Abstract
Study Objectives:
Obstructive sleep apnea (OSA) has been associated with increased perioperative morbidity and mortality. We initiated a protocol designed to screen patients preoperatively and monitor them postoperatively. The goal was to identify patients who were at risk for oxygen desaturation after discharge from the postanesthesia recovery room (PACU).
Methods:
Patients without previously diagnosed OSA presenting to the preoperative evaluation clinic were assessed over a 10.5-month period using a validated prediction rule to identify patients thought to be at high risk of OSA (sleep apnea clinical score, SACS ≥ 15). Following surgery, patients were monitored in the PACU for significant respiratory events: apnea, increased FiO2 requirement, pain-sedation mismatch, or episodes of desaturation. Patients were placed in 3 groups based on their SACS and the presence or absence of recurrent PACU respiratory events (group 1: SACS < 15, no recurrent events; group 2: SACS ≥15, no recurrent events; and group 3: SACS ≥ 15, recurrent events.) The number of oxygen desaturations ≥ 4% per hour, the oxygen desaturation index (ODI), was calculated for each patient for 24 to 48 hours after PACU discharge. An ODI > 10 was the threshold chosen to indicate a high frequency of oxygen desaturation.
Results:
The percentage of patients with ODI > 10 differed significantly across the 3 study groups (12%, 37%, and 57%, for groups 1–3, p = 0.005). Mean ODI in group 1 was significantly different from groups 2 and 3 (5.8 compared to 10.0 group 2 and 11.4 group 3 with p = 0.001).
Conclusions:
We have shown that combining preoperative screening is useful for identifying patients at risk for oxygen desaturation after PACU discharge.
Citation:
Gali B; Whalen FX; Gay PC; Olson EJ; Schroeder DR; Plevak DJ; Morgenthaler TI. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med 2007;3(6):582-588.
Keywords: Sleep apnea, obstructive, postoperative complications, recovery room, hypoventilation, sleep-disordered breathing, oximetry, preoperative evaluation, sedation
Obstructive sleep apnea (OSA) is a common disorder that continues to be undiagnosed in many patients,1 including a number who require surgery. There is an associated increase in perioperative morbidity and mortality in patients with OSA.2–5 In addition, patients with OSA are noted to have a higher incidence of prolonged length of postoperative hospital stay, which may be related to increased incidence of complications.6
Analgesic agents utilized during the perioperative period can decrease pharyngeal tone as well as depress ventilatory responses to hypoxia and hypercarbia.2,3 The American Society of Anesthesiologists has recently published practice guidelines that include recommendations regarding the perioperative management of OSA.7 The guidelines include presumptive diagnosis of OSA based on elevated body mass index, increased neck circumference, snoring, craniofacial abnormalities affecting the airway, daytime hypersomnolence, and abnormalities on airway exam. Although these findings are prevalent in patients with OSA, the added risk of coexistent disease markers has not been validated in the clinical setting, and the relative sensitivity and specificity is not known. The American Society of Anesthesiologists recommends close attention to airway management, extubation when the patient is fully awake and strong, and use of regional anesthesia whenever possible. The American Academy of Sleep Medicine Practice Review from 2003 has similar recommendations.8 The American Society of Anesthesiologists guidelines include postoperative care of patients who are suspected to be at high risk with observed pulse oximetry or in a monitored setting, as well as caution with the use of opioids. The American Academy of Sleep Medicine guidelines recommend careful attention during the first 24 hours and caution that patient-controlled analgesia may not be appropriate.
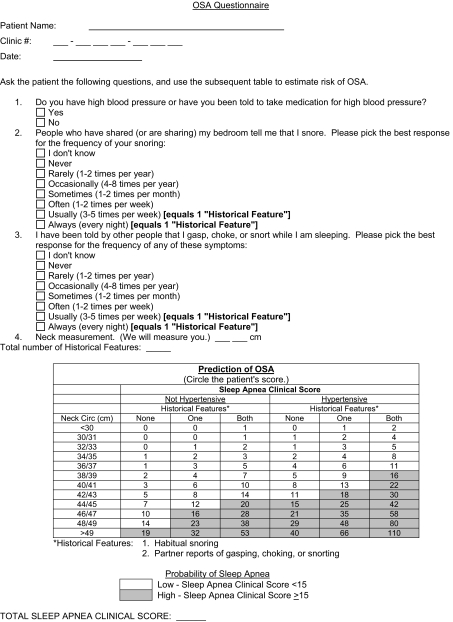
Identifying patients at risk prior to surgery as well as determining how to best monitor patients at risk for postoperative cardiorespiratory events are major concerns. Clinical prediction formulas can help identify patients and have good sensitivities (> 85%) but typically have had low specificities (<55%).9 One of these clinical prediction formulas was developed by Flemons et al and requires neck circumference, history of hypertension, and reported clinical symptoms to generate a likelihood ratio (Figure 1).10 Using this formula, a sleep apnea clinical score (SACS) of 15 or higher has a likelihood ratio of 5.17 and a posttest probability of 81% that a patient has OSA.10 When used to evaluate patients undergoing evaluation for sleep apnea in another outpatient setting, the Flemons criteria had a sensitivity of 76% and a positive predictive value of 73%.9
Figure 1.
Flemons Criteria (Preoperative Evaluation Clinic evaluation form). OSA refers to obstructive sleep apnea.
The postanesthesia care unit (PACU) is an area specifically for cardiopulmonary monitoring of postoperative patients, as well as pain management. Our goal was to apply a preoperative instrument to identify patients at risk of perioperative cardiorespiratory events and monitor these patients in the PACU for evidence of immediate postoperative respiratory episodes. We wished to determine if combining preoperative evaluation with PACU observations is predictive of oxygen desaturation in the first 24 to 48 hours postoperatively. Our hypothesis was that patients identified as high risk by preoperative screening and subsequent respiratory events in the PACU would be more likely to have significant oxygen desaturation postoperatively than would be patients who did not have preoperative indicators or PACU events.
METHODS
This protocol began as a clinical practice improvement initiative at our institution to better identify patients at risk for having OSA. Patients presenting to preoperative evaluation clinic for inpatient surgery were assessed with the Flemons criteria, which includes historical features (habitual snoring, choking, or gasping), hypertension, and neck circumference (Figure 1). A SACS was generated for each patient, and patients with scores of 15 or higher were categorized as “high risk.” The practice initiative occurred between September 29, 2004, and August 10, 2005. Following Institutional Review Board approval, all patients who consented to chart review for investigational purposes were eligible for enrollment. Only those patients without a previous diagnosis of OSA were included in the investigation.
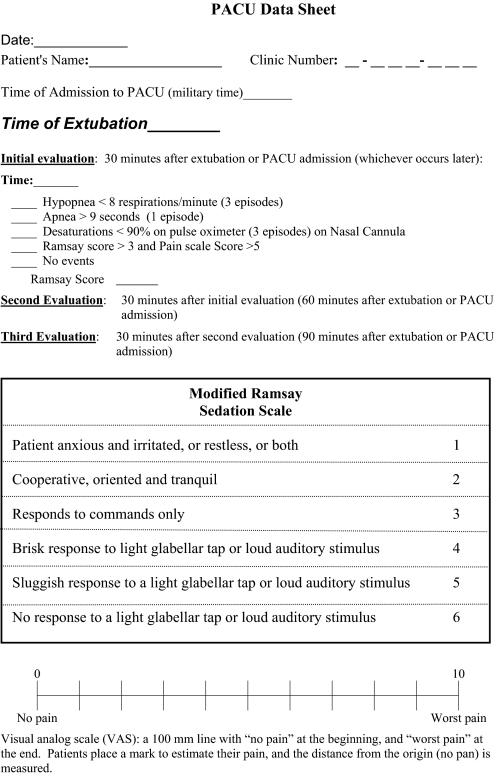
On the day of surgery, the anesthesiologist was aware of the patient's risk stratification based upon the SACS. Alteration of the type of sedation, anesthetic, and method of airway management was not required for the purpose of this practice initiative. After the operation, patients were assessed in the PACU for recurrent respiratory events that included bradypnea (< 8 respirations/minute); apnea, defined by a complete cessation of breathing on respiratory waveform and witnessed by PACU nurse (> 9 seconds); desaturations < 90% on pulse oximeter while on supplemental oxygen via nasal cannula of 4 liters or less for 30 seconds; inability to wean to a nasal cannula; and pain-sedation mismatch (high pain score with high sedation score using Ramsay > 3 and visual analog scale > 5, Figure 2).11,12 Pain-sedation mismatch was included as an indicator because these patients may be at increased risk for respiratory complications if given additional analgesics despite a high sedation score after discharge from the PACU. Patients were assessed by PACU nurses at 3 time points in their PACU stay: 30, 60, and 90 minutes after extubation or PACU admission. The evaluation was continuous for the duration of the stay in PACU, and events were observed by PACU nurses during their assessments of the patients. If patients experienced repeated events at 2 or 3 of these intervals or at PACU discharge (Group 3, see below), they were sent to a monitored bed, and pulse oximetry was recorded. Patients who had high SACS but had no events, no repeated events, or events that resolved before PACU discharge (Group 2 below) were sent to the floor, and pulse oximetry was recorded. Patients who preoperatively had been determined to be low risk (Group 1 below) were not routinely assessed in the PACU for events and were sent to the floor with routine care. Because we were attempting to formulate a practice initiative, we focused on the high-risk group of patients.
Figure 2.
Postanesthesia Care Unit (PACU) Evaluation Form
Using the baseline SACS and PACU monitoring results, patients were classified into 1 of 3 mutually exclusive groups: Group 1 patients were those who were at low risk of having OSA (SACS < 15), Group 2 patients had a high risk of having OSA (SACS ≥ 15) but had no recurrent PACU events,
Group 3 patients had a high risk of having OSA (SACS≥15) and had recurrent PACU events.
During the last phase of our practice initiative, for comparative purposes, a sample of 30 consecutive consenting patients who were considered to be low risk (SACS < 15) had PACU events monitored and underwent postoperative recording of pulse oximetry. These “low-risk” patients were considered to be the control group, and their data were compared with that of the “high-risk” (SACS ≥ 15) group of patients.
Pulse oximetry was obtained on 41 Nellcor 595 oximeters (Nellcor, Pleasonton, Calif.) with a sampling rate of 2 seconds. Data were analyzed using SCORE software system (version 1.1a Mallinckrodt 1999), and an oxygen desaturation index (ODI) was calculated. ODI was defined as the number of desaturations per hour of recording, and a desaturation was defined as a decrease in saturation of 4% or greater for 10 seconds or more. An ODI was calculated for each patient for 24 to 48 hours after PACU discharge. The length of oximetry recording varied from 24 to 48 hours and was primarily affected by hospital length of stay. An ODI above 10 was chosen to indicate a high frequency of oxygen desaturation.
Data collected included age, sex, type of operation, type of anesthetic (general vs spinal for the surgical procedure and postoperative catheter for analgesia after surgery), American Society of Anesthesiologists Classification, body mass index, and SACS. Postoperative data collected included PACU events (Figure 2), length of hospitalization, recorded pulse oximetry, and unplanned intensive care unit (ICU) admissions. Unplanned ICU admissions included those patients who were not sent directly to the ICU from the PACU, but, instead were those who, at the discretion of the treating physician, required ICU admission at other times in the hospitalization. Characteristics were compared across the risk groups using the Kruskal-Wallis test for age, body mass index, and neck circumference and using the χ2 test for all other characteristics. The percentage of patients with an ODI higher than 10 was summarized for each of these patient groups using a point estimate and 95% confidence interval (CI). ODI was compared across risk groups using the Kruskal-Wallis test, with ODI as a continuous variable and the χ2 test with ODI as a dichotomous variable (≤ 10 vs > 10). In all cases, a p value ≤ 0.05 was used to denote statistical significance.
RESULTS
Initiation of the protocol began with screening patients in the preoperative evaluation clinic to determine SACS in order to gain comfort with this assessment tool. A total of 2206 patients were screened, and data from 22 were excluded from analysis due to missing perioperative information or cancellation of surgery. Of these, 1923 had a low SACS and 251 had a high SACS. The frequency of unplanned ICU admission for low those patients with a SACS was 0.5%, compared with 8.8% for those with a high SACS, which was significantly different (p < 0.001, RR = 16.9, 95% CI 8.2–35.2). Thus, SACS was able to identify patients at higher risk of unplanned ICU admission.
After full implementation of the protocol, including preoperative and postoperative segments, complete data (preoperative, PACU, and oximetry for 24 hours or longer) was obtained on 115 of 195 high-risk patients, defined by a SACS of ≥ 15 without a known diagnosis of OSA. The remaining 80 patients were among the earliest in the clinical pathway, and the oximetry either malfunctioned or was not collected as intended, due to unfamiliarity with the clinical practice protocol. Complete data were obtained on 25 of the 30 consecutive low-risk patients (SACS < 15) studied toward the end of the project.
Table 1 depicts the demographics of the population divided by preoperative risk and PACU events. None of the patients with a low SACS had recurrent PACU events (group 1). The patients with a high SACS were divided into those without recurrent PACU events (group 2) and those with recurrent events (group 3). Compared with low-risk patients, patients at high risk for OSA (group 2 and 3) had higher body mass index (p < 0.001), but there was not a significant difference in body mass index between the high SACS without recurrent events and those with recurrent events. There was also a significant difference in neck circumference between the low-risk and the high-risk groups (p = 0.001), but no significant difference between the 2 high-risk groups. There was a significantly higher number of patients receiving postoperative regional analgesia in the high-risk group without recurrent PACU events (group 2), compared with the high-risk groups with recurrent events (group 3) (p = 0.019). There were no other significant baseline differences between the high-risk and low-risk groups (Table 1).
Table 1.
Patient Demographics According to Risk Groupa
| Characteristic | Group |
p Valueb | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| No recurrent events with SACS < 15 (N=25) | No recurrent events with SACS ≥ 15 (N=92) | Recurrent events with SACS ≥ 15 (N=23) | ||
| Sex, n (%) | 0.274 | |||
| Male | 18 (72) | 78 (85) | 20 (87) | |
| Female | 7 (28) | 14 (15) | 3 (13) | |
| Age, mean ± SD, y | 59.6 ± 8.5 | 59.9 ± 9.7 | 59.8 ± 9.9 | 0.792 |
| ASA Score, n (%) | 0.073 | |||
| 1 | 2 (8) | 1 (1) | 2 (9) | |
| 2 | 18 (72) | 51 (55) | 12 (52) | |
| 3 | 5 (20) | 40 (43) | 9 (39) | |
| BMI, mean ± SD, kg/m2 | 29.9 ± 4.5 | 35.9 ± 7.5 | 36.4 ± 7.6 | < 0.001c |
| 20.0–24.9, no. (%) | 2 (8) | 2 (2) | 0 (0) | |
| 25.0–29.9, no. (%) | 13 (52) | 15 (16) | 5 (22) | |
| 30.0–34.9, no. (%) | 8 (32) | 36 (39) | 6 (26) | |
| ≥ 35.0, no. (%) | 2 (8) | 39 (42) | 12 (52) | |
| Neck circumference, mean ± SD, cm | 40.4 ± 3.5 | 45.3 ± 2.7 | 44.9 ± 2.5 | < 0.00d |
| Anesthesia, n (%) | 0.082 | |||
| General | 22 (88) | 71 (77) | 22 (96) | |
| Spinal | 3 (12) | 21 (23) | 1 (4) | |
| Postoperative regional analgesia, n (%) | 0.036e | |||
| Yes | 13 (52) | 64 (70) | 10 (43) | |
| No | 12 (48) | 28 (30) | 13 (57) | |
| Type of operation, n (%) | 0.199 | |||
| Plastic | 0 (0) | 0 (0) | 1 (4) | |
| Colorectal | 3 (12) | 4 (4) | 1 (4) | |
| Otorhinolaryngology | 1 (4) | 2 (2) | 2 (9) | |
| GYN | 2 (8) | 4 (4) | 2 (9) | |
| Urologic | 7 (28) | 20 (22) | 4 (17) | |
| Orthopedic | 12 (48) | 62 (67) | 13 (57) | |
Flemons criteria was used to classify patients as having low (SACS < 15) or high (SACS ≥ 15) risk of OSA. SACS refers to Sleep Apnea Clinical Score. Recurrent events were defined as described in text.
Characteristics are compared across risk groups using the Kruskal-Wallis test for age, body mass index, and neck circumference and using the χ2 test for all other characteristics. For type of operation, the categories compared were orthopedic, urologic, and other, with the other category including plastic, colorectal, otorhinolaryngologic, and gynecologic.
Pairwise group comparisons: 1 vs 2 p<0.001; 1 vs 3 p<0.001; 2 vs 3 p = 0.745
Pairwise group comparisons: 1 vs 2 p<0.001; 1 vs 3 p<0.001; 2 vs 3 p = 0.359
Pairwise group comparisons: 1 vs 2 p = 0.101; 1 vs 3 p = 0.555; 2 vs 3 p = 0.019
Surgical procedures included orthopedic, urologic, gynecologic, colorectal, plastics, and otorhinolaryngoscopic procedures (Table 1). This case mix and patient demographics are typical of those seen at the hospital in which this data were collected. All were inpatients with hospital length of stay ranging from 1 to 10 days. Unplanned ICU admission during hospitalization occurred in 5 of the 115 (4.3%) patients in the high-risk group and 0 of the 25 patients in the low-risk group (p = 0.59).
Among the patients with complete data, the percentage of patients with ODI > 10 following PACU discharge differed significantly across the 3 study groups (p = 0.005), with group 1 having an incidence of ODI > 10 of 12%, (95% CI 3%-31%), group 2 having an incidence of 37% (95% CI 27%-48%), and group 3 having an incidence of 57% (95% CI 34%-77%). The incidence of ODI > 10 was significantly higher in groups 2 and 3, compared with group 1 (p = 0.017 and p = 0.001, respectively). Although the observed difference in the incidence of ODI > 10 for group 2 and group 3 was in the hypothesized direction, this difference was not statistically significant (p = 0.088). Mean ODI was significantly different between group 1 (5.8 with median 5.3) and pairwise comparison to groups 2 (10.0 with median 8.3) and 3 (11.4 with median 10.3) (p = 0.001 and p < 0.001, respectively). There was not a significant difference between the mean ODI in groups 2 and 3 (p = 0.113).
DISCUSSION
In our clinical practice improvement project, patients with a preoperative indicator of increased risk (SACS score ≥ 15) and recurrent immediate postoperative respiratory events (group 3) had the highest incidence of an ODI > 10 after surgery. Although there was not a statistically significant difference between high-risk patients without recurrent PACU events and those with recurrent PACU events, there was a higher mean and median ODI in those with recurrent events. Patients without a preoperative indication of increased risk and no PACU events (group 1) had the lowest incidence of ODI > 10 and were unlikely to require unplanned ICU admissions during the postoperative period.
Because OSA is not uncommon, it is vitally important to develop risk-stratification methods so that resources may be used wisely to safeguard patients undergoing surgical procedures. Death and cardiorespiratory complications have been associated with OSA during the postoperative period, and some have advocated intensive monitoring be used for most patients with OSA.2 In a retrospective study, patients undergoing hip and knee replacements with OSA were found to have a 24% incidence of complications, compared with 9% of those without OSA, including cardiac events, complications requiring transfer to and ICU, and respiratory events requiring support such as continuous positive airway pressure or intubation.5 Serious complications (cardiac and respiratory) have been reported in up to 8.7% of patients undergoing uvulopalatopharyngoplasty, the most common surgical treatment for OSA.13; in addition, mortality has been reported to be 1.6% of those patients undergoing UPPP.13 One of the most powerful factors associated with prolonged length of stay in patients undergoing gastric bypass surgery is OSA.6
We intended this clinical practice improvement project to systematically alert anesthesiologists to OSA risk and, secondly, to assist in gathering data about resources needed to adequately monitor patients following discharge from the PACU for future planning. Our results suggest that those patients in groups 2 or 3 are most at risk for having respiratory events, as defined by ODI greater than10; even patients who snore or have a high body mass index but a low-risk score (Group 1 patients) are unlikely to require postoperative monitoring. Our findings should be confirmed in a larger group of patients, including other those at institutions.
It may be suggested that utilization of both a preoperative screening and PACU monitoring is unnecessary. However, we felt that it made sense to combine a validated clinical prediction rule stratifying risk for OSA with a physiologic observation of the effects of surgery and analgesia on respiratory events (addition of a challenge to respiration) postoperatively. The Flemons criterion was selected for initial assessment of patients because of our familiarity with the tool, its simplicity, and its relatively favorable specificity and sensitivity in identifying patients with OSA. Use of other screening and monitoring tools may provide greater utility in identifying patents at risk in the perioperative period. One screening tool, the Berlin Questionnaire, shows promise in identifying patients with OSA.14 Future investigators may consider using the Berlin Questionnaire to identify patients at risk for the having OSA in the perioperative period and may wish to compare results with the use of the Flemons score. The PACU was chosen as the physiologic triage point because of available respiratory monitoring, low patient-to-nurse ratio, and the quantity of opioid drugs used during the postoperative period.
We chose to use the ODI as a surrogate marker for postoperative apnea-related events.15 An ODI > 10 was used as a cut-off for classifying patients as having clinically significant episodes of hypoxia. ODI from continuous pulse oximetry has been evaluated as 1 method to determine which patients should receive polysomnography for the diagnosis of OSA. In the outpatient setting, an ODI of 10 or above has been reported to have sensitivities of 71% to 85% and specificities of 90% to 93%; overall, the ODI correlates well with a polysomnographically determined apnea-hypopnea index.16–18 Although postoperative desaturation events correlate with obstructive apneas, other respiratory events such as central apneas contribute. However, Rosenberg et al19 reported that only a fraction of obstructive and central apneas are associated with desaturation events, so the ODI likely underestimates the apnea-hypopnea index.
Table 2.
Oxygen-Desaturation Index According to Risk Groupa
| ODI | 1 | 2 | 3 | p Valueb |
|---|---|---|---|---|
| No recurrent events with SACS < 15 (n=25) | No recurrent events with SACS ≥ 15 (n=92) | Recurrent events with SACS ≥ 15 (n=23) | ||
| Continuous | <0.001c | |||
| Mean ± SD | 5.8 ± 4.2 | 10.0 ± 6.7 | 11.4 ± 5.3 | |
| Median (IQR) | 5.3 (2.9, 7.4) | 8.3 (5.1, 13.0) | 10.3 (5.1, 15.1) | |
| Categorical, n (%) | 0.005d | |||
| ODI ≤ 10 | 22 (88) | 58 (63) | 10 (43) | |
| ODI > 10 | 3 (12) | 34 (37) | 13 (57) |
Flemons criteria was used to classify patients as having low (SACS < 15) or high (SACS ≥ 15) risk of obstructive sleep apnea (OSA). SACS refers to Sleep Apnea Clinical Score. Recurrent events were defined as described in text.
The Oxygen desaturation index (ODI) is compared across risk groups using the Kruskal-Wallis test, with ODI treated as a continuous variable and the χ2 test with ODI dichotomized (≤ 10 vs >10).
Pairwise group comparisons: 1 vs 2 p = 0.001; 1 vs 3 p<0.001; 2 vs 3 p = 0.113
Pairwise group comparisons: 1 vs 2 p = 0.017; 1 vs 3 p = 0.001; 2 vs 3 p = 0.088
Other than neck circumference, which is part of the SACS, there were no other baseline demographics that were significantly different between the low-SACS and high-SACS groups. Neck circumference did not differ between the patients with high SACS without PACU events and the patients with high SACS with PACU events.
There was, however, a significant difference in the number of patients with and without recurrent PACU events among those who were in the high-SACS groups and had some form of postoperative regional analgesia. Postoperative analgesia is provided via a regional catheter that is placed specifically to aid in postoperative pain control. Fewer patients with a high SACS and who had postoperative regional analgesia had recurrent PACU events, as compared with those patients who had high SACS but no postoperative regional analgesia. It may be that those patients who had only intravenously administered analgesics in the PACU (as opposed to those who had postoperative regional analgesia) were more likely to have the immediate effects of respiratory depression secondary to intravenously administered opioid analgesics. This difference may be related to the opioid-sparing properties of regional analgesia and avoidance of the systemic respiratory effects that occur with the use of intravenously administered narcotics.20,21 The ODI was not significantly different between the high-SACS groups without and with recurrent events, perhaps suggesting that SACS is more predictive of a possibility of sleep-related disturbances than events.
There are several limitations to our study. First, there was substantial data loss. Because our protocol was instituted as a practice initiative, complete data were not retrievable in every patient; as clinicians grew familiar with the protocol and with the data collection, the quality of data collection improved, but we do not believe that data loss introduced any systematic bias in our data. After the protocol had been in place for several months, complete data were obtained on a consistent basis.
Secondly, the surgeons and anesthesiologists were not blinded to the patients' SACS. This may have altered their anesthetic management toward safer practices in patients with higher risk.7 This would be expected to reduce the postoperative complication rate and reduce the likelihood of unplanned ICU transfer. However, we would regard risk reduction as a positive and desired effect of the protocol, and, even with this, the value of a “low-risk” score was predictive of no need for the ICU. Thus, the information gained from the protocol remains clinically useful in terms of planning postoperative care and monitoring.
Although there was limited data collection for the study group, there was a strong association between high SACS and unplanned ICU admission. Despite the limited data collection, this was a significant finding based on our initial evaluation of all patients enrolled in our protocol. This is an area that will need further investigation to determine if this can help guide our identification of patients who may be at risk in the perioperative period.
We have demonstrated a clinical practice protocol that determines the risk of repetitive oxygen desaturation events and stratifies risk of an unplanned ICU transfer. Although our study provides a starting point for risk stratification, several questions for postoperative risk assessment and monitoring needs remain. For example, will special monitoring and intervention for high-risk patients in the postoperative period prevent patients with postoperative hypoxemia from having cardiorespiratory-related complications and death? Additional studies are needed to determine if there is a direct relationship between ODI in the first 24 to 48 hours after surgery and the incidence of cardiorespiratory complications and death. If so, the level of monitoring and personnel required to prevent perioperative complications remains to be defined. Our investigation does not provide information on when a patient who is thought to be at risk for postoperative hypoxemia or has documented postoperative hypoxemia should receive a formal sleep study, though, most sensibly, patients identified as high risk should be referred for evaluation by a sleep specialist. Very importantly, all medical institutions will need to know if the safe perioperative care of patients with OSA or who are at high risk for having OSA will require additional ICU resources.
ACKNOWLEDGMENTS
Authors would like to acknowledge and thank Laurie Meade and Lavonne Liedl for their invaluable work conducting the day-to-day activities of this project.
Laurie Meade, Study Coordinator, Mayo Clinic, Department of Anesthesiology, Rochester, MN
Lavonne Liedl, Study Coordinator, Mayo Clinic, Department of Anesthesiology, Rochester, MN
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ohayon MM, Guilleminault C, Paiva T, et al. An international study on sleep disorders in the general population: methodological aspects of the use of the Sleep-EVAL system. Sleep. 1997;20:1086–92. doi: 10.1093/sleep/20.12.1086. [DOI] [PubMed] [Google Scholar]
- 2.Boushra NN. Anaesthetic management of patients with sleep apnoea syndrome. Canadian J Anaesth. 1996;43:599–616. doi: 10.1007/BF03011774. [DOI] [PubMed] [Google Scholar]
- 3.Catley DM, Thornton C, Jordan C, Lehane JR, Royston D, Jones JG. Pronounced, episodic oxygen desaturation in the postoperative period: its association with ventilatory pattern and analgesic regimen. Anesthesiology. 1985;63:20–8. doi: 10.1097/00000542-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Reeder MK, Muir AD, Foex P, Goldman MD, Loh L, Smart D. Postoperative myocardial ischaemia: temporal association with nocturnal hypoxaemia. Br J Anaesth. 1991;67:626–31. doi: 10.1093/bja/67.5.626. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne GH, Svahn J, Capella RF, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: body mass index, length of surgery, sleep apnea, asthma, and the metabolic syndrome. Obes Surg. 2004;14:1042–50. doi: 10.1381/0960892041975460. [DOI] [PubMed] [Google Scholar]
- 7.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. quiz 1117–8. [DOI] [PubMed] [Google Scholar]
- 8.Meoli AL, Rosen CL, Kristo D, et al. Upper airway management of the adult patient with obstructive sleep apnea in the perioperative period—avoiding complications. Sleep. 2003;26:1060–5. doi: 10.1093/sleep/26.8.1060. [DOI] [PubMed] [Google Scholar]
- 9.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 10.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279–85. doi: 10.1164/ajrccm.150.5.7952553. [DOI] [PubMed] [Google Scholar]
- 11.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel MD. Management of agitation in the intensive care unit. Clin Chest Med. 2003;24:713–25. doi: 10.1016/s0272-5231(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 13.Harmon JD, Morgan W, Chaudhary B. Sleep apnea: morbidity and mortality of surgical treatment. South Med J. 1989;82:161–4. [PubMed] [Google Scholar]
- 14.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg J, Kehlet H. Postoperative episodic oxygen desaturation in the sleep apnoea syndrome. Acta Anaesth Scand. 1991;35:368–9. doi: 10.1111/j.1399-6576.1991.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 16.Chiner E, Signes-Costa J, Arriero JM, Marco J, Fuentes I, Sergado A. Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax. 1999;54:968–71. doi: 10.1136/thx.54.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai WH, Flemons WW, Whitelaw WA, Remmers JE. A comparison of apnea-hypopnea indices derived from different definitions of hypopnea. Am J Respir Crit Care Med. 1999;159:43–8. doi: 10.1164/ajrccm.159.1.9709017. [DOI] [PubMed] [Google Scholar]
- 18.Series F, Kimoff RJ, Morrison D, et al. Prospective evaluation of nocturnal oximetry for detection of sleep-related breathing disturbances in patients with chronic heart failure. Chest. 2005;127:1507–14. doi: 10.1378/chest.127.5.1507. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg J, Rasmussen GI, Wojdemann KR, Kirkeby LT, Jorgensen LN, Kehlet H. Ventilatory pattern and associated episodic hypoxaemia in the late postoperative period in the general surgical ward. Anaesthesia. 1999;54:323–8. doi: 10.1046/j.1365-2044.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–80. doi: 10.1054/jpai.2002.123652. [DOI] [PubMed] [Google Scholar]
- 21.Horlocker TT, Kopp SL, Pagnano MW, Hebl JR. Analgesia for total hip and knee arthroplasty:a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14:126–35. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]