and CD4 for 2 min induces comparable amounts of p23 in all 3 populations. This confirms that there is adequate Src family kinase activity in tolerant T cells to fully activate TCR zeta.
Figure 2.
TCR-zeta chain phosphorylation.
Purified naïve, adaptively tolerant, or preactivated T cells were stimulated for 1 min with varying concentrations of anti-TCRβ-biotin mAb (0, 1, or 10μg/ml) and anti-CD4-biotin mAb at either 0 or 10 μg/ml, followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-ζ, mAb and run on SDS-PAGE. Western blots were performed and probed with anti-phosphotyrosine mAb and then reprobed with anti-Zap70 mAb or anti-ζ mAb.
Recruitment of ZAP-70 to TCRζ and Its Activation by Phosphorylation
Once the zeta or epsilon chain is phosphorylated on both tyrosines in a pair of ITAM motifs, it can recruit Zap-70 to bind stably to the receptor complex (Figure 2 - row 1). Surprisingly, tolerant T cell receptors bind more Zap-70 (following co-precipitation with anti-TCRζ) than either naïve or pre-activated T cells. There are two possible reasons for this. One is because the tolerant cells express significantly more Zap-70, as measured by Western blotting of total cell lysates (Figure 2 - row 5). The other is that tolerant cells have more p21 activated zeta chains in their resting state (Figure 2 - row 4). The Zap-70 bound to p21, however, does not become stably activated by tyrosine phosphorylation (Figure 2 - row-2). Following TCR and CD4 cross-linking, all 3 populations show an enhancement in the binding of Zap-70, but again the tolerant cells bind much more. Some of this Zap-70 is tyrosine phosphorylated, but, surprisingly, the total amount is the same for all 3 populations (Figure 2 - row 2). This is also seen on the tyrosine phosphorylation blot of total cell lysates (p70 bands in Figure 1). This suggests that in activated tolerant T cells the bound Zap-70 molecules have less tyrosine phosphorylation per molecule or that a cohort of the molecules bound to p23 is not phosphorylated (similar to the Zap-70 molecules bound to p21).
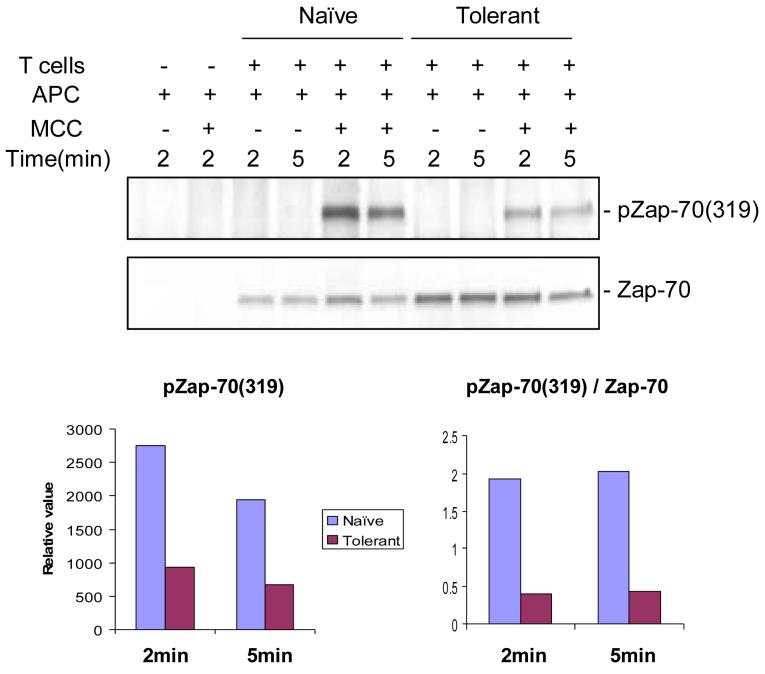
Immunoprecipitation of Zap-70 after TCR and CD4 cross-linking also revealed the same amount of total phosphorylation in all three cell populations, and a similar amount of phosphorylation was observed at the individual sites when blotting total lysates with antibodies specific for Y292, Y319, and Y493 [22]. However, an in vitro kinase assay using the exogenous substrate cdb3 revealed a selective decrease in activity in anti-Zap-70 immunoprecipitates from the tolerant cells (1/4 of that for naïve cells) [22]. In addition, if the tolerant cells were stimulated with antigen and APC, instead of antibody cross-linking, a significant decrease in total tyrosine phosphorylation of Zap-70 was observed (Figure 3). In this comparison between naïve and tolerant T cells, the tyrosine phosphorylation of site 319 was 2.9 fold lower (34%) in the tolerant cells without normalization to Zap-70, and 4.8 fold lower (21%) with normalization. Our conclusion from all these studies is that the tolerant cells have a partial impairment in Zap-70 function.
Figure 3.
Zap70 phosphorylation.
Purified naïve and adaptively tolerant T cells were stimulated for 2 min or 5 min with the P13.9 cell line (MHC-II+, B7.1+, ICAM-1+) that had been prepulsed with or without Ag (20μM MCC 81-104). Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-Zap70, and run on SDS-PAGE. Western blots were performed and probed with an anti-phospho-Zap70 mAb specific for the tyrosine phosphorylation site Y319 on Zap70, and then reprobed with anti-Zap70 mAb. The density of each band was determined using GelPro software. The relative values for Zap70 (Y319) phosphorylation are expressed in the lower left figure, and the values normalized to the total level of Zap70 expression are shown in the lower right figure.
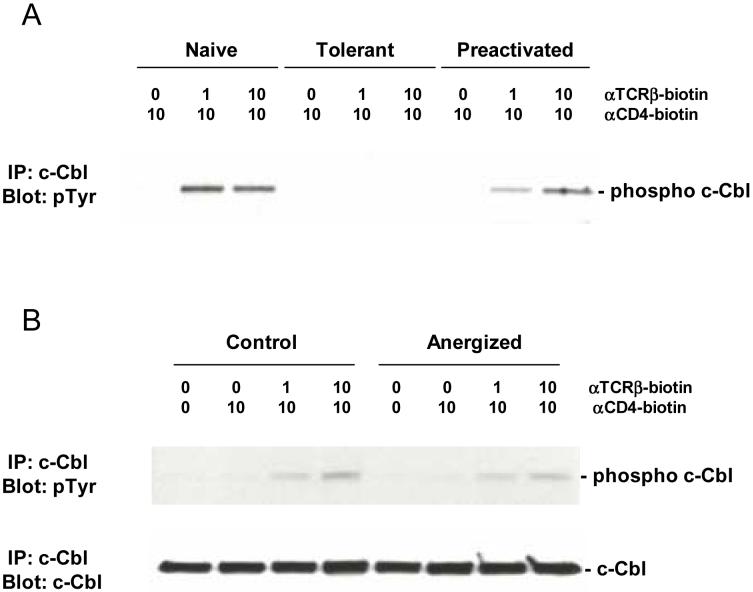
The reason for the increase in total Zap-70 in the tolerant cells is not known. cCbl can bind to Y292 on Zap-70 after it is phosphorylated at this site, and act as an E3 ubiquitin ligase to target Zap-70 for degradation by the proteosome [41,42]. Normally cCbl is tyrosine phosphorylated following TCR stimulation, but this is blunted in tolerant T cells (Figure 4A and the p120 band in Figure 1). If cCbl requires this phosphorylation for its E3 ligase activity, then its impaired phosphorylation in tolerant cells in the face of chronic TCR stimulation might reduce the turnover of Zap-70 and account for the increase in its steady state level.
Figure 4.
cCbl phosphorylation.
Purified naïve, adaptively tolerant, or preactivated 5C.C7 T cells in (A) or control and anergized A.E7 T cells in (B) were stimulated for 1 min with varying concentrations of anti-TCRβ-biotin mAb (0, 1, or 10μg/ml) and anti-CD4-biotin mAb at 10 μg/ml, followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-cCbl antiserum, and run on SDS-PAGE. Western blots were performed and probed with anti-phosphotyrosine mAb and then reprobed with anti-cCbl mAb.
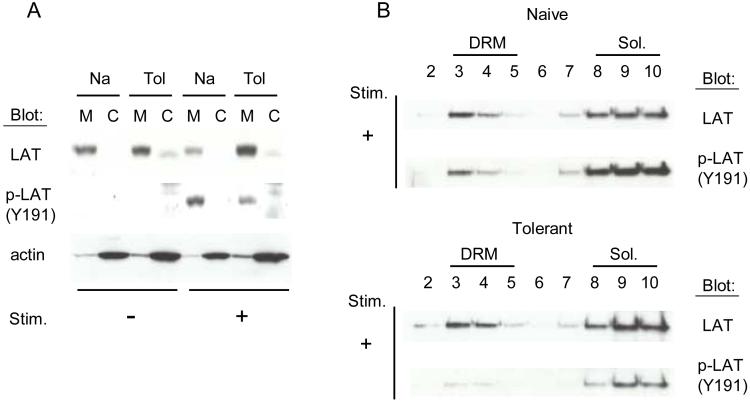
LAT Phosphorylation and Localization to the Plasma Membrane
The major downstream substrate of Zap-70 in vivo is the adaptor molecule LAT. This molecule is post-translationally modified by palmitoylation, which allows LAT to attach to the inner leaflet of the plasma membrane and localize in detergent resistant membranes (DRM) referred to as lipid rafts [43]. The total amount of LAT was similar in all 3 cell populations. In both naïve and tolerant T cells, LAT is localized almost exclusively in the membrane fraction of the cell and this does not change after stimulation with anti-TCR and anti-CD4 cross-linking (Figure 5A). The portion that is distributed in DRMs is also similar after stimulation (Figure 5B). In contrast, the amount of LAT phosphorylation at site Y191 (Y195 in mouse) that takes place following stimulation is significantly less for molecules both in and out of the lipid rafts. Examination of each of the individual tyrosine phosphorylation sites on LAT with anti-phospho-peptide specific antibodies (Y136, Y175, Y195, and Y235) revealed that all the sites were similarly affected [22]. This impairment represents the primary block in the TCR signaling pathway that we have observed in the tolerant T cells. The mechanism for how this comes about, however, is unknown. We are currently looking for a ZAP-70 inhibitor that blocks at this step.
Figure 5.
LAT localization and phosphorylation.
A, Purified naïve and adaptively tolerant T cells were unstimulated or stimulated for 1 min with anti-TCRβ-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml), followed by cross-linking with streptavidin. Samples were lysed, and cytoplasmic and membrane fractions were separated [67]. Western blots were done with an anti-phospho-LAT antiserum specific for the tyrosine phosphorylation site Y191 on LAT and then reprobed with an anti-LAT mAb or an anti-actin mAb.
B, Purified naïve and adaptively tolerant T cells were stimulated for 1 min with anti-TCRβ-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml), followed by cross-linking with streptavidin. Samples were lysed, and Detergent Resistant Membranes (DRM) and Triton soluble membrane fractions (Sol.) were separated on a sucrose gradient [43]. Western blots were done with an anti-phospho-LAT antiserum specific for the tyrosine phosphorylation site Y191 on LAT and then reprobed with an anti-LAT mAb.
PLC-γ1 Phosphorylation and Mobilization of Downstream Events
The amount of PLC-γ1 is comparable in all 3 T cell populations. It binds to LAT at site Y136 after this amino acid has been phosphorylated by ZAP-70. PLC-γ1 is then activated in the receptor complex by tyrosine phosphorylation at amino acid Y783. This phosphorylation event is greatly impaired in the tolerant T cells 1 to 2 minutes after TCR and CD4 cross-linking. In assays for both total tyrosine phosphorylation in immunoprecipitates of PLC-γ1 and specific anti-phospho-Y783 phosphorylation in Western blots of total cell lysates, the activation level was only 5-10% of that seen in either naïve or pre-activated T cell populations [22]. As a consequence of this impairment, the downstream mobilization of intracellular calcium by IP3 production from PIP2 cleavage is profoundly reduced. Whether this in turn prevents the dephosphorylation of NF-AT by calcinuerin and prevents its shift to the nucleus is currently under investigation.
Inhibition of PLC-γ1 activation also reduces DAG formation. This should impair PKC-θ activation and mobilization of NF-κB to the nucleus. We have only examined the degradation of IκBα in tolerant T cells and found this to be impaired. DAG is also required for activation of RasGRP1. In conjunction with the inhibition of LAT phosphorylation, which should reduce plasma membrane bound Ras activation via Grb2-SOS, we expected to see a profound impairment in the activation of the ERK/MAP kinase pathway. However, the inhibition of ERK phosphorylation was relatively mild (2-3 fold), suggesting that the tolerant cells have other ways of stimulating this pathway [44].
Potential negative regulatory molecules increased in the tolerant cells
In addition to Fyn discussed above, adaptively tolerant T cells show modest increases in CD5 surface levels and increases in the cytoplasmic levels of the tyrosine phosphatases Shp2 and to a lesser extent Shp1 [unpublished data]. These molecules could be involved in negative feedback regulation and so we have begun to test their roles in adaptive tolerance by crossing the Rag2o/o 5C.C7 TCR transgenic mouse to CD5-deficient and the Shp1 moth eaten viable mutations. Both affect thymic selection, reducing the number of CD4+ T cells that mature. However, purification of the resting subset in the periphery and transferring it into the CD3εo/o host expressing pigeon cytochrome c revealed a completely normal expansion and tolerance induction process suggesting that despite their augmentation, these two negative signaling molecules are not critical for the induction or maintenance of adaptive tolerance [unpublished data].
COMPARISON WITH OTHER MODELS OF T CELL ANERGY
Biochemical studies on the induction and maintenance of T cell anergy in a variety of different systems have not been extensively carried out, because of a limitation in the number of cells available for analysis. Nonetheless, there have been enough differences described to warrant a preliminary comparison among the different models. In Table 1 we summarize the changes in expression of many of the proteins thought to be involved in the tolerance process and in Table 2 we summarize the impairments in activation of some of these proteins (usually as an assessment of phosphorylation of critical amino acids). The only extensive signaling comparison that has been performed in the same laboratory is between a murine clonal anergy model and an adaptive tolerance model restimulated under the same conditions [22]. We will first describe that comparison and then focus on individual signaling molecules and their altered status in particular anergic systems.
Table 1.
Changes in Signaling Molecules Observed in T or B cells Anergized in Different Ways
| adaptive tolerance | clonal anergy | costimulation blockade | superantigen model | ionomycin model | peptide model | oral tolerance | Treg | B cell anergy | |
|---|---|---|---|---|---|---|---|---|---|
| Expressed level of | |||||||||
| TCR/BCR | → | → | nd | nd | → | nd | → | → | ↓ |
| Lck/Lyn | → | →/↓ | nd | nd | → | nd | → | nd | → |
| Fyn | ↑ | →/↑ | nd | nd | → | nd | nd | nd | nd |
| c-Cbl | → | → | nd | nd | nd | nd | nd | nd | nd |
| Zap-70/Syk | ↑ | → | nd | nd | → | → | → | → | → |
| LAT | → | → | nd | nd | → | → | → | nd | nd |
| PLCγ1/2 | → | →/↓ | → | → | →/↓ | → | → | → | nd |
| DGKα | → | ↑ | nd | nd | ↑ | nd | ↑ | nd | nd |
| Cbl-b | ↑ | →/↑ | nd | nd | ↑ | ↑ | nd | nd | nd |
| GRAIL | → | ↑ | nd | nd | ↑ | ↑ | nd | nd | nd |
| PD1 | ↑ | nd | nd | nd | nd | nd | nd | nd | nd |
| CD5 | ↑ | nd | nd | nd | nd | nd | nd | nd | ↑ |
| SHP2 | ↑ | nd | nd | nd | → | nd | nd | nd | nd |
| p27kip1 | → | → | ↑ | * | nd | * | * | * | nd |
→, similar expression
↑, increased expression
↓, decreased expression
nd, not determined
*, does not decrease after activation
Table 2.
Changes in the Phosphorylation and/or Activation of Signaling Molecules Observed in T or B cells Anergized in Different Ways
| adaptive tolerance | clonal anergy | costimulation blockade | superantigen model | ionomycin model | peptide model | oral tolerance | Treg Cell | B cell anergy | |
|---|---|---|---|---|---|---|---|---|---|
| Phosphorylation or Activation of | |||||||||
| TCR-ζ/Ig-α/β | - | - | nd | -/ ++ | - | - | + | nd | ++ |
| Zap-70/Syk | ++ | + | nd | ++ | - | - | ++ | + | ++ |
| LAT | ++ | + | nd | ++ | ++ | ++ | ++ | + | nd |
| PLCγ1/2 | ++ | - | ++ | ++ | ++ | ++ | ++ | + | nd |
| [Ca++]i | ++ | - | ++ | ++ | ++ | nd | ++ | + | + |
| ERK | + | ++ | ++ | -/ ++ | nd | nd | - | -/ + | - |
| JNK | nd | ++ | nd | ++ | nd | nd | - | ++ | ++ |
| p38 | nd | ++ | nd | - | nd | nd | nd | - | nd |
| PKC-θ/IκBα | ++ | ++ | nd | nd | nd | nd | nd | + | ++ |
- , no defect
+ , slight defect
++ , defect
nd, not determined
Clonal Anergy vs Adaptive Tolerance
The response to PCC was measured in the murine T cell clone A.E7 after induction of clonal anergy by stimulation with plate bound anti-TCRβ for 16h in the absence of anti-CD28 or APCs, followed by washing and 7 days of rest in culture medium. These clonally anergic T cells showed no changes in their levels of TCR, CD4, and Zap-70, in contrast to the increases seen in adaptively tolerant T cells for CD4 and Zap-70. On stimulation by streptavidin cross-linking with anti-TCR and anti-CD4, Zap-70 phosphorylation of clonally anergic cells after 2 minutes was only slightly less than that of a non-anergized control population (<2 fold different) [22]. This small decrease was mostly seen in phosphorylation at site Y292, but it was not accompanied by a decrease in the tyrosine phosphorylation of cCbl (Figure 4B), as was seen in adaptively tolerant T cells (Figure 4A). Zap-70 kinase activity was unimpaired in vitro and the tyrosine phosphorylation of its in vivo substrates, LAT and PLC-γ1, was only decreased 30-55%, compared to a 90-95% decrease in adaptively tolerant T cells. As a consequence, activation of calcineurin and dephosphorlyation of NF-AT were normal. Instead, the signaling pathways affected in clonal anergy were the activation of MAP kinases and the mobilization of NF-κB to the nucleus. Previous studies [45] had shown that activation of Ras to the GTP-bound state was impaired and recent studies [46] have shown that this is a consequence of an increase in diacylglycerol kinase (DGK)-alpha levels which rapidly depletes DAG by phosphorylation and prevents activation of RasGRP1. The effect on NF-κB is presumably from suboptimal mobilization of PKC-θ to the plasma membrane. Why LAT/Grb2/SOS activation of Ras is not adequate for MAP kinase activation is unclear. Perhaps it is antagonized by the Ras-GAP protein CAPRI, which only requires increased calcium levels for its activation at the plasma membrane [30]. Finally, the block in IL-2 production in clonal anergy can be reversed with a DGK inhibitor [46]. By contrast, this inhibitor has no effect on adaptively tolerant T cells and there is no evidence for increased expression at the mRNA level [unpublished data].
Human Clonal Anergy and the Role of Fyn
The original studies of anergy induction in alloreactive human T cell clones revealed the preferential association of the Src-family tyrosine kinase Fyn in immunoprecipitates with TCRζ when the cells were stimulated with alloantigen alone [47]. In contrast, if B7 costimulation was also present, then Lck and Zap-70 were preferentially precipitated instead of Fyn. In addition, after the cells were induced into the anergic state, the activity of the Fyn protein was significantly increased. An increase in the amount or activity of Fyn has been seen in a number of other anergy models (Table 1). These observations led Boussiotis and colleagues to persue the role of Fyn in maintaining the clonal anergic state [48]. They found that Fyn was constitutively phosphorylated and associated with enhanced binding of cCbl through Fyn’s SH3 domain. cCbl also became tyrosine phosphorylated during anergy induction. Thus, it too was constitutively active and associated with the CrkL-C3G guanine nucleotide exchange protein for Rap1, which in turn was found mostly in its active GTP-bound form. Active Rap1 bound Raf1 and was postulated to sequester it away from Ras, preventing activation of the MEK-1/ERK pathway and subsequent IL-2 production. They also observed a block in Ras-GTP formation in anergic cells, similar to what was found in murine clonal anergy. Therefore, they postulated that it was the ratio of Ras-GTP to Rap1-GTP that determines whether the ERK pathway gets activated [48]. Generalization of this model, however, has been controversial. First, T cell clones from both the Fyn and CrkL knock out mice can still be anergized [unpublished data, 46], suggesting that the Rap1 activation pathway was not critical and that augmented DGK-α and its limitation on Ras-GTP formation is sufficient for the anergic phenotype. Furthermore, initial attempts to inhibit lymphocyte activation in a transgenic mouse expressing a constitutively active form of Rap1-V12 were not successful, although this mouse did show enhancement in T cell integrin activation [49]. A more recent study, using a transgenic mouse expressing a different and more potent constitutively active mutation (Rap1-63E), was successful at inhibiting peripheral CD4 T cell function [50]. The phenotype, however, was quite complex. No block in ERK activation or proliferation was seen in thymocytes and the block in peripheral T cell function and signaling were largely mediated by an enhanced frequency of CD4+, CD103+, CD25+ regulatory T cells. Removal of these cells left a population of CD4+, CD25- T cells manifesting less than a 2 fold impairment in IL-2 production and proliferation, and it was unclear whether all the Foxp3+ Tregs had been removed. Overall, these results suggest that the Rap1 activation pathway is not a major component in the maintenance of T cell clonal anergy.
Costimulatory Receptor Blockade and p27kip1
Models that use antibodies and solubilized receptors to inhibit costimulation during activation of primary T cells were initially viewed as an extension of clonal anergy models, because they were assumed to be allowing signal 1 to be delivered in the absence of signal 2 [9]. However, subsequent biochemical studies indicated that this state of anergy is very different [51]. Spleens stimulated with soluble anti-CD3 in the presence of CTLA4-Ig to block B7 molecules results in an anergic state in which PLC-γ1 activation and increases in intracellular calcium are blocked, in addition to inhibitory effects on the Ras/MAP kinase pathway [51]. Thus this state seems more akin to adaptive tolerance or a combination of this and clonal anergy.
This model has also been used to support a role for the cell cycle inhibitor p27kip1 in anergy induction [52-54]. Knock out or mutant mice lacking the cyclin-Cdk-binding domain of p27kip1 show no effects on proliferation or IL-2 production following initial activation, but later rounds of division and cytokine production are enhanced. These mice are also resistant to anergy induction by costimulatory blockade and this was correlated with continued cell cycle progression because of a failure to phosphorylate Smad3 [52]. In contrast, clonal anergy induction was not impaired in p27kip1-deficient mice [55] and the cell cycle arrest caused by the drug Sanglifehrin A does not result in anergy induction [56]. Thus, again these two types of anergy appear to be different.
Anergy Induced by Superantigens
Two basic models have been employed to study anergy induced by superantigens. The first involved injection of Mls-1a+ spleen cells into Mls-1a negative mice and study of the responding Vβ6+ T cells [10]. Biochemical studies utilized a Vβ8.1+ transgenic mouse expressing this Mls-1a-reactive TCRβ chain on 95% of its T cells [57]. The second model involved injection of the bacterial superantigen SEB, which induces anergy in all Vβ8+ T cells [11,12]. In this case biochemical studies were done on SEB-stimulated splenic T cells [58] or spleen cells from a TCR transgenic expressing 90%Vβ8+ T cells [59]. The Mls-1a studies revealed a block in the tyrosine phosphorylation of two proteins, p38 and p75, following anti-CD3 stimulation [57]. The former is likely to have been LAT and the latter was possibly Slp-76. The SEB studies revealed an impairment in the tyrosine phosphorylation of p36 (LAT?) and p70 (Zap-70?) proteins as well as a p150 protein following SEB stimulation [58]. The direct immunoprecipitation of Zap-70 showed that indeed its phosphorylation was impaired in the anergized T cells. In addition, in the TCR transgenic model, activation of PLC-γ1 and generation of IP3 were impaired resulting in a decreased intracellular calcium response [59]. No impairment of Erk1 phosphorylation was observed. The anergic state could be overcome by a calcium ionophore and its induction prevented by a constitutively active calcineurin transgene. Finally, in a variant model with multiple injections of the superantigen SEA, the inductions of both AP-1 and NF-κB p65 binding to their response elements in the IL-2 locus were blocked [60]. This model, however, is more complicated as it displays both suppressive and anergic components [61]. So a more direct assay of the NF-κB pathway in SEB-induced anergy is still needed.
A knock out of the E3 ubiquitin ligase Cbl-b has also been examined in a model of multiple injections of SEB [62]. Following one injection of SEB in WT mice, both Cbl-b and GRAIL are up-regulated [8,62]. After the second injection, the normal hyporesponsive, cytokine-production state seen in WT mice fails to materialize in the cells from the Cbl-b knock out mouse and many of the animals die from a cytokine storm [62]. This has been interpreted as evidence for a critical role of Cbl-b in maintaining the superantigen-induced anergic state. However, the system is quite complicated with both regulatory T cells and anergy affecting both CD4 and CD8 effector cells. Which components are altered by the Cbl-b deficiency is not clear. In addition, the elimination of a general negative feedback mechanism may simply lower the threshold for activation of the whole T cell signaling machinery (as seen in the augmentation of the primary expansion in the KO) and allow a normally blunted response to now be adequate to cause immunopathology [13].
In general, the biochemical results in the superantigen models are very similar to the findings for adaptive tolerance discussed earlier. Zap-70 and LAT phosphorylation are significantly decreased resulting preferentially in impairment of signaling in the calcium/calcineurin and NF-κB pathways. However, there are a few reported discrepancies. For example, the failure to phosphorylate Zap-70 in the initial SEB-induced anergy experiments resulted from a failure to recruit it to the TCR complex because of an impairment in TCRζ phosphorylation [58]. This suggests that the Src-family kinases were not activated. However, a direct examination of this in the SEB/TCR transgenic model found both Lck activation and TCRζ phosphorylation [59], consistent with the state of adaptive tolerance. Another variable is the impact on MAP kinase activation. SEB-induced anergic cells had no impairment of Erk1 phosphorylation, whereas this pathway was partially blocked in adaptive tolerance. In the Mls-1a-induced anergy model, however, both Erk and Jnk phosphorylation were significantly impaired following TCR cross-linking, while phosphorylation of p38 (after resting the cells for 3 hours to alleviate stress-induced activation) was significantly enhanced [63]. This dichotomy was confirmed with in vitro kinase assays. Interestingly, inhibiting p38 activation with the drug SB203580 partially reversed the block in proliferation and IL-2 production as well as inhibited IL-10 production by the anergic T cells. Because over-expression in a T cell hybridoma of the Tab1 protein, an activator of p38, reproduced a state similar to that of Mls-1a-induced anergy, including impairment of Erk phosphorylation, the authors proposed that augmentation of p38 was the primary cause of the inhibition of Erk activation. This mechanism, however, could not explain the Jnk inhibition they observed and the level of Tab1 protein subsequently turned out to be the same in naïve and anergic T cells. Furthermore, blocking p38 with SB203580 in the adaptive tolerance model did not reverse the block in IL-2 production and even inhibited the residual production at high concentrations [unpublished data]. Thus, the importance of changes in MAP kinase signaling for maintaining superantigen-induced anergy seems moot. For the most part, then, we would conclude that superantigen-induced anergic states in vivo are closely related to adaptive tolerance and involve a major impairment of the calcium/calcineurin pathway and a varying degree of impairment in the Ras/MAPkinase pathways.
Ionomycin-induced anergy and E3 ubiquitin ligases
The early finding that cyclosporin A had the ability to inhibit clonal anergy induction led Jenkins and colleagues to test whether calcium signaling alone could induce the anergic state [64]. The calcium ionophore, ionomycin, was capable of transiently creating an unresponsive state after overnight exposure, but this state decayed over a 4 day period and the CD4+ clone regained its ability to proliferate and produce IL-2. Nonetheless, Macian and colleagues pursued the molecular events occurring under this treatment of Th1 clones and determined a key role for NF-AT activation in the absence of AP-1 interactions, which resulted in the induction of a unique set of genes involved in the negative regulation [7]. These included several tyrosine phosphatases and DGK-α (which we now know plays a critical role in the maintenance of clonal anergy) [46]. Also in clonal anergy induction, there is an up-regulation of the transcription factors Egr2 and Egr3, downstream of NF-AT, which appears to be an important part of this negative regulatory program [65]. Another part of the induced program included the up-regulation of the E3 ubiquitin ligases, Cbl-b, Itch, and Grail as well as the ubiquitin binding protein Tsg101 [8]. On subsequent restimulation or even just homotypic cell adhesion in high density cultures, membrane translocation of Itch and Nedd4 to the detergent insoluble fraction was triggered, followed by monoubiquitination and the partial degradation of PKC-θ and RasGAP via endosomal sorting to lysosomes. Grail is already located in endosomal compartments, but its substrates there are not known. In addition, these E3 ligases synergized with the elevated levels of Cbl-b to inactivate PLC-γ1and completely degrade it. As a consequence calcium signaling through the TCR was fully inhibited, even in primary T cells. Although T cell/APC synapses initially formed in these anergic cells, the structures were not stable, with disintegration of the outer LFA-1 ring occurring after about 20 minutes. Interestingly, Itch and Cbl-b knock outs were resistant to ionomycin-induced anergy induction and did not down-regulate PLC-γ1 or PKC-θ. Their immunological synapses, however, were only partially stabilized.
A recent paper has claimed that the palmitoylation of LAT is impaired in ionomycin-induced anergy and that this critical signaling molecule fails to localize properly in lipid rafts for phosphorylation following TCR activation [66]. This result is controversial, however, as the original studies of Heissmeyer et al. found LAT to be totally membrane associated with a normal distribution in the detergent insoluble fraction [8]. The same is true in adaptive tolerance (Figure 5). In humans with Rheumatoid arthritis, however, anergic T cells isolated from the synovial joint fluid were found to have decreased LAT at their plasma membranes as a result of oxidative stress interfering with its palmitoylation [67].
Initially ionomycin-induced anergy was thought to mimic clonal anergy. However, both its functional and biochemical negative consequences are much more profound. In this state cytokine/chemokine inhibition includes IL-4, IFN-γ, and MIP-1α, while these are not much affected in clonal anergy [7,21]. The two states do have in common an increase in the level of DGK-α [46]; however, ionomycin-induced anergy has profound negative effects on PLC-γ1 activity stemming from increases in the E3 ligases [8], while the calcium/calcineurin signaling pathway is fairly normal in clonal anergy [68]. In addition, introduction into an anergic Th1 clone of a dominant negative Cbl protein (via an adenovirus vector) did not reverse the block in IL-2 production [46]. PLC-γ1 activity is down in adaptive tolerance; but the level of this protein is not affected [22] and T cells harboring the Cbl-b mutation can still be anergized [unpublished data]. Other proteins, such as Ras-GAP, which are also down-regulated in ionomycin-induced anergy [8], are in fact up-regulated in adaptive tolerance [unpublished data]. Finally, synapse formation is impaired even at the beginning of APC contact in adaptive tolerance [unpublished data], as opposed to only after 20 minutes in ionomycin-induced anergy [8]. Thus, it appears that ionomycin-induced anergy has a number of unique features that make it worthy of consideration as a separate category of anergy. It is possible that the strong 16 hour pharmacologic signal turns on all possible negative feedback mechanisms via the massive activation of the AP-1-independent/NF-AT transcription factor pathway as suggested by Rao and colleagues [7].
TCR transgenic mice immunized with soluble peptide antigens
This model exists in two forms. TCR transgenic mice are either directly injected iv. with soluble peptide ligands [13,66] or a cohort of cells (1-5 million) is transferred to a syngeneic non-transgenic recipient, which 24 hours later is injected with the peptide ligand [69]. Biochemistry experiments have been done mostly in the first model [66], because the second yields very few cells for analysis. In the first model, however, it is often difficult to be sure that all the cells are equally stimulated, because the large numbers of cells with the same receptor compete with one another for pMHC complexes. Despite these caveats, these two models are the most commonly used because they are easy to perform in vivo. The controls in these experiments were naïve mice and mice given LPS along with the peptide or mice given the antigen in adjuvant. The first molecule described to be involved in anergy in this system was CTLA-4 [70]. Administration of blocking anti-CTLA-4 antibody at the time of peptide injection prevented anergy induction. Subsequent studies with CTLA40/0 mice, however, gave mixed results with no effect on anergy induction in some cases [13,71] and a significant inhibition in others [72]. In different models, no effect was observed in adaptive tolerance using the CTLA4o/o[73], whereas blocking antibodies interfered in the induction by low dose antigen of a hyporesponsive state in primed CD4+ T cells [74] and in an anti-CD3 induced unresponsive state in spleen cells [75]. Because CTLA4 is found constitutively on Tregs as well as activated CD4+ effectors, and because it can negatively signal the T cell as well as activate the APC to make IDO, it is possible that the different outcomes in different models will relate to ways in which the anergic state is induced and maintained[76,77].
The peptide models have also been used to demonstrate up-regulation of Cbl-b during anergy induction [62]. The Cbl-b knock out mutation introduced onto several different TCR transgenic backgrounds resulted in a failure to induce soluble peptide anergy and instead generated a hyper-responsive state following repeated peptide injection. For Grail, a dominant negative version was retrovirally transduced into the bone marrow cells of TCR transgenic mice and used to reconstitute lethally irradiated syngeneic mice. Following peptide immunization, isolated GFP+, TCR+ transgenic T cells were found to be resistant to anergy induction [78]. Finally, peptide induction of anergy was abrogated in Egr3o/o mice [65]. Overall, it appears that many aspects of this soluble peptide model are different from either clonal anergy or adaptive tolerance and that therefore it should be viewed as a unique entity.
CD8 models of anergy
The first study of anergy in CD8+ T cells was done in murine T cell clones and showed a functional phenotype similar to that of CD4+ T cell clones [79]. The anergic cells had reduced IL-2 production and proliferation on antigen stimulation, but the differentiated effector functions of IFN-γ production and CTL killing were normal. The state could be reversed by stimulation with IL-2. No biochemical studies have ever been done in this system. In contrast, one adaptive tolerance model has been examined for its biochemical defects. In this system naïve female Rago/o CD8+ TCR transgenic T cells specific for the male H-Y antigen plus H-2Db were transferred into lethally irradiated Rago/o females reconstituted with 5 million bone marrow cells from CD3εo/o males [80]. The anergic cells did not flux calcium at all and the block was at the level of tyrosine phosphorylation. The Src kinases p56lck and p59fyn were hardly active and the zeta chains were not phosphorylated or associated with the rest of the TCR. Instead, constitutive CD3ε phosphorylation was observed and Zap-70 was bound to it and also constitutively phosphorylated. Whether this was adequate to phosphorylate LAT was not examined, but possibly not, since there was no activation of the calcium pathway [81]. This anergic state appears to be very different than anything ever seen for CD4+ T cells. It is not clear, however, whether this is a lineage difference or simply a reflection of this particular model.
Another anergic state called AINR has been described in CD8+ T cells that have been fully activated in the absence of CD4+ T cell help [82]. These cells lose their ability to proliferate and make IL-2, but retain the ability to make IFN-γ and to kill. The state is reversible with IL-2 stimulation like CD4+ T cell clonal anergy and biochemically the cells show a block in all three MAP kinase pathways. The status of PLC-γ1 and the calcium pathway, however, have not been reported.
Oral Tolerance and a Transplant Model of Anergy
Addition of antigen by an oral or nasal route has been reported to induce a hyporesponsive state involving regulatory T cells at low antigen concentrations or deletion and anergy at high antigen doses [83]. Although potentially very complex, activation of the remaining T cells has been studied in a few models. In a well studied high dose antigen model [19], stimulation of orally tolerant, OVA-specific, TCR transgenic CD4+ T cells with anti-TCR and anti-CD4 mAbs revealed a failure to phosphorylate ZAP-70, LAT and PLC-γ1 resulting in reduced calcium responses and a failure to mobilize NF-AT1 and 2 to the nucleus. Erk and Jnk activation, however, were normal. Although TCRζ chain phosphorylation was slightly impaired, Lck kinase activity was normal. Overall this pattern looks very similar to our adaptive tolerant state, which may emerge from the repeated presence of antigen entering from the gut. However, these cells were also impaired in the down-regulation of p27kip1 following IL-2R stimulation, which prevented the cells from dividing. The site of that inhibition was beyond STAT5a activation. In another transgenic model specific for OVA [7], spleen and lymph node CD4+ T cells from high dose, 5 day orally tolerized mice were stimulated with an agonist peptide and the up-regulation of 13 genes, found to be elevated in ionomycin-induced anergy, were also detected here. This included DGK-α and several phosphatases. It will be interesting to learn which of these many genes are critical for this anergic state.
Infusion of donor lymphocytes prior to organ transplantation has been known to improve graft survival [84,85]. A CD8+ TCR transgenic model of this form of tolerance was studied in a mouse cardiac allograft situation, where a single infusion of F1 donor spleen cells could eliminate the MLR and reduce the CTL response [20]. The recovered T cells did not proliferate or make IL-2 and IFN-γ on restimulation with alloantigen. Biochemically the activation of tyrosine phosphorylation with anti-TCR and anti-CD8 was impaired in the tolerant cells compared to naïve cells. ZAP-70 and LAT phosphorylation were completely absent and Lck autophosphorylation was greatly reduced suggesting a block in signaling at the earliest TCR events. This is again different from adaptive tolerance, where Lck activity is not affected very much.
T Regulatory Cells
The initial characterization of CD4+CD25+ T regulatory cells (Tregs) revealed that they did not proliferate or make IL-2 when stimulated with anti-TCR and anti-CD28 in vitro [86,87]. They would proliferate if IL-2 was added to the stimulus, but after expansion they still retained their anergic phenotype. Initial experiments showed a partial impairment in anti-CD3-induced calcium flux and a more profound defect when stimulated with peptide and splenic APC [88]. Subsequent experiments also showed a partial inhibition of Ras activation (Ras-GTP) and Erk phosphorylation with a modest decrease in PLC-γ1 activation and a generalized reduction in tyrosine phosphorylation, both in terms of magnitude and shortened kinetics [89,90], although one group has reported a decrease in JNK rather than ERK phosphorylation [91]. Total PLC-γ1, PKC-θ, and Ras were the same as in CD25 negative cells, suggesting that enhanced function of E3 ligases was not responsible for the effect. The block in proliferation and IL-2 production could largely be overcome by the addition of PMA and ionomycin and partially reversed by adding the DGK-α inhibitor to anti-CD3 plus anti-CD28. The latter stimulus enhanced the appearance of c-Rel and c-Jun in the nucleus but not c-Fos. The more profound inhibition in IL-2 production than would seem warranted by the modest reduction in TCR signaling might be explained by the interference of Foxp3 with NF-AT’s pairing with AP-1 that is required for a productive T cell activation program [92].
B Cell Anergy
One of the earliest models of anergy was described by Goodnow and colleagues in the double HEL B cell receptor transgenics (BCR and antigen) [93]. This state can be induced in 4 days in vivo and is manifested by a 90% down-regulation of the IgM BCR and a block in tyrosine phosphorylation through the IgD receptor [94]. Activation of the NF-κB and JNK pathways are blocked, but ERK phosphorylation is normal [94]. The calcium/calcineurin pathway was dampened, but sufficient NF-AT signaling was maintained to possibly induce a negative regulatory program in vivo similar to ionomycin-induced T cell anergy. This signaling pattern seems to be related to the rapid turnover of BCRs in response to antigen and depends heavily on the cholesterol levels in the membrane [95]. When cholesterol is removed with methyl-β-cyclodextrin, BCRs recycle to the surface and normal activation of the NF-κB pathway is restored. Thus, while receptor sequestration in anergic B cells from chronic antigen stimulation accomplishes a similar desensitization to antigen as seen in adaptive T cell tolerance, the biochemical mechanism is quite different.
CONCLUSION
Table 1 lists most of the molecules that have been implicated in anergy induction or its maintenance. Everything from BCR turnover to a transcription factor-induced negative regulatory program has been invoked as the cause in one study or another. Enhancement of phosphatases, kinases, E3 ligases, cell cycle inhibitors, and surface molecules mediating coinhibtion have all been implicated. What seems clear is that all these possible mechanisms do not operate in every model system. This apparent plethora of ways to keep lymphocytes in check has undoubtedly evolved to optimize the effectiveness of the immune response as it walks the fine line between destruction of the pathogen and prevention of immunopathology. Just as there are many different peripheral tolerance mechanisms (deletion, anergy, and regulation); so there appear to be a large number of different molecular mechanisms to carry them out. Although this complexity has made the unraveling of the puzzle particularly difficult, in the long run this could be advantageous for pharmacologic intervention by providing numerous possible targets for chemical intervention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–9. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–12. [PubMed] [Google Scholar]
- [4].Boussitis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993;178:1753–63. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- [6].Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- [7].Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–31. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- [8].Heissmeyer V, Macian F, Im S, Varma R, Feske S, Venuprasad K, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nature Immunol. 2004;5:255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- [9].Wells AD, Walsh MC, Sankaran D, Turka LA. T cell effector function and anergy avoidance are quantitatively linked to cell division. J Immunol. 2000;165:2432–43. doi: 10.4049/jimmunol.165.5.2432. [DOI] [PubMed] [Google Scholar]
- [10].Rammensee HG, Kroschewski R, Frangoulis B. Clonal anergy induced in matureVβ6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1992;339:541–4. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- [11].Rellahan BL, Jones LA, Kruisbeek AM, Fry AM, Matis LA. In vivo induction of anergy in peripheral Vβ8+ T cells in staphylococcal enterotoxin B. J Exp Med. 1990;172:1091–100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kawabe Y, Ochi A. Selective anergy of Vβ8+, CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1065–70. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frauwirth KA, Alegre ML, Thompson CB. CTLA-4 is not required for induction of CD8+ T cell anergy in vivo. J Immunol. 2001;167:4936–41. doi: 10.4049/jimmunol.167.9.4936. [DOI] [PubMed] [Google Scholar]
- [14].Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- [15].Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–8. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- [16].Lanoue A, Bona C, von Boehmer H, Sarukhan A. Condition that induce tolerance in mature CD4+ T cells. J Exp Med. 1997;185:405–14. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alder AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–55. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- [18].Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–39. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- [19].Asai K, Hachimura S, Kimura M, Toraya T, Yamashita M, Nakayama T, et al. T cell hyporesponsiveness induced by oral administration of ovalbumin is associated with impaired NFAT nuclear translocation and p27kip1 degradation. J Immunol. 2002;169:4723–31. doi: 10.4049/jimmunol.169.9.4723. [DOI] [PubMed] [Google Scholar]
- [20].McKay DB, Irie HY, Hollander G, Ferrara JLMF, Strom TB, Li YS, et al. Antigen-induced unresponsiveness results in altered T cell signaling. J Immunol. 1999;163:6455–61. [PubMed] [Google Scholar]
- [21].Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- [22].Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–91. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- [23].Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–17. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–12. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- [26].Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. Regulation of T cell receptor signaling by a Src-family protein tyrosine kinase (p59FYN) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- [27].Chan AC, Iwashima M, Turck CW, Weiss A. Zap70: a 70-kD protein-tyrosine kinase that associates with the TCRζ-chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- [28].Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the Zap70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- [29].Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the RAS pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- [30].Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- [31].Sommers CL, Samelson LE, Love PE. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays. 2004;26:61–7. doi: 10.1002/bies.10384. [DOI] [PubMed] [Google Scholar]
- [32].Randriamampita C, Trautmann A. Ca2+ signals and T lymphocytes; “New mechanisms and functions in Ca2+ signaling”. Biol Cell. 2004;96:69–78. doi: 10.1016/j.biolcel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [33].Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- [34].Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- [35].Altman A, Villalba M. Protein kinase C-θ (PKCθ): it’s all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- [36].Stilo R, Liguoro D, Di Jeso B, Formisano S, Consiglio E, Leonardi A, et al. Physical and functional interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB essential modulator. J Biol Chem. 2004;279:34323–31. doi: 10.1074/jbc.M402244200. [DOI] [PubMed] [Google Scholar]
- [37].DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible I[unk]B phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garrity PA, Chen D, Rothenberg EV, Wold BJ. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994;14:2159–69. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- [40].Pitcher LA, Young JA, Mathis MA, Wrage PC, Bartok B, van Oers NSC. The formation and functions of the 21- and 23-kDa tyrosine-phosphorylated TCRζ subunits. Immunological Reviews. 2003;191:47–61. doi: 10.1034/j.1600-065x.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- [41].Davanture S, Leignadier J, Milani P, Soubeyran P, Malissen B, Malissen M, et al. Selective defect in antigen-induced TCR internalization at the immune synapse of CD8 T cells bearing the Zap-70(Y292F) mutation. J Immunol. 2005;175:3140–9. doi: 10.4049/jimmunol.175.5.3140. [DOI] [PubMed] [Google Scholar]
- [42].Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120 c-Cbl in T lymphocytes. J Exp Med. 1996;183:301–6. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–46. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- [44].Chau LA, Madrenas J. Phospho-LAT-independent activation of the ras-mitogen-activated protein kinase pathway: a differential recruitment model of TCR partial agonist signaling. J Immunol. 1999;163:1853–8. [PubMed] [Google Scholar]
- [45].Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–78. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- [46].Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-α. Nature Immunol. 2006;7:1166–73. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- [47].Boussiotis VA, Barber DL, Lee BJ, Gribben JG, Freeman GJ, Nadler LM. Differential association of protein tyrosine kinases with the T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J Exp Med. 1996;184:365–376. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–28. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- [49].Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–58. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- [50].Li L, Greenwald RJ, Lafuente EM, Tzachanis D, Berezovskaya A, Freeman GJ, et al. Rap1-GTP is negative regulator of Th cell function and promotes the generation of CD4+CD103+ regulatory T cells in vivo. J Immunol. 2005;175:3133–39. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- [51].Wells AD, Liu QH, Hondowicz B, Zhang J, Turka LA, Freeman BD. Regulation of T cell activation and tolerance by phospholipase Cγ-1-dependent integrin avidity modulation. J Immunol. 2003;170:4127–33. doi: 10.4049/jimmunol.170.8.4127. [DOI] [PubMed] [Google Scholar]
- [52].Li L, Iwamoto Y, Berezovskaya A, Boussiotis VA. A pathway regulated by cell cycle inhibitor p27kip1 and checkpoint inhibitor Smad3 is involved in the induction of T cell tolerance. Nature Immunol. 2006;7:1157–65. doi: 10.1038/ni1398. [DOI] [PubMed] [Google Scholar]
- [53].Rowell EA, Walsh MC, Wells AD. Opposing roles for the cyclin-dependent kinase inhibitor p27kip1 in the control of CD4+ T cell proliferation and effector function. J Immunol. 2005;174:3359–68. doi: 10.4049/jimmunol.174.6.3359. [DOI] [PubMed] [Google Scholar]
- [54].Rowell EA, Wang L, Hancock WW, Wells AD. The cyclin-dependent kinase inhibitor p27kip1 is required for transplantation tolerance induced by costimulatory blockade. J Immunol. 2006;177:5169–76. doi: 10.4049/jimmunol.177.8.5169. [DOI] [PubMed] [Google Scholar]
- [55].Powell JD, Bruniquel D, Schwartz RH. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27kip1. Eur J Immunol. 2001;31:3737–46. doi: 10.1002/1521-4141(200112)31:12<3737::aid-immu3737>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [56].Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- [57].Bhandoola A, Cho EA, Yui K, Saragovi HU, Greene MI, Quill H. Reduced CD3-mediated protein tyrosine phosphorylation in anergic CD4+ and CD8+ T cells. J Immunol. 1993;151:2355–67. [PubMed] [Google Scholar]
- [58].Migita K, Eguchi K, Kawabe Y, Tsukada T, Ichinose Y, Nagataki S. Defective TCR-mediated signaling in anergic T cells. J Immunol. 1995;155:5083–87. [PubMed] [Google Scholar]
- [59].Kimura M, Yamashita M, Kubo M, Iwashima M, Shimizu C, Tokoyama K, et al. Impaired Ca/calcineurin pathway in in vivo anergized CD4 T cells. Int Immunol. 2000;12:817–24. doi: 10.1093/intimm/12.6.817. [DOI] [PubMed] [Google Scholar]
- [60].Sundstedt A, Sigvardsson M, Leanderson T, Hedlund G, Kalland T, Dohlsten M. In vivo anergized CD4+ T cells express perturbed AP-1 and NFκB transcription factors. Proc Natl Acad Sci USA. 1996;93:979–84. doi: 10.1073/pnas.93.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, et al. Essential Role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–77. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [63].Ohkusu-Tsukada K, Tominaga N, Udono H, Yui K. Regulation of the maintenance of peripheral T-cell anergy by TAB1-mediated p38α activation. Mol Cell Biol. 2004;24:6957–66. doi: 10.1128/MCB.24.16.6957-6966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jenkins MK, Pardoll DM, Mizuguchi J, Chused TM, Schwartz RH. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–13. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nature Immunol. 2005;6:472–80. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- [66].Hundt M, Tabata H, Jeon M, Hayashi K, Tanaka Y, Krishna R, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–22. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [67].Gringhuis SJ, Leow A, Papendrecht-van der Voort EAM, Remans PHJ, Breedveld FC, Verweij CL. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164:2170–79. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- [68].Mondino A, Whaley CD, DeSilva DR, Li W, Jenkins MK, Mueller DL. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J Immunol. 1996;157:2048–57. [PubMed] [Google Scholar]
- [69].Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–29. [PubMed] [Google Scholar]
- [70].Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–17. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- [71].Frauwirth KA, Alegre ML, Thompson CB. Induction of T cell anergy in the absence of CTLA-4/B7 interaction. J Immunol. 2000;164:2987–93. doi: 10.4049/jimmunol.164.6.2987. [DOI] [PubMed] [Google Scholar]
- [72].Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- [73].Inobe M, Schwartz RH. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J Immunol. 2004;173:7239–48. doi: 10.4049/jimmunol.173.12.7239. [DOI] [PubMed] [Google Scholar]
- [74].Mirshahidi S, Huang CT, Sadegh-Nasseri S. Anergy in peripheral memory CD4+ T cells induced by low avidity engagement of T cell receptor. J Exp Med. 2001;194:719–31. doi: 10.1084/jem.194.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest. 2001;108:895–903. doi: 10.1172/JCI13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Takahashi T, Tagami T, Yamazaki S, Ude T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA-4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- [78].Seroogy CM, Soares L, Ranheim EA, Su L, Holness C, Bloom D, et al. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- [79].Otten GR, Germain RN. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–31. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- [80].Guillaume S, Tuosto L, Tanchot C, Bartolo VD, Acuto O, Rocha B. Proximal changes in signal transduction that modify CD8+ T cell responsiveness in vivo. Eur J Immunol. 2003;33:2551–56. doi: 10.1002/eji.200324196. [DOI] [PubMed] [Google Scholar]
- [81].Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, et al. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–90. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- [82].Tham EL, Mescher MF. Signaling alterations in activation-induced nonresponsive CD8 T cells. J Immunol. 2001;167:2040–48. doi: 10.4049/jimmunol.167.4.2040. [DOI] [PubMed] [Google Scholar]
- [83].Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].El-Malik F, Malik ST, Varghese Z, Sweny P, Moorhead JF. The enhancing and sensitizing effects of donor blood components, including dendritic cells, in a rat renal allograft model. Transplantation. 1984;38:213–6. doi: 10.1097/00007890-198409000-00003. [DOI] [PubMed] [Google Scholar]
- [85].Lenhard V, Renner D, Hansen B, Opelz G. Suppression of antibody response and prolongation of skin graft survival by multiple blood transfusions in the rat. Transplantation. 1985;39:424–9. doi: 10.1097/00007890-198504000-00017. [DOI] [PubMed] [Google Scholar]
- [86].Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- [88].Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nature Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- [89].Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–94. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- [90].Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, et al. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–73. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Su Leon, Creusot RJ, Gallo EM, Chan SM, Utz PJ, Fathman CG, et al. Murine CD4+CD25+ regulatory T cells fail to undergo chromatin remodeling across the proximal promoter region of the IL-2 gene. J Immunol. 2004;173:4994–5001. doi: 10.4049/jimmunol.173.8.4994. [DOI] [PubMed] [Google Scholar]
- [92].Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- [93].Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- [94].Healy JI, Goodnow CC. Positive versus negative signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1998;16:645–70. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- [95].Blery M, Tze L, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-κB in B cell clonal anergy. J Exp Med. 2006;203:1773–83. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]