Abstract
After peripheral nerve axotomy, vasoactive intestinal peptide (VIP) gene expression is upregulated in neurons, whereas ciliary neurotrophic factor (CNTF) accumulates extracellularly at the lesion site. Although CNTF-induced VIP gene expression has been reported in cultured sympathetic neurons and neuroblastoma cells, it still remains to be determined if CNTF and VIP play interrelated roles in nerve injury. The corneal endothelium, like sympathetic neurons, derives from the neural crest. Previously, we demonstrated that a sublethal-level of oxidative stress induces CNTF release from corneal endothelial (CE) cells in situ. Here, we show that human CE cells express the 53kDa ligand-binding α subunit of the CNTF receptor (CNTFRα). We further demonstrate that CNTF induces VIP immunoreactivity in human donor corneas. To determine if the increase in VIP immunoreactivity was reflected by an increase in gene expression, donor human corneas were bisected and treated with CNTF or vehicle, and analyzed by real-time RT-qPCR. Two experiments using different sets of bisected corneas indicated that CNTF induced increases in VIP mRNA levels of 6.5-fold ±2.2 (N=7 corneas) and 2.3-fold ±0.6 (N=10 corneas), (mean±SEM), respectively. Whereas VIP is produced as a CE autocrine factor against oxidative stress, the present study suggested that oxidative stress-released CNTF plays a role in protecting CE cells against oxidative stress injury by upregulating VIP expression.
Keywords: VIP, CNTF, Corneal endothelial cells, gene transcription
In cultured sympathetic neurons, ciliary neurotrophic factor (CNTF) induces the synthesis of vasoactive intestinal peptide (VIP) [14, 18]. The induced VIP synthesis is primarily the result of modulation of the VIP gene transcription at its cytokine responsive element (CyRE) via CNTF-induced binding of the transcription factor STAT to the CyRE [24, 25]. VIP, a 28-amino acid neuropeptide, is a well-recognized neuroprotective factor in a variety of systems [16, 28] and its expression is upregulated following axotomy of peripheral neurons [4, 9, 23], including the sympathetic neurons [23]. Axotomy- induced VIP expression in the sympathetic neuron is impaired in mice lacking leukemia inhibitory factor (LIF) [23], a cytokine that shares the same signaling pathway as CNTF. It is also known that following axotomy, a significant amount of CNTF accumulates extracelluarly at the lesion site [20]. Native CNTF lacks a classical signal sequence for secretion [22] and is considered a lesion factor released only after injury [20]. Although indirect evidences support the presence of injury-induced CNTF release [5, 29, 30], its mechanism and physiological significance have remained unknown. Likewise, the physiological significance of CNTF-induced VIP expression in sympathetic neurons is not established.
CNTF was discovered in an extract of eye tissues consisting of ciliary body, iris, and choroid and characterized as a survival factor for the chick ciliary ganglion neurons [2,3]. It has since been shown to exert neurotrophic activities in various neuronal injury models including axotomy-induced motor neuron degeneration and retinal ganglion cell apoptosis in vivo [19,26]. Recently, because the beneficial effects of CNTF treatment have been observed in a variety of animal models of photoreceptor cell degeneration [reviewed in reference #31], a phase I (safety) human clinical trial has been conducted, in which encapsulated cells that were engineered to secrete CNTF were implanted into the vitreous of the eyes of retinitis pigmentosa patients [21].
Like the sympathetic neurons, the corneal endothelial (CE) cells, which express the neuron-specific enolase [1], originate from the neural crest [8]. VIP mRNA and immunoreactivity are expressed by the CE cells and exogenous VIP promotes the survival of CE cell under acute oxidative stress in human and bovine corneoscleral explants [11]. CE cells that have survived H2O2-induced oxidative stress in bovine corneoscleral explant cultures release endogenous CNTF in a complex with CNTFRα, the CNTF binding subunit of the CNTF receptor [12]. In the eye, CE cells are situated in proximity to the CNTF-rich ciliary body and iris.
The present study investigated the possibility that CNTF can induce VIP expression in CE cells in corneoscleral explant cultures established from donor human eyes and corneas. Although these corneas were found not suitable for transplantation due to the advanced age of the donors and/or less than optimal CE cell density, they were nonetheless without disease and would allow studies of the physiological significance of CNTF induction of VIP expression in the future.
We first demonstrated the presence of CNTFRα in CE cells from fresh donor human eyes. Human eyes with postmortem times of less than 24 h were obtained from the Maryland Eye Bank (Baltimore). Human corneoscleral explants were dissected as previously described [10,11]. CE cells were scraped off corneas using a razor blade and extracted in RIPA buffer. As a positive control of CE cell CNTFRα, CE cells from bovine eyes obtained from the local abattoir and used within six h of sacrifice was also extracted [11,12]. CE cell extract was electrophoresed in tris-glycine polyacrylamide gradient gels (8-16%), transferred onto nitrocellulose membranes, and immunostained with an affinity purified goat anti-human CNTFRα primary antibody (R & D Systems) and an anti-goat IgG-alkaline phosphatase conjugate secondary antibody (Calbiochem). CNTFRα on nitrocellulose membranes was detected by a chromogenic method, using an alkaline phosphatase substrate solution made from Fast Red TR/Naphthol AS-MX tablets (Sigma).
CNTFRα was detected in CE cell extracts from all 12 human eyes examined. Fig. 1 showed that CE cell extracts from bovine and human eyes contained an anti-human CNTFRα antibody-reactive molecule with an approximate molecular mass of 53 kDa.
Fig. 1.
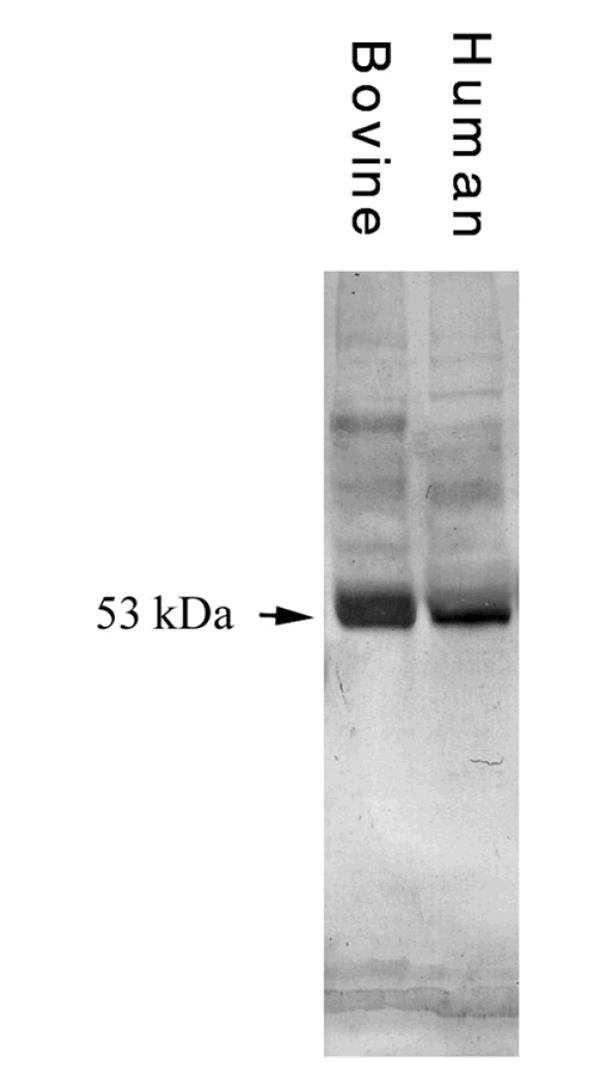
Western blot of anti-human CNTFRα-reactive molecules in bovine and human CE cell extracts. Each lane contained 80μg protein. CNTFRα was detected in CE extracts from all eyes examined: 12 human and six bovine in six, and three experiments, respectively.
Since CNTF induction of VIP expression is observed in sympathetic neurons and neuroblastoma cells, the possibility that CNTF/CNTFRα signaling pathway in CE cells, which are also neural crest-derived, leads to VIP upregulation was examined. Human corneoscleral explants were dissected from fresh donor eyes, rinsed in DPBS, incubated in 10 ml medium A (antibiotics [100 U/ml penicillin, 100 μg/ml streptomycin sulfate]-supplemented DMEM) for 30 min (23°C), transferred to 10 ml medium B (L-glutamine [0.292 mg/ml]-supplemented medium A), and incubated for 24 h (37°C) [10]. Explants were quartered and incubated in 10 ml complete medium B (fetal calf serum [5%] and fungizone [0.25 μg/ml amphotericin B]-supplemented medium B) in the absence or presence of 2.1 × 10-9 M rat CNTF (Alomone Labs) for 48 h (37°C). Following previously reported procedures [11], explants were cut into 6 μm sections and immunostained with an anti-VIP rabbit serum (ICN Biochemicals), used at 1:100 dilution in 1% BSA/PBS. Rabbit IgG (10 μg/ml) was used as a negative control. VIP immunoreactivity was detected with the chromogenic method (above).
As shown in Fig. 2, the CNTF treatment of the human corneoscleral explants produced an increase in the level of VIP immunoreactivity in the corneal endothelium. The same result was obtained in five experiments, using five fresh donor eyes.
Fig. 2.
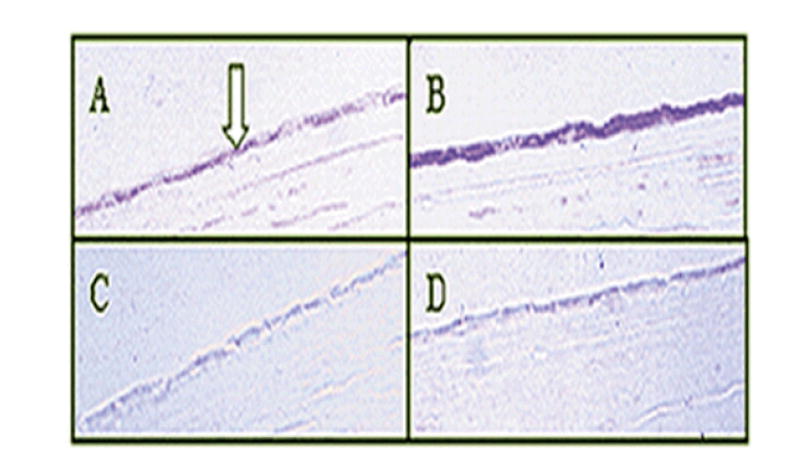
CNTF upregulated VIP immunoreactivity in human CE cells in situ. Corneoscleral explants treated with 0 M (A & C) and 2.1 × 10-9 M (B & D) CNTF were sectioned and stained with either anti-VIP (A&B) or rabbit IgG (C&D) as a control. The same result was obtained in five experiments, using five fresh eyes from four donors. Arrow: CE cells.
Eyes with extensive CE cell damage caused by ocular disease, trauma, and surgery require corneal transplantation. Potential donor corneas are commonly stored in Optisol-GS at 4°C for several days before transplantation [7]. If CNTFRα is expressed in CE cells in stored corneas, then the possibility exists that CE cells in transplanted corneas are responsive to modulation by the endogenous CNTF derived from the recipient eyes. CNTF-rich ciliary body and iris of the recipient eye [2], while situating in the proximity of the cornea may release CNTF in response to the surgical injury. We examined 14 human donor corneas that had been stored in Optisol-GS for varying length of time. While persistently expressed in CE cells of stored corneas, CNTFRα level in CE cells decreased dramatically after one month in storage (data not shown). There was no correlation between the CNTFRα level and the age of the donor, and there was a general correlation between the level of CNTFRα and the storage time of the corneas.
Previously, we have demonstrated VIP immunoreactivity in CE cells in fresh donor and bovine eyes by immunocytochemistry and Western blots, and that VIP mRNA is present in these fresh CE cells by in situ hybridization [11]. To demonstrate VIP gene expression in CE cells of corneas stored in Optisol-GS, we isolated total RNA (RNA- Bee) from pooled CE cells from 11 corneas (storage time=two weeks - two months) and subjected the RNA to reverse transcription (RT) using a kit (Ambion) and PCR, using 5% of the RT reaction product and the following primers : 5’-CTTGAGTCTCTTATGGGAAAACGTGT-3’ (forward, on exon 4) and 5’- GTGAAGACTGCATCTGAGTGACG-3’ (reverse, on exon 5) and Ready-To-Go PCR beads (Amersham) in 25 μl. After a 94° C (3 min) treatment, 35 cycles were performed: 94°C (60 sec), 60°C (60 sec), and 72° C (60 sec), followed by one cycle of 10 min at 72° C. As a positive control, RNA was extracted from CNTF (0.5 ng/ml, 24 h)-treated human neuroblastoma cell line NBFL [24]. Two negative controls were conducted: a reagent control in which the RT reaction was conducted in the absence of RNA and a PCR control in which RNA was not subjected to RT. The RT-PCR products were electrophoresed (2% agarose gels), stained with ethidium bromide.
As shown in Fig. 3 A, a specific band at the appropriate size (86 bp) was observed in human CE cell and neuroblastoma cell samples. Sequencing of the 86 bp band from human CE cell samples (Biopolymer Lab, University of Maryland) revealed it to be identical to the sequence for human VIP (nucleotides 373-458). This product could not have been due to genomic DNA contamination because the primers were on different exons.
Fig. 3.
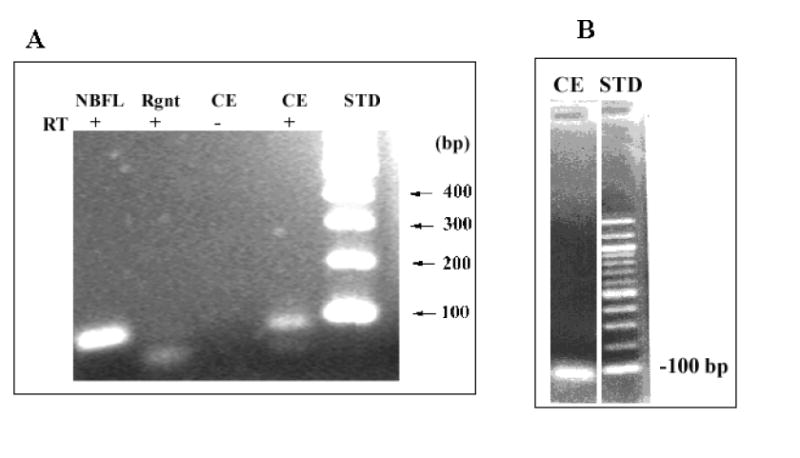
VIP mRNA in CE cells from donor human corneas stored in Optisol. RT-PCR product (A) and real-time RT-qPCR product (B) of VIP mRNA were electrophoresed (2% agarose). For A- Rgnt: reagent negative control, RT-: RNA not subjected to RT, STD: DNA standard, bp: base pair, NBFL: human neuroblastoma cell line as positive control. CE 86 bp product was sequenced. B: In the presence of SYBR green, the RT product of CE RNA was subjected to PCR.
Thus, donor human corneas stored in Optisol-GS were used to determine if the increase in VIP immunoreactivity was reflected by an increase in gene expression. Donor human corneas were bisected and treated with CNTF or vehicle, and analyzed by real-time RT-qPCR.
As illustrated in the Scheme above, human donor corneas, removed from Optisol-GS, were incubated (37°C) in medium B (3.5 ml, 24 h) and then bisected. The two halves from the same cornea were incubated in 3.5 ml of either complete medium B alone or that plus 0.1 nM human CNTF (R & D Systems) for 24 h.
Scheme.
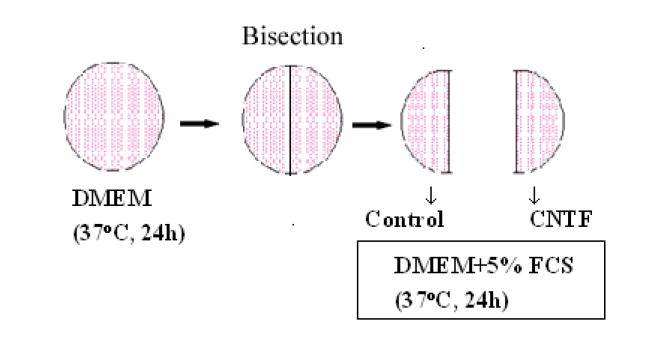
CNTF treatment of donor human corneas
Total RNA (200-300 ng) isolated from CE cells of each of the corneal halves was subjected to RT (volume= 20 μl), using a kit from Invitrogen (Super Script First-strand synthesis system). Following the RT reaction, the residual RNA in the mixture was removed by addition of 1μl RNase H (2 unit/μl) and incubation (37°C, 20 min). Real-time qPCR was conducted using duplicate samples each contained 20% of the RT product. For quantifying VIP mRNA levels, VIP primers used were the same as those used in RT-PCR (above). For endogenous standard and normalization of VIP mRNA levels in samples, mRNA of two house-keeping (cyclophilin B and β-actin) genes, were quantified. When using human cyclophilin B as the standard (primers: 5’-TGG CAC AGG AGG AAA GAG CAT C-3’ [forward] and 5’-AAA GGG CTT CTC CAC CTC GAT C-3’ [reverse]), samples and controls in 25 μl contained 6 pmoles of each of the respective primers and 12.5 μl of a 2X master solution (iQSYBRGreen Supermix [Bio-Rad]) and the following cycling condition in the BioRad Cycler 1: 95 °C (3.5 min), 40 cycles of 95 °C (30 sec), 60°C (30 sec), 72°C (30 sec), followed by one cycle of 72°C (5 min). VIP mRNA RT-PCR product was electrophoresed (2% agarose gel) and, in a series of dilutions, was used to determine the relative efficiency of the PCR. When using human β-actin as standard (primers: 5-GACAGGATGCAGAAGGAGATCACT-3’ [forward], 5-TCAGGAGGAGCAAGGATCTTGA-3’ [reverse]), samples and controls in 20 μl contained 3 pmoles of each of the respective primers and 10 μl of a 2X master solution (SYBR®, Applied Biosystems) and the cycling conditions in the 5700 Sequence Detection System (Applied Biosystems) were: 50 °C (2 min), 95°C (10 min), 40 cycles of 95 °C (15 sec), 60°C (1 min). Whereas the Ct is defined as the number of cycles required for reaching a point at which the fluorescent signal from the PCR reaction product is significantly higher than the background, the Ct is inversely related to the level of the specific mRNA whose cDNA is being amplified by the primer pair in the PCR reaction. Thus, VIP mRNA level relative to the endogenous mRNA standard is 2Ct (standard) / 2Ct (VIP). Normalization of the relative VIP mRNA level found in the CNTF-treated corneal half against that found in its paired-control half gives the fold of CNTF stimulation: 2Ct (standard) / 2Ct (VIP) found in control half over 2Ct(standard) / 2Ct (VIP) found in the CNTF-treated half, i. e., 2(Ct[VIP]-Ct [standard])control-(Ct[VIP]-Ct [standard])CNTF-treated.
Electrophoresis of RT-qPCR products from VIP primers showed a single band of 86 bp VIP cDNA (Fig. 3B) distributed in all samples. In the present study, two experiments on two different sets of bisected corneas were performed. The first, on 7 bisected corneas, utilized cyclophilin B as an endogenous standard (“housekeeping gene”) in real-time RT-qPCR. In this experiment, CNTF induction of the VIP mRNA was 6.54± 2.25-fold (mean±SEM, 7 corneas) (Fig. 4). In the second experiment, to confirm the effect of CNTF, real-time RT-qPCR was conducted using the mRNA level of an alternative housekeeping gene, β-actin. In the paired control vs CNTF-treated corneal halves, CNTF induction of the VIP mRNA in this case was 2.32±0.59-fold (mean±SEM, 10 corneas) (Fig. 4). Thus, in two independent experiments using different endogenous standards, CNTF induced significant increases in VIP mRNA, indicating that the increase in VIP immunor eactivity was mirrored by an increase in VIP gene transcripts.
Fig. 4.
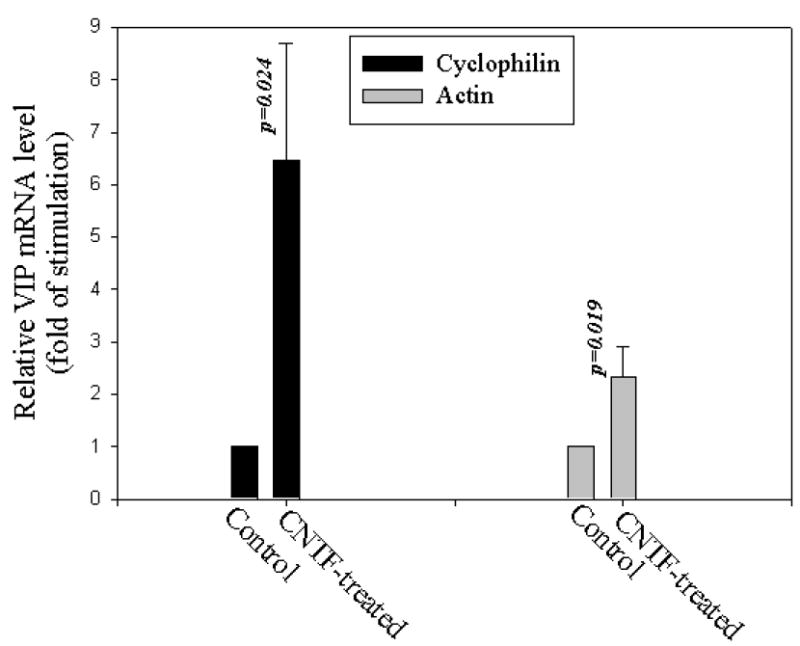
CNTF- induced VIP mRNA in CE cells in stored donor human corneas demonstrated by real-time RT-qPCR. VIP mRNA level over that of the endogenous mRNA standard (cyclophilin B or β- actin) found in each of the two corneal halves (control vs CNTF-treated) of the same cornea was normalized against that of the control half and values derived from both the control and the CNTF-treated halves were given on Y-axis. CNTF treatment (0.1 nM, 24 h) increased VIP mRNA levels by (mean±SEM) 6.54±2.25 (N=7 corneas, p=0.024; cyclophilin B) and 2.32±0.59 (N=10 corneas, p=0.019; β-actin) folds.
The present study demonstrated ex vivo induction of VIP gene in a transplantable tissue. CE cells in donor human corneas expressed functional CNTFRα and exogenous CNTF at 0.1 nM and 2.1 nM induced VIP mRNA (Fig. 4) and protein expression (Fig. 2), respectively. In cultured sympathetic neurons, the maximal effect of CNTF in inducing the expression of VIP is (5-25) ng/ml or 0.2-1.0 nM [24]. The donor human cornea may prove to be the first tissue model suitable for studying the physiological significance of CNTF/CNTFRα modulation of VIP gene expression, a phenomenon that has been observed, thus far, only in cultured neuronal and neuroblastoma cells. In this model, autocrine trophic factors CNTF and VIP act in concert to protect CE cells against the oxidative stress. Central to the theme of this model is CNTF induction of VIP expression.
The cornea is a major site of light refraction in the eye. The transparency of the cornea is maintained by CE cells, which function as a physical barrier to fluid movement into the cornea and also actively pumps fluid out of the cornea [27]. These CE cell functions can be lost as a result of damage caused by ocular disease, trauma, and surgery. Potential donor corneas are commonly stored in Optisol-GS at 4°C for several days before transplantation [7]. The persistent expression of CNTFRα in CE cells in stored corneas demonstrated in the present study suggested that, following transplantation surgery, CE cells in transplanted corneas might be responsive to modulation by the endogenous CNTF derived from the CNTF-rich ciliary body and iris [2] of recipient eyes. Increased VIP expression in CE cells of the donor cornea may result in increased release of VIP, a survival f actor of CE cells [11], and therefore the survival of the CE cells, which in turn is essential for the survival of the transplanted cornea [15].
In addition to being a trophic factor, VIP is a well-known immunosuppressive agent [6]. CE cells form the anterior border of the anterior chamber, a site of immune privilege in which VIP immunoreactivity has been found [13, 17]. We hypothesize that CE cells that have survived the initial oxidative stress release CNTF to upregulate VIP gene expression to protect themselves from further oxidative stress and to ensure an adequate supply of VIP for the anterior chamber.
Acknowledgments
Supported in part by NIH EY11607 (SWMK), NIH EY15304, career development grant from the Research to Prevent Blindness, Inc, (New York), and V. Kunn Rasmussen Foundation (Denmark) (SLB), NIH HD04612, HD06576, HD34475 (JAW), and by USUHS R075LK, NIH R29NS35839 (AJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamis AP, Molnar ML, Tripathi BJ, Emmerson MS, Stefansson K, Tripathi RC. Neuronal-specific enolase in human corneal endothelium and posterior keratocytes. Exp Eye Res. 1985;41:665–668. doi: 10.1016/0014-4835(85)90039-9. [DOI] [PubMed] [Google Scholar]
- 2.Adler R, Landa K, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204:1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- 3.Adler R, Varon S. Neuronal survival in intact ciliary ganglia in vivo and in vitro: CNTF as a target surrogate. Dev Biol. 1982;92:470–475. doi: 10.1016/0012-1606(82)90192-0. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 5.Cao W, Wen R, Li F, Lavail MM, Steinberg RH. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res. 1997;65:241–248. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- 6.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 7.Frueh BE, Bohnke M. Prospective, randomized clinical evaluation of Optisol vs organ culture corneal storage media. Arch Ophthalmol. 2000;118:757–760. doi: 10.1001/archopht.118.6.757. [DOI] [PubMed] [Google Scholar]
- 8.Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- 9.Kashiba H, Senba E, Ueda Y, Tohyama M. Co-localized but target-unrelated expression of vasoactive intestinal polypeptide and galanin in rat dorsal root ganglion neurons after peripheral nerve crush injury. Brain Res. 1992;582:47–57. doi: 10.1016/0006-8993(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 10.Koh SW, Yeh TH, Morris SM, Leffler M, Higginbotham EJ, Brenneman DE, Yue BY. Vasoactive Intestinal peptide stimulation of human trabecular meshwork cell growth. Invest Ophthalmol Vis Sci. 1997;38:2781–2789. [PubMed] [Google Scholar]
- 11.Koh SWM, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000;41:4085–4092. [PubMed] [Google Scholar]
- 12.Koh SWM. Ciliary neurotrophic factor released by corneal endothelium surviving oxidative stress ex vivo. Invest Ophthalmol Vis Sci. 2002;43:2887–2896. [PubMed] [Google Scholar]
- 13.Koh SW, Rutzen A, Coll T, Hemady R, Higginbotham E. VIP immunoreactivity in human aqueous humor. Curr Eye Res. 2005;30:189–194. doi: 10.1080/02713680490908715. [DOI] [PubMed] [Google Scholar]
- 14.Lewis SE, Rao MS, Symes AJ, Dauer WT, Fink JS, Landis SC, Hyman SE. Coordinate regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- 15.Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Exp Ophthalmol. 2005;33:642–657. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 16.Moody TW, Hill JM, Jensen RT. VIP as a trophic factor in the CNS and cancer cells. Peptides. 2003;24:163–177. doi: 10.1016/s0196-9781(02)00290-5. [DOI] [PubMed] [Google Scholar]
- 17.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 18.Rao MS, Tyrrell S, Landis SC, Patterson PH. Effects of ciliary neurotrophic factor (CNTF) and depolarization on neuropeptide expression in cultured sympathetic neurons. Dev Biol. 1992;150:281–293. doi: 10.1016/0012-1606(92)90242-9. [DOI] [PubMed] [Google Scholar]
- 19.Sendtner A, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 20.Sendtner M, Stockli KA, Thoenen H. Synthesis and localization of ciliary neurotrophic factor in sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992;118:1436–1453. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- 24.Symes A, Rao MS, Lewis SE, Landis SC, Hyman SE, Fink JS. Ciliary neurotrophic factor coordinately activates transcription of neuropeptide genes in a neuroblastoma cell line. Proc Natl Acad Sci USA. 1993;90:572–576. doi: 10.1073/pnas.90.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symes A, Lewis S, Corpus L, Rajan P, Hyman SE, Fink JS. STAT proteins participate in the regulation of the vasoactive intestinal peptide gene by the ciliary neurotrophic factor family of cytokines. Mol Endocrinol. 1994;8:1750–1763. doi: 10.1210/mend.8.12.7708062. [DOI] [PubMed] [Google Scholar]
- 26.van Adel BA, Arnold JM, Phipps J, Doering LC, Ball AK. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–234. doi: 10.1002/neu.20117. [DOI] [PubMed] [Google Scholar]
- 27.Waring GO, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium: normal and pathologic structure and function. Ophthalmology. 1982;89:531–590. [PubMed] [Google Scholar]
- 28.Waschek JA. Vasoactive intestinal peptide: An important trophic factor and developmental regulator? Dev Neurosci. 1995;17:1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- 29.Watt JA, Bone S, Pressler M, Cranston HJ, Paden CM. Ciliary neurotrophic factor is expressed in the magnocellular neurosecretory system of the rat in vivo: evidence for injury-and activity-induced upregulation. Exp Neurol. 2006;197:206–214. doi: 10.1016/j.expneurol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Wen R, Song Y, Cheng T, Matthes MT, Yasumura D, LaVail MM, Steinberg RH. Injury-induced upregulation of bFGF and CNTF mRNAs in the rat retina. J Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen R, Song Y, Kjellstrom S, Tanikawa A, Liu Y, Li Y, Zhao L, Bush RA, Laties AM, Sieving PA. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci. 2006;26:13523–13530. doi: 10.1523/JNEUROSCI.4021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


