Abstract
Recent clinical research indicates that d-amphetamine is effective in treating cocaine and methamphetamine dependence. There is concern, however, with the use of d-amphetamine as a pharmacotherapy because acute administration of d-amphetamine decreases inhibition in cocaine-using individuals and may increase drug-taking behavior. The purpose of the present experiment was to determine whether acute d-amphetamine pretreatment would alter the reinforcing, subject-rated, and cardiovascular effects of d-amphetamine. To this end, 7 human volunteers first sampled doses of oral d-amphetamine (0, 8, and 16 mg). These doses engender moderate drug taking and were selected to avoid a ceiling or floor effect. Volunteers were then allowed to self-administer these sampled doses using a modified-progressive ratio procedure in two sessions in which they received pretreatments with either 0 or 15 mg oral d-amphetamine 2 hours prior to completing the modified progressive-ratio procedure. d-Amphetamine produced prototypical stimulant-like effects (e.g., increased ratings of stimulated, elevated blood pressure) and maintained responding on the modified progressive-ratio schedule. Pretreatment with 15 mg oral d-amphetamine also produced prototypical stimulant-like effects, but failed to alter break points for d-amphetamine on the modified progressive-ratio procedure relative to placebo pretreatment. These results indicate that acute d-amphetamine pretreatment does not increase stimulant self-administration.
Keywords: d-Amphetamine, Human, Reinforcement, Pretreatment, Subject-Rated Effects, Progressive-Ratio, Agonist Pharmacotherapy
1. Introduction
Recent clinical trial results indicate that d-amphetamine is effective for the treatment of cocaine and amphetamine dependence (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003). The use of d-amphetamine as an agonist replacement therapy for cocaine or amphetamine dependence may reduce illicit drug use, as well as associated harms (Shearer et al., 2002). In the seminal trial, cocaine dependent patients were randomly assigned to receive d-amphetamine (15 or 30 mg/day) or placebo for 25 weeks (Grabowski et al., 2001). During the fifth week, the d-amphetamine dose was doubled. Patients maintained on 30/60 mg/day d-amphetamine used markedly less cocaine as determined by benzoylecgonine-free urines during the trial than patients maintained on either 15/30 mg/day d-amphetamine or placebo. These investigators, as well as others, have replicated this finding (Grabowski et al., 2004; Shearer et al., 2003).
The use of d-amphetamine as an agonist pharmacotherapy to manage stimulant dependence has met with resistance due to its significant abuse potential. For example, epidemiological findings indicate that amphetamine isomers are diverted and misused (Johnston et al., 2006; McCabe et al., 2005). In the first study, approximately 4% of 10th and 12th graders reported past 30-day use of pharmaceutical amphetamines (e.g., d-amphetamine [Dexedrine®] or d,l-amphetamine [Adderall®]) in 2005 (Johnston et al., 2006). These use rates were actually greater than those for cocaine or methamphetamine in the same population. The second study found that past month use of prescription stimulants (including d- and d,l-amphetamine) was reported by 2.1% of students sampled at random from 119 colleges (McCabe et al., 2005). Use of prescription stimulants in that sample was associated with greater amounts of drug use and increased involvement in risky behaviors.
Findings from human laboratory studies are consistent with epidemiological data, and indicate that d-amphetamine has considerable abuse potential in populations that abuse stimulant drugs. (e.g., Oliveto et al., 1998; Stoops et al., 2004). In the first study, cocaine-abusing volunteers were taught to discriminate oral cocaine (80 mg/70 kg) from placebo (Oliveto et al., 1998). After acquiring the discrimination, a range of doses of d-amphetamine (5-20 mg/70 kg) was tested to determine if they shared discriminative-stimulus effects with cocaine. d-Amphetamine dose dependently increased cocaine-appropriate responding and the highest dose tested completely substituted. d-Amphetamine and cocaine produced a similar constellation of subject-rated effects. In the second study, stimulant-abusing volunteers sampled doses of oral d-amphetamine (8, 16 and 24 mg), oral methylphenidate (16, 32, and 48 mg) and placebo, and were then allowed to self-administer the sampled dose on a modified progressive-ratio procedure (Stoops et al., 2004). Active doses of d-amphetamine and methylphenidate maintained higher break points on the modified progressive-ratio procedure than placebo, and produced prototypical stimulant-like behavioral effects.
Another concern regarding the use of an agonist replacement in the management of stimulant dependence is the possibility that d-amphetamine may prime or increase drug taking. Results from research with rats and nonhuman primates indicate that acute administration of d-amphetamine can reinstate extinguished stimulant self-administration behavior (de Wit and Stewart, 1981; Gerber and Stretch, 1975). In humans with histories of stimulant abuse, acute administration of d-amphetamine has also been shown to impair inhibitory control (Fillmore et al., 2003). Such impaired inhibitory control has been hypothesized to lead to greater impulsivity and increased drug taking.
Although a number of other studies have examined the effects of acute pretreatment agents on drug-taking behavior (e.g., Barrett et al., 2006; Bigelow et al., 1977; de Wit and Chutuape, 1993; Foltin and Fischman, 1994; Spiga et al., 2001), little is known about the effects of putative agonist replacement therapies on subsequent stimulant self-administration. We are not aware of any human laboratory studies in which the effects stimulant drugs were examined following pretreatment with d-amphetamine. One human laboratory study, however, examined the effects of pretreatment with cocaine on the reinforcing effects of stimulants (Donny et al., 2004). In that experiment, volunteers sampled doses of intravenous cocaine (0, 15, or 30 mg/70 kg) and were then allowed to choose between the sampled dose and descending amounts of money following administration of an acute cocaine “priming” dose (0, 15, or 30 mg/70 kg) (Donny et al., 2004). Regardless of pretreatment condition, choice for cocaine increased as the available money alternative decreased. Cocaine pretreatment increased the value of money at which cocaine was chosen relative to pretreatment with placebo, suggesting that the reinforcing effects of cocaine were enhanced by a priming dose of cocaine. These findings indicate that acute pretreatment with an agonist replacement pharmacotherapy can alter the behavioral effects of stimulant drugs.
The purpose of the present experiment was to determine the effects of acute administration of d-amphetamine on the reinforcing, subject-rated and physiological effects of d-amphetamine in an attempt to better characterize the effects of acute administration of a putative agonist replacement therapy on subsequent stimulant taking. To this end, seven volunteers first sampled doses of oral d-amphetamine (0, 8, and 16 mg). These doses engender moderate drug taking (Rush et al., 2001; Stoops et al., 2004) and were selected to ensure there was no ceiling or floor effect that would preclude observation of d-amphetamine pretreatment effects. In subsequent sessions, volunteers were allowed to work to receive these doses on a modified progressive-ratio procedure following pretreatment with placebo or 15 mg oral d-amphetamine, which is a behaviorally active dose (Rush et al., 1998; 2003). We chose to use d-amphetamine as both the compound for self-administration and pretreatment agent because it is efficacious as an agonist replacement medication for stimulant dependence (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003) and because the ability to observe a pharmacological interaction is enhanced when the pretreatment agent is pharmacologically similar to the drug in question (Walsh et al., 2000).
2. Method
2.1. Volunteers
Seven healthy adult volunteers (4 males, 3 females) were recruited through newspaper advertisements, flyers and word of mouth to complete this experiment. One of the volunteers was African American and Hispanic and the rest were Caucasian. Volunteers ranged in age from 19 to 25 years (mean = 22) and in weight from 52 to 100 kg (mean = 72). Volunteers scored between 0 and 3 (mean = 2) on the Drug Abuse Screening Test (DAST; Skinner, 1982). Volunteers reported consuming 0 to 15 alcohol-containing beverages per week (mean = 7) and scored between 2 and 6 (mean = 3) on the Michigan Alcohol Screening Test (MAST; Selzer, 1971). All volunteers reported use of stimulant drugs (e.g., caffeine, nicotine, or amphetamine) prior to their participation in this experiment. This inclusion criterion was selected based on previous human behavioral pharmacology research in which oral d-amphetamine was administered (Fillmore et al., 2005; Kelly et al., 2006; Stoops et al., 2006). Two additional volunteers completed the experiment, but failed to respond for d-amphetamine or placebo on the modified progressive-ratio procedure under any condition. Two other volunteers were enrolled but were excluded from further participation prior to completion. One of these volunteers was released for non-compliance and the other was lost to follow up. Data from these four participants were excluded from analysis.
Volunteers were paid for participating. Half of the money was paid to volunteers at the end of each experimental session. The other half of the money accumulated during participation and was paid when volunteers completed the entire study.
2.2. General Procedures
The Institutional Review Board of the University of Kentucky Medical Center approved the conduct of this study and all volunteers gave their sober, written informed consent prior to enrolling. Prior to participation, all potential volunteers completed a comprehensive medical-history questionnaire, drug-use questionnaire, a mini-mental status examination and vital sign assessment and were evaluated by a physician. Routine clinical laboratory blood and urine chemistry tests as well as an electrocardiogram were conducted on all potential volunteers. Potential volunteers with histories of serious physical disease, current physical disease (e.g., impaired cardiovascular functioning, chronic obstructive pulmonary disease, etc.), seizure, head trauma or CNS tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I, DSM IV), were excluded from participation.
Volunteers were instructed to abstain from taking all psychoactive drugs (with the exception of tobacco) throughout the study, caffeinated products and solid food for 4 h before a scheduled session and alcohol for 12 h before and after a scheduled session. Drug urine screens were conducted at the outset of every session for amphetamine, benzodiazepines, barbiturates, cocaine, opioids and THC to ensure that each volunteer was compliant with the drug use restrictions (Abuscreen ONTRAK TesTstiks, Varian Diagnostics, Palo Alto, CA). Volunteers also provided an expired air sample, which was assayed for the presence of alcohol using an Alco-Sensor breathalyzer (Intoximeters, St. Louis, MO). All expired air samples provided by participants had to be negative for an experimental session to continue. No volunteers tested positive for the presence of drugs other than those administered experimentally or THC throughout the experimental protocol. All volunteers also underwent a field sobriety test at the outset of each session to ensure that they were not currently intoxicated. If volunteers successfully completed this sobriety test, they were permitted to participate in the scheduled experimental session.
Female volunteers had to report using an effective form of birth control in order to participate and must not have been pregnant. Female volunteers were also screened for pregnancy (urine HCG; Mainline Technology, Ann Arbor, MI) prior to each session to ensure that they did not continue in the study if pregnant. None of the female volunteers tested positive for pregnancy throughout the experimental protocol.
Volunteers enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky Medical Center. Volunteers participated in a total of nine experimental sessions. Volunteers were informed that during their participation, they would receive various drugs including placebo or stimulants. Volunteers were told that the purpose of the study was to determine how these drugs affect mood and behavior and whether they would be willing to work to receive the drugs. Other than receiving this general information, volunteers were unaware of the type of drug administered, what they were supposed to do, or what outcomes were expected. The overall timeline for the experiment is presented in Table 1.
Table 1.
Experiment Timeline. This table represents an example of the experimental timeline. Order of d-amphetamine sampling doses was randomized and the order of pretreatment drug administration (i.e., placebo or d-amphetamine) was counterbalanced across volunteers to ensure that there was no effect of order of conditions.
| Day | Session | Session Type | Self-Administration Drug | Pretreatment |
|---|---|---|---|---|
| 1 | Practice | |||
| 2 | Practice | |||
| 3 | Session 1 | Sampling | placebo | |
| 4 | Session 2 | Self-Administration | placebo | placebo |
| 5 | Session 3 | Self-Administration | placebo | 15 mg d-amphetamine |
| 6 | Session 4 | Sampling | 8 mg d-amphetamine | |
| 7 | Session 5 | Self-Administration | 8 mg d-amphetamine | placebo |
| 8 | Session 6 | Self-Administration | 8 mg d-amphetamine | 15 mg d-amphetamine |
| 9 | Session 7 | Sampling | 16 mg d-amphetamine | |
| 10 | Session 8 | Self-Administration | 16 mg d-amphetamine | placebo |
| 11 | Session 9 | Self-Administration | 16 mg d-amphetamine | 15 mg d-amphetamine |
2.2.1. Practice Sessions
Prior to beginning the experiment proper, volunteers completed two “practice” sessions. These practice sessions were used to familiarize volunteers with the modified progressive-ratio procedure, subject-rated drug-effect questionnaires and daily laboratory routine, all of which are described below. Experimental medications were not administered on these days.
2.2.2. Experimental Sessions
Experimental sessions were approximately 7.5 hours long. The daily timeline for each type of experimental session is presented in Table 2. On each session day, volunteers arrived at the laboratory at approximately 0800. Volunteers consumed a light breakfast with a decaffeinated beverage and completed the pre-session tasks between 0800 and 0830. On self-administration days only, volunteers ingested the pretreatment drug (placebo or 15 mg d-amphetamine) at 0830, completed assessments, described below, at 0900, 0930, 1000 and 1030, and then completed the modified progressive-ratio procedure between 1030 and 1100. On sampling and self-administration days, volunteers ingested capsules at approximately 1130 and completed the subject-rated drug-effect questionnaires at 1230, 1330, 1430 and 1530. This experimental timeline was devised based on the time course of the effects of d-amphetamine observed in previous experiments in our laboratory (e.g., Lile et al., 2005; Rush et al., 2001). That is, on self-administration days, the d-amphetamine pretreatment dose was allowed to reach its estimated peak effect (i.e., approximately two hours following administration) during completion of the modified progressive-ratio procedure. Sessions were generally conducted daily and arrival time was held constant for each volunteer.
Table 2.
Timeline for Daily Experimental Sessions.
| Sampling Sessions (Sessions 1, 4 and 7) | |
|---|---|
| 0800 | Arrival |
| 0800-0830 | Sobriety Test, Baseline Measures, Urine Screen |
| 0830 | Cardiovascular Measures |
| 0900 | Cardiovascular Measures |
| 0930 | Cardiovascular Measures |
| 1000 | Cardiovascular Measures |
| 1030 | Cardiovascular Measures |
| 1130 | Sampling Dose (0, 8, or 16 mg d-Amphetamine) |
| 1230 | 1 Hour Post-Drug Administration Measures |
| 1330 | 2 Hour Post-Drug Administration Measures |
| 1430 | 3 Hour Post-Drug Administration Measures |
| 1530 | 4 hour Post-Drug Administration Measures |
|
Self-Administration Sessions (Sessions 2-3, 5-6 and 8-9) | |
| 0800 | Arrival |
| 0800-0830 | Sobriety Test, Baseline Measures, Urine Screen |
| 0830 | Cardiovascular Measures, Pretreatment Dose (0 or 15 mg d-Amphetamine) |
| 0900 | Measures |
| 0930 | Measures |
| 1000 | Measures |
| 1030 | Measures, Modified Progressive-Ratio Procedure |
| 1130 | Self-Administered Dose (0, 8, or 16 mg d-Amphetamine) |
| 1230 | 1 Hour Post-Drug Administration Measures |
| 1330 | 2 Hour Post-Drug Administration Measures |
| 1430 | 3 Hour Post-Drug Administration Measures |
| 1530 | 4 hour Post-Drug Administration Measures |
Testing of each of the self-administered dose conditions described below consisted of three separate sessions: 1) a sampling session, 2) a self-administration session following pretreatment with placebo and 3) a self-administration session following pretreatment with 15 mg d-amphetamine (see Table 1).
2.2.2.1. Sampling Sessions
A sampling session was conducted to acquaint volunteers with the effects of each drug dose that would be available for self-administration in a later session (i.e., 0, 8 and 16 mg d-amphetamine). After the pre-drug questionnaires were completed and physiological measures recorded, volunteers were instructed to pay attention to and make notes about the effects of the drug, because in a future session they would be offered the opportunity to work to receive that drug again. Volunteers were also instructed that each capsule contained one eighth of the total drug dose (i.e., 0, 1 and 2 mg d-amphetamine per capsule). Volunteers ingested eight identical capsules at approximately 1130 and then completed the subject-rated drug-effect questionnaires and physiological measures at hourly intervals for 4 h.
2.2.2.2. Self-Administration Sessions
Self-administration sessions differed from sampling sessions only in that volunteers had to earn capsules by responding on a modified progressive-ratio procedure (Comer et al., 1997; 1998; Rush et al., 2001; Stoops et al., 2004; 2005a; 2005b). In addition, volunteers received the d-amphetamine pretreatment dose (0 or 15 mg) two hours prior to completing the modified-progressive-ratio procedure. This pretreatment dose was visually distinct (i.e., red capsule) from the sampled/self-administered capsules (i.e., blue and white capsules). Volunteers were instructed that the pretreatment drug was unrelated to the sampled dose, which they would be allowed to work for later in the session. Following administration of the pretreatment dose, volunteers completed the battery of subject-rated drug-effect questionnaires and physiological measures every half hour for two hours. Volunteers then completed the Modified Progressive-Ratio Procedure. The remainder of the session was identical to Sampling Sessions.
2.3. Modified Progressive-Ratio Procedure
The modified progressive-ratio procedure has been used previously and is a sensitive measure of drug reinforcement in humans (e.g., Comer et al., 1997, 1998; Rush et al., 2001; Stoops et al., 2004; 2005a; 2005b). During each progressive-ratio procedure, volunteers were able to respond on a computer mouse to earn all, or some, of the capsules that were administered during the preceding sampling session. A total of eight opportunities were available to volunteers to self-administer the previously sampled dose. Prior to each of the eight opportunities to earn a capsule, volunteers were asked if they wanted to work for one of the capsules administered during the previous sampling session. Volunteers responded by clicking either a YES or NO presented on a computer screen. If the volunteer responded YES, they were then required to click the mouse a predetermined number of times to earn the capsule. To earn the first capsule, volunteers were required to click the mouse 25 times. The number of responses required to earn each additional capsule doubled (i.e., 50, 100, 200, 400, 800, 1600 and 3200 responses). To receive all eight capsules, volunteers had to click the mouse a total of 6,375 times. If the volunteer responded NO at any time when they were asked if they wanted to work for one of the capsules administered during the previous sampling, the task was terminated. The dependent measure on this procedure was the number of capsules earned.
Volunteers ingested all of the capsules they earned after completing the modified progressive-ratio procedure. As described above, each capsule contained 12.5% of the total dose of the test drug administered during the preceding sampling session. Thus, if a volunteer responded for all eight capsules during a progressive-ratio procedure, he/she earned the total dose received during the preceding sampling session. After ingesting any capsules earned on the modified progressive-ratio procedure, volunteers completed questionnaires and cardiovascular measures at hourly intervals for 4 h. If a volunteer did not respond for any capsules, he/she still completed the questionnaires and cardiovascular measures as scheduled to ensure that he/she chose to not self-administer any capsules in an attempt to shorten the session.
2.4. Subject-Rated Drug-Effect Questionnaires and Cardiovascular Measures
The subject-rated drug-effect questionnaires were administered on an Apple Macintosh computer (iMac, Cupertino, CA) in fixed order. The cardiovascular measures were recorded using a Dinamap Vital Signs monitor (Johnson and Johnson, Alexandria, TX). The assessments were completed as noted in Table 2.
2.4.1. Addiction Research Center Inventory (ARCI)
The 49-item short form of the true-false inventory (Martin et al., 1971) yielded information on five scales:, Amphetamine (A), Benzedrine-Group (BG), Morphine-Benzedrine Group (MBG), Lysergic Acid Diethylamide (LSD) and Pentobarbital, Chlorpromazine, Alcohol Group (PCAG).
2.4.2. Adjective Rating Scale
The adjective rating scale consisted of 32 items and contained two subscales: Stimulant and Sedative (Oliveto et al., 1992). Each subscale consisted of 16 adjectives. Volunteers rated each item using the computer mouse to point to and select among one of five response options: Not at All, A Little Bit, Moderately, Quite a Bit and Extremely (scored numerically from 0 to 4, respectively). Responses to individual items were summed to produce a composite score for each subscale. The maximum possible score on each subscale was 64.
2.4.3. Drug-Effect Questionnaire
A 20-item drug-effect questionnaire was used in this experiment (Rush et al., 2003). Items were presented one at a time. Volunteers rated each of the items using a 5-point scale similar to the one described above.
2.5. Drug Administration
All drug doses were administered in a double-blind fashion. Capsules were ingested with approximately 150 ml water. During each sampling session, volunteers orally ingested eight blue and white capsules. During self-administration sessions, volunteers orally ingested one red capsule containing placebo or 15 mg d-amphetamine and the number of capsules earned during the modified progressive-ratio procedure.
Drug doses were prepared using commercially available d-amphetamine (Barr Laboratories, Pomona, NY). Each sampled d-amphetamine capsule contained 0 mg (placebo), 1 mg (8 mg dose), or 2 mg (16 mg dose) d-amphetamine. These doses engender moderate drug taking and were selected to ensure there was no ceiling or floor effect that would preclude observation of d-amphetamine pretreatment effects (Rush et al., 2001; Stoops et al., 2004). Each d-amphetamine pretreatment capsule contained 0 mg (placebo) or 15 mg d-amphetamine. Cornstarch was used to fill the remainder of all the capsules. Placebo capsules contained only cornstarch. Drug administration procedures were designed to ensure that volunteers swallowed the capsules and did not open them in their mouths to taste the contents (Abreu and Griffiths, 1996).
2.6. Data Analysis
Statistical analyses of group data were conducted to examine drug effects on the progressive-ratio task, subject-rated drug-effect questionnaires and cardiovascular measures. For all statistical analyses, effects were considered significant for p ≤ 0.05.
Data from the progressive-ratio task (i.e., number of capsules earned) were analyzed with a two-factor repeated-measure analysis of variance (ANOVA). The factors for this ANOVA were Sampled Dose (i.e., 0, 8, or 16 mg d-amphetamine) and Pretreatment Dose (i.e., 0 or 15 mg d-amphetamine).
Data from the sampling sessions were analyzed to determine the acute effects of d-amphetamine on the subject-rated drug-effect questionnaires and cardiovascular measures. Data were analyzed using a two factor repeated-measures ANOVA with Sampling Dose (0, 8 and 16 mg d-amphetamine) and Time (Pre- and 1, 2, 3 and 4 h post-dose) as the factors. If a statistically significant effect (i.e., interaction of Sampling Dose and Time) was observed, Tukey’s Honestly Significant Difference (HSD) post hoc tests were then used to compare the active sampling doses with placebo at each time point. Analyses with only significant main effects of Time will not be reported here.
Data from self-administration sessions collected following administration of the pretreatment dose (i.e., 0 or 15 mg d-amphetamine), but before administration of the dose earned on the progressive ratio (i.e., every half hour following administration through completion of the progressive ratio), were initially analyzed using a three factor repeated-measures ANOVA with Replication (1, 2, or 3), Dose (0 and 15 mg d-amphetamine) and Time (Pre-, 0.5, 1, 1.5 and 2 h post-pretreatment dose) as the factors. There was generally no effect of replication, so data were collapsed across this factor. If a statistically significant effect (i.e., interaction of Pretreatment Dose and Time) was observed, Tukey’s HSD post hoc tests were then used to compare the active pretreatment dose with placebo at each time point. Analyses with only significant main effects of Time will not be reported here.
During self-administration sessions, subjects determined the amount of drug they ingested using the modified progressive-ratio schedule. Consequently, subjects ingested varying amounts of drug during the self-administration session. Because volunteers ingested varying amounts of drug, subject-rated drug-effect-questionnaire, performance and cardiovascular data from the self-administration sessions were not analyzed statistically.
3. Results
3.1. Modified Progressive-Ratio Performance
Statistical analysis revealed a significant main effect of Sampling Dose (F2,12 = 7.8) for the Number of Capsules Earned on the modified progressive-ratio procedure (Figure 1). Both 8 and 16 mg d-amphetamine increased responding on the modified progressive-ratio procedure relative to placebo. The main effect of Pretreatment Dose, as well as the interaction of Sampling Dose and Pretreatment Dose, failed to attain statistical significance.
Figure 1.
Dose-response function for d-amphetamine for number of capsules earned on the Modified Progressive-Ratio Procedure as a function of pretreatment condition. X-axis: total d-amphetamine dose (mg). The dose per capsule was 0 mg d-amphetamine (placebo [PL]), 1 mg d-amphetamine (8 mg dose) and 2 mg d-amphetamine (16 mg dose). The maximum number of capsules earned was 8. Brackets indicate one S.E.M. Unidirectional brackets were used for clarity.
3.2. Subject-Rated and Cardiovascular Effects of Sampled d-Amphetamine
3.2.1. ARCI
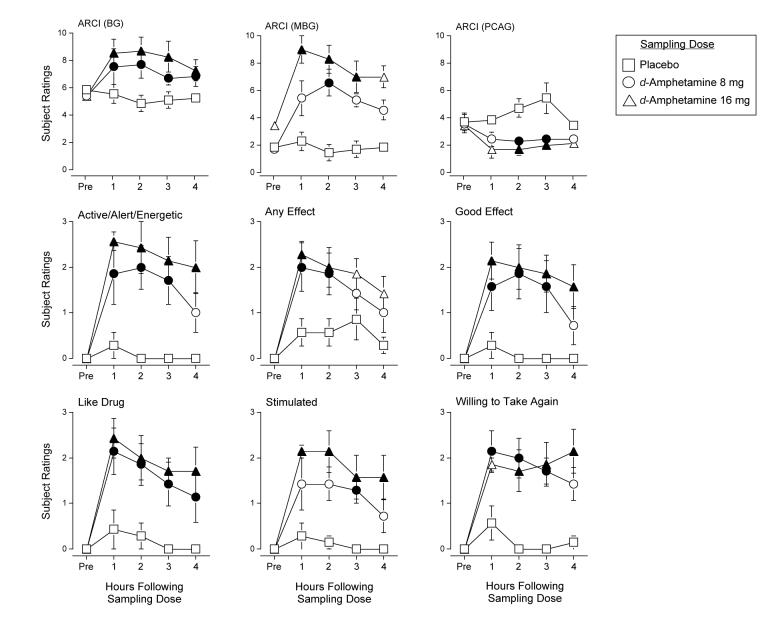
Statistical analysis revealed a significant interaction of Sampling Dose and Time (F8,48 values > 2.4) on scores on the BG, MBG and PCAG scales of the ARCI. Post hoc tests revealed that active doses of d-Amphetamine increased scores on the BG and MBG scales of the ARCI and decreased scores on the PCAG scale of the ARCI in a dose-related fashion relative to placebo between 1 and 4 hours following drug administration (Figure 2).
Figure 2.
Dose- and Time-response functions for sampling doses of d-amphetamine for scores on the BG, MBG, and PCAG scales of the ARCI and subject ratings of Active/Alert/Energetic, Any Effect, Like Drug, Good Effects, Stimulated and Willing to Take Again from the Drug-Effect Questionnaire during Sampling Sessions. X-axis: hours following drug administration. The maximum score on the subject-rated measures was 4. Filled symbols are significantly different from placebo at that time point. Brackets indicate one S.E.M.
A significant main effect of Sampling Dose (F2,12 = 3.6) and Time (F4,24 = 3.4) was observed on the A scale of the ARCI, but the interaction of Sampling Dose and Time did not reach statistical significance. Active doses of d-amphetamine increased scores on the A scale of the ARCI relative to placebo from 1 to 4 hours following drug administration (data not shown).
3.2.2. Adjective Rating Scale
Statistical analysis revealed a significant interaction of Sampling Dose and Time (F8,48 = 5.0) on scores on the Stimulant Subscale of the Adjective Rating Scale. Post hoc tests revealed that active doses of d-amphetamine increased scores on the Stimulant Subscale of the Adjective Rating Scale relative to placebo from 1 to 4 hours following drug administration. A significant main effect of Sampling Dose (F2,12 = 6.9) was observed on the Sedative Subscale of the Adjective Rating Scale. Active doses of d-amphetamine decreased scores on the Sedative Subscale relative to placebo independent of time (data not shown).
3.2.3. Drug-Effect Questionnaire
Statistical analysis revealed a significant interaction of Sampling Dose and Time (F8,48 values > 2.8) on ten items from the Drug-Effect Questionnaire: Active/Alert/Energetic, Any Effect, Good Effects, Like Drug, Performance Improved, Restless, Rush, Stimulated, Willing to Take Again and Talkative. Post hoc tests revealed that active doses of d-amphetamine increased scores on these measures between 1 and 4 hours following drug administration relative to placebo. Figure 2 shows representative data for volunteer ratings of Active/Alert/Energetic, Any Effect, Like Drug, Good Effects, Stimulated and Willing to Take Again.
A significant main effect of Sampling Dose (F2,12 = 4.2) was observed for subject ratings of Sluggish from the Drug-Effect Questionnaire. Active d-Amphetamine decreased ratings on this measure relative to placebo independent of time (data not shown).
3.2.4. Cardiovascular Measures
Statistical analysis revealed a significant interaction of Sampling Dose and Time (F8,48 values > 2.5) for Systolic and Diastolic Blood Pressure and Heart Rate. Post hoc tests revealed that active doses of d-amphetamine increased these measures between 1 and 4 hours following drug administration relative to placebo but the degree to which these cardiovascular indices were acutely increased were not considered clinically significant (data not shown).
3.3. Subject-Rated and Cardiovascular Effects of d-Amphetamine Pretreatment
3.3.1. ARCI
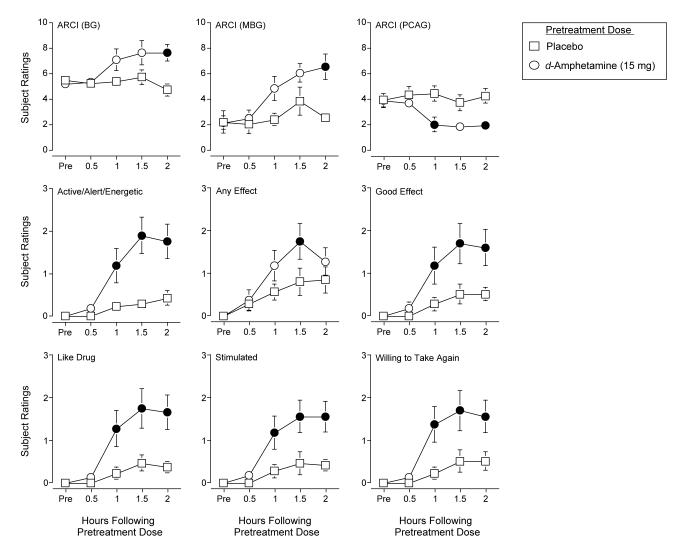
Statistical analysis revealed a significant interaction of Pretreatment Dose and Time (F4,24 values > 3.2) on the A, BG, MBG and PCAG scales of the ARCI. Post hoc tests revealed that the active pretreatment dose of d-amphetamine increased scores on the A, BG, and MBG scales of the ARCI between 1 and 2 hours following drug administration relative to placebo. Post hoc tests revealed that the active pretreatment dose of d-amphetamine decreased scores on the PCAG scale of the ARCI between 1 and 2 hours following drug administration relative to placebo. Figure 3 shows representative data for scores on the BG, MBG, and PCAG scales of the ARCI.
Figure 3.
Dose- and Time-response functions for pretreatment doses of d-amphetamine for scores on the BG, MBG, and PCAG scales of the ARCI and subject ratings of Active/Alert/Energetic, Any Effect, Like Drug, Good Effects, Stimulated and Willing to Take Again from the Drug-Effect Questionnaire following administration of the pretreatment dose during Self-Administration Sessions. X-axis: hours following drug administration. The maximum score on the subject-rated measures was 4. Filled symbols are significantly different from placebo at that time point. Brackets indicate one S.E.M.
3.3.2. Adjective Rating Scale
Statistical analysis revealed a significant interaction of Pretreatment Dose and Time (F4,24 = 8.1) on the Stimulant Subscale of the Adjective Rating Scale. Post hoc tests revealed that the active pretreatment dose of d-amphetamine increased scores on the Stimulant Subscale of the Adjective Rating Scale from 1 to 2 hours following drug administration relative to placebo (data not shown).
3.3.3. Drug-Effect Questionnaire
Statistical analysis revealed a significant interaction of Pretreatment Dose and Time (F4,24 values > 3.0) on ten items from the Drug-Effect Questionnaire: Active/Alert/Energetic, Any Effect, Good Effects, Like Drug, Willing to Pay For, Performance Improved, Rush, Stimulated, Willing to Take Again and Talkative. Post hoc tests revealed that the active pretreatment dose of d-amphetamine generally increased subject ratings on these items between 1 and 2 hours following drug administration relative to placebo. Figure 3 shows representative data for subject ratings of Active/Alert/Energetic, Any Effect, Like Drug, Good Effects, Stimulated and Willing to Take Again.
3.3.4. Cardiovascular Measures
Statistical analysis revealed a significant interaction of Pretreatment Dose and Time (F6,36 values > 6.5) on Systolic and Diastolic Blood Pressure, as well as Heart Rate. Post hoc tests revealed that the active pretreatment dose of d-amphetamine increased these measures from 1 to 2 hours following drug administration relative to placebo, but the degree to which these cardiovascular indices were acutely increased were not considered clinically significant (data not shown).
4. Discussion
Consistent with prior human research, d-amphetamine functioned as a reinforcer as measured by the modified progressive-ratio procedure, produced positive subject-rated effects (e.g., increased ratings of Like Drug and Good Effects and scores on the MBG, BG and A scales of the ARCI), and elevated cardiovascular measures (Comer et al., 1996; Rush et al., 2001; Stoops et al., 2004). The behavioral effects of d-amphetamine peaked at approximately two hours following administration, in agreement with previous human laboratory research (e.g., Chait et al., 1985; Kelly et al., 1993; Rush et al., 1998) and the time course for peak plasma levels following oral administration (Angrist et al., 1987). Thus, the peak effects of the d-amphetamine pretreatment dose occurred during completion of the modified progressive-ratio procedure in Self-Administration Sessions.
The current findings support those of previous studies that have used the modified progressive-ratio procedure and demonstrated that it is sensitive to the reinforcing effects of a number of drugs including heroin, caffeine, marijuana, pentobarbital, d-amphetamine, and methylphenidate (Comer et al., 1997; Griffiths et al., 1989; Haney et al., 1997; McLeod and Griffiths, 1983; Rush et al., 2001; Stoops et al., 2004). Results from those studies have also demonstrated that the progressive-ratio procedure is sensitive to manipulation of both pharmacological (e.g., dose or pretreatment agent) (e.g., Comer et al., 1997; 2005) and environmental variables (e.g., alternative reinforcers or behavioral requirements following drug administration) (e.g., Stoops et al., 2005a; 2005b). The reason that pretreatment with d-amphetamine did not alter d-amphetamine self-administration in the present study is unknown. One possible explanation is that the pretreatment dose of d-amphetamine (i.e., 15 mg) was insufficient. It should be noted that previous studies that have demonstrated that the progressive-ratio procedure is sensitive to pharmacological pretreatment agents have been conducted with opioid drugs (e.g., Comer et al., 2005).
One previous human laboratory study has demonstrated that acute pretreatment with a stimulant drug can enhance subsequent stimulant reinforcing effects (Donny et al., 2004). The reason for the discrepancy between the present results and that study is unknown but could be due to differences in methodology (e.g., use of d-amphetamine versus cocaine, use of non-drug abusers versus use of cocaine abusing/dependent volunteers, use of a modified progressive-ratio procedure versus a choice procedure). Worth noting is that, although the data were not analyzed statistically, visual inspection revealed that the subject-rated effects of self-administered d-amphetamine were enhanced following pretreatment with 15 mg d-amphetamine compared to placebo. These data are consistent with previous research that has demonstrated that the reinforcing effects of stimulant drugs are more difficult to manipulate with pharmacological pretreatment than the subject-rated effects in human volunteers (see discussion in Haney et al., 2006).
Although the one previous human study that examined the effects of acute stimulant drug pretreatment on stimulant self-administration revealed a priming effect, another possibility is that pretreatment with d-amphetamine could have decreased d-amphetamine self-administration. A number of nonhuman laboratory studies have demonstrated that acute stimulant pretreatment can attenuate the reinforcing effects of stimulant drugs (e.g., Barrett et al., 2004). Because either an enhancement or reduction in drug taking was a possible outcome, the doses of d-amphetamine that were selected were expected to engender moderate drug taking. In fact, the availability of both doses of d-amphetamine resulted in moderate levels of drug self-administration, indicating that the lack of an effect of d-amphetamine pretreatment was not due to a floor or ceiling effect.
One parsimonious explanation for the negative results is that a higher dose of d-amphetamine would be required to modify d-amphetamine self-administration under the modified progressive-ratio procedure. Only a single active pretreatment dose was tested, which is a limitation of the present experiment. Although this dose of d-amphetamine administered during self-administration sessions was behaviorally active (e.g., increased ratings of Like Drug and Good Effects, increased heart rate and blood pressure), it approximates the threshold dose to produce reliable behavioral effects (Chait, 1993), suggesting that even higher doses might be necessary to modify d-amphetamine self-administration. There is an extensive pre-clinical literature that has demonstrated that higher pretreatment doses are more effective at decreasing stimulant self-administration than low doses (e.g., Barrett et al., 2004; Harrod et al., 2001; Newman and Beardsley, 2006). Consistent with this notion, the dose of d-amphetamine administered in the present study (i.e., 15 mg) was not effective at modifying drug taking behavior when administered to stimulant-dependent patients (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003).
Despite the evidence that d-amphetamine is efficacious as an agonist replacement pharmacotherapy for cocaine and methamphetamine dependence (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003), clinicians may be reluctant to prescribe it due to concern about its abuse potential and the possibility of increased toxicity when combined with an abused stimulant (Shearer et al., 2002). Although not analyzed statistically, visual inspection of the data indicate that pretreatment with d-amphetamine enhanced the cardiovascular effects of self-administered d-amphetamine, but not to a clinically significant degree. Another concern is that d-amphetamine has been shown to reinstate drug-seeking behavior in animal models (de Wit and Stewart, 1981; Gerber and Stretch, 1975) and to impair inhibitory control (Fillmore et al., 2003), which might lead to increased drug use. The findings of the present study lend support to the use of d-amphetamine as an agonist replacement pharmacotherapy for stimulant dependence because a behaviorally active dose of d-amphetamine did not increase subsequent d-amphetamine self-administration. The present findings are also consistent with clinical trial data in that acute administration of d-amphetamine does not increase drug taking as verified by urine drug screens conducted during initial treatment (e.g., the first few weeks) (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003). Moreover, non-human laboratory and clinical trial data demonstrate that prolonged d-amphetamine treatment decreases stimulant drug use, suggesting that the acute dosing regimen used in the present study might not have been sufficient to decrease drug-taking behavior (Grabowski et al., 2001; 2004; Negus and Mello 2003a; 2003b; Shearer et al., 2001; 2003).
There are several limitations to the present experiment that need to be acknowledged. First, this experiment examined the effects of d-amphetamine in non-drug dependent volunteers. Moreover, pretreatment with d-amphetamine was acute, whereas when used clinically, d-amphetamine has been administered chronically to cocaine or amphetamine dependent individuals (Grabowski et al., 2001; 2004; Shearer et al., 2001; 2003). Future research should better model the clinical condition by examining the effects of chronic treatment with a range of d-amphetamine doses on cocaine or methamphetamine self-administration in dependent individuals.
Acknowledgement
This research was supported by Grant DA 021155 from the National Institute on Drug Abuse (C.R.R.). The authors wish to thank Frances Wagner, RN, John Blackburn, Michelle Gray, Neena Khanna, Derek Roe and Kristi Yingling for their expert medical and technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu ME, Griffiths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacology. 1996;125:255–7. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- Angrist B, Corwin J, Bartlik B, Cooper T. Early pharmacokinetics and clinical effects of oral d-amphetamine in normal subjects. Biol Psychiatry. 1987;22:1357–68. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Griffiths RR, Liebson IA. Pharmacological influences upon human ethanol self-administration. Adv Exp Med Biol. 1977;85B:523–38. doi: 10.1007/978-1-4615-9038-5_33. [DOI] [PubMed] [Google Scholar]
- Chait LD. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav Pharmacol. 1993;4:191–9. [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. The discriminative stimulus and subjective effects of d-amphetamine in humans. Psychopharmacology. 1985;86:307–12. doi: 10.1007/BF00432219. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine maintained humans. Behav Pharmacol. 1997;8:677–90. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Comer SD, Haney M, Foltin RW, Fischman MW. Amphetamine self-administration by humans: modulation by contingencies associated with task performance. Psychopharmacology. 1996;127:39–46. doi: 10.1007/BF02805973. [DOI] [PubMed] [Google Scholar]
- Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent subjects. Psychopharmacology. 2005;181:664–75. doi: 10.1007/s00213-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4:29–36. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology. 2004;172:316–23. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend. 2005;77:151–9. doi: 10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–52. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–61. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–6. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–81. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Reinforcing effects of caffeine in coffee and capsules. J Exp Anal Behav. 1989;52:127–40. doi: 10.1901/jeab.1989.52-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–12. [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–21. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–9. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975-2005. Volume I: Secondary school students (NIH Publication No. 06-5883) National Institute on Drug Abuse; Bethesda, MD: 2006. [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: Dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–72. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PEA, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus effects of d-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–14. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: Prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR. Human progressive-ratio performance: maintenance by pentobarbital. Psychopharmacology. 1983;79:4–9. doi: 10.1007/BF00433007. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003a;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003b;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology. 2006;185:142–9. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–9. [PubMed] [Google Scholar]
- Oliveto AH, McCance-Katz E, Singha A, Hameedi F, Kosten TR. Effects of d-amphetamine and caffeine in humans under a cocaine discrimination procedure. Behav Pharmacol. 1998;9:207–17. [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing volunteers. J Clin Psychopharmacology. 2001;21:273–86. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shearer J, Sherman J, Wodak A, Van Beek I. Substitution therapy for amphetamine users. Drug Alcohol Review. 2002;21:179–85. doi: 10.1080/09595230220139082. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, Mattick RP, Van Beek I, Lewis J, Hall W, et al. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction. 2001;96:1289–96. doi: 10.1046/j.1360-0443.2001.96912898.x. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–41. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Spiga R, Huang DB, Meisch RA, Grabowski J. Human methadone self-administration: Effects of diazepam pretreatment. Exp Clin Psychopharmacol. 2001;9:40–6. doi: 10.1037/1064-1297.9.1.40. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PEA, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–43. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: Influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005a;177:349–55. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: Influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005b;182:186–93. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addict Behav. 2006 doi: 10.1016/j.addbeh.2006.08.006. In Press; EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology. 2000;150:361–73. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]