Abstract
The gastrointestinal (GI) tract is innervated by intrinsic enteric neurons and by extrinsic projections, including sympathetic and parasympathetic efferents as well as visceral afferents, all of which are compromised by age to different degrees. In the present review, we summarize and illustrate key structural changes in the aging innervation of the gut, and suggest a provisional list of the general patterns of aging of the GI innervation. For example, age-related neuronal losses occur in both the myenteric plexus and submucosal plexus of the intestines. These losses start in adulthood, increase over the rest of the lifespan, and are specific to cholinergic neurons. Parallel losses of enteric glia also occur. The extent of neuronal and glial loss varies along an oral-to-anal gradient, with the more distal GI tract being more severely affected. Additionally, with aging, dystrophic axonal swellings and markedly dilated varicosities progressively accumulate in the sympathetic, vagal, dorsal root, and enteric nitrergic innervation of the gut. These dramatic and consistent patterns of neuropathy that characterize the aging autonomic nervous system of the GI tract are candidate mechanisms for some of the age-related declines in function evidenced in the elderly.
1. Introduction
Aging is associated with a variety of gastrointestinal (GI) disorders (Talley et al., 1992; Majumdar et al., 1997). For example, with advancing age comes an increased incidence of motility or transit related problems, including delays in gastric emptying and longer intestinal transit time with associated fecal stasis (e.g., Jost, 1997; O’Mahony et al., 2002; Hays and Roberts, 2006; Norton, 2006). Furthermore, other diseases that afflict the elderly can precipitate GI disorders (Bassotti et al., 1998). These age-related GI problems have significant impact on both the well being of aging individuals and on health care costs. The causes of the GI disturbances of the elderly are unclear, but the symptoms suggest that an underlying etiology might be the loss or functional distortion of the neural mechanisms of the gut that normally contribute to GI function and health.
The GI tract is innervated by intrinsic neurons of the enteric nervous system (ENS) and by the axons of extrinsic sympathetic, parasympathetic, and visceral afferent neurons. Both the intrinsic and the extrinsic innervation, to different degrees, are affected by age. Earlier experimental reports describing the changes in the innervation of the GI tract during healthy aging have been extensively reviewed, summarized, interpreted, and discussed elsewhere (e.g., Santer and Baker, 1993; Hall, 2002; Wade, 2002; Wiley, 2002; Saffrey et al., 2004; Wade and Cowen, 2004; Wade and Hornby, 2005); thus, these observations are not simply repeated in the present survey. Rather, the goals of the present survey are threefold: First, we summarize recent observations, including those from our laboratory as well as those reported by others, and place these findings on age-related changes in the innervation of the GI tract in context with the earlier material covered by the previous reviews. Key structural changes in the aging autonomic innervation of the gut are illustrated. Second, we suggest a provisional list of general patterns of aging of the GI innervation. Third, and lastly, we consider methodological issues that need to be addressed in order to accurately and fully characterize the neurobiology of the aging gut.
The review is limited to the stomach and the small and large intestines. Age-related changes in the innervation of the esophagus have also been documented (e.g., Meciano Filho et al., 1995; Wu et al., 2003), but the functional and structural differences of that organ (e.g., striated musculature) put it beyond the scope of the present survey.
2. Intrinsic innervation of the GI tract: the myenteric and submucosal plexuses
2.1. Myenteric plexus
The myenteric plexus (MP), the outer of the two major ENS plexuses, is the network of neurons situated between the muscle layers of the GI tract, and is primarily involved in the initiation and control of smooth muscle motor patterns such as peristalsis. Loss of neurons in the aged MP was first described by Santer and Baker (1988) in the rat, and verified as a general phenomenon of aging in the intestines of numerous species including guinea pig (e.g., Gabella, 1989), humans (e.g., De Souza et al., 1993), and mice (El-Salhy et al., 1999). Most relevant to the current review, cell death in the MP of the small and large intestines has been demonstrated in several strains of rats raised under a wide range of housing conditions (Fischer 344: Phillips and Powley, 2001; Phillips et al., 2003, 2004b, 2006a,b; Sprague-Dawley: Cowen et al., 2000; Thrasivoulou et al., 2006; Wistar: Santer and Baker, 1988); Figure 1. Because species, strain, staining method, and sampling strategy all potentially influence the patterns of results observed and complicate comparisons among experiments, we report, in the course of this survey, some of the more critical variables for the individual experiments. We also consider the effects of these variables more explicitly in Section 7.
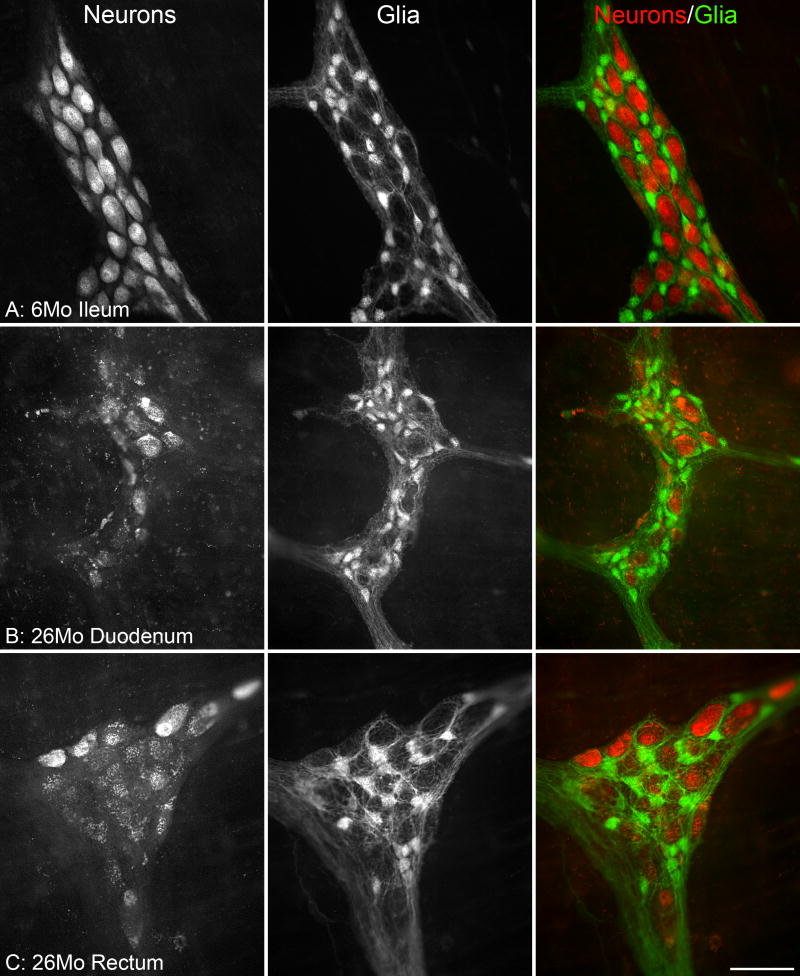
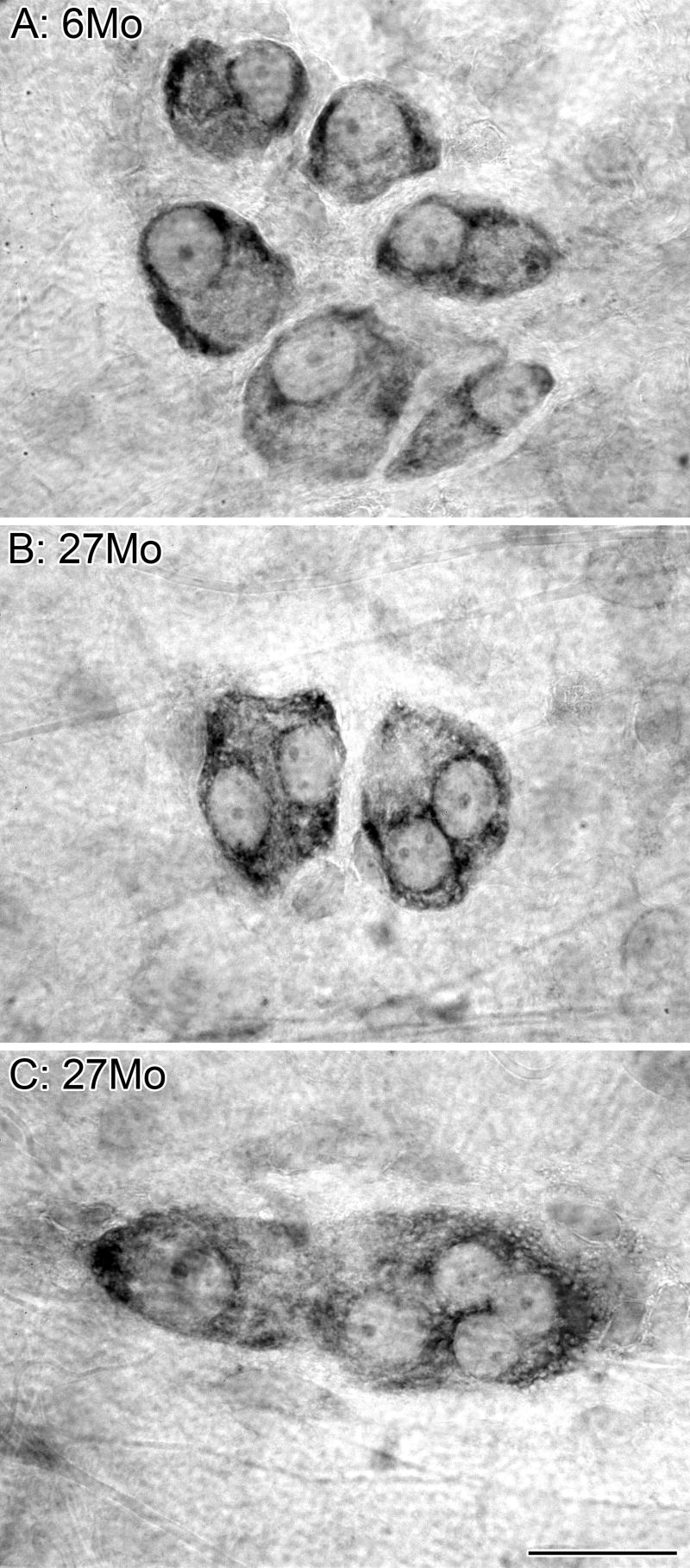
Figure 1.
Dramatic age-related changes occur in the myenteric plexus. Immunostaining of neurons (left column, HuC/D) and glia (middle column, S-100) in the myenteric plexus. The merged images (right column) illustrate the relationship of neurons (red) and glia (green). At 6 months of age, HuC/D strongly labeled the nuclei of neurons with weaker labeling of the cytoplasm and no labeling of the neuronal processes, and S-100 strongly labeled the soma of the glia with weaker labeling of processes (A). At 26 months of age, there was a reduction in the presence of the HuC/D antigen in the neurons of the small (B) and large (C) intestines. The complete loss of HuC/D staining of some neurons at 26 months is in stark contrast to the strong labeling of other neurons within the same ganglion, and is consistent with the observation that age-related neuron loss is specific to a subpopulation of neurons. The complete deterioration of some neurons within a ganglion is indicated by the presence of granular debris or lipofuscin within unstained silhouettes outlined by the glial scaffolding. Scale bar = 50 μm in C (applies to A-C).
Although initial quantitative studies examining age-associated changes in the number of neurons in the MP of the intestines reported losses in aged subjects, it still remained to be determined at what point in the lifespan the loss of neurons begins and what the pattern of loss is. To address these two questions, we quantified cell loss, using the stain Cuprolinic Blue (Phillips et al., 2004a), in the small (duodenum, jejunum, and ileum) and large (colon and rectum) intestines over most of the lifespan (i.e., at 3, 12, 21, 24 and 27 months of age) of ad libitum fed virgin male Fischer 344 (F344) rats (Phillips and Powley, 2001), and then replicated the findings in the colon of F344 rats at 6, 12, 18, 24 and 27 months of age (Phillips et al., 2006a), using the same pan-neuronal marker; Figure 2. We observed that neuron loss starts in adulthood and progresses in essentially a linear manner over the remainder of the rodent’s lifespan. A similar pattern of loss has been reported, using the neuronal marker PGP 9.5, for the ileum of ad libitum fed Sprague-Dawley rats (Thrasivoulou et al., 2006), with a significant loss of neurons by 13 months of age (compared to 6-month-old rats) that progressed to a total loss of 51% of the neurons by 24 months. Unlike our observations, however, Thrasivoulou and co-workers did not find a loss in neurons between 6 and 12 months of age, but instead cell death began abruptly between 12 and 13 months.
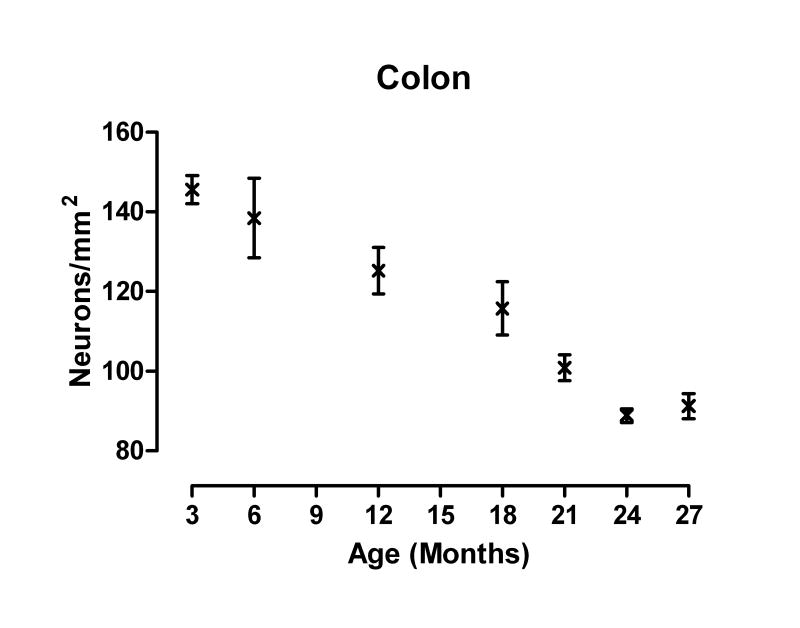
Figure 2.
Age-related neuronal loss in the myenteric plexus of the colon was evident as early as 12 months of age and progressed in a linear manner with increasing age (combined data from Phillips and Powley, 2001; Phillips et al., 2003, 2006a). A runs test following linear regression analysis verified that the distribution of the data was not significantly nonlinear (runs test, p = 0.54). A mean ± S.E.M of 248 ± 19 neurons/cm2/month are lost with age (goodness of fit, r2 = 0.71). The mean (± S.E.M) density of neurons, which were corrected for age-related changes in tissue area, was 146 (3.6), 138 (10), 125 (5.6), 116 (6.6), 101 (3.3), 89 (1.7), and 91 (3.2) neurons/mm2 at 3, 6, 12, 18, 21, 24, and 27 months of age, respectively. The analysis was based on a sample size of 12, 6, 12, 6, 5, 16, and 12 rats at 3, 6, 12, 18, 21, 24, and 27 months of age, respectively.
Finally, El-Salhy et al. (1999) reported, using PGP 9.5, neuron loss between 3 and 12 months of age in the duodenum and colon of ad libitum fed NMRI/Bom mice, but no additional loss for both regions at 24 months, suggesting that cell death was finished by middle-age. No additional neuronal loss after 12 months is at odds with our findings and those of Thrasivoulou et al. (2006) in the rat, and may be either an important observation illuminating species differences or a reflection of something mundane, such as differences in sampling protocols (e.g., corrected areal counts compared to neurons per ganglion estimates to characterize changes with age; see Section 7.2.3 for additional discussion). Future studies, using comparable protocols, are needed to address such differences.
In contrast to the consistent finding of age-related losses in the intestines, the integrity of the MP of the stomach over the lifespan remains less clear. We found, using Cuprolinic Blue, that there were no changes in the numbers of MP neurons innervating the forestomach, corpus, and antrum of F344 rats between the ages of 3 and 24 months. We did, however, note a significant loss of neurons in the forestomach, but not until approximately 27 months of age (Phillips and Powley, 2001), which is close to the end of the F344 rat’s lifespan (Turturro et al., 1999). Conversely, El-Salhy et al. (1999) report, using PGP 9.5, that there is a significant decrease in the number of neurons per ganglion in the antrum of 12-month-old NMRI/Bom mice compared to 3-month-olds. The number of neurons per ganglion in the antrum of 24-month-old mice was similar to 12-month-old mice indicating no additional cell loss after one year of age (see, however, comments in Sections 7.2.1 and 7.2.2 about the efficacy of different pan-neuronal markers for the study of aging).
2.2. Submucosal plexus
The submucosal plexus (SMP), the second of the two major ENS plexuses, is the network of neurons located in the submucosa between the smooth muscle layers and mucosa. In the intestines, submucosal neurons are organized as plexuses, i.e. networks of ganglia linked by connectives, however in the stomach the ganglia are both sparser and smaller and lack conspicuous connectives. For simplicity, though, we use SMP to refer to the submucosal networks throughout the entire GI tract. The SMP coordinates reflexes such as secretion and absorption, as well as motor control of smooth muscle. By comparison with the number of observations on the MP, still more limited information is available on changes with age in the neuronal population of the SMP.
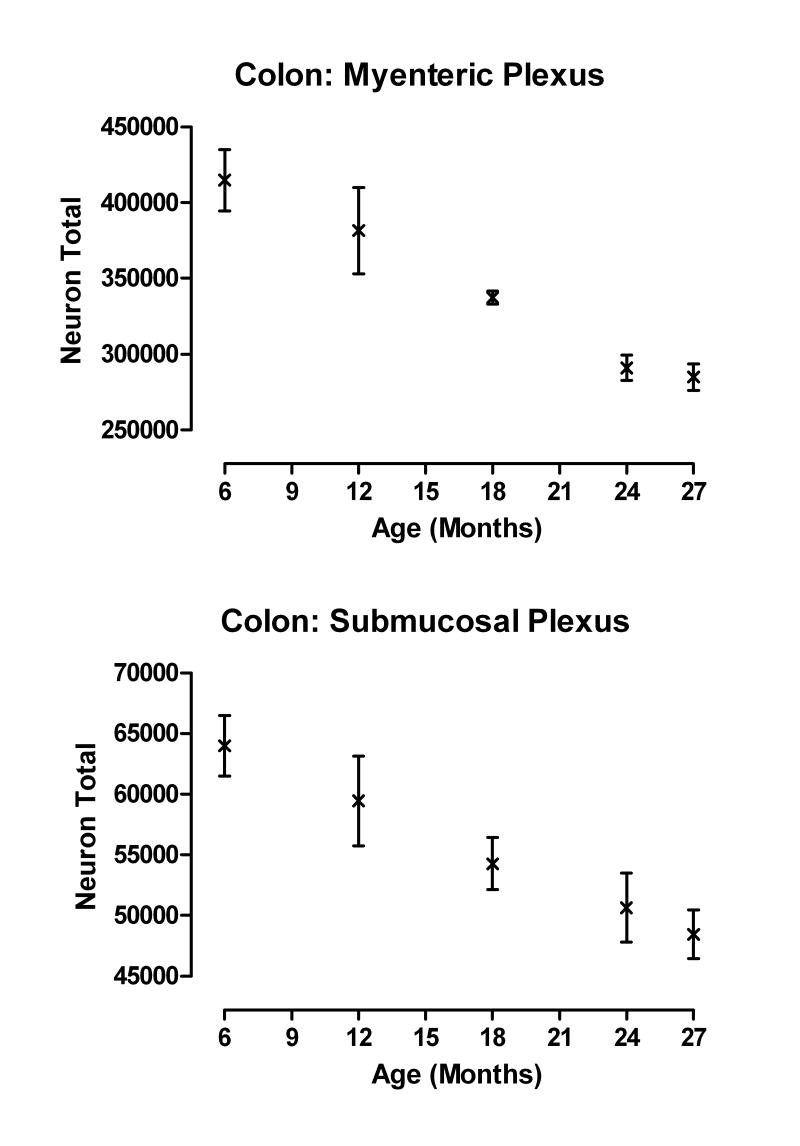
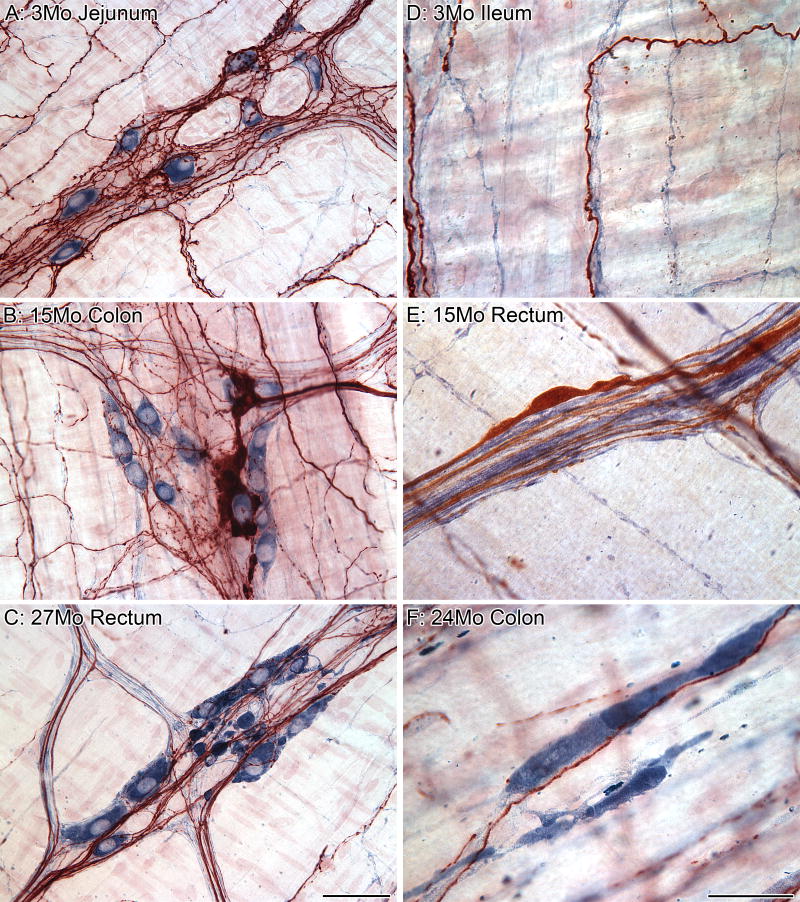
In the NIA F344 rat, we observed, using Cuprolinic Blue stained SMP whole mounts, that the SMP undergoes a progressive loss of neurons with age and that this pattern of neuronal loss is similar to what we have reported previously for the MP of the colon; Figure 3. Cell loss was evident in the SMP at 12 months of age and progressed linearly with increasing age for both the proximal and distal colon (Phillips et al., 2006a). By 27 months of age, approximately 24 % of the neurons had been lost as compared to adult rats (6 months of age). Comparable observations on the SMP of the stomach and small intestine remain to be done.
Figure 3.
The pattern of neuronal loss with age was similar for both the myenteric and submucosal plexuses (data from Phillips et al., 2006a). Total neuronal estimates for both plexuses are from the same rats with 6 rats/age sampled.
Prior to our observations on the F344 rat SMP, only one published full report had looked at age-related changes in the SMP. El-Salhy et al. (1999) found significant loss of PGP 9.5-positive neurons in the SMP of the antrum, duodenum, and colon of NMRI/Bom mice. Similar to their findings in the MP, El-Salhy and co-workers noted significant losses of SMP neurons in all three regions by 12 months of age with no additional loss in older mice (i.e., 24 months of age).
2.3. Chemical phenotype of neurons lost with age
Different functional classes of enteric neurons can be distinguished on the basis of their chemical coding (Furness, 2000). Thus, determining whether age-related neuronal loss is distributed across all phenotypes, or is specific to one or more subtypes, is a good starting point for understanding this cell loss (Figure 4A,D). Nitrergic neurons (i.e., those using nitric oxide synthesized by nitric oxide synthase) and cholinergic neurons (i.e., those using acetylcholine synthesized by choline acetyltransferase) represent two separate subpopulations that collectively account for the entire population in the MP, a pattern that is especially clear-cut for the rat (Mann et al., 1999; Nakajima et al., 2000). The majority of the nitrergic neurons in the MP are inhibitory motor neurons that innervate the smooth muscle, whereas cholinergic neurons are functionally diverse, and include interneurons, intrinsic sensory neurons and excitatory motor neurons that innervate smooth muscle.
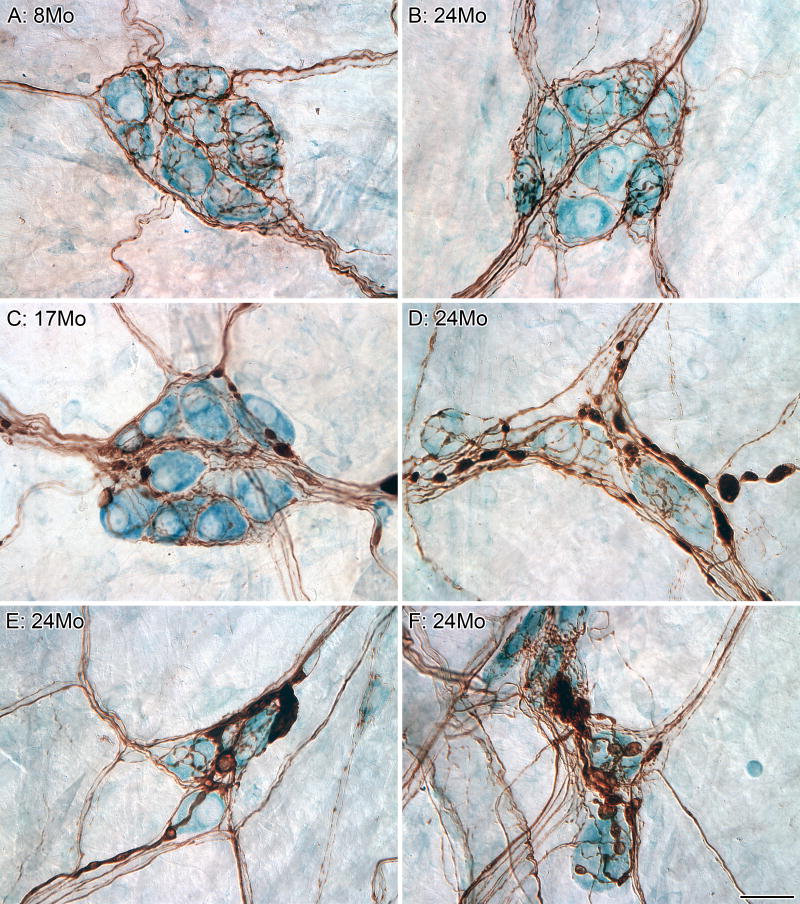
Figure 4.
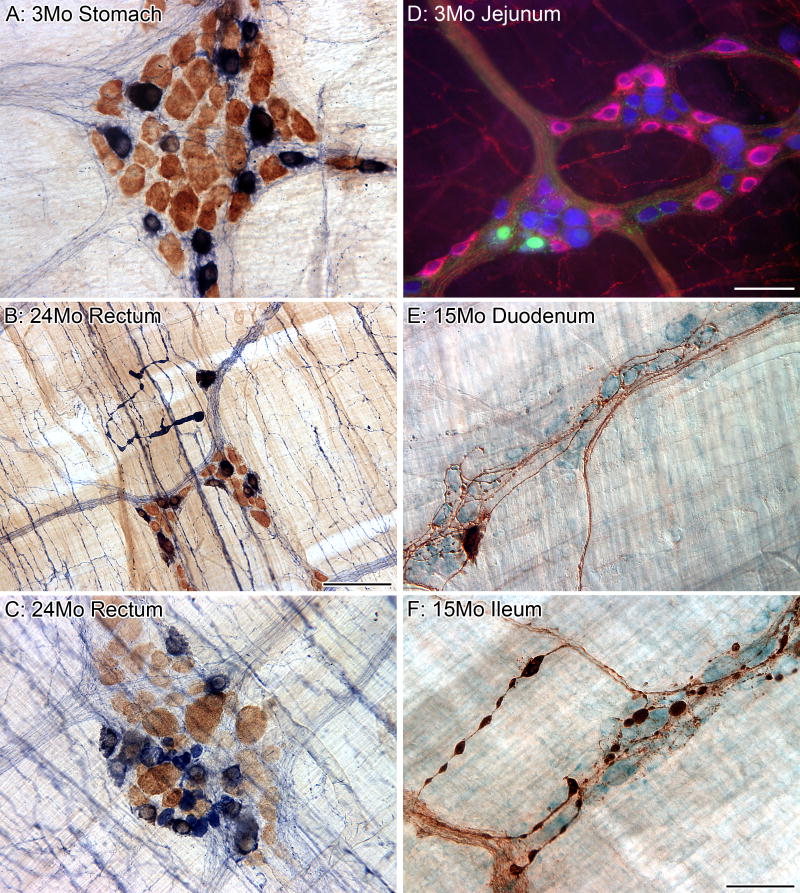
The myenteric plexus can be broken down into different subpopulations based upon the neurochemical phenotype of the neurons, and aging appears to affect the subpopulations differently. All of the neurons in the myenteric plexus were labeled using the putative pan-neuronal marker HuC/D (brown), and the subpopulation of neurons that are nitrergic were labeled using the histochemical reaction NADPHd (dark blue; A-C). There is no loss of nitrergic neurons throughout the gastrointestinal tract with age; however, markedly swollen nitrergic axons are routinely found in the smooth muscle (B). In addition, swollen nitrergic axons and dystrophic varicosities were also found in the myenteric ganglia (C; HuC/D neurons brown; nitrergic neurons dark blue with nuclei in silhouette; varicosities solid dark blue elements, some as large as neuronal somata) of aged rats. The majority of the myenteric neurons (blue, HuC/D) in the small intestine of the rat that are positive for the calcium binding protein calbindin (green) are not part of the nitrergic (red, nNOS) phenotype (D). Calbindin-positive (brown) neurons are a small subpopulation of the total population of neurons (light blue, Cuprolinic Blue) within the myenteric plexus (E). We have identified markedly swollen axons in the myenteric plexus of middle-age (15 months of age, F) and aged (24 months of age, not shown) Fischer 344 rats that are positive for calbindin (large brown varicosities and brown axons; blue neuronal counterstain, Cuprolinc Blue). Scale bars = 50 μm in F (applies to A,E,F), D (applies to C,D); 100 μm in B.
In separate groups of male F344 rats, we quantified the total population of neurons, stained using Cuprolinic Blue, and the subpopulation of nitrergic neurons, stained using nicotinamide adenine dinucleuotide phosphate diaphorase (NADPHd). Areal estimates of neuron density from aged rats (i.e., 24 months of age) for both stains were corrected using a factor derived from differences in the total areas of intestinal whole mounts between the young (3 months of age) and aged rats to account for differences in organ area resulting from growth. There were no losses of NADPHd-positive neurons in the aged stomach, small intestine, and large intestine, but there were significant age-related losses of Cuprolinic Blue-labeled neurons in both the small and large intestines (Phillips et al., 2003). Sparing of NADPHd-positive neurons has also been reported for the ileum (Belai et al., 1995; Cowen et al., 2000) and proximal colon (Belai et al., 1995) of aged (24-26-month-old) Sprague-Dawley rats, and the jejunum (Santer, 1994) of aged (24-30-month-old) Wistar rats.
Although nitrergic neurons do not die with age, it appears that they are not unscathed by age. We observed that aged rats exhibited numerous swollen NADPHd-positive axons in the muscle layers (Figure 4B) and within individual ganglia (Figure 4C) of the small and large intestines (Phillips et al., 2003, 2006b). Further, Cowen et al. (2000) reported a reduction in the staining intensity of nerve bundles positive for NADPHd, and Takahashi et al. (2000) found decreased expression of nitric oxide synthase in the colon MP of aged Fischer (F344 X Brown Norway) F1 rats.
From the studies looking at nitrergic neurons, it was clear that NADPHd-negative neurons (i.e., by elimination, the cholinergic neurons) are the subpopulation or phenotype that dies with age. Using an antibody to choline acetyltransferase, Cowen et al. (2000) confirmed this conclusion in the ileum by demonstrating a loss of 64% of the cholinergic neurons in 24 month old rats.
Cholinergic neurons can be further subdivided based on their expression of one of the calcium-binding proteins, calretinin and calbindin. Calcium-binding proteins are thought to be primarily involved in the sensing and regulation of calcium pools critical for synaptic plasticity (Schwaller et al., 2002). Neurons positive for calretinin form a much larger proportion of myenteric neurons compared to calbindin-positive neurons (Thrasivoulou et al. 2006); Figure 4D,E. Both of the subpopulations distinguished by the expression of calcium binding proteins seem either to die with age or to have down-regulated expression of the peptides. In the rat ileum, approximately 30% of the neurons positive for calretinin are lost by 17 months of age (Thrasivoulou et al., 2006) with similar findings reported for the guinea-pig ileum (Abalo et al., 2005). Calbindin-positive neurons also die with a loss of over 50% of the neurons in the ileum of 17-month-old rats (Thrasivoulou et al., 2006). While we have not quantitatively evaluated changes with age in the calbindin neurons in the MP of F344 rats, we have observed the presence of markedly swollen calbindin-positive axons in the MP ganglia and connectives of middle-aged rats; Figure 4F. No comparable observations describing age-related changes in the different chemical phenotypes of the SMP presently exist.
Currently, it is not clear why neurons positive for acetylcholine and for calcium-binding proteins are particularly sensitive to the insults of aging; however, Thrasivoulou et al. (2006) have put forth the interesting hypothesis that these neurons are vulnerable as a result of calcium dyshomeostasis, which may be linked to elevated intra-neuronal levels of reactive oxygen species. Furthermore, caloric restriction, a manipulation that reduces the accumulation of reactive oxygen species, has been shown to limit or even prevent age-related losses of myenteric neurons (Cowen et al., 2000). Another intriguing pattern is the fact that cholinergic neurons are particularly susceptible to age-related damage in both the ENS and the CNS (McKinney and Jacksonville, 2005; Schliebs and Arendt, 2006).
2.4. Changes in the fine structure of ganglia with age
With a loss of neurons and glia (see Section 3) with age in the MP, there should be a decrease in ganglionic area, that is to say, as neurons and glia die off, individual ganglia effectively shrink in upon themselves and become smaller in area. Thus, we evaluated the ganglionic area of myenteric ganglia in which the boundaries of a ganglion were delineated by stained neurons and glia (Phillips et al., 2004b); Figure 1. Average ganglion size was smaller in aged (26-month-old) F344 rats compared to adult (5-6-month-old) F344 rats for the duodenum, jejunum, colon and rectum (see Section 7.2.3 for discussion of issues identifying a ganglion in the MP of the rat). A similar reduction in myenteric ganglionic size has been reported for the ileum of the aged guinea-pig (Abalo et al., 2005).
Further evidence for a reduction in ganglionic area with age is found in the SMP (Phillips et al., 2006a). As illustrated in Figure 5, the presence of atrophic ganglia is qualitatively obvious in the SMP. Neurons within a SMP ganglion are typically separated by small interstices of neuropil consisting of axons, dendrites and enteric glia; Figure 5A. In aged F344 rats (27-month-old), however, in some ganglia observed, surviving neurons were tightly aggregated and the neuropil spaces were almost completely reduced, as though the ganglia had collapsed in upon themselves; Figure 5B,C. From the extensive loss of neuropil, it would appear that aging ganglia must sustain a loss of dendrites, supporting enteric glia, and perhaps innervation from extrinsic axons in addition to the loss of neurons.
Figure 5.
A reduction in ganglionic area occurs with age. Cuprolinic Blue labeled neurons in the submucosal plexus of the colon. Neurons were well defined with stained Nissl substance outlining the unstained nuclei. At 6 months of age, neurons within a ganglion were evenly spaced, providing room for unstained axons, dendrites, and glia (A). In the plexus of 27-month-old rats, ganglia were often observed that appeared to have collapsed in upon themselves (B,C), with little or no evidence of neuropil, i.e., the ganglionic tissue surrounding the neuronal cell bodies (e.g., glial cells, nerve fibers and processes of ganglion neurons). Scale bar = 25 μm in C (applies to A-C).
3. Enteric glia
Enteric glia are prominently located within the ganglia of both the MP and SMP where they interdigitate with neurons; Figure 1A. While located primarily within the ganglia, glia are also present in the interconnecting nerve strands of both plexuses, and within the villi of the mucosa where they make close contacts with the epithelial cell layer (Rühl, 2005). Classically, enteric glia were thought to act only as structural support for neurons, but recently the list of their candidate functions has expanded to include metabolic, trophic, and protective support of neurons (Gershon and Rothman, 1991; Cabarrocas et al., 2003; Rühl et al., 2004; Nasser et al., 2006). Additionally, it is clear that glia play an important role in the health of the enteric innervation of the GI tract (Bush et al., 1998; Cabarrocas et al., 2003; Steinkamp et al., 2003; Rühl et al., 2004; Nasser et al., 2006).
In the GI tract, S100 is considered to be exclusively localized in enteric glial cells (Ferri et al., 1982) and appears to be a good candidate for a pan-glial marker (Hoff et al., 2005). We, therefore, used S100 to evaluate changes with age in the enteric glial population in the MP of young (5-6 months of age) and aged (26 months of age) F344 rats (Phillips et al., 2004b). We observed a significant decrease in the number of S100-positive glia in the duodenum, jejunum, ileum, and colon of aged rats; Figure 1B. The mean number of glia in the MP of the rectum was reduced in aged rats compared to their younger counterparts, however, the loss was not significant for this region of the large intestine; Figure 1C. There was a significant loss of neurons, stained using the antibody to HuC/D, for all five of the regions from the GI tract in the same ganglia from which the enteric glial cells were sampled and quantified.
The loss of glia with age appears to parallel the loss of neurons, however, there is some evidence that neuron death may precede the loss of glia. Throughout the GI tract the number of glia in a ganglion outnumbers the number of neurons. If glial and neuronal losses occur concurrently and in strict parallel, then the ratio of glia to neurons should stay constant across age. What we observed, though, was that the ratio of glia to neurons was consistently larger in aged rats throughout the GI tract, especially in the duodenum. Such a pattern is tentative, however, because HuC/D was used to visualize the neurons of the MP (Phillips et al., 2004b), so the decrease in neuron number may reflect both neuron loss with age and a decrease in antigenicity (see Section 7.2.2). Conceivably, too, age-associated stresses of neuronal loss and dysregulation might stimulate a reactive proliferation of glia that would offset and mask any second process promoting glial losses. Changes in the number of enteric glial cells in the SMP and mucosal villi during aging have so far not been described.
4. Extrinsic innervation of the gastrointestinal tract: sympathetic, parasympathetic and sensory innervation
The gut’s extrinsic innervation is supplied by neurons located in prevertebral ganglia, the brainstem, and peripheral afferent ganglia. Though some observations on the effects of aging on these different sites are available (Baker and Santer, 1988b; Schmidt et al., 1990; Sturrock, 1990; Gai et al., 1992; Soltanpour et al., 1996; Soltanpour and Santer, 1997; Bergman and Ulfhake, 1998; Santer et al., 2002; Schmidt, 2002a), these same ganglia and brainstem nuclei also contain neurons that project to non-GI viscera. The absence of practical techniques to readily distinguish those neurons within a nucleus that innervate the gut from those that innervate non-gut tissues, makes it difficult to survey target- or organ-specific neuronal subpopulations. Thus, studies of the aging of the extrinsic innervation of the GI tract have not examined neuronal somata and have instead been largely limited to examinations of the extrinsic axons as they course through gastrointestinal tract tissues. As outlined below, dilated and dystrophic profiles occur in both the efferent and afferent extrinsic projections to the gut.
4.1. Sympathetic innervation
The noradrenergic fibers within the wall of the GI tract originate from cell bodies located within the prevertebral sympathetic ganglia. The celiac-mesenteric ganglia provide fibers to the stomach, small intestine and, to some extent, the proximal large intestine. The inferior mesenteric ganglia provide fibers to the large intestine, and the remaining noradrenergic fibers to the rectum originate from the pelvic ganglia. The noradrenergic innervation from these prevertebral ganglia supplies an extensive network of fibers to the smooth muscle wall, ganglia of the MP and SMP, and arteries of the GI tract (Furness and Costa, 1974). Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the biosynthesis of dopamine and noradrenaline, so we routinely use an antibody to TH to selectively visualize the sympathetic innervation of the ENS.
Tyrosine hydroxylase immunoreactive (TH-IR) fibers extensively innervate the MP of the stomach, small intestine, and large intestine of F344 rats (Phillips et al., 2006b). Within ganglia, varicose TH-IR fibers encircle neurons, but the somata of the myenteric neurons themselves are rarely positive for TH; Figure 6A. In addition, TH-IR fibers form networks in the regions adjacent to ganglia (Figure 6D), travel from ganglia to ganglia within the connectives (Figure 6E), and heavily innervate the circular layer of the smooth muscle. The SMP of the colon of F344 rats is similarly innervated by TH-IR fibers (Phillips et al., 2006a); Figure 7A,B.
Figure 6.
There was no clear relationship between the age-related changes seen in nitrergic and sympathetic neurites. The innervation of nitrergic neurons (dark blue, NADPHd) by sympathetic axons (red-brown, TH) in the ganglia of the myenteric plexus appears, based on the amount of contacts of the varicosities on sympathetic axons with the cytoplasm and nucleus of the nitrergic neurons, to range from sparse to dense (A). In the whole mounts of the myenteric plexus from aging rats, markedly swollen sympathetic axons were found in ganglia with healthy nitrergic innervation (B) and conversely, markedly swollen nitrergic varicosities (dense, dark blue varicosities) were found in ganglia with intact sympathetic innervation (C); however, ganglia with both were never observed in our samples. Sympathetic (red-brown, TH) and nitrergic (dark blue) axons frequently traveled together in the same connectives (D). Markedly swollen sympathetic axons were found coursing through the connective with other sympathetic axons and nitrergic axons of normal diameter (E). Conversely, markedly swollen nitrergic axons in close association with sympathetic axons of normal diameter were frequently found throughout the myenteric plexus (F). Scale bars = 50 μm in C (applies to A-C); 25 μm in F (applies to D-F).
Figure 7.
A distinct axonopathy represented by markedly swollen sympathetic axons was commonly found in the innervation of the submucosal plexus. All of the ganglia (blue neuronal counterstain, Cuprolinc Blue) within the submucosal plexus were innervated by sympathetic (brown, TH) axons (A-F). Cases of well-labeled sympathetic axons that were healthy in appearance (i.e., identical to the innervation seen at younger ages; A) were routinely found in aged tissue (B). In contrast, however, swollen sympathetic axons and terminals also became both more common and more pronounced with age (C-F). Ganglia were often observed to be innervated by sympathetic axons that were both markedly swollen and healthy looking (C,D). Scale bar = 25 μm in F (applies to A-F).
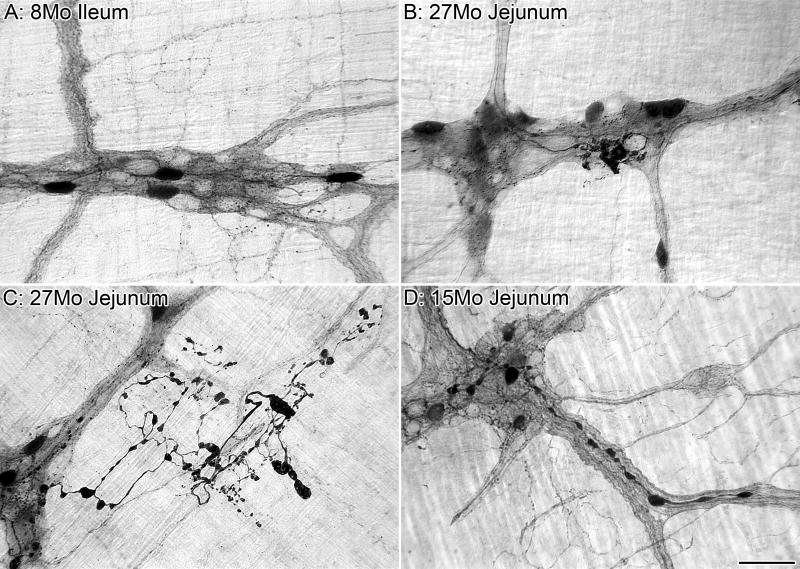
Using gloxylic acid fluorescence to visualize noradrenergic axons in the MP of the proximal jejunum of Wistar rats, Baker and Santer (1988a) observed a decline in the level of fluorescence of the noradrenergic axons and a breakdown in plexus regularity between the ages of 12 and 18 months. These investigators also noted the presence of swollen axons and bright, round structures at 24 months of age, which they interpreted as indicative of degenerating axons. Similarly, we found that TH-IR axons innervating the MP and SMP of 24-month-old F344 rats exhibit axonopathies and other signs of deterioration as evidenced by the presence of markedly swollen axons and terminals, as well as a decrease in TH staining of some of the surviving processes; Figure 6B,E. Neuropathies of the TH-IR fibers were evident as early as 8 months of age in the SMP of the colon and 15 months of age in the MP of the small and large intestine, and became progressively more common and pronounced with age; Figure 7C-F. Similar axonopathies consisting of dilated or swollen varicosities were noted in the lamina propria and along blood vessels within the SMP. In contrast, markedly swollen TH-IR fibers were not observed in the circular smooth muscle of aged rats. There was, however, a decrease in the staining intensity of some of these fibers suggesting that there is a down regulation in available TH to the circular muscle with age.
The presence of swollen axons within the GI tract originating from both intrinsic nitrergic neurons (cf. Section 2.3; Figure 4) and extrinsically located prevertebral ganglia (cf. TH-labeling results above) provided us with the opportunity to attempt to examine the temporal relationship between the neuropathies of the intrinsic and extrinsic innervation of the MP in the hopes of illuminating the aging sequence. We considered the straightforward hypothesis that as any particular MP ganglion underwent age-related neuronal loss there would be simultaneous and correlated compensatory changes and reactive axonopathies in both the sympathetic and nitrergic innervation of that ganglion. Ruling out any easy explanation, no obvious relationship between dystrophic sympathetic and nitrergic axons and terminals was found (Phillips et al., 2006b). Either markedly swollen sympathetic terminals (Figure 6B) or markedly swollen nitrergic terminals (Figure 6C) were found within an aged ganglion, but the two neuropathies were not typically observed in the same sites. In most cases, when swollen axons of one type were observed, the axons of the other were “healthy” in appearance (i.e., similar in appearance to labeling seen in rats younger than 12 months of age). A similar lack of association between the intrinsic and extrinsic axonopathies was observed for the connectives (Figure 6E) and the muscle layers (Figure 6F) where both types of neurites travel in close association with each other. The lack of an apparent relationship between the two neuropathies may suggest that they are temporally out of phase, with one leading (and dissipating) before the other occurs, or, alternatively may indicate that the two neuropathies are simply orthogonal.
4.2. Vagal innervation
The vagus nerve supplies both sensory (afferent fibers) and motor (efferent fibers) functions. The neurites of vagal afferents form extensive networks of endings in the smooth muscle, MP, and mucosa (Powley et al., 1994; Berthoud et al., 1995, 1997). Vagal afferent endings in the smooth muscle are known as intramuscular arrays, or IMAs, and those in the myenteric ganglia are known as intraganglionic laminar endings, or IGLEs. The distribution and morphology of the two endings is consistent with IMAs being stretch receptors and IGLEs being tension receptors (Phillips and Powley, 2000; Powley and Phillips, 2002; Neuhuber et al., 2006). Vagal sensory innervation of the SMP has not been described in any detail. Vagal sensory innervation of the mucosa consists of terminal arborizations mainly between the crypts and the villous lamina propria, with terminal branches reaching the basal lamina but not penetrating it so as to make direct contact with epithelial cells (Powley et al., 1994; Berthoud et al., 1995; Powley et al., 2005). The morphology of the vagal terminals in the mucosa is described as free nerve endings. Vagal motor innervation of the GI tract is restricted to the MP where individual efferent fibers collateralize extensively throughout the plexus, with terminal swellings (i.e., presumptive synaptic contacts) in close approximation with individual myenteric neurons (Holst et al., 1997).
Utilizing the tracers wheat germ agglutinin-horseradish peroxidase (WGA-HRP) and cholera toxin subunit B-horseradish peroxidase (CTB-HRP) to label respectively the vagal sensory and motor innervation of the stomach, we described the pattern of innervation for the two branches of the vagus nerve in both young (3 months of age) and aged (24 months of age) F344 rats (Phillips and Powley, 2001). The density and distribution of gastric IMAs and IGLEs were stable across both ages. Similarly, we found no age-related difference in the number of myenteric neurons encircled by vagal efferents (a semi-quantitative measure of efferent innervation of the MP). Both HRP conjugates are instructive because they label selectively and completely the endings in the stomach (Phillips et al., 1997; Wang and Powley, 2000; Phillips and Powley, 2001) and thus provide an impression of possible changes with age at a global level, but they are limited by their inability to illuminate fine structural changes of individual fibers and terminals (Powley and Phillips, 2005).
More recently, we applied a battery of alternative tracer protocols, which provide more detailed information about the morphology of individual vagal endings (Powley and Phillips, 2005) to aging vagal afferents. Biotinylated dextran tetramethylrhodamine (mini-ruby; Molecular Probes) was injected into the nodose ganglia of young (3 months of age) and aged (22 months of age) F344 rats (n = 6/age) to provide preliminary information about the morphology of individual vagal sensory endings in the smooth muscle and MP of the aged stomach and the mucosal villi of the aged small intestine. In aged rats, we observed markedly swollen varicosities along the length of parent neurites terminating as IGLEs in the MP of the stomachs; Figure 8A. In addition, vagal sensory innervation of the villi of young rats consisted of free nerve endings (Figure 8B), whereas the innervation of villi of aged rats was contracted into smaller plates of dystrophic neurites; Figure 8C. More observations need to be made on the vagal sensory innervation of the aged MP and villi and expanded to include the sensory innervation of the smooth muscle and vagal motor innervation of the MP, but it appears that there are aged-related changes in the vagal innervation of the GI tract at the level of the individual terminals that are not seen when using HRP conjugates for general surveys (Phillips and Powley, 2001).
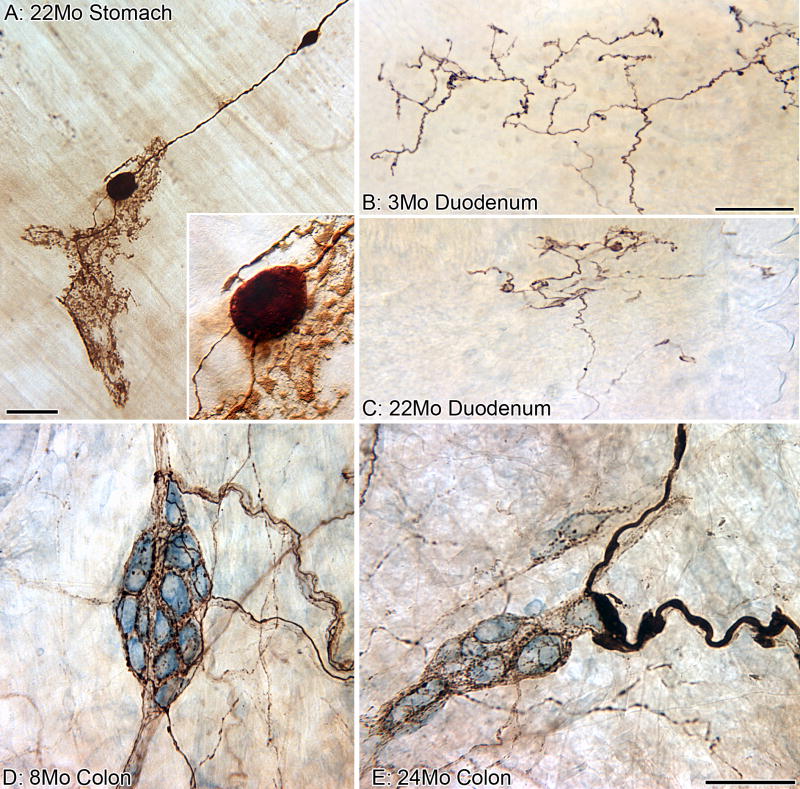
Figure 8.
Visceral afferent innervation of the gastrointestinal tract evidenced signs of deterioration with age. A parent neurite with two markedly swollen varicosities along its length (brown, labeled using the anterograde tracer TMR-B) terminates as a putative vagal mechanoreceptor known as an “intraganglionic laminar ending” (IGLE) in the myenteric plexus (A). The insert is a higher power view of the larger of the two swollen varicosities shown in panel A; small lucent vesicles (mitochondria?) can be seen around the circumference of the bulbous varicosity. In young Fischer 344 rats (B), vagal afferents (brown, TMR-B) course into small intestinal mucosal villi as relatively un-branched neurites and then ramify into plates of free nerve endings immediately subjacent to the epithelium (chemoreceptors?). In old rats (C), however, the vagal afferents in the villi contract into smaller plates of dystrophic neurites. In both panels B and C, the gut lumen is at the top, and the epithelial wall separates the endings from the lumen. Visceral afferents (brown, CGRP), which most likely originate from the dorsal root ganglia, densely innervate the ganglia (blue neuronal counterstain, Cuprolinc Blue) of the submucosal plexus (D) and were typically “healthy” in appearance throughout the lifespan of the Fischer 344 rat; however, swollen axons and terminals were uncommonly found in the submucosal plexus of some aged rats (E). Scale bar = 50 μm in A, E (applies to D,E), C (applies to B,C); 18 μm in A (applies to Insert).
4.3. Visceral afferent innervation
Primary afferent sensory fibers originating from cell bodies in dorsal root ganglia project along the mesenteric arteries to enter the GI tract and provide a perivascular network of fibers to the arterial system, around neurons in the ganglia of the MP and SMP, and within the villi of the mucosa. The role of dorsal root afferents in the control of GI function echoes this pattern of innervation, and includes regulation of blood flow, neuronal excitability, motility, and secretion (Green and Dockray, 1987; McCulloch and Cook, 1989; Vanner, 1994; Sternini and Anderson, 1992; Chiocchetti et al., 2006).
The majority of the spinal afferents innervating the GI tract of the rat stain positive for alpha-calcitonin gene-related peptide (CGRP; Sternini and Anderson, 1992). The extrinsic origin of CGRP-positive fibers to the GI tract has been confirmed by either surgically severing the extrinsic nerves to the GI tract or treating subjects with the neurotoxin capsaicin to destroy unmyelinated sensory fibers, and observing that both procedures result in the elimination of CGRP-positive fibers in the GI tract (Lee et al., 1987; Sternini et al., 1987; Green and Dockray, 1988). While CGRP is a useful and convenient marker for extrinsic primary afferents in the GI tract, CGRP is not entirely exclusive to afferents originating from the dorsal root ganglia. A minor component of the afferent innervation of the GI tract is derived from CGRP-containing neurons located in the nodose ganglia (Lee et al., 1987; Green and Dockray, 1988; Sternini and Anderson, 1992; Wang and Neuhuber, 2003). Furthermore, in mice, CGRP is a marker for the Dogiel type II class of enteric neuron that label preferentially with calbindin in the guinea pig (Furness et al., 2004), and CGRP has been suggested to be involved in transmission from these neurons (Grider, 1994, 2003).
We used a monoclonal antibody to alpha-CGRP (4901; CURE/Digestive Research Center; Wong et al., 1993) to examine the dorsal root afferent innervation of the aging GI tract. Neurons in the MP throughout the entire GI tract from the stomach to the rectum were densely innervated by CGRP-immunoreactive (CGRP-IR) neurites forming dense networks around the ganglion cells. A similar pattern of innervation of SMP neurons by CGRP-IR fibers was observed in the large intestine (Phillips et al., 2006a); Figure 8D. Innervation of the MP and SMP by CGRP-IR fibers was relatively stable across the age-range studied (i.e., 8 to 24 months of age), however, we did notice a small number of swollen dystrophic CGRP-IR fibers; Figure 8E. These swollen visceral afferents were never observed in adult rats (8 months of age) and were extremely rare in middle-aged (16 months of age) and aged (24 months of age) rats. Intact CGRP-IR fibers throughout the lifespan of the rodent are consistent with a previous report that cervical and lumbar dorsal root ganglion neurons are spared with age (Bergman and Ulfhake, 1998). The relative sparing with age of the visceral afferents when compared with the dramatic age-related changes reported above for the sympathetic innervation suggests that the former are not as severely affected by the insults of age or at least are on a different timetable of deterioration than the sympathetics.
These observations on the age-related changes of the dorsal root afferent innervation of the GI tract are limited by the constraints of the technique we used to visualize the fibers (i.e., CGRP’s lack of specificity for only dorsal root afferents) and the incompleteness of our observations (i.e., the SMP was only examined in the large intestine and the mucosa of aged rodents was never sampled). To characterize possible terminal neuropathies with age unequivocally and precisely, protocols that result in the selective labeling of single or subsets of visceral sensory fibers, tracer protocols similar to those described above to label individual vagal terminals, will be needed.
5. Effects of disease on the pattern of innervation
For initial surveys of the effects of aging on the GI tract, there are compelling reasons to examine healthy patterns of aging uncomplicated by disease, malnutrition or debilitating factors. Many aging individuals, however, are afflicted by a multiplicity of disorders and, furthermore, many diseases are associated with secondary GI complications that are known to produce autonomic neuropathies (Wakabayashi et al., 1989, 1993, 1999; Spangeus et al., 1999; Spangeus and El-Salhy, 2001) and to precipitate GI tract disorders (Bassotti et al., 1998). Thus, there is a need to begin to broaden the initial surveys to include models of aging complicated by disease.
In Parkinson’s disease (PD), for example, an accumulation of Lewy bodies has been described in the sympathetic ganglia, sacral parasympathetic nuclei, and dorsal motor nucleus as well as the myenteric and submucosal plexus of the enteric nervous system (Wakabayashi et al., 1988, 1989, 1990; Gai, 1992; Wakabayashi and Takahashi, 1997). Because postmortem studies of patients with PD have shown neuronal loss in the sites where Lewy bodies are produced, Lewy body formation is considered a marker for neuronal degeneration (Jellinger, 1991). Lewy bodies are thought to reflect altered neurofilament metabolism and/or transport due to neuronal damage and subsequent degeneration, causing an accumulation of altered cytoskeletal elements. Immunohistochemically, Lewy bodies stain with a variety of antibodies including, in particular, antibodies to alpha-synuclein (Spillantiniet al., 1997). The normal function of alpha-synuclein is incompletely known, but aberrant aggregation of alpha-synuclein has been detected in PD, and abnormal processing of alpha-synuclein is thought to lead to pathological changes in its binding properties and function. Alpha-synuclein is expressed predominantly in neurons of the central and peripheral nervous system, with very intense labeling of the cell bodies in the dorsal motor nucleus of the vagus and the myenteric and submucosal plexus (Li et al., 2002; Bloch et al., 2006; Braak et al., 2006).
Thus, alpha-synuclein would appear, based on the role of Lewy bodies in autonomic dysfunction of the GI tract of PD sufferers, to be a good place to take a “first look” in aging. We, therefore examined the expression of the protein in the MP of the small intestine from a small number of adult (8 months of age), middle-aged (15 months of age), and aged (27 months of age) F344 rats and C57Bl/6 mice. Gut whole mounts were processed using an immunohistochemical protocol to visualize alpha-synuclein (S63320; BD Transduction Laboratories). We observed a subpopulation of neurons in the MP of the small intestine that were positive for alpha-synuclein; Figure 9A. In middle-aged and aged rodents, there was an accumulation of alpha-synuclein-positive material within the MP in close approximation to alpha-synuclein-positive neurons; Figure 9B. Additionally, alpha-synuclein-positive axons that were disorganized in appearance were observed with dystrophic swelling along their length; Figure 9C,D.
Figure 9.
A subpopulation of neurons in the myenteric plexus are positive for alpha-synuclein which is a constituent of, and marker for, Lewy bodies commonly found in individuals diagnosed with Parkinson’s disease. In adult Fischer 344 rats, myenteric neurons and neurites positive for alpha-synuclein are “healthy” in appearance (i.e., not dilated, swollen, or dystrophic; A). In aged rats, build-up and accumulation of alpha-synuclein-positive material was found within the myenteric ganglia around alpha-synuclein-positive neurons (B). Additionally, in aged rats, some alpha-synuclein-positive axons were highly disorganized in appearance, with abnormally large and dystrophic swellings along their lengths (C). We have observed similar alpha-synuclein-positive axonopathies in the innervation of the myenteric plexus of aging mice (D). Scale bar = 50 μm in D (applies to A-D).
In addition to alpha-synuclein, other markers also may be useful indices of the neuropathies of aging and may provide clues as to the mechanisms underlying the disorders. Lipofuscin granules, for example, appear to accumulate with age in at least some subpopulations of neurons of the ENS (cf. Corns et al., 2002; Brehmer et al., 2004; Abalo et al., 2005), presumably in response to oxidative stress.
Findings where the neuropathies of aging are associated with one or more co-morbidities raise two main hypotheses about the interaction of disease with normal aging of the ENS that should be assessed. First, the autonomic neuropathies of PD might be concentrated on the same elements of the autonomic nervous system that prove particularly vulnerable to normal aging--a disease such as PD might simply accelerate the basic pattern of aging. Such a possibility would be consistent with the indications that diabetic and alcoholic neuropathies of the sympathetic nervous system seem to accelerate some of the changes seen with aging (Schmidt, 2002a,b). Second, alternatively, the disease-related neuropathies might involve losses and disturbances quite unlike, and independent of, those of healthy aging. In either case, the neuropathies of disease and the neuropathies of normal aging might interact in super-additive ways or they might produce losses that combine in more additive patterns.
6. Patterns of aging
Taken together, the general consensus of the findings reviewed above establishes a provisional list of patterns of aging for the innervation of the GI tract:
-
Enteric neurons in the aging GI tract die.
-
◦
Losses occur in both the MP and the SMP.
-
◦
The degree of loss varies by organ with neurodegeneration in the large intestine > small intestine > stomach.
-
◦
Both in the small and large intestines, losses follow consistent temporal progressions.
-
▪
Neurodegeneration begins in adulthood.
-
▪
Once initiated, neuron loss continues throughout middle- and old-age.
-
▪
-
◦
-
Effects of aging vary by neuronal chemical phenotype.
-
◦
Neuronal loss is specific to cholinergic neurons, with cholinergic neurons positive for calcium binding proteins being particularly sensitive to the insults of aging.
-
◦
Nitrergic neurons do not die, but are adversely affect by age (e.g., the presence of markedly swollen axons and decreased expression of nitric oxide synthase).
-
◦
Enteric glia die with age, and their loss parallels the degenerative changes described above for the intrinsic neuronal population.
Sympathetic innervation of the MP and SMP deteriorates with age (e.g., the presence of markedly swollen axons and decreased expression of tyrosine hydroxylase).
Visceral afferents are not as severally affected as the sympathetics or at least are on a different timetable of deterioration.
Neuropathies that occur during healthy aging and age-associated diseases share common pathways and markers (e.g., alpha-synuclein).
7. Methodological Issues in GI Gerontology
In the discussion above, we alluded to several methodological issues and complicating factors that affect comparisons made between various species and strains of animals at different ages. These methodological considerations are now addressed more fully.
7.1. The Experimental Model: Importance of Species, Strain and Nutrition
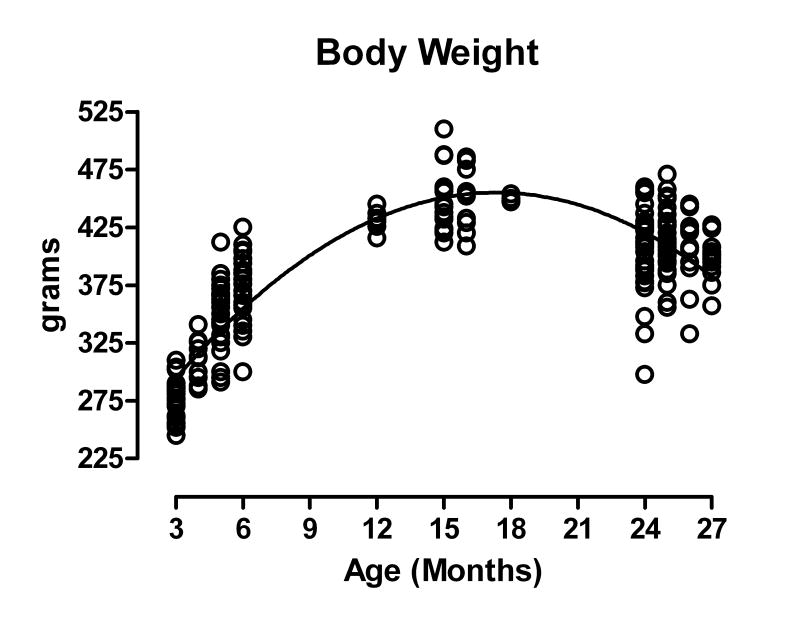
Unless investigators work with a strain or species that is well characterized in terms of its general health, its GI status (proneness to GI tumors, etc.), and more specifically its longevity and survivorship functions, it is difficult to describe aging patterns in anything but a qualitative manner. General categories such as “young” or “old” without available lifespan curves are too imprecise to permit quantitative comparisons. Because of such concerns, our initial surveys have focused on the Fischer 344 (F344) rat from the National Institute on Aging (NIA) colony maintained by Harlan Laboratory (Indianapolis, IN). The NIA F344 rat offers an exceptionally powerful tool for addressing issues of aging (Hazzard, 1991; Sprott, 1991; Hazzard et al., 1992; Masoro, 1991). There is, for example, an extensive normative physiological data base on the NIA F344 rat (see for example Maeda et al., 1985; Yu et al., 1985; Turturro et al., 1999; Black et al., 2003; also see Figure 10) including, most importantly, survival characteristics and growth curves. Additionally, it is practical with the F344 to get representative cohorts of animals for the full lifespan to establish timetables by which age-related changes in the innervation of the GI tract develop and evolve. Also, with this rat model it is feasible to do an extensive series of experiments and make cross-experiment comparisons on the same populations in the same laboratory, thus facilitating direct experiment-to-experiment comparisons.
Figure 10.
The growth curve of ad libitum fed, virgin male Fischer 344 rats obtained for anatomical studies from the National Institute on Aging colony maintained by Harlan Laboratory (Indianapolis, IN, USA). Mean body weight peaked at 15 months of age (449.1 g), remained stable until 18 months of age (449.8 g), and then slowly declined (397.1 g at 27 months of age). Age (n) = 3 (25), 4 (11), 5 (24), 6 (24), 12 (6), 15 (16), 16 (10), 18 (6), 24 (33), 25 (31), 26 (13), and 27 (13) months of age.
The use of any particular animal model, though, raises issues of generality. Species differences are to be expected, but there are also differences in enteric organization among strains of the same species (e.g., Wu et al., 2003; Gulley et al., 2005). Similarly, investigators have documented strain-specific differences in the impact of age on other elements of the autonomic nervous system; Schmidt and colleagues (1998), for example, have shown that strain affects the extent (both rate and degree) to which neuropathies occur with age. Thus, one concern is that our findings on the NIA F344 rat are specific to only that strain and do not reflect general patterns of aging. Nevertheless, it is clear that many of our observations in the aged F344 rat such as neuron loss with age, the sparing of nitrergic neurons with age, and deterioration of the sympathetic projections to the aged gut are consistent with findings in different strains of rat and other species such as mice and guinea pigs; observations made in animals kept under very different housing conditions than the NIA F344 rat. However, now that we have additional detail about the normal pattern of aging of gut innervation in the F344, the major new findings should be replicated in other strains of rodents maintained by the NIA (Miller and Nadon, 2000; Nadon, 2004) under housing conditions similar to those of the F344 rat. Investigators employing other animal models must similarly consider issues of generality.
Nutrition also affects the aging patterns of the gut innervation. Cowen and colleagues (2000; also Johnson et al., 1998) have shown that the neuronal loss (~50% by 24 months) in the MP of the ileum of the ad libitum-fed Sprague Dawley rat can be prevented by chronic caloric restriction. Indeed, the investigators observed that diet restriction from six months of age can prevent the expected loss of neurons, even out to 30 months of age. These results underscore that fact that nutrition (and perhaps other maintenance or environmental factors) can influence the pattern of aging of GI innervation. In addition to underscoring how different models might display different patterns of age-related changes, the impact of caloric restriction on cell loss suggests the possibility, mentioned above, that oxidative stress may be responsible for the loss of vulnerable cholinergic neurons in the GI tract.
7.2. Difficulties studying age-related changes in cell numbers
Surveys of enteric cell numbers are subject to a number of sampling and staining issues that can distort and compromise comparisons, and these methodological problems are often exaggerated when comparisons are made across ages.
7.2.1. Pan-neuronal markers
Of all the putative pan-neuronal markers used to establish age-related loss in the ENS, the stain Cuprolinic Blue and antibodies to HuC/D appear to be the markers of choice at the present time. Both are similarly inclusive in labeling the entire population of neurons (Phillips et al., 2004a; Ganns et al., 2006), and each has been used extensively to document age-related changes in the ENS (Phillips and Powley, 2001; Phillips et al., 2003, 2004b; Abalo et al., 2005; Phillips et al., 2006a,b; Thrasivoulou et al., 2006). Because each has its own strengths and weaknesses, together they provide a complementary set of markers to study age-related changes in the total neuronal population of the ENS.
The fact that Cuprolinic Blue non-selectively stains single-stranded RNA means that the down regulation of expression of any single gene or modest number of genes over the course of the lifespan should not compromise the staining. Additionally, staining with Cuprolinic Blue is quick, permanent, and compatible with most, if not all, immunohistochemical protocols. However, while Cuprolinic Blue is powerful in permanent labeling studies, it is not a fluorescent molecule, so it cannot be used in fluorescent protocols that are necessary to determine age-related changes in subpopulations.
HuC/D is a useful marker because it can be immunohistochemically detected in fluorescent labeling protocols, has a low signal-to-noise with very little background, and is robust over a broad range of protocols. On the other hand, detection of neurons in the aged using HuC/D appears to be influenced by down regulation of the molecule so that counts of HuC/D-positive neurons in aging appear to reflect both a loss of neurons and a lack of expression of the marker in potentially surviving neurons.
7.2.2. Reduced binding of antibodies rather than neuron loss
The potential confusion of down regulation versus cell loss is not limited to HuC/D or pan-neuronal markers. Indeed, work with several markers (Takahashi et al., 2000; Phillips et al., 2004b; Abalo et al., 2005; Thrasivoulou et al., 2006; see, for instance, the neuron silhouettes in Figure 1B,C) suggests that any marker that relies on staining a single protein may result in inflated estimates of neuronal loss, and such limitations need to be considered when interpreting the findings from studies using antibodies.
Though down regulation of the expression of a cell marker is probably the more common problem, cell labeling—or the lack of cell labeling—can also be influenced by changes in tissue permeability to staining agents and such changes in penetration could be affected by aging and growth of the GI tract.
7.2.3. Inventorying innervation patterns as gastric and intestinal volume change with age
A complicating factor in obtaining inventories of neurons or neuronal elements is the fact that the volume of the GI tract and the density of its innervation practically preclude complete cell counts or neurite surveys. Sampling methods that minimize bias and error are needed. Sampling, however, must also be normalized for tissue or organ size because the GI tracts of many species, including the rat, continue to grow through middle-age. Thus, inventories of the innervation of the gut typically need to address sampling issues and correction calculations.
Complications of obtaining estimates of absolute number or of density per unit area as well as the recent developments in the protocols of “unbiased” stereology have highlighted the need to validate one’s counting methods. In this regard, a particularly useful feature of the GI tract is that the wall of the digestive tube approximates a set of two-dimensional plexuses or monolayers, particularly when the organs are divided into tissue layers and prepared as whole mounts. When it is practical to work with whole mounts and when the elements counted are distributed in a monolayer, neuronal and glial somata for example, it is not necessary to employ stereology, which is designed to sample through multiple consecutive (histological or optical) sections of a three-dimensional volume. However, obtaining valid estimates of element number may require the use of a stereological protocol when (1) the whole mount is not stretched or flattened into a monolayer, (2) the elements of interest in the tissue are neurites, varicosities or other smaller features that might overlay one another, or (3) GI specimens can’t be collected as whole mounts but rather must be sectioned.
Assuming that a valid counting protocol has been chosen, comparisons between animals varying in age still need to be based on counts normalized for differences in organ size. Given a fixed number of enteric neurons or other elements to be counted, as animals grow, changes in organ size and surface area result in a “dilution” of the density of neurons or other elements whenever one uses counts of neurons-per-unit-area to compare neuron numbers between different ages (i.e., the dilution effect can generate a calculation that erroneously suggests an apparent age-related loss of neurons). There are three common ways to handle this dilution effect.
First, experimenters can estimate the total number of neurons within an organ and then compare such totals for each age. However, determining an estimate of the areal density for neurons that is representative for the entire organ is problematic because the density of neurons within an organ can--and commonly does--vary dramatically over the length and circumference of the region of interest. In addition, if comparable sites are to be sampled in organs that differ in size with age, then the experimenter must still find a valid means of correcting for differences in organ size. Furthermore, such a “whole-organ” strategy is more ambiguous when the question under investigation deals with a more circumscribed region of the gut wall. For such delimited areas, boundaries or transition zones are often harder to specify--particularly in wholemounts or other limited tissue samples.
A second means of addressing the dilution effect, which we and others (e.g., Gabella, 1989; Johnson et al., 1998; Cowen et al., 2000) have adopted most frequently, is to determine a size correction factor for any particular region of interest. Typically, the cross-sectional area of the region is determined for each age under investigation, and ratios of the cross-sectional areas are calculated. For each age group, counts of neurons are then multiplied by the appropriate ratio or age-specific correction factor. Using the same F344 rat tissue samples, we have compared the neuronal density derived by counting all of the neurons in a known area with the neuronal density derived from areal count samples adjusted using a correction factor and found the correction factor to be an accurate and valid way to control for differences in tissue area and stretch (Phillips et el., 2003). The region sampled needs to be well defined (e.g., the region of the duodenum consisting of the first 2 to 4 cm of the small intestine distal to the pyloric sphincter), and care needs to be taken to sample from exactly the same regions for each age.
Finally, a third way that we and others (El-Salhy et al., 1999; Phillips et al., 2004b; Abalo et al., 2005; Phillips et al., 2006a) have tried to normalize neuronal counts and to correct for dilution effects with organ growth, is to use stretch-insensitive measures such as the number of neurons per ganglion or number of neurons per ganglionic area. These estimates appear to be the most problematic of the three strategies because even though it is easy to find clear cut and distinct examples of ganglia, in many instances the boundaries between a particular ganglion and the connectives of the plexus or the boundaries between two ganglia are blurred and hard to define unequivocally. Furthermore, it is often hard to justify an unambiguous set of criteria that cover the variations one encounters. Whereas many ganglia may be compact and coherent, others can be more diffuse or distributed, making boundary decisions more difficult. In some regions, and particularly in the submucosal plexus, a substantial fraction of putative ganglia consist of only one, two, or three neurons. Finally, and even more critically, strategies to measure losses that attempt to correct for dilution by examining individual ganglia make an unwarranted assumption that may confuse any survey: this assumption is that any losses will be distributed uniformly across all ganglia. If aging or a disease process eliminates some ganglia while sparing others, it would be possible to observe no change in the spared ganglia and to erroneously conclude that there was no impact of age or disease. While the pitfalls of these stretch-insensitive strategies appear to make them the least attractive methods for normalization, they may serve nicely as ways of having a second estimate to compare against counts derived with one of the alternate normalization techniques.
8. Future directions
Why both the intrinsic and extrinsic innervation of the ENS deteriorates with age, and how to either slow (cf., for example, caloric restriction: Johnson et al., 1998; Cowen et al., 2000) or prevent such changes remains to be answered. Similarly, the conditions that result in selective deterioration of certain elements (e.g., cholinergic neurons and sympathetic axons) while other aspects of the ENS are relatively spared (e.g., nitrergic neurons and visceral afferents) need to be fully characterized before a comprehensive understanding of how the nervous innervation of the GI tract ages can be realized. Whether the age-related losses in the autonomic innervation of the gut are simply accelerated by diseases that afflict the elderly or whether such co-morbidities produce distinctly different and independent neuropathies also needs to be investigated more thoroughly.
Furthermore, whether the functional GI tract disorders are the consequence of the accumulating neuropathies in the gut needs to be examined. Finally, if there is such an association, whether the functional disorders are proportional to the neural losses and dystrophies or whether compensatory mechanisms can initially offset and mask the functional effects of the neural losses until some critical threshold loss is exceeded (cf., for example, nigral cell death in Parkinson’s: Mattson et al., 1999; Honig and Rosenberg, 2000; McKinney and Jacksonville, 2005; Honig and Rosenberg, 2000; Mattson and Magnus, 2006) are additional questions that need to be considered.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH DK61317 and DK27627). Helpful comments on an earlier draft of the material were provided by Lindsey A. Schier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abalo R, Jose Rivera A, Vera G, Isabel Martin M. Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil. 2005;17:123–132. doi: 10.1111/j.1365-2982.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- Baker DM, Santer RM. A quantitative study of the effects of age on the noradrenergic innervation of Auerbach’s plexus in the rat. Mech Ageing Dev. 1988a;42:147–158. doi: 10.1016/0047-6374(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Baker DM, Santer RM. Morphometric studies on pre- and paravertebral sympathetic neurons in the rat: changes with age. Mech Ageing Dev. 1988b;42:139–145. doi: 10.1016/0047-6374(88)90069-3. [DOI] [PubMed] [Google Scholar]
- Bassotti G, De Giorgio R, Stanghellini V, Tonini M, Barbara G, Salvioli B, Fiorella S, Corinaldesi R. Constipation: a common problem in patients with neurological abnormalities. Ital J Gastroenterol Hepatol. 1998;30:542–548. [PubMed] [Google Scholar]
- Belai A, Cooper S, Burnstock G. Effect of age on NADPH-diaphorase-containing myenteric neurones of rat ileum and proximal colon. Cell Tissue Res. 1995;279:379–383. doi: 10.1007/BF00318495. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396:211–222. [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Black BJ, Jr, McMahan CA, Masoro EJ, Ikeno Y, Katz MS. Senescent terminal weight loss in the male F344 rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R336–342. doi: 10.1152/ajpregu.00640.2001. [DOI] [PubMed] [Google Scholar]
- Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Brehmer A, Blaser B, Seitz G, Schrodl F, Neuhuber W. Pattern of lipofuscin pigmentation in nitrergic and non-nitrergic, neurofilament immunoreactive myenteric neuron types of human small intestine. Histochem Cell Biol. 2004;121:13–20. doi: 10.1007/s00418-003-0603-7. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- Chiocchetti R, Grandis A, Bombardi C, Lucchi ML, Dal Lago DT, Bortolami R, Furness JB. Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum: a morphometric and neurochemical investigation. Cell Tissue Res. 2006;323:183–196. doi: 10.1007/s00441-005-0075-2. [DOI] [PubMed] [Google Scholar]
- Corns RA, Hidaka H, Santer RM. Neurocalcin-alpha immunoreactivity in the enteric nervous system of young and aged rats. Cell Calcium. 2002;31:53–58. doi: 10.1054/ceca.2001.0261. [DOI] [PubMed] [Google Scholar]
- Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza RR, Moratelli HB, Borges N, Liberti EA. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39:183–188. doi: 10.1159/000213532. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Sandstrom O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107:93–103. doi: 10.1016/s0047-6374(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol. 1974;69:2–51. [PubMed] [Google Scholar]
- Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res. 2004;317:1–12. doi: 10.1007/s00441-004-0895-5. [DOI] [PubMed] [Google Scholar]
- Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989;96:1487–1493. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Gai WP, Blumbergs PC, Geffen LB, Blessing WW. Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology. 1992;42:2106–2111. doi: 10.1212/wnl.42.11.2106. [DOI] [PubMed] [Google Scholar]
- Ganns D, Schrodl F, Neuhuber W, Brehmer A. Investigation of general and cytoskeletal markers to estimate numbers and proportions of neurons in the human intestine. Histol Histopathol. 2006;21:41–51. doi: 10.14670/HH-21.41. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett. 1987;76:151–156. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Grider JR. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol. 1994;266:G1139–1145. doi: 10.1152/ajpgi.1994.266.6.G1139. [DOI] [PubMed] [Google Scholar]
- Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther. 2003;307:460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- Gulley S, Sharma SK, Mansour M, Sullivan CN, Moran TH, Sayegh AI. Strain differences in myenteric neuron number and CCK(1) receptor mRNA expression may account for differences in CCK induced c-Fos activation. Brain Res. 2005;1058:109–119. doi: 10.1016/j.brainres.2005.07.074. [DOI] [PubMed] [Google Scholar]
- Hall KE. Aging and neural control of the GI tract. II Neural control of the aging gut: can an old dog learn new tricks? Am J Physiol Gastrointest Liver Physiol. 2002;283:G827–832. doi: 10.1152/ajpgi.00162.2002. [DOI] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hazzard DG. Relevance of the rodent model to human aging studies. Neurobiol Aging. 1991;12:645–649. doi: 10.1016/0197-4580(91)90115-z. [DOI] [PubMed] [Google Scholar]
- Hazzard DG, Bronson RT, McClearn GE, Strong R. Selection of an appropriate animal model to study aging processes with special emphasis on the use of rat strains. J Gerontol. 1992;47:B63–64. doi: 10.1093/geronj/47.3.b63. [DOI] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hoff S, Wegner M, Schemann M, Ruhl A. Immunohistochemical characterization of glial cells in the human enteric nervous system (ens) Gastroenterology. 2005;128(Suppl 2):A-42. [Google Scholar]
- Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am J Med. 2000;108:317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Schemann M, Santer RM, Cowen T. The effects of age on the overall population and on sub-populations of myenteric neurons in the rat small intestine. J Anat. 1998;192(Pt 4):479–488. doi: 10.1046/j.1469-7580.1998.19240479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost WH. Gastrointestinal motility problems in patients with Parkinson’s disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging. 1997;10:249–258. doi: 10.2165/00002512-199710040-00002. [DOI] [PubMed] [Google Scholar]
- Lee Y, Shiotani Y, Hayashi N, Kamada T, Hillyard CJ, Girgis SI, MacIntyre I, Tohyama M. Distribution and origin of calcitonin gene-related peptide in the rat stomach and duodenum: an immunocytochemical analysis. J Neural Transm. 1987;68:1–14. doi: 10.1007/BF01244635. [DOI] [PubMed] [Google Scholar]
- Li JY, Henning Jensen P, Dahlstrom A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Meciano Filho J, Carvalho VC, de Souza RR. Nerve cell loss in the myenteric plexus of the human esophagus in relation to age: a preliminary investigation. Gerontology. 1995;41:18–21. doi: 10.1159/000213658. [DOI] [PubMed] [Google Scholar]
- Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- Majumdar AP, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc Soc Exp Biol Med. 1997;215:134–144. doi: 10.3181/00379727-215-44120. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB, Southwell BR. Choline acetyltransferase immunoreactivity of putative intrinsic primary afferent neurons in the rat ileum. Cell Tissue Res. 1999;297:241–248. doi: 10.1007/s004410051352. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Use of rodents as models for the study of “normal aging”: conceptual and practical issues. Neurobiol Aging. 1991;12:639–643. doi: 10.1016/0197-4580(91)90114-y. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- McCulloch CR, Cooke HJ. Human alpha-calcitonin gene-related peptide influences colonic secretion by acting on myenteric neurons. Regul Pept. 1989;24:87–96. doi: 10.1016/0167-0115(89)90214-0. [DOI] [PubMed] [Google Scholar]
- McKinney M, Jacksonville MC. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL. Maintaining aged rodents for biogerontology research. Lab Anim (NY) 2004;33:36–41. doi: 10.1038/laban0904-36. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat. 2000;18:31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol. 2006;495:529–553. doi: 10.1002/cne.20898. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Raab M, Berthoud HR, Worl J. Innervation of the mammalian esophagus. Adv Anat Embryol Cell Biol. 2006;185:1–73. [PubMed] [Google Scholar]
- Norton C. Constipation in older patients: effects on quality of life. Br J Nurs. 2006;15:188–192. doi: 10.12968/bjon.2006.15.4.20542. [DOI] [PubMed] [Google Scholar]
- O’Mahony D, O’Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527. doi: 10.2165/00002512-200219070-00005. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004a;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004b;209:19–30. doi: 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2006a doi: 10.1016/j.neurobiolaging 2006.05.019. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006b;211:673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Methods and New Frontiers in Neuroscience: Advances in Vagal Afferent Neurobiology. Vol. 28. CRC Press; Boca Raton, Florida: 2005. pp. 123–145. [Google Scholar]
- Powley TL, Holst MC, Boyd DB, Kelly JB. Three-dimensional reconstructions of autonomic projections to the gastrointestinal tract. Microsc Res Tech. 1994;29:297–309. doi: 10.1002/jemt.1070290407. [DOI] [PubMed] [Google Scholar]
- Ruhl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- Ruhl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004;16(Suppl 1):44–49. doi: 10.1111/j.1743-3150.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125:899–906. doi: 10.1016/j.mad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Santer RM. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994;49:115–121. doi: 10.1016/0165-1838(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Santer RM, Baker DM. Enteric neuron numbers and sizes in Auerbach’s plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Santer RM, Baker DM. Enteric system. In: Amenta F, editor. Aging of the Autonomic Nervous System. CRC Press; Baca Raton, Florida: 1993. pp. 213–225. [Google Scholar]
- Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002;96:73–81. doi: 10.1016/s1566-0702(01)00366-6. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Schmidt RE. Age-related sympathetic ganglionic neuropathology: human pathology and animal models. Auton Neurosci. 2002a;96:63–72. doi: 10.1016/s1566-0702(01)00372-1. [DOI] [PubMed] [Google Scholar]
- Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol. 2002b;50:257–292. doi: 10.1016/s0074-7742(02)50080-5. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Dorsey DA, Beaudet LN, Plurad SB, Parvin CA, Bruch LA. Vacuolar neuritic dystrophy in aged mouse superior cervical sympathetic ganglia is strain-specific. Brain Res. 1998;806:141–151. doi: 10.1016/s0006-8993(98)00678-7. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Chae HY, Parvin CA, Roth KA. Neuroaxonal dystrophy in aging human sympathetic ganglia. Am J Pathol. 1990;136:1327–1338. [PMC free article] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Soltanpour N, Baker DM, Santer RM. Neurons and microvessels of the nodose (vagal sensory) ganglion in young adult and aged rats: morphometric and enzyme histochemical studies. Tissue Cell. 1996;28:593–602. doi: 10.1016/s0040-8166(96)80062-0. [DOI] [PubMed] [Google Scholar]
- Soltanpour N, Santer RM. Vagal nuclei in the medulla oblongata: structure and activity are maintained in aged rats. J Auton Nerv Syst. 1997;67:114–117. doi: 10.1016/s0165-1838(97)00097-0. [DOI] [PubMed] [Google Scholar]
- Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- Spangeus A, Kand M, El-Salhy M. Gastrointestinal endocrine cells in an animal model for human type 2 diabetes. Dig Dis Sci. 1999;44:979–985. doi: 10.1023/a:1026664715280. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Development of animal models of aging at the National Institute of Aging. Neurobiol Aging. 1991;12:635–638. doi: 10.1016/0197-4580(91)90113-x. [DOI] [PubMed] [Google Scholar]
- Steinkamp M, Geerling I, Seufferlein T, von Boyen G, Egger B, Grossmann J, Ludwig L, Adler G, Reinshagen M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology. 2003;124:1748–1757. doi: 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Sternini C, Anderson K. Calcitonin gene-related peptide-containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res. 1992;9:45–59. doi: 10.3109/08990229209144762. [DOI] [PubMed] [Google Scholar]
- Sternini C, Reeve JR, Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987;93:852–862. doi: 10.1016/0016-5085(87)90450-1. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. A comparison of age-related changes in neuron number in the dorsal motor nucleus of the vagus and the nucleus ambiguus of the mouse. J Anat. 1990;173:169–176. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Qoubaitary A, Owyang C, Wiley JW. Decreased expression of nitric oxide synthase in the colonic myenteric plexus of aged rats. Brain Res. 2000;883:15–21. doi: 10.1016/s0006-8993(00)02867-5. [DOI] [PubMed] [Google Scholar]
- Talley NJ, O’Keefe EA, Zinsmeister AR, Melton LJ., 3rd Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895–901. doi: 10.1016/0016-5085(92)90175-x. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Vanner S. Corelease of neuropeptides from capsaicin-sensitive afferents dilates submucosal arterioles in guinea pig ileum. Am J Physiol. 1994;267:G650–655. doi: 10.1152/ajpgi.1994.267.4.G650. [DOI] [PubMed] [Google Scholar]
- Wade PR. Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;283:G489–495. doi: 10.1152/ajpgi.00091.2002. [DOI] [PubMed] [Google Scholar]
- Wade PR, Cowen T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004;16(Suppl 1):19–23. doi: 10.1111/j.1743-3150.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Wade PR, Hornby PJ. Age-related neurodegenerative changes and how they affect the gut. Sci Aging Knowledge Environ. 2005;2005:pe8. doi: 10.1126/sageke.2005.12.pe8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol (Berl) 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Lewy bodies in the enteric nervous system in Parkinson’s disease. Arch Histol Cytol. 1989;52(Suppl):191–194. doi: 10.1679/aohc.52.suppl_191. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol (Berl) 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol. 1993;60:609–612. [PubMed] [Google Scholar]
- Wakabayashi K, Toyoshima Y, Awamori K, Anezaki T, Yoshimoto M, Tsuji S, Takahashi H. Restricted occurrence of Lewy bodies in the dorsal vagal nucleus in a patient with late-onset parkinsonism. J Neurol Sci. 1999;165:188–191. doi: 10.1016/s0022-510x(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl) 2003;207:363–371. doi: 10.1007/s00429-003-0351-4. [DOI] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- Wiley JW. Aging and neural control of the GI tract: III. Senescent enteric nervous system: lessons from extraintestinal sites and nonmammalian species. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1020–1026. doi: 10.1152/ajpgi.00224.2002. [DOI] [PubMed] [Google Scholar]
- Wong HC, Tache Y, Lloyd KC, Yang H, Sternini C, Holzer P, Walsh JH. Monoclonal antibody to rat alpha-CGRP: production, characterization, and in vivo immunoneutralization activity. Hybridoma. 1993;12:93–106. doi: 10.1089/hyb.1993.12.93. [DOI] [PubMed] [Google Scholar]
- Wu M, Van Nassauw L, Kroese AB, Adriaensen D, Timmermans JP. Myenteric nitrergic neurons along the rat esophagus: evidence for regional and strain differences in age-related changes. Histochem Cell Biol. 2003;119:395–403. doi: 10.1007/s00418-003-0526-3. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]