Summary
Background
Cycloxygenase-2 (COX-2) overexpression and subsequent prostaglandin E2 (PGE2) production are frequently associated with human non-small cell lung cancer (NSCLC) and are involved in tumor proliferation, invasion, angiogenesis, and resistance to apoptosis. Here we report that ciglitazone downregulates PGE2 in NSCLC cells.
Methods
PGE2 ELISA assay and COX-2 ELISA assay were performed for measuring PGE2 and COX-2 respectively in NSCLC. The mRNA level of COX-2 was measured by semi-quantitative RT-PCR. The transient transfection experiments were performed to measure COX-2 and PPRE promoter activity in NSCLC. Western blots were unitized to measure PGE synthase (PGES) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) protein expression.
Results
COX-2 ELISA assays suggested that ciglitazone-dependent inhibition of PGE2 occurs through the suppression of COX-2. Ciglitazone treatment suppressed COX-2 mRNA expression and COX-2 promoter activity while upregulating peroxisome proliferator-response element (PPRE) promoter activity. Ciglitazone did not modify the expression of enzymes downstream of COX-2 including PGES and 15-PGDH. Utilization of a dominant-negative PPARγ showed that the suppression of COX-2 and PGE2 by ciglitazone is mediated via non-PPAR pathways.
Conclusion
Taken together, our findings suggest that ciglitazone is a negative modulator of COX-2/PGE2 in NSCLC.
Introduction
Tumor COX-2 and its metabolite prostaglandin E2 (PGE2) play important roles in regulating tumor invasion, angiogenesis and apoptotic resistance [1-3]. Elevated COX-2 expression has been documented in a variety of malignancies including colon, gastric, esophageal, prostate, pancreatic, breast, and lung carcinomas [3-11]. Consistent with findings related to COX-2, PGE2 is also known to possess properties that promote malignant growth. For example, PGE2 stimulates angiogenesis, invasiveness and inhibits immune surveillance (7). Specific inhibition of COX-2 or PGE2 led to significant in vivo tumor reduction in murine lung cancer models [1]. Targeted inhibition of COX-2 and/or PGE2 are now regarded as poteintial strategies to stop completely the occurance or progress of cancers. However, inhibition with COX-2 inhibitors such as coxibs has been recently reported to be associated with increased cardiovascular risk [12]. Therefore we sought to identify reagents other than coxibs which might decrease COX-2/PGE2 levels in NSCLC cells.
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the PPAR family of ligand-activated nuclear receptors. Thiazolidinediones (TZDs), a class of anti-diabetic and insulin sensitizing drugs, also known as PPARγ ligands, include troglitazone, ciglitazone, rosiglitazone and pioglitazone. The role of TZDs in the progression of cancer has been the subject of extensive study during the past several years. Recent evidence indicates that ciglitazone exhibits antiproliferative activities against a variety of human cancer cell lines including prostate, colon, breast, thyroid, melanoma and lung ([13] [14] [15]. Further, ciglitazone is reported to induce differentiation and apoptosis in non small cell lung cancer [16]. In addition, the TZDs have been found to decrease lung cancer induced angiogenesis and to limit proliferation [17]. We recently have found that different TZDs have the capacity to modulate arachidonic acid metabolism by a variety of pathways[18]. The aim of our present study was to investigate the mechanism of ciglitazone’s capacity to modulate PGE2 levels in lung adenocarcinoma cells.
Materials and Methods
Cell Culture and Reagents
Human A427 (obtained from Dr. J.A. Radosevich, Northw estern University) and A549 (American Type Culture Collection, Manassas, VA) are maintained in RPMI 1640 medium (Mediatech Inc., Herndon, VA) supplemented with 4.5 g/L glucose and 4 mM L-glutamine, 100 units/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA), and 10% fetal calf serum (Gemini Bio-Products, Woodland, CA). Cell cultures were grown in 5% CO2 atmosphere at 37 °C.
PGE2 ELISA Assay
Cells were plated in 6-well plates in RPMI containing 10% FBS medium and cultured overnight. Next day, cells were treated with 10 μM ciglitazone (Axxora LLC, San Diego, CA) for 24 hours. DMSO was utilized here as diluent. Arachidonic acid (15 μM) was added to the culture medium one hour before collecting the culture medium for PGE2 assay. PGE2 levels in the culture medium were determined by PGE2 EIA kits (Cayman Chemicals, Ann Arbor, MI). Cells were washed with PBS and were lysed with RIPA buffer for the total protein measurement using Bradford reagent (Biorad, Hercules, CA).
COX-2 ELISA Assay
Cells were plated in a six-well plate (0.2-0.3 × 106 cells/well) in RPMI medium containing 10% FBS and treated as described above. The lysates were stored at -80 °C for protein isolation. COX-2 protein was measured by the Human Cycloxygenase-2 Enzyme Immunometric Assay Kit (Assay Designs, Ann Arbor, MI) using 30 μg of each protein sample.
Western Blot Analysis of Cellular Proteins
NSCLC cells were cultured in a 6-well plate for 24 hours. Cells were washed with PBS once and lysed with a lysis buffer containing 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1x complete protease inhibitor mixture (Roche Diagnostics Corp., Indianapolis, IN), 1 mM Na3Vo4, 1 mM NaF. The protein content was measured using Bradford reagent (Biorad). An equal amount (20 μg) of the whole cell protein was run and separated by SDS-PAGE and transferred on polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA). For determining the expression of all three prostaglandin E synthase and 15-PGDH expression in ciglitazone treatment, polyclonal antibodies (Cayman Chemical) against Prostaglandin E Synthase-1 (microsomal), Prostaglandin E Synthase (cytosolic), Prostaglandin E Synthase-2 (microsomal) and 15-PGDH were used. Glyceraldehyde-3-Phosphate Dehydrogenase Monoclonal Antibody (Advanced Immuno Chemicals Inc., Long Beach, CA) at a concentration of 0.2-2 μg/10 ml in TBS (100 mM Tris-HCl, 1.5 M NaCl, pH 7.4) with 5% nonfat milk. The blots were subsequently incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1 μg/10 ml. Detection of proteins on the blots was achieved using the Amersham ECL Detection System (Amersham Biosciences, Piscataway, NJ).
Semi-quantitative RT-PCR
Total RNA was isolated from cells treated with the 10μM ciglitazone for 24 hours using RNeasy Mini kit (Qiagen, Valencia, CA). Reverse transcription was performed using 2 μg total RNA and SuperScript II RNase H Reverse transcriptase (Invitrogen Corp.). COX-2 was amplified using COX-2 primers and following the instructions of the manufacturer. β-Actin gene expression was used as a standard. The sequence of the primer is:
5′-TCCTATTATACTAGAGCCCTTCCT-3′ and
5′TTCCACAATCTCATTTGAATCAGG-3′.
Transient transfection
The COX-2 promoter construct (-1432/+59) was a generous gift of Dr. Tadashi Tanabe (National Cardiovascular Research Institute, Osaka, Japan). This construct contains COX-2 promoter sequences in a firefly luciferase reporter construct. The PPRE promoter construct (tk-PPRE × 3-luciferase) was a kind gift of Dr. Tontonoz (University of California, Los Angeles). The dn.PPARγ construct was a generous gift of Dr. VK Chatterjee (Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge). Cells were seeded at a density of 3 × 106 cells/well (A549) in a 6 well plate in RPMI medium containing 10% FBS and cultured overnight. One μg of each PPRE, COX-2 or dn. PPARγ constructs was transfected in NSCLC. The renilla luciferase reporter pRL-CMV plasmid (Promega, Madison, WI) 0.01 μg/well was cotransfected as an internal control. Effectene transfection reagent (Qiagen) according to the manufacturer’s protocol. Ciglitazone (10 μg) was added 24 hours after the transfection in a serum free medium for another 24 hours. Luciferase activities were measured using the dual-luciferase reporter assay kit (promega). Here relative luciferase units were calculated as the ratio of firefly luciferase to renella lucifearse for the promoter activities. For dominant-negative PPARγ (dn. PPARγ) overexpression, 1 μg of the PCDNA3 control vector or 1μg of dn. PPARγ vector was transfected in each well. The ciglitazone was added 24 hours after the transfection in a serum free medium for another 24 hours. Arachidonic acid (15 μM) was added 1 hour before harvesting the medium. Culture medium was collected for measuring PGE2 levels by using PGE2 EIA kit (Cayman Chemicals).
Statistical Analysis
Probability values were calculated using two-tailed non paired Student’s t test. Tests of statistical significance were significant if P < 0.05.
Results
Ciglitazone inhibit PGE2 production in NSCLC
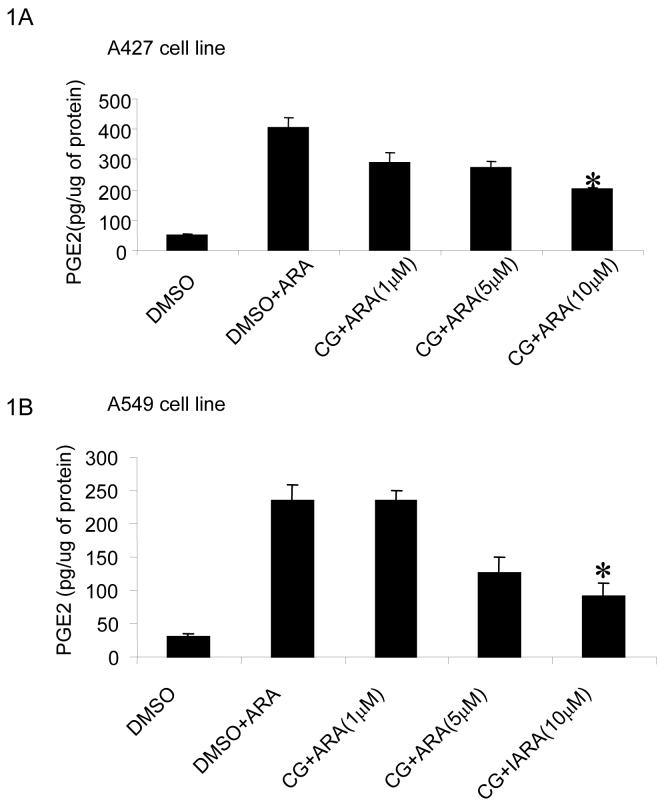
Two NSCLC cell lines, A427 and A549 were utilized to access the impact of ciglitazone on PGE2 production. A dose response study has been performed to detect the optimal concentration of ciglitazone for suppressing PGE2 in NSCLC. Treatment with ciglitazone (1-10 μM) for 24 hours dose-dependently inhibited PGE2 levels in both A427 (Fig 1A) and A549 (Fig 1B) cell lines. Here we also counted the number of cells in the plates after treating them with DMSO or ciglitazone for 24 hrs and found that 10 μM ciglitaozone showed an optimal effect on PGE2 in these cell lines without causing cellular cytotoxicity.
Fig 1.
Ciglitazone decreases PGE2 release by A427 and A549 cells: Tumor cells were treated with ciglitazone (1-10 μM) for 24 hours in a 6 well plate in serum free DMEM. Arachidonic acid (15 μM) was applied one hour prior to collecting the conditioned culture medium (50 μl) for assessment of PGE2 using a PGE2 enzyme immunoassay kit. Ciglitazone decreased PGE2 in a dose dependent manner in A427 (A) and A549 cells (B). (* P < 0.05). All data are representative of five independent experiments.
Ciglitazone inhibits COX-2 expression
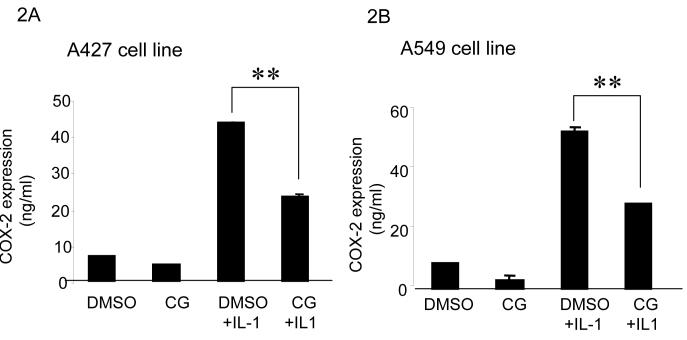
Two NSCLC cell lines, A427 and A549 were selected for studies. Here we tested whether ciglitazone has the capacity to inhibit IL-1β induced COX-2 expression in NSCLC cells. COX-2 ELISA assays showed that COX-2 levels were markedly increased when stimulated with IL-1β (280 units/ml) in both cell lines (Fig 2A and 2B). In both cell lines, ciglitazone (10 μM) significantly suppressed IL-1β induced COX-2 expression (Fig 2A and 2B).
Fig 2.
Ciglitazone inhibits COX-2 expression in NSCLC cells: Ciglitazone (10 μM, 24 hours) decreases IL-1β induced COX-2 expression by 2 fold in both A427 (2A) and A549 (B) cell lines as determined by COX-2 ELISA Assay (** p< 0.01). All data are representative of three independent experiments.
Ciglitazone significantly suppresses COX-2 mRNA and promoter activity while increasing PPRE activity
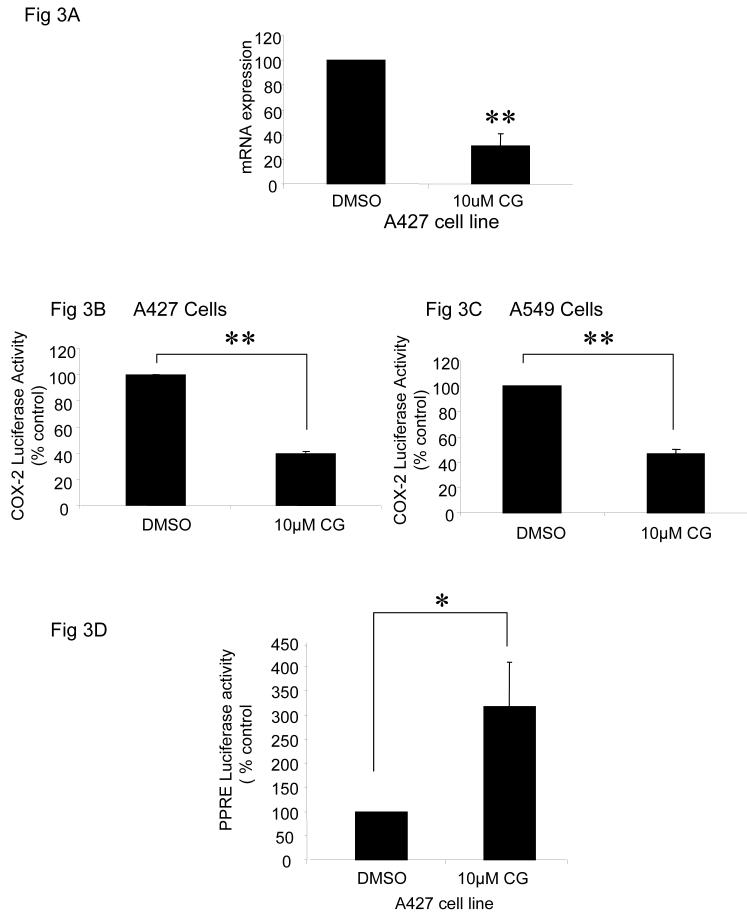
Regulation of COX-2 may occur either at the transcriptional or posttranscriptional level. To determine whether ciglitazone inhibits COX-2 mRNA levels, real time RT-PCR was performed in the A427 cell line. Ciglitazone treatment significantly decreased COX-2 mRNA expression by 60% (Fig 3A).
Fig 3.
Ciglitazone decreases COX-2 mRNA expression and COX-2 promoter activity, and upregulates PPRE activity. Ciglitazone treatment (24 hrs) decreased COX-2 mRNA expression by approximately 60% (** p< 0.01); (A). This suppression of COX-2 mRNA was associated with the suppression of its promoter activity in NSCLC cells (** p< 0.01) (B and C). To determine whether ciglitazone modifies PPRE activity, A427 cells were transfected with phRG-TK and PPRE-luciferase constructs for 24 hours followed by the addition of ciglitazone (10 μM) or DMSO for additional 24 hours. Ciglitazone treatment significantly increased PPRE activity by 3 fold (* p< 0.05) in A427 cells (D). All data are representative of three independent experiments.
To determine whether this decrease of COX-2 mRNA is associated with a decrease in the promoter activity, a human COX-2 promoter construct was transiently transfected in both A427 and A549 cell lines. In both cell lines, ciglitazone (10 μM for 2 hours) significantly decreased COX-2 promoter activity (3B and 3C). Ciglitazone treatment did not alter COX-2 mRNA stability (data not shown). Thus these results suggest that ciglitazone suppresses COX-2 expression by inhibiting COX-2 gene expression.
In order to understand whether ciglitazone increases PPARγ activity, we performed transient transfection experiments using a PPRE promoter construct and found that 24 hour treatment with ciglitazone significantly increased PPRE promoter activity by about 3 fold (3D).
TZDs do not alter the expression of PGE synthases and 15-PGDH in NSCLC cells
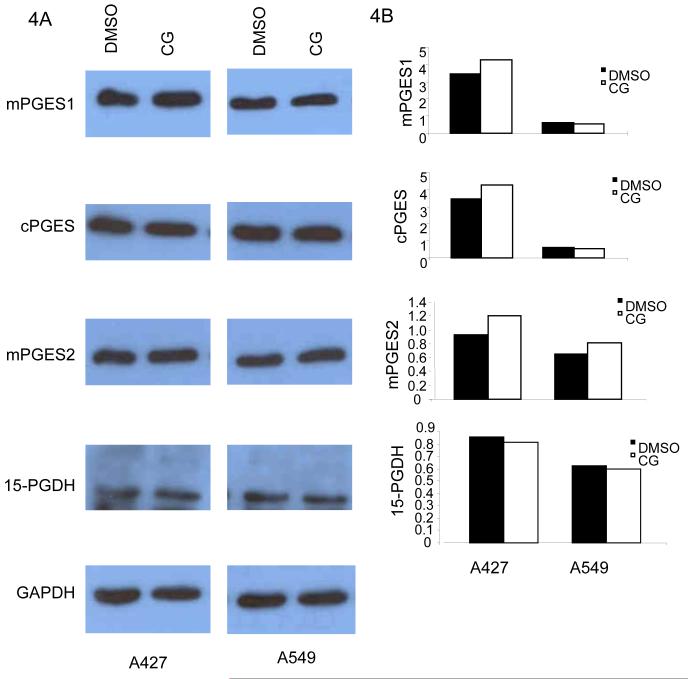
In order to further understand the mechanism underlying ciglitazone-mediated suppression of PGE2 in NSCLC cells, the enzymes downstream events of COX-2 that regulate PGE2 production were assessed. Here three enzymes possessing PGE synthetic activity have been identified. Microsomal PGES (mPGES1) is an enzyme downstream of COX-2 that affects PGE2 production while cytosolic PGES (cPGES) is functionally coupled with COX-1 only and mPGES2 is functionally coupled with both COX-1 and COX-2. As described previously, the first step of metabolism of PGE2 is catalyzed by the 15-PGDH enzyme, which produces biologically inactive 15-keto-prostaglandins [19]. Here we determined if these three synthase enzymes (mPGES1, cPGES, mPGES2) and 15-PGDH were altered by TZDs in A427 and A549 cell lines (Fig 4A and B). Western blot analysis of the mPGES1, cPGES, mPGES2 and 15-PGDH enzymes revealed no ciglitazone mediated change in their expression in NSCLC.
Fig 4.
Ciglitazone mediated suppression of COX-2 and PGE2 are PGES and 15-PGDH independent: The cells were treated with ciglitazone or DMSO for 24 hours in serum free medium. Ciglitazone (10 μM) failed to inhibit mPGES1, cPGES, mPGES2 and 15-PGDH expressions as compared to the DMSO control in both A427 and A549 cells (4) suggesting that the ciglitazone suppressed PGE2 via PGES and 15-PGDH independent ways. The bottom panel of GAPDH expression (A) shows the equal loading of the protein samples. Densitometric analysis of the Western blot is shown in B. The expression of PGES and 15-PGDH are normalized for the expression of GAPDH. All data are representative of three independent experiments.
Suppression of COX-2 and PGE2 by ciglitazone is PPARγ independent
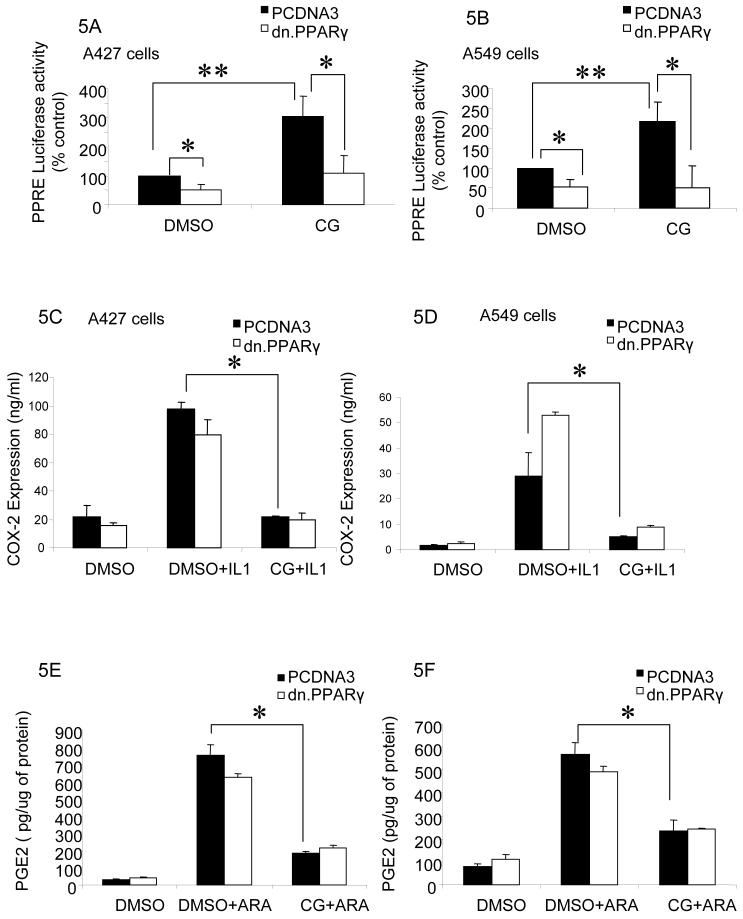
Because ciglitazone is known as a PPARγ ligand, we determined whether the suppressive effect of ciglitazone on COX-2 and PGE2 is PPARγ dependent. Here, a dominant negative PPARγ plasmid [20] construct was transfected in both A427 and A549 cells. Ciglitazone treatment clearly shows a significant increase in PPRE activity, which was suppressed by expressing dn. PPARγ. In addition, expression of dn. PPARγ significantly diminishes the basal PPRE activity in both cell lines (Fig 5A, 5B). This clearly shows that dn. PPARγ expression is blocking both basal and ciglitazone mediated induction of PPRE-luciferase activity. When dn. PPARγ was overexpressed, ciglitazone still continued to decrease COX-2 (Fig 5C and 5D) and PGE2 (Fig 5E and 5F) significantly in NSCLC cells suggesting that the effect of ciglitazone on both COX-2 and PGE2 is PPARγ independent.
Fig 5.
Ciglitazone decreases COX-2 expression and PGE2 production in a PPARγ independent manner: Both A427 and A549 cells were transiently transfected with a dn. PPARγ construct for 24 hrs followed by the addition of ciglitazone (10 μM, 24 hrs). Expression of dn. PPARγ suppressed both basal level as well as ciglitazone induced PPRE activity in both A427 (A) and A549 cells (B). Consistent with the findings in figure 2, COX-2 ELISA assays show that IL-1β treatment significantly increases COX-2 protein expression and addition of ciglitazone significantly decreases COX-2 expression (* P < 0.05). Introduction of a dn. PPARγ [20] did not significantly change the suppressive effect of ciglitazone on COX-2 in both A427 (C) and A549 (D) cells. PGE2 is inhibited by ciglitazone treatment in both cell lines (E and F) as shown previously (* P < 0.05). Here the suppression effect of ciglitazone on PGE2 is also PPARγ expression independent as there is no significant changes in suppression of PGE2 production by ciglitazone observed in dn. PPARγ [20] expressing NSCLC cells (E and F). Data are one representative experiment out of three independent experiments.
Discussion
Recent investigations support the importance of COX-2 and PGE2 in the pathogenesis of lung cancer [5, 21, 22]. Because COX-2 and PGE2 appear to be important in modulating apoptosis resistance, angiogenesis, invasion and cell mediated immunity, agents targeting the arachidonic acid pathway have attracted attention for cancer prevention and therapy. The recent predominant focus has been directed to the coxibs which had originally been developed for arthritis and pain control. Despite the interest and clinical utility of the coxibs, there may be advantages for using alternative agents that have COX-2 inhibitory effects or modulate other aspects of arachidonic acid metabolism because an increased cardiovascular risk has been associated with the use of some coxibs. In addition, other agents that could have the capacity to inhibit COX-2 or decrease PGE2 production may also have additional antitumor properties that could be advantageous. We recently showed that pioglitazone and rosiglitazone decrease PGE2 in non-small cell lung cancer cells by upregulating 15-hydroxyprostaglandin dehydrogenase [18]. Here, we consider ciglitazone because it has previously been documented to have differentiation and apoptosis inducing effects in NSCLC [16]. Ciglitazone is known to inhibit cell proliferation in several cancer cell lines including melanoma, prostate, colon, and breast cancers [13, 14, 23]. Based upon these previous studies we therefore assessed the capacity of ciglitazone to decrease COX-2 and PGE2 in NSCLC.
Our studies demonstrate that ciglitazone significantly decreases COX-2 expression and PGE2 production in NSCLC cell lines. In addition to COX-2, PGES and 15-PGDH also regulate PGE2 levels [19] [24]. However, neither PGES nor 15-PGDH expression were affected by ciglitazone treatment of NSCLC cell lines. This suggests that the decrease of PGE2 by ciglitazone is primarily via downregulation of COX-2 expression. Ciglitazone treatment decreased COX-2 mRNA expression as well as its promoter activity suggesting that the suppression of COX-2 occurs at the transcriptional level. The COX-2 promoter studies (data not shown) confirmed that NF-kB, C/EBP and CRE sites alone are not responsible for ciglitazone-mediated suppression of COX-2 in NSCLC. However, it is possible that ciglitazone may inhibit the COX-2 promoter by suppressing transcriptional factors binding to at least two of these cis-elements [25].
In addition, recent studies have provided evidence that transcriptional repression is associated with histone deacetylation [26, 27]. Based on these studies it is also speculated that ciglitazone could suppress COX-2 via a histone deacetylase mechanism.
Ciglitazone treatment increased PPARγ activity as indicated by increased PPRE activity. However while TZDs are widely known as ligands for PPARγ, they may mediate receptor-independent effects as previously demonstrated [28]. For example, by utilizing the embryonic stem cells from PPARγ null mice, Chawla et al found that neither macrophage differentiation nor anti-inflammatory effects of synthetic PPARγ ligands are PPARγ receptor dependent [29]. In addition ciglitazone was found to activate MAP kinase cascades in astrocytes and preadipocytes through PPARγ independent mechanisms [28]. In order to understand whether the suppression of COX-2 and PGE2 by ciglitazone is PPARγ dependent in NSCLC, we performed experiments utilizing a dn. PPARγ plasmid vector [20]. Here we report that the ciglitazone mediated decrease of COX-2 and PGE2 is independent of PPARγ activation as shown in dn. PPARγ overexpressing A427 and A549 cell lines. There are several possible mechanisms that could explain the PPARγ independent effects. For examples, based on its demonstrated activity in central nervous system inflammation, ciglitazone may decrease COX-2 and PGE2 expression by suppressing JAK-STAT activation in NSCLC [30] [31]. Other studies have found that ciglitazone may directly affect the ERK signaling pathway to regulate COX-2 expression [32] [28]. These studies suggest that the impact of ciglitzone may vary in accord with the cellular system utilized to assess the PPARγ independent effects
Conclusion
In summary, ciglitazone inhibits COX-2 expression and PGE2 production via a PPARγ independent pathway. In addition to the capacity to decrease COX-2 and PGE2 production, these TZD agents have been found to impact multiple pathways regulating the malignant phenotype in lung cancer [33]. This study supports the rationale for further evaluation of the TZDS in lung cancer prevention and combination therapies.
Acknowledgments
We thank Dr. Peter Tontonoz, Department of Pathology and Laboratory Medicine, University of California, Los Angeles for his valuable suggestions. We thank Dr. Tadashi Tanabe, National Cardiovascular Research Institute, Osaka, Japan for providing us the COX-2 promoter deletion constructs.
Sources of support: UCLA Specialized Programs of research excellence In Lung Cancer NIH P50 CA 90388; NIH RO1 CA111851; UC Tobacco-Related Disease Research Program (13RT-0031); Medical Research Funds From The Department of Veteran Affairs; The Flight Attendant Medical Research Institute (FAMRI) Young Clinical Scientist Awards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 2.Dohadwala M, Luo J, Zhu L, et al. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 4.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 7.Shao J, Sheng H, Inoue H, Morrow JD, DuBois RN. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J Biol Chem. 2000;275:33951–33956. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- 8.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–676. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 9.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 10.Chan G, Boyle JO, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 11.Dannenberg AJ, Zakim D. Chemoprevention of colorectal cancer through inhibition of cyclooxygenase-2. Semin Oncol. 1999;26:499–504. [PubMed] [Google Scholar]
- 12.Rainsford KD. Introduction - The coxib controversies. Inflammopharmacology. 2005;13:331–341. doi: 10.1163/156856005774415628. [DOI] [PubMed] [Google Scholar]
- 13.Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer Res. 2004;24:2765–2771. [PubMed] [Google Scholar]
- 14.Mossner R, Schulz U, Kruger U, et al. Agonists of peroxisome proliferator-activated receptor gamma inhibit cell growth in malignant melanoma. J Invest Dermatol. 2002;119:576–582. doi: 10.1046/j.1523-1747.2002.01861.x. [DOI] [PubMed] [Google Scholar]
- 15.Nunez NP, Liu H, Meadows GG. PPAR-gamma ligands and amino acid deprivation promote apoptosis of melanoma, prostate, and breast cancer cells. Cancer Lett. 2006;236:133–141. doi: 10.1016/j.canlet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- 17.Li M, Lee TW, Mok TS, Warner TD, Yim AP, Chen GG. Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem. 2005;96:760–774. doi: 10.1002/jcb.20474. [DOI] [PubMed] [Google Scholar]
- 18.Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM. Pioglitazone and Rosiglitazone decrease PGE2 in non-small cell lung cancer cells by upregulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007 doi: 10.1124/mol.106.033357. [DOI] [PubMed] [Google Scholar]
- 19.Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;68-69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 20.Gurnell M, Wentworth JM, Agostini M, et al. A dominant-negative peroxisome proliferator-activated receptor gamma (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma-mediated adipogenesis. J Biol Chem. 2000;275:5754–5759. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- 21.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 22.Hosomi Y, Yokose T, Hirose Y, et al. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000;30:73–81. doi: 10.1016/s0169-5002(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 23.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 24.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Uto T, Fujii M, Hou DX. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 transcription by 6-(methylsulfinyl) hexyl isothiocyanate, a chemopreventive compound from Wasabia japonica (Miq.) Matsumura, in mouse macrophages. Biochem Pharmacol. 2005;70:1772–1784. doi: 10.1016/j.bcp.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Lim S, Caramori G, et al. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99:8921–8926. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon AM, Ramauge M, Dessouroux A, Pierre M. MAP kinase cascades are activated in astrocytes and preadipocytes by 15-deoxy-Delta(12-14)-prostaglandin J(2) and the thiazolidinedione ciglitazone through peroxisome proliferator activator receptor gamma-independent mechanisms involving reactive oxygenated species. J Biol Chem. 2002;277:29681–29685. doi: 10.1074/jbc.M201517200. [DOI] [PubMed] [Google Scholar]
- 29.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 31.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol. 2006;176:5050–5059. doi: 10.4049/jimmunol.176.8.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong MA, Lee KW, Yoon DY, Lee HJ. Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbol-ester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in cultured human mammary epithelial cells. Ann N Y Acad Sci. 2007;1095:458–466. doi: 10.1196/annals.1397.049. [DOI] [PubMed] [Google Scholar]
- 33.Li MY, Lee TW, Yim AP, Chen GG. Function of ppargamma and its ligands in lung cancer. Crit Rev Clin Lab Sci. 2006;43:183–202. doi: 10.1080/10408360600552587. [DOI] [PubMed] [Google Scholar]