Abstract
Selective exposure to x-irradiation during infancy, from postnatal days (PND) 2–11 in the rat, results in severe hippocampal granule cell hypoplasia. Preweanling (PND 17–18) rats, which suffer such hippocampal granule-cell agenesis, show deficits in patterned single alternation (PSA), a form of memory-based learning. Deficits in short-term memory along with increased arousal have been suggested as characteristic of children diagnosed with attention deficit–hyperactivity disorder (ADHD). We report here on the ameliorating effects of d-amphetamine, a drug commonly used in the treatment of ADHD, before Ritalin, on PSA, after infantile (PND 2–15) exposure to x-irradiation. After i.p. injections of 0.3 mg/kg d-amphetamine, the onset and magnitude of the PSA memory-based discrimination in the x-irradiated preweanling rats was restored to about the level of controls. These results, showing alleviation of x-irradiation-related deficits in short-term memory by d-amphetamine injections, along with our earlier and present results, showing substantial deficits after x-irradiation alone, encourage the hypothesis that hippocampal granule-cell hypoplasia, which would occur in humans prenatally and is Altman’s model of “minimal brain dysfunction” [Altman, J. (1986) in Learning Disabilities and Prenatal Risk, ed. Lewis, M. (Univ. of Illinois Press, Urbana), pp. 241–304], may be a factor in at least some forms of ADHD and may provide a basis for an animal model of the disease.
Keywords: dentate gyrus, hippocampus, granule cells, minimal brain dysfunction
In 1986 it was proposed (1) that, in the rat, treatments (teratogens) that disrupt the normal (mainly postnatal) development of microneurons (granule cells) in the brain, particularly in the dentate gyrus of the hippocampus and the cerebellum, provide models for “minimal brain dysfunction” (MBD), a term used by Joseph Altman in reference to hippocampal and cerebellar hypoplasia. In 1990, one of us (2) proposed that, in the context of an early theory relating frustration to persistence or to discrimination learning (Fig. 1), the deleterious effects of specific teratogens, such as infantile x-irradiation or early postnatal exposure to alcohol on the early postnatal development of a family of learned behavioral dispositions could be understood. These behavioral effects include (i) the present case: patterned single alternation (PSA), a form of short-term, memory-based discrimination learning, and (ii) a subsequent experiment (unpublished) on the effect of postnatal exposure to alcohol on the partial-reinforcement extinction effect (PREE), a test of relative resistance to extinction (persistence). Further, they represent an animal model for some of the symptoms often associated with the diagnosis of attention deficit–hyperactivity disorder (ADHD) in children. The essence of this suggestion is that normal children (and untreated preweanling experimental animals) learn, more readily than those with ADHD or MBD, (i) to discriminate on the basis of short-term memories based on single-alternating reward (R) and nonreward (N), the subject of the present paper, and (ii) to acquire persistence on the basis of quasi-random presentations of R and N. In the present case—of forming discriminations based on short-term memory—our model suggests that in learning a discrimination based on alternating Rs and Ns, pups exposed to x-irradiation of hippocampal granule cells will be affected by unusually strong anticipation of frustrating events and the subsequent prolonged conflict (3). Specifically, in moving through the normal four developmental stages in learning a memory-based discrimination (or in learning to persist) in a given context, experimental animals, like children with ADHD (2, 4) get locked into the third stage, which leaves them in conflict.
Figure 1.
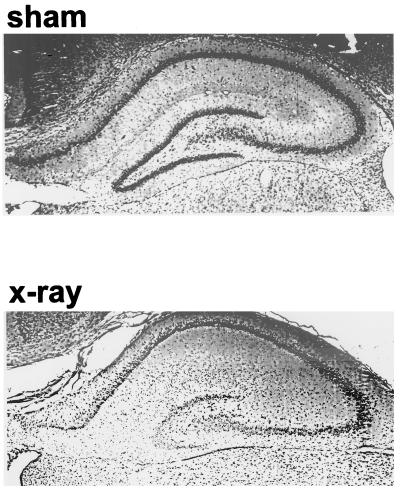
Photomicrographs of the 21-day-old hippocampus. (Upper) A sham-irradiated animal shows no damage to the dentate gyrus. (Lower) X-irradiated hippocampus with an approximately 80% decrease in dentate gyrus granule cells.
Previous work has shown that the normal mitotic development of the granule cells in the cerebellum, hippocampus, and olfactory bulbs can be disrupted by focal x-irradiation during infancy (e.g., ref. 5). By shielding the other areas of the brain from damage, it has been shown that disruption in hippocampal granule-cell growth alone (up to an 80% decrease in number of cells in the hippocampus) coincides with attenuation of PSA in the 16- to 17-day-old rat pup (6) and in a reduction of the PREE in the 20- to 21-day-old rat (7). This hippocampal granule cell agenesis is, in Altman’s (1) terms, “microneuronal hypoplasia,” a putative model for at least some forms of ADHD.
PSA has been shown to be dependent on age and the length of the intertrial interval (ITI), occurring at postnatal day (PND) 11 at 8 s (but not longer) ITI and at PND 17–18 at 60 s ITI (8). This emergence of PSA at longer and longer ITIs closely parallels the development of the granule cells of the dentate gyrus and their mossy fiber connections to pyramidal cells of the hippocampus (9, 10). If this experiment represents a reasonable animal model of ADHD, then treatments that improve memory and attention in children diagnosed with ADHD, such as with d-amphetamine or methylphenidate (Ritalin), should also improve performance on our behavioral tests. The present research examined the effects of d-amphetamine (Sigma) on PSA in the 17- to 18-day-old rat pup after subjection to x-irradiation in early infancy, the suggestion being that d-amphetamine would restore the control level of PSA discrimination after infant exposure to x-irradiation.
METHODS
Subjects.
Subjects were 42 Sprague–Dawley rats from our laboratory breeding colony at the University of Texas at Austin. The colony is maintained on a 14-h light/10-h dark cycle with ad lib access to food and water. On PND 3, litters were culled to eight pups.
X-Irradiation Treatment.
The source of the x-irradiation was a Norelco PG 140 kV x-ray unit. Pups were exposed to x-irradiation by using the procedures of Brunner, Haggbloom, and Gazzara (ref. 10; see also ref. 6). On PND 2 and 3 the pups received a dose of 2 Gy. On subsequent days (days 5, 7, 9, 11, 13, and 15) they received 1.5 Gy. Pups were wrapped and placed in plastic tubing and put into lead-shielded Plexiglas boxes. On PND 11, 13, and 15, pups were exposed to ether for 40 s before the procedure to keep them from moving. The area of irradiation (width of slot corresponding to the hippocampus) increased from 5.5 to 12.0 mm as the rat aged, to allow for appropriate age-specific shielding of the granule cells of the olfactory bulbs and of the cerebellum. Sham controls were wrapped, placed in plastic tubing, and exposed to ether when appropriate, and placed in shielded boxes. They were not, however, exposed to x-irradiation.
Behavioral Apparatus and Procedure.
The behavioral procedures were similar to those that are standard in our laboratory. PSA learning was tested in a Plexiglas straight runway. The runway consisted of a start box (13 × 7.5 × 12 cm), an alley (60 × 7.5 × 12 cm), and goal box (17 × 25 × 12 cm). All had smooth Plexiglas floors and were covered with hinged, clear Plexiglas lids. The goal box was bisected by a metal gate into rear (reward, 15 × 25 × 12 cm) and front (nonreward, 8.5 × 25 × 12 cm) sections. An anesthetized dam was placed in the reward section. The alley was separated from both the start box and goal box by manually operated opaque Plexiglas sliding doors. Odors were expelled by an exhaust fan (115 V, 50 Hz, 10-cm diameter) mounted on the rear wall of the reward section of the goal box. The alley was lined with photocells providing a measure or running time (converted to speed). A Plexiglas enclosure (12 × 12 × 18 cm) served as a holding box during the ITI.
Twenty-four hours before runway training, on PND 16, pups were removed from the litter, weighed, food- and water-deprived, and placed in individual Plexiglas compartments (14 × 10 × 14.5 cm) in an incubator heated to 31–33°C. Approximately 18 h before the first session, they were all fitted with oral cannulas according to the procedure of Hall and Rosenblatt (11). After cannulation the pups were returned to the holding boxes.
To orient the pups to the runway apparatus, goal-box training was conducted approximately 12 h before the first session. This began by placing the pups to be trained into the runway apparatus with all doors and the metal gate between front and rear portions of the goal boxes raised, allowing them to explore the apparatus freely for 10 min. They were then confined to the combined goal box for an additional 10 min. In the presence of an anesthetized dam, three 0.03-ml infusions of infant diet (the reward-to-be) were delivered through the oral cannula, by means of a Harvard (model 906) infusion pump. Each pup was first placed on the dam’s ventrum and, contingent on attaching to a nipple, was given the first milk infusion. For the next two infusions the pup was placed in the front section of the goal box and had to crawl toward the dam and attach to a nipple. After goal-box training, pups were put back into the heated holding boxes for the balance of the deprivation period.
Runway training was conducted in sets of two pups. Pups were quasi-randomly assigned to groups depending on exposure to x-irradiation and drug treatment. Training consisted of 240 trials (3 sessions of 40 trials on each of 2 days; 60-s ITI). The time between sessions was 4 h (beginning at 9:00 a.m., 1:00 p.m., and 5:00 p.m.). Before each session the pups had their bladders voided via anogenital stimulation and were weighed. A lactating dam was anesthetized with sodium pentobarbital (Nembutal, Abbott Laboratories, Chicago; 32.5 mg/kg body weight) and returned to the litter until the start of the session so that her nipples would be well suckled. This dam was age-matched so that the experimental pups were as close to the age of her actual pups as possible. Rewarded (R) and nonrewarded (N) trials were single-alternated such that odd-numbered trials were R and even-numbered trials were N. On all trials the pup was released from the start box and allowed to traverse the alley and enter the goal box. Upon breaking the photobeam near the goal box, on an R trial, the metal gate opened automatically, giving the pup access to the anesthetized dam. The opaque Plexiglas door between the alley and the goal box was then closed. When the pup attached to the nipple, the tip of the infusion tube was gently inserted into the cannula, the infusion pump was activated, and a reward of 0.03 ml was delivered over 9 s. Time spent in the goal box was approximately 30 s. On N trials the metal gate was not raised, the pup was kept in the front portion of the goal box for 30 s, did not reach the dam, and received no milk infusion. Between trials, the pup remained in the ITI box for 60 s.
At the end of the first five of the six sessions, pups were weighed and returned to the heated holding boxes. After the third session of the first day the pups were given a supplemental feeding while attached to the anesthetized dam outside of the runway apparatus. The supplemental feeding (0.6 ml; equivalent to 20 infusions) was given to prevent excessive weight loss during the extended intersession interval. After the final session (third session of the second day) cannulas were removed and pups were placed back in their home litters.
Time measures (converted to speeds) were taken over the 60-cm alley. If a pup did not traverse the first segment of the alley in 30 s it was gently pushed into the goal-box area and rewarded or not rewarded according to the trial, and a time of 30 s was recorded. The entire procedure was computerized on a screen to cue the experimenter in every aspect of its sequence, and the data were collected and stored on an IBM-AT using a Professional fortran program.
Drug Treatment.
Pups in the amphetamine condition were administered 0.3 mg/kg per ml d-amphetamine sulfate (Sigma) i.p., 10 min before each session. The amphetamine was mixed in physiological saline (vehicle control) before each experimental session.
Analysis.
There were four experimental conditions: x-ray/saline, x-ray/amphetamine, sham/amphetamine, and sham/saline. Times to traverse the runway were converted to speed (cm/s) and blocked into averages of five R trials and five N trials. There were, then, 24 R trial and 24 N trial blocks. Because previous work has shown that differences in groups arise only on the second day of training, only the last 12 blocks were used in the analysis. Four separate 2 (reward) × 12 (trials) multiple ANOVA tests were run. This was done because there are more degrees of freedom than observations when using a 2 (x-ray) × 2 (drug) × 2 (reward) × 12 (trials) design.
Neuroanatomical Procedures.
Neuroanatomical procedures were the same as in Diaz-Granados et al. (6). Pups were sacrificed with Nembutal and perfused transcardially with PBS and then with neutral-buffered formalin. The whole brain was extracted and stored in neutral-buffered formalin before embedding in paraffin. Paraffinized brains were sectioned at 3 μm, with every 55th and 60th section kept serially, and stained with cresyl violet. The dentate gyrus was examined with a Zeiss microscope at ×40 with the aid of a camera lucida, a bitpad (Summagraphics, Fairfield, CT, model MM1210) and a Professional fortran program. Data were stored on an IBM-AT. All cells and significant fragments of cells (defined as more than 50% of the membrane and nuclei visible) were included in the analysis. Only those rats in the x-irradiation conditions that showed at least an 80% decrease in dentate gyrus granule cells (as compared with sham irradiated) were used in the analysis.
RESULTS
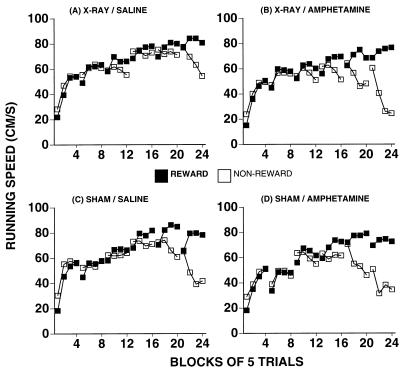
Results are based on 31 of the 42 rats. Eleven pups were dropped because of insufficient damage caused by the focal x-irradiation. Fig. 1 shows the typical damage to the dentate gyrus that results from the x-irradiation protocol. Table 1 presents the statistical analyses of the behavioral results. The x-irradiated rats receiving saline injections (n = 7; Fig. 2A) began to show PSA discrimination at the very end of the fifth session.
Table 1.
P values for block × group
| Group | Block
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| X-ray/saline | .870 | .870 | .151 | .396 | .089 | .215 | .06 | .017 | .936 | .018 | .032 | .008 |
| Sham/saline | .017 | .084 | .110 | .018 | .604 | .006 | .003 | .020 | .881 | .006 | .001 | .004 |
| X-ray/amp | .008 | .102 | .088 | .031 | .642 | .050 | .001 | .035 | .227 | .002 | .000 | .000 |
| Sham/amp | .020 | .035 | .001 | .109 | .559 | .002 | .005 | .001 | .007 | .001 | .001 | .001 |
Table shows block where each group begins to show a difference between R and N trials. P values that were significant are marked in boldface. All groups except the x-ray/saline group begin patterning earlier than block 20. In particular, the sham/saline and x-ray/amp groups show identical onset and patterning behavior. The sham/amp group may even show an earlier onset of patterning.
Figure 2.
(A) X-irradiated animals receiving saline injections begin to pattern late (blocks 21–24). (B) Amphetamine (0.3 mg/kg) restores PSA in x-irradiated animals to the level of sham-treated animals. They start patterning as early as block 16. Sham-irradiated animals receiving saline (C) and sham-irradiated animals receiving d-amphetamine (D) also pattern much earlier than x-irradiated animals receiving saline. These groups also show PSA as early as block 16.
In contrast, all other groups showed the discrimination much earlier. Sham-irradiated animals receiving saline (n = 8; Fig. 2D) began to show PSA discrimination at the end of the fourth session. Sham-irradiated animals receiving amphetamine (n = 8; Fig. 2D) showed PSA discrimination as early as blocks 14 and 15 of the fourth session, in the last three blocks of the fifth session, and throughout the sixth session. Most important, the x-irradiated animals receiving d-amphetamine injections (n = 8; Fig. 2B) had the same onset of the PSA discrimination as the shams receiving saline. They showed PSA discrimination at the end of the fourth session.
DISCUSSION
As in Diaz-Granados et al. (6), our results were that x-irradiation reduces the PSA, memory-based discrimination. Although the x-ray/saline group shows this memory-based learning in the last block of the fifth session and in the last three blocks of the sixth session, the other three groups show earlier onsets. The two control groups (sham/saline and sham/amphetamine) and, particularly, the x-ray/amphetamine group begin to show the PSA discrimination at the end of the fourth session and are clearly discriminating R from N trials throughout the fifth and sixth sessions. d-amphetamine restores the onset of PSA discrimination to the level of control groups and does not effect an increase in overall running speed. In an earlier report (6), we showed an elimination of PSA at a 60-s ITI but not at a 30-s ITI. In this experiment, the x-irradiation does not eliminate the PSA discrimination at a 60-s ITI, but causes a delay in its onset. This difference from these findings is most likely a result of the difference in age of the pups. At 60 s ITI the emergence of PSA discrimination depends on the age of the animal (8), and the 17- to 18-day-old pups learn better than the 16- to 17-day-olds. In this experiment there also was no observed effect on running speed, as there was earlier (6), probably because of the age difference in the two experiments: the 17- to 18-day-old pups may be running at close to their maximum speed, making hyperactivity from x-irradiation less possible to observe by using PSA discrimination learning as a test.
This experiment provides further evidence that hippocampal microneuronal damage may be a contributing factor in ADHD, which currently is treated with psychostimulants, such as d-amphetamine and methylphenidate (Ritalin). In the brain, these drugs act at the cellular level to increase the duration of action of both the dopaminergic and noradrenergic systems (e.g., ref. 12). This has contributed to the hypothesis that these systems are involved in ADHD. The dopaminergic system, which is enhanced by amphetamine treatment, has been implicated in encoding of prediction of reward (13), which is consistent with the hypothesis that the granule-cell-depleted rats in this experiment have some deficiency in encoding reward. Similarly, the noradrenergic system, because of its role in attention and, in general, cognitive function, has been implicated in many of the symptoms that characterize ADHD children (see ref. 14 for a review). Although these symptoms have been associated mainly with noradrenergic projections to the prefrontal cortex, the results from our animal model, consistent with Altman’s concept of microneuronal hypoplasia (1), now suggest that noradrenergic projections to the hippocampus also may play a role in the disorders suffered by these children.
ABBREVIATIONS
- PND
postnatal day
- ADHD
attention deficit–hyperactivity disorder
- ITI
intertrial interval
- PSA
patterned single alternation
References
- 1.Altman J. In: Learning Disabilities and Prenatal Risk. Lewis M, editor. Urbana: Univ. of Illinois Press; 1986. pp. 241–304. [Google Scholar]
- 2.Amsel A. Cognit Emotion. 1990;4:239–268. [Google Scholar]
- 3.Douglas V I, Parry P A. J Abnorm Child Psychol. 1983;11:313–326. doi: 10.1007/BF00912094. [DOI] [PubMed] [Google Scholar]
- 4.Amsel A. Frustration Theory: An Analysis of Dispositional Learning and Memory. Cambridge, U.K.: Cambridge Univ. Press; 1992. [Google Scholar]
- 5.Bayer S A, Brunner R L, Hine R, Altman J. Nature (London) 1973;242:222–224. doi: 10.1038/newbio242222a0. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Granados J L, Greene P L, Amsel A. Behav Neurosci. 1992;106:211–225. doi: 10.1037//0735-7044.106.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Granados J L, Greene P L, Amsel A. Behav Neural Biol. 1994;61:251–259. doi: 10.1016/s0163-1047(05)80008-1. [DOI] [PubMed] [Google Scholar]
- 8.Stanton M E. Anim Learn Behav. 1983;11:415–423. [Google Scholar]
- 9.Bayer S A. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- 10.Brunner R L, Haggbloom S J, Gazzara R A. Physiol Behav. 1974;13:485–494. doi: 10.1016/0031-9384(74)90278-9. [DOI] [PubMed] [Google Scholar]
- 11.Hall W G, Rosenblatt J S. Behav Biol. 1977;24:413–427. doi: 10.1016/s0091-6773(78)90723-x. [DOI] [PubMed] [Google Scholar]
- 12.Glowinski J, Axelrod J. J Pharmacol Exp Ther. 1965;149:43–49. [PubMed] [Google Scholar]
- 13.Schultz W, Dayan P, Mantague P R. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 14.Arnsten A F T, Steere J C, Hunt R D. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]