Abstract
Human lens β-crystallin contains four acidic (βA1 → βA4) and three basic (βB1 → βB3) subunits. They oligomerize in the lens, but it is uncertain which subunits are involved in the oligomerization. We used a two-hybrid system to detect protein–protein interactions systematically. Proteins were also expressed for some physicochemical studies. The results indicate that all acidic–basic pairs (βA–βB) except βA4-βBs pairs show strong hetero-molecular interactions. For acidic or basic pairs, only two pairs (βA1–βA1 and βA3–βA3) show strong self-association. βA2 and βA4 show very weak self-association, which arises from their low solubility. Confocal fluorescence microscopy shows enormous protein aggregates in βA2- or βA4-crystallin transfected cells. However, coexpression with βB2-crystallin decreased both the number and size of aggregates. Circular dichroism indicates subtle differences in conformation among β-crystallins that may have contributed to the differences in interactions.
Keywords: β-Crystallin, Two-hybrid system, Protein–protein interaction, Confocal fluorescence microscopy, Circular dichroism
1. Introduction
Human lens proteins can be grouped into the α-crystallin family and the β/γ-crystallin superfamily [1]. α-Crystallin is a polymer consisting of two subunits, αA and αB. The β/γ-crystallin superfamily comprises oligomeric β-crystallin and monomeric γ-crystallin; they are more heterogeneous than α-crystallin, each containing many subunits: seven in β-crystallin and six in γ-crystallin. The seven isoforms of β-crystallin are divided into four acidic (βA1, βA2, βA3, and βA4) and three basic (βB1, βB2, and βB3) polypeptide subunits, which associate to either homo- or hetero-oligomers with sizes ranging from 50 to 200 kDa in the lenses [2]. βA1 and βA3 are isoforms having identical sequences except for an additional 17 amino acids at the N-terminus for βA3. How oligomers are formed in vivo is not known, but detection of dimers in some β-crystallins in vitro may imply that the dimers are building blocks for oligomerization [3–5]. Although many studies on the dimerization and oligomerization of some β-crystallins have been reported [3,5–7], we do not know whether all β-crystallins form dimers or which dimers, homo or hetero, are more likely to form. A systematic study of the most heterogeneous β-crystallin family should be beneficial in the understanding of crystallin subunit interactions, which would allow further study of the more complex oligomerization in the eye lens. Dimerization or subunit interaction can be studied by many methods; previous studies with a two-hybrid system assay indicate that it is ideal for protein–protein interaction [4,8–10]. Such interactions are usually studied by some spectroscopic measurements, requiring time-consuming protein expression and purification processes. The two-hybrid system obviates this requirement and gives quantitative estimates of interactions. In the present study, we subcloned all of the seven β-crystallins to the two-hybrid system vectors and investigated the interactions. The results indicate that hetero pairs (acidic–basic) of β-crystallins interact strongly but that homo pairs (acidic–acidic or basic–basic) of β-crystallins interact less strongly. When we expressed all seven β-crystallins and subjected them to some biophysical studies, we found some differences, especially in conformation, among them.
2. Materials and methods
2.1. Mammalian two-hybrid system
The Clontech's Mammalian Two-Hybrid System Assay Kit II was used (Clontech, Palo Alto, CA) [4,11]. There are three vectors in the system. The first test proteins (bait) were fused into the GAL4 DNA-BD in the pM vector and the second test proteins (prey) were fused into the VP16-AD in the pVP16 vector. The third vector pG5SEAP contains a reporter construct encoded with secreted alkaline phosphatase (SEAP). β-Crystallin genes were obtained from various sources: βA3 (A3-PET3a) from Dr. Kirsten Lampi (Oregon Health and Science University), βA2 (A2-pOTB7) and βB1 (B1-pCMV.SPORT6) from American Type Culture Collection (ATCC, Manassas, VA), and βA4 (EX-U1349-B01) and βB3 (EX-T1356-B01) from GeneCopeia (Germantown, MD). βA1 (A1-PET3a) was prepared from βA3 by mutation to remove 17 amino acids in the N-terminus. The βB2 gene was obtained from our previous work [8]. The β-crystallin genes were subcloned into pM and pV16 vectors with the forward and reverse primers (Table 1). The underlined sequences are for restriction enzymes EcoRI and XbaI, respectively, for all β-crystallin genes except for βB1, for which the two enzymes are BamHI and XbaI. The sequences of the DNA inserts were verified by sequence analysis.
Table 1.
Primers for cloning of β-crystallin genes to the two-hybrid system vectorsa
| Forward primers | Reverse primers | |
|---|---|---|
| βA1 | TTGAATTCGCTCAGACCAACCCTACC | GCTCTAGACTACTGTTGGATTCG |
| βA2 | TTTTTTGAATTCGTGAGCAGCGCCCCC | GCTCTAGACTAGTGCTGGACTCT |
| βA3 | TTTGAATTCGAGACCCAGGCTGAG | GCTCTAGACTACTGTTGGATTCG |
| βA4 | GGAATTCACCCTGCAATGCACA | GCTCTAGATCACTGCTGGATCCT |
| βB1 | CGGGATCCGTTCTCAGGCTGCAAAG | TTTCTAGATCACTTGGGGGGCTCT |
| βB2 | CAGGAATTCATGGCCTCAGATCAC | CATGGTCTAGAGGGCACTAGTTGG |
| βB3 | AGAATTCGCGGAACAGCACGGA | AGTCTAGATCAGCTGCTGGGGAA |
The underlined sequences are EcoRI and XbaI restriction enzyme sites for 5′ and 3′ primers, respectively, for all β-crystallins except for βB1-crystallin, for which the two enzymes are BamHI and XbaI.
HeLa cells were cotransfected with Lipofectamine 2000 (Invitrogen, Rockville, MD). After culture for 48 h, SEAP activity was measured in the supernatants by reading the fluorescence of the substrate MUP (4-methylumbelliferyl phosphate) at 360/449 nm [11]. The readings were normalized with the readings of the basal control (cotransfection of pM and pVP16). The pairs of p53-T and p53-CP were used as the positive and negative controls, respectively; the SV40 large T antigen was known to interact with p53, and the polyoma virus coat protein was known not to interact with p53 [12]. To determine whether protein concentrations varied among the cotransfections, cultured cells were lysed and protein concentrations were determined; the results indicated very little variation. The change in SEAP activity was not caused by difference in protein expression.
2.2. Confocal fluorescence study of fusion protein of GFP and βA2-crystallin
Laser scanning microscope (LSM) images were obtained as described in our recent report [13]. The GFP or its red variant RED vectors were used: pAcGFP-C1 and pDsRED monomer-C1 (Clontech, Palo Alto CA). The pAcGFP1-C1 vector is encoded with a green fluorescent protein (GFP) (λex = 475 nm and λem = 505 nm), and the pDsRED-Monomer-C1 is encoded with the red fluorescent protein DsRed (λex = 557 nm and λem = 585 nm). Various β-crystallin genes (βA2, βA4, and βB2) were subcloned into either pAcGFP-C1 or pDsRED monomer-C1 with appropriate primers (Table 2). The plasmids were cotransfected into HeLa cells. After culture for 48 h, microscopy images of living cells were acquired with a Zeiss Laser Scanning Confocal Microscope (510 META Axioplan 2, Carl Zeiss, Inc., Tornwood, NY).
Table 2.
Primers for subcloning β-crystallin genes to GFP and DsRED vectorsa
| Forward primers | Reverse primers | |
|---|---|---|
| βA2 | TTTTCTCGAGTGAGCAGCGCCCCCGC | CGAATTCCTAGTGCTGGACTCTCC |
| βA4 | AACAAGCTTTGACCCTGCAATGC ACA | GGAATTCTCACTGCTGGATCCTGCG |
| βB2 | GAACCTCGAGCTGCCTCAGATCACCAG | GGTCTAGAATTCCTAGTTGGAGGGGTG |
The underlined sequences are restriction enzyme sites for 5′ and 3′ primers, XhoI and EcoRI, respectively, for βA2 and ββ2. The corresponding restriction enzyme sites for βA4 are HindIII/EcoRI.
2.3. Expression of β-crystallins
The QIAexpression Type IV Kit (Qiagen, Valencia, CA) was used in the cloning, expression, and purification of various 6xHis-tagged β-crystallins as described elsewhere [4]. Briefly, the β-crystallin genes in pM plasmids (e.g., pM-βA1) were amplified by PCR using Pfu DNA polymerase (Stratagene) with the forward/reverse primers (Table 3). Two restriction sites, BamHI or KpnI and HindIII or KpnI, were included in the 5′ and 3′ primers. The PCR products and pQE-30 vector were doubly digested by the two enzymes. The digested genes and vector were then ligated by DNA ligase under standard conditions. The β-crystallin cDNA inserts were verified by sequencing analysis.
Table 3.
Primers for cloning of β-crystallin genes to expression pQE30 vectora
| Forward primers | Reverse primers | |
|---|---|---|
| βA1 | TTTGGATCCGCTCAGACCAACCCTACC | TTTGGTACCCTACTGTTGGATTCGGCG |
| βA2 | TTTGGATCCTTGAGCAGCGCCCCCG | TTTGGTACCCTAGTGCTG GACTCT |
| βA3 | TTTGGATCCGAGACCCAGGCTGAGCAG | TTTGGTACCCTACTGTTGGATTCGGCG |
| βA4 | AAAGGTACCACCCTGCAATGCACA | CCCAAGCTTTCACTGCTGGATCCT |
| βB1 | TTTGGATCCTCTCAG GCTGCAAAG GCC | TTTAAGCTTTCACTTGGGGGGCTC |
| βB2 | CGGGGTACCCCGGCCTCAGATCACCAG | CCCAAGCTTGGGGTTGGAGGGGTGGAA |
| βB3 | AAAGGTACCGCGGAACAGCACGGA | AAGAAGCTTTCAGCTGCTGGGGAA |
The underlined sequences are restriction enzyme sites for 5′ and 3′ primers, respectively: BamHI/KpnI for BA1-,BA2-, and βA3-crystallin; KpnI/HindIII for βA4-, βB2-, and βB3-crystallins; and BamHI/HindIII for βB1-crystallin.
The expression constructs containing various β-crystallin genes were transformed into E. coli strain M15 [pREP4]. Cell culture was performed to induce expression of various proteins by a standard protocol. The 6xHis-tagged β-crystallins were purified by Ni-NTA affnity chromatography with protocol for either soluble proteins (βA1, βA3, βB1, βB2, and βB3) or insoluble proteins (βA2 and βA4). The purified β-crystallins were dialyzed against 50 mM phosphate buffer, pH 7.4.
Protein concentrations were determined by measuring absorption at 280 nm: A0.1% = 2.85 for βA1-, 2.12 for βA2-, 2.62 for βA3-, 2.43 for βA4-, 2.05 for βB1-, 1.76 for βB2-, and 2.24 for βB3-crystallin [14].
2.4. SDS–PAGE, FPLC size-exclusion chromatography, and CD
SDS–PAGE was performed in a slab gel (12% acrylamide) under reducing conditions according to the method of Laemmli [15].
Size-exclusion chromatography was carried out in FPLC equipped with FPLC director software, using a superose-12 column (Pharmacia, Piscataway, NJ).
CD spectra were obtained with an Aviv Circular Dichroism Spectrometer (model 60 DS, Aviv Associates, Lakewood, NJ). Five scans were recorded, averaged, and followed by a polynomial-fitting program. The CD was expressed as a unit of deg-cm2–dmol−1.
3. Results
3.1. Two-hybrid assay for protein–protein interactions
Protein–protein interactions among the various β-crystallins are shown in Fig. 1. The increase in SEAP activity was uniformly observed for the hetero pairs (βA–βB) (about 40-fold increase), except that the pairs involving βA4 showed a smaller increase and the pairs of βA1–βB3 and βA3–βB3 showed a tremendous increase (about 130-fold). Among the homo pairs (self-association), either acidic β-crystallins (βA–βA) or basic β-crystallins (βB–βB), only the pairs involving βA1 or βA3 showed a modest increase (about 40-fold). The extremely small increase of SEAP activity for βA2- or βA4-crystallin was caused by their low solubility as confirmed later in the expression experiment and confocal fluorescence microscopy.
Fig. 1.
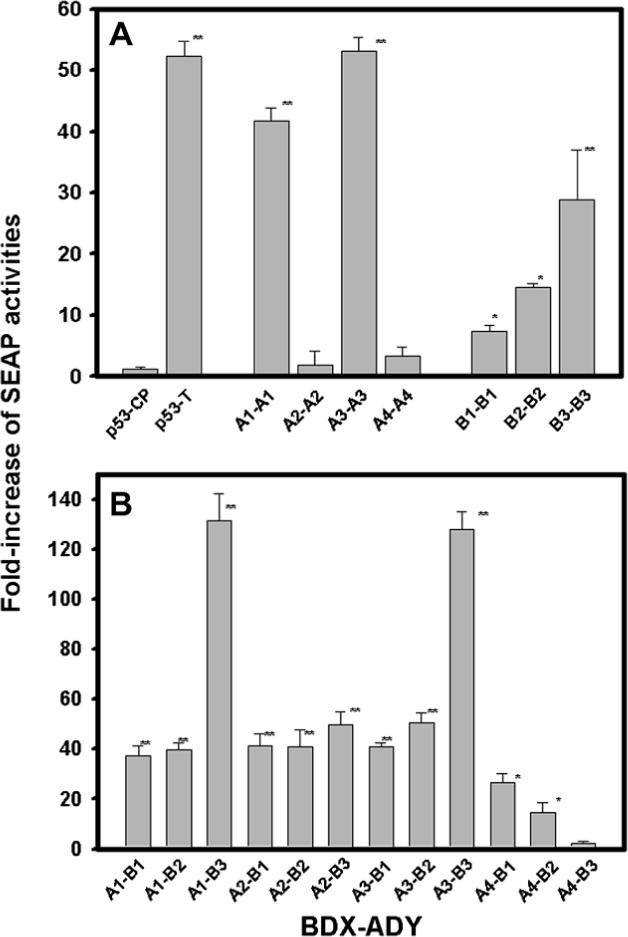
SEAP activities of pairs of various β-crystallins. The β-crystallin genes were fused into either the pM vector containing the DNA-binding domain (BD) or a pVP16 vector containing the transcript activity domain (AD). The pairs of various genes were co-transfected to HeLa cells with pG5SEAP reporter vector. (A) Homo pairs of βA–βA or βB–βB and (B) hetero acidic–basic pairs of βA–βB. The culture media were assayed for SEAP activity. The fold increases of SEAP activities were normalized with the basal control (pM and pVIP19 w/o DNA inserts). The negative and positive controls (p53-CP and p53-T) were included. Statistical significance was calculated by the paired t-test. Significant increases were observed for the pairs from control p53-CP (*P < 0.05 and **P < 0.005).
3.2. Confocal fluorescence microscope study
Cells co-transfected with GFP-βA2 and RED-βA2 show a large number of protein aggregates (Fig. 2A and B). In contrast, cells cotransfected with GFP-βA2 and RED-βB2 show fewer cells with protein aggregates (Fig. 2C and D). The corresponding cotransfected cells of the homo pair (GFP-βA4 and RED-βA4) and the hetero pair (GFP-βA4 and RED-βB2) are shown in Fig. 2E–H. The aggregation of βA4-crystallin is more intense than the βA2-crystallin; the co-expression with βB2-crystallin did not eliminate aggregation, but the size of the protein aggregates decreased significantly. The percentage of cells with protein aggregates in the βA2- and βB2-crystallin cotransfected cells decreased more than 3-fold from the βA2-crystallin transfected cells but decreased only insignificantly for βA4-crystallin (Fig. 3). These results indicate that βA2-crystallin, but not all βA4-crystallin, becomes soluble when co-expressed with βB2-crystallin, consistent with the results of the two-hybrid system assay. These observations are also consistent with a previous report that expression of soluble βA4 requires co-expression with βB1-crystallin [6].
Fig. 2.
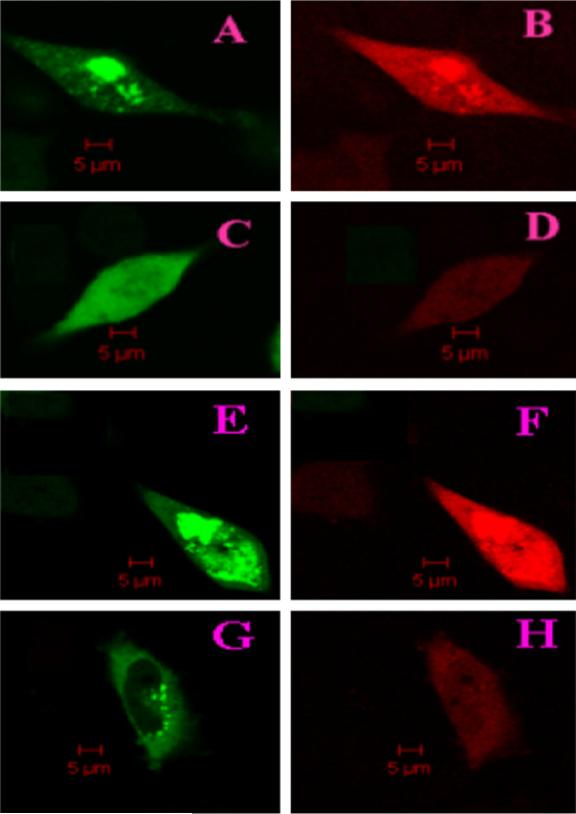
Confocal fluorescence microscopy images cells cotransfected with pairs of GFP or RED fusion β-crystallins. (A) GFP channel image of cells cotransfected with GFP-βA2 and RED-βA2, (B) RED channel image of cells cotransfected with GFP-βA2 and RED-βA2, (C) GFP channel image of cells cotransfected with GFP-βA2 and RED-βB2, and (D) RED channel image of cells cotransfected with GFP-βA2 and RED-βB2. (E–H) The corresponding images of cells cotransfected with βA4- and βB2-crystallin.
Fig. 3.

Cells with aggregates decrease greatly for βA2-crystallin but only little for βA4-crystallin in the cells cotransfection with βB2-crystallin. The mean and standard deviation is shown as percentage ± S.D. and represents an average of four independent experiments. For each experiment, cells were counted from 20 to 50 confocal LSM images. A significance decrease was observed for cells of (βA2+βB2) (*P = 0.02) but not for cells of (βA4+βB2) (**P = 0.3).
3.3. SDS–PAGE and FPLC size-exclusion chromatography, and CD
Protein expression indicates that all basic β-crystallins are soluble, but that two of the acidic β-crystallins (βA2 and βA4) are insoluble. The expressed β-crystallins, except βA4-crystallin, showed a single band with a molecular mass between 24 and 30 kDa on SDS–PAGE (Fig. 4). Degradation was observed for βA4-crystallin, consistent with a previous report [6].
Fig. 4.

SDS–PAGE of various β-crystallins. Gel electrophoresis was performed in 12% polyacrylamide gel. Lane 1, markers; lane 2−8, βA1-, βA2-, βA3-, βA4, βB1-, βB2-, and βB3-crystallin, respectively.
Size-exclusion chromatography showed that all β-crystallins except βB1-crystallin were dimers in dilute solutions (<1.0 mg/ml) (Fig. 5). βB1-crystallin appears to be a mixture of monomer and dimer; the elution profile is wider than that of other β-crystallins. βA2- and βA4-crystallins were insoluble; after treatment with GdHCl and dialysis, concentrations of reconstituted proteins were low: < 0.05 mg/ml for βA2- and ∼0.15 mg/ml for βA4-crystallin. No spectroscopic measurements or FPLC gel filtration could be performed for βA2-crystallin, but far-UV CD and FPLC could be made for βA4-crystallin. βA4-crystallin could refold to a dimer, but some degraded species were found, which also was shown in SDS–PAGE gel. FPLC gel filtration also indicates that acidic β-crystallins (A1 and A3) are susceptible to oligomerization; samples kept even at 4 °C for a month show many species with high molecular weight (data not shown).
Fig. 5.

FPLC size-exclusion chromatography of various human lens recombinant β-crystallins in the Sepharose-12 column. The reference proteins bovine albumin (67k), ovalbumin (52k), and human recombinant γC-crystallin (21k) are indicated in the upper x-axis. βA2-crystallin was not measured because the concentration was too dilute.
CD spectra are shown in Fig. 6. Both far- and near-UV CD spectra show great differences among β-crystallins, reflecting their different secondary and/or tertiary structures. The far-UV CD intensity is similar and rather low for βA1- and βA3-crystallin; this is expected since they have identical sequences except for an additional 17 amino acids at the N-terminus for βA3. βA4-crystallin shows some differences in increased intensity and shifting of the trough band position. For the three basic β-crystallins, their far-UV CD intensities are greater than those of βA–crystallins, but the negative band position of βB1-crystallin shifts to around 210 nm, suggesting it may have a different content of secondary structure. Using the PROSEC program [16], the content of various secondary structures (α-helix, β-sheet, β-turn, and random coil) was calculated (Table 4). The predicted secondary structures for all β-crystallins using the PROF program [17] are also included in the table and are comparable to the CD data.
Fig. 6.

CD spectra of β-crystallins. (A) Far-UV region and (B) near-UV region. Protein concentrations are 0.1−0.5 mg/ml. Cell path lengths are 1 mm for far-UV region and 10 mm for near-UV region. βA2-crystallin (far-UV and near-UV regions) and βA4-crystallin (near-UV region) were not measured because their concentrations were too dilute.
Table 4.
Percent of secondary structures of β-crystallinsa
| α-Helix | β-Sheet | β-Turn | Random coil | (other) | |
|---|---|---|---|---|---|
| βA1 | 6 (12)b | 34 (32) | 32 | 28 | (57) |
| βA2c | − (4) | − (39) | - | - | (58) |
| βA3 | 6 (5) | 30 (34) | 34 | 30 | (61) |
| βA4 | 4 (5) | 49 (34) | 19 | 27 | (61) |
| βB1 | 8 (7) | 37 (32) | 23 | 32 | (61) |
| βB2 | 9 (4) | 37 (34) | 26 | 28 | (62) |
| βB3 | 10 (7) | 46 (33) | 18 | 27 | (61) |
The content of secondary structures was calculated from CD data by PROSEC program [14].
The numbers in the brackets are the secondary structures predicted by PROF program [15]. The program gave only the α-helix and β-sheet; the remaining structures were given as other.
Concentration of βA2-crystallin was too dilute for CD measurement.
The near-UV CD signals arise mainly from the three aromatic amino acids (Trp, Tyr, and Phe). They reflect not only the amounts of the aromatic amino acids but also their local environments. While the amounts of aromatic amino acids for all β-crystallins are almost the same, their near-UV CD spectra and thus locations are quite different.
4. Discussion
The significant increase in SEAP activity for some acidic–acidic pairs and most hetero acidic–basic pairs of β-crystallins indicates they interact strongly. We do not know whether those β-crystallins expressed in the two-hybrid system are dimers or oligomers, but we tend to believe that β-crystallins with weak interactions are in a dimeric state and those with strong interactions are in an oligomeric state. The results are consistent with a previous report that βA3-crystallin, which shows strong self-interaction, formed homo-oligomers, and that the βA1-βB1 pair also shows strong interactions and formed hetero-oligomers [5]. Hetero-oligomerization is believed to result from subunit exchange from two different dimers, such as between homodimeric βB2- and βA3-crystallins [7]. βA1- and βA3-crystallin are the same polypeptide except that βA3-crystallin has an additional 17 amino acid residues in the N-terminus. Thus, it is not surprising that their far- and near-UV CD spectra are almost the same. However, βA3 has more SEAP activity than βA1-crystallin, suggesting involvement of the 17 AA segment in the self-association. This is consistent with the report that the N-terminal extension of βB1-crystallin has a role in higher assembly [18]. The high self-association for βA1-, βA3-, and βB3-crystallin also translated to high hetero-association for βA1-βB3 and βA3-βB3. The extremely small increases in SEAP activity of βA2- and βA4-crystallins arise from their low protein solubility; protein expression experiments indicate that both βA2- and βA4-crystallin have a very low solubility, and confocal microscopy shows the presence of large protein aggregates. The insolubility of βA2- and βA4-crystallin most likely arises from misfolding. Co-expression with the basic β-crystallins increased SEAP activity and prevented insolubilization of βA2-crystallin. However, this phenomenon was not so prominent for βA4-crystallin; co-expression with basic β-crystallins did not increase SEAP activity or decrease protein aggregates as much as for βA2-crystallin. The results are consistent with a previous report that βB1-crystallin enhances solubility of βA4-crystallin [19]. The ability of βB2- or βB1-crystallin to solubilize βA2-or βA4-crystallin is not unique among lens crystallins; αA-crystallin increases the solubility of αB-crystallin [13]. The mechanism underlying these effects is likely to be subunit exchange [7,20].
In human lenses, the earlier studies of size-exclusion chromatography separated β-crystallins into three fractions: β1-,β2-, and β3-crystallin with molecular mass ranging from 50 to 200 kDa [21,22]; each fraction contains at least two major polypeptide subunits with molecular mass of 24 and 27 kDa. A later report designated the three fractions as βH-, βL1-, and βL2-crystallin [23]; βH-crystallin contains βA3/βA1, βA4, βB1, and βB2, and βL1- and βL2-crystallin fractions contain βA3/βA1, βA4, βB1, βB2, and βB3. It seems surprising that each of the three fractions contains the same subunits except that βH-crystallin fraction does not have βB3 subunit. We now know that β-crystallin includes seven gene products; it is logical to assume that β-crystallin subunits with stronger interactions tend to become larger oligomers. In the bovine lenses, the corresponding three fractions are also designed as βH-, βL1-, and βL2-crystallin; both βH- and βL1-crystallin contain βA3 and βA4 subunits, and βL2-crystallin contains βB2 and βB3 subunits [2]. This study indicates that the acidic β-crystallin subunits are more prone to higher oligomerization than the basic β-crystallin subunits. This is consistent with the results of our two-hybrid system assays and expression experiments; acidic βA1- and βA3-crystallin show higher activity than the basic β-crystallins. It is not known why βA2 subunit was not detected in any of the three fractions in either human or bovine lenses.
The nature of the interaction between β-crystallins, as detected by the two-hybrid system, was demonstrated partially from domain interactions of β-sheets comprising many β-strands [11]. The domain interaction is similar to the inter-molecular interaction in αA-crystallin [24] or αB-crystallin [25] and the intra-molecular interaction in γD-crystallin [26]. All β-crystallins have high content of β-sheet conformation, facilitating domain association. In addition, a charge or hydro-phobic interaction may also contribute to the overall interaction. Two pairs (βA1-βB3 and βA3-βB3) stand apart from the others, showing more than a 120-fold increase in SEAP activity; the β-sheet domain interaction alone cannot account for this large increase.
CD data indicate there may be a subtle difference in the structure or conformation among β-crystallins. The far-UV CD spectrum of βB1-crystallin appears to differ from other basic β-crystallins and is not similar to acidic β-crystallins. In fact, the near-UV CD spectrum of βB1-crystallin also differs from that of other basic β-crystallins. Near-UV CD signal arises from electronic transitions of the three aromatic acids (Phe, Trp, and Tyr) and reflects the conformational environment of individual aromatic amino acids and thus protein tertiary structure. The great difference of the near-UV CD between βB1-and βB2-crystallin can be attributed to their different tertiary structures as manifested by the X-ray crystallographic data. The crystallographic structures of only two human lens β-crystallins have been reported: truncated βB1-crystallin (PDB id; 1OKI) [18] and wild-type βB2-crystallin (1YTQ) [27]. The wild type βB1-crystallin could not be crystallized. The crystallographic structures of both β-crystallins consist of four Greek key motifs: two motifs in the N-terminal domain and two motifs in the C-terminal domain. The βB2-crystallin dimer is formed by domain swapping of two subunits; the N-terminal domain of one subunit assembles with the C-terminal domain of the other subunit, but truncated βB1-crystallin domains are paired intramolecularly, and the domain–domain interface is buried. The βB1-crystallin dimer is formed through a different interface, and the oligomer is formed by dimer–dimer interaction. The structural differences in βB1-crystallin may underlie the observations of different patterns for this crystallin in CD spectra and FPLC elution profiles.
In conclusion, our studies demonstrate the presence of homo- and hetero-molecular interactions among the β-crystallins. These interactions are responsible for the oligomerization observed in the lens β-crystallins; posttranslational modifications or site-specific mutations [4,28,29] are likely to disrupt the interactions and thus lens structure.
Acknowledgements
The authors thank Dr. Kirsten Lampi for her generous gift of the βA3 clone. Technical assistance from Mark Hanson is gratefully acknowledged. This work was supported by grants from the National Institutes of Health (EY13968) and the Massachusetts Lions Eye Research Fund.
Abbreviations
- CD
circular dichroism
- GFP
green fluorescence protein
- SEAP
secreted alkaline phosphatase
References
- 1.Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu. Rev. Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 2.Slingsby C, Bateman OA. Quaternary interactions in eye lens beta-crystallins: basic and acidic subunits of beta-crystallins favor heterologous association. Biochemistry. 1990;29:6592–6599. doi: 10.1021/bi00480a007. [DOI] [PubMed] [Google Scholar]
- 3.Hejtmancik JF, Wingfield PT, Sergeev YV. Beta-crystallin association. Exp. Eye Res. 2004;79:377–383. doi: 10.1016/j.exer.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Liu BF, Liang JJ. Interaction and biophysical properties of human lens Q155* betaB2-crystallin mutant. Mol. Vis. 2005;11:321–327. [PubMed] [Google Scholar]
- 5.Bateman OA, Sarra R, van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic beta-crystallin oligomers and hetero-oligomers. Exp. Eye Res. 2003;77:409–422. doi: 10.1016/s0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Vinader L, Onnekink C, van Genesen ST, Slingsby C, Lubsen NH. In vivo heteromer formation. Expression of soluble betaA4-crystallin requires coexpression of a heteromeric partner. Febs J. 2006;273:3172–3182. doi: 10.1111/j.1742-4658.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- 7.Hejtmancik JF, Wingfield PT, Chambers C, Russell P, Chen HC, Sergeev YV, Hope JN. Association properties of betaB2- and betaA3-crystallin: ability to form dimers. Protein Eng. 1997;10:1347–1352. doi: 10.1093/protein/10.11.1347. [DOI] [PubMed] [Google Scholar]
- 8.Fu L, Liang JJ. Detection of protein–protein interactions among lens crystallins in a mammalian two-hybrid system assay. J. Biol. Chem. 2002;277:4255–4260. doi: 10.1074/jbc.M110027200. [DOI] [PubMed] [Google Scholar]
- 9.Boelens WC, Croes Y, de Ruwe M, de Reu L, de Jong WW. Negative charges in the C-terminal domain stabilize the alphaB-crystallin complex. J. Biol. Chem. 1998;273:28085–28090. doi: 10.1074/jbc.273.43.28085. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Welsh MJ. Identification of a site of Hsp27 binding with Hsp27 and alpha B-crystallin as indicated by the yeast two-hybrid system. Biochem. Biophys. Res. Commun. 1999;255:256–261. doi: 10.1006/bbrc.1999.0174. [DOI] [PubMed] [Google Scholar]
- 11.Liu BF, Liang JJ. Domain interaction sites of human lens betaB2-crystallin. J. Biol. Chem. 2006;281:2624–2630. doi: 10.1074/jbc.M509017200. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. Faseb J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- 13.Liu BF, Anbarasu K, Liang JJ. Confocal fluorescence resonance energy transfer microscopy study of protein–protein interactions of lens crystallins in living cells. Mol. Vis. 2007;13:854–861. [PMC free article] [PubMed] [Google Scholar]
- 14.Mach H, Middaugh CR, Lewis RV. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 1992;200:74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Yang JT, Wu CS, Martinez HM. Calculation of protein conformation from circular dichroism. Meth. Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]
- 17.Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 18.Van Montfort RL, Bateman OA, Lubsen NH, Slingsby C. Crystal structure of truncated human betaB1-crystallin. Protein Sci. 2003;12:2606–2612. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin-Vinader L, Onnekink C, van Genesen ST, Slingsby C, Lubsen NH. In vivo heteromer formation. Expression of soluble betaA4-crystallin requires coexpression of a heteromeric partner. Febs J. 2006;273:3172–3182. doi: 10.1111/j.1742-4658.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- 20.Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J. Biol. Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- 21.Liang JN, Andley UP, Chylack LT., Jr. Spectroscopic studies on human lens crystallins. Biochim. Biophys. Acta. 1985;832:197–203. doi: 10.1016/0167-4838(85)90332-2. [DOI] [PubMed] [Google Scholar]
- 22.Zigler JS, Jr., Horwitz J, Kinoshita JH. Human beta-crystallin. I. Comparative studies on the beta 1, beta 2 and beta 3-crystallins. Exp. Eye Res. 1980;31:41–55. doi: 10.1016/0014-4835(80)90089-5. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Hanson SR, Lampi KJ, David LL, Smith DL, Smith JB. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp. Eye Res. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- 24.Koteiche HA, McHaourab HS. Folding pattern of the alpha-crystallin domain in alphaA-crystallin determined by site-directed spin labeling. J. Mol. Biol. 1999;294:561–577. doi: 10.1006/jmbi.1999.3242. [DOI] [PubMed] [Google Scholar]
- 25.Muchowski PJ, Wu GJ, Liang JJ, Adman ET, Clark JI. Site-directed mutations within the core alpha-crystallin" domain of the small heat-shock protein, human alphaB-crystallin, decrease molecular chaperone functions. J. Mol. Biol. 1999;289:397–411. doi: 10.1006/jmbi.1999.2759. [DOI] [PubMed] [Google Scholar]
- 26.Kosinski-Collins MS, Flaugh SL, King J. Probing folding and fluorescence quenching in human gammaD crystallin Greek key domains using triple tryptophan mutant proteins. Protein Sci. 2004;13:2223–2235. doi: 10.1110/ps.04627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MA, Bateman OA, Jaenicke R, Slingsby C. Mutation of interfaces in domain-swapped human betaB2-crystallin. Protein Sci. 2007 doi: 10.1110/ps.062659107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human beta B1 alters the elongated structure of the dimer. Exp. Eye Res. 2001;72:279–288. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R, Srivastava K, Srivastava OP. Truncation of motifs III and IV in human lens betaA3-crystallin destabilizes the structure. Biochemistry. 2006;45:9964–9978. doi: 10.1021/bi060499v. [DOI] [PubMed] [Google Scholar]


