INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by snoring, repetitive narrowing or collapse of the pharyngeal airway during sleep with associated blood gas disturbances (hypercapnia and hypoxemia), plus fragmented sleep. Multiple pathophysiological factors contribute to OSA pathogenesis, including the arousal threshold to respiratory stimuli and chemical control instability (loop gain).1,2 However, the interaction between compromised pharyngeal anatomy in OSA patients compared to healthy controls3–5 and a state-dependent inability of the upper airway dilator muscles (UAMs) to protect pharyngeal patency during sleep are believed to be particularly important underlying mechanisms.1,6
Given the importance of UAMs in maintaining patency, decreased UAM function could theoretically contribute to pharyngeal closure. Skeletal muscle myopathies result in muscular weakness or wasting not caused by nerve disorders.7 Some are genetic in origin (e.g., muscular dystrophy), others can be caused by inflammatory (e.g., polymyositis) or endocrine problems (e.g., hyperthyroidism). Indeed, UA myopathy may be important in OSA pathogenesis in some rare syndromes.8–10 However, as will be discussed in detail below, the airway does not obstruct during wakefulness. This fact strongly supports the role of state-related reductions in neural drive to UAMs rather than pharyngeal myopathy being fundamentally important in OSA pathogenesis.
UPPER AIRWAY MYOPATHY IS NOT AN IMPORTANT FACTOR IN OSA PATHOGENESIS
Evidence Against Myopathy
In contrast to the presence of UAM weakness in OSA, the force generated via a tongue protrusion and the fatigue properties of the tongue are not different between OSA patients and matched controls during wakefulness.11 Indeed, there is evidence to suggest that while the UAMs appear to be highly trained, their ability to produce force and tension is elevated rather than diminished compared to snorers.12,13 Furthermore, myopathic disorders are diagnosed clinically with motor unit potentials that are small and of short duration.7 Recent single motor unit data in the genioglossus muscle of OSA patients do not support the presence of these myopathic changes.14,15
State-Related Reductions in Neural Drive to Upper Airway Muscles
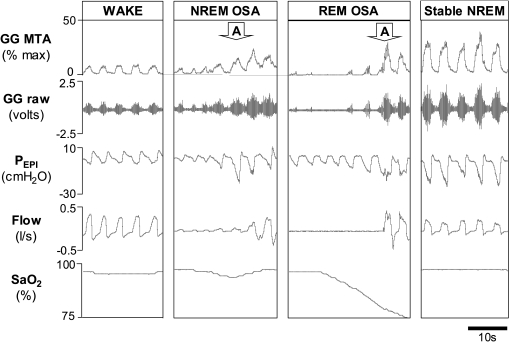
During wakefulness, patients with OSA appear to compensate for their anatomical compromise through protective reflex mechanisms which increase UAM activity to levels higher than that observed in healthy individuals.1,6 During sleep onset the electromyographic (EMG) activity of UAMs is reduced even in healthy individuals16; this results in important reductions in airway caliber and reduced ventilation in the OSA patient with compromised UA anatomy. Furthermore, there are several studies that highlight the role of state-dependent reductions in neural drive to UAMs as a critical pathogenic mechanism in OSA. That is, the UAMs are not weak, but drive to the muscles is reduced during sleep. Inhibitory input from sleep regulating neurons to UAMs, independent of central respiratory drive and negative airway pressure, appears to contribute to this effect.17 In addition, oxygen desaturation and event duration worsen during REM sleep in OSA.18 Consistent with reduced central respiratory drive to UAMs during REM sleep, when CPAP is applied to minimize UA negative pressure inputs to the largest UAM, the genioglossus, EMG activity is reduced from NREM to REM sleep.19 Finally, the protective reflex response to negative pressure stimuli is also markedly reduced during REM sleep,20,21 further supporting the importance of state-related changes in UAM control. Indeed, these step-wise, state-related decreases in UAM activity (from wakefulness to NREM sleep to REM sleep) coincident with respiratory events can be seen in Figure 1. In this characteristic example, neural drive to genioglossus (based on EMG activity) yields a respiratory event early in the sleep period (NREM OSA) and more pronounced reductions in muscle activity associated with further deterioration in ventilation during REM sleep.
Figure 1.
Respiratory events associated with state-related reductions in upper airway muscle activity and recovery associated with increased activity: What happened to the myopathy? A polysomnographic example of an OSA patient instrumented with fine wire electrodes into the genioglossus muscle (GG) to measure electromyographic activity during an overnight research study. Note the decreased GG muscle tone from wakefulness during the start of the NREM respiratory event (NREM OSA), which was further reduced at the start of the more severe respiratory event during REM sleep (REM OSA). Also note that despite the reduced muscle tone at the beginning of these events the muscle is capable of responding. The apparent myopathy has vanished as can be seen in the elevated GG levels coincident with stable breathing during NREM sleep (stable NREM) and progressive increases in GG activity during the respiratory events which culminate in arousal from sleep (indicated with the downward pointing arrows). GG MTA = 100 ms moving time average of the genioglossus electromyogram, GG raw = raw genioglossus electromyogram, Pepi = epiglottic pressure, Flow = airflow measured via pneumotachograph, and SaO2= arterial blood oxygen saturation measured via finger plethysmography.
Ability of Upper Airway Muscles to Respond to Maintain Stable Sleep
While neural drive and UAM responsiveness appear to be reduced from NREM to REM sleep compared to wakefulness early in the respiratory event, these muscles may respond to respiratory stimuli (Figure 1), particularly when combinations of stimuli are provided.22–24 Indeed, as highlighted by Younes,25 most OSA patients do experience stable periods of breathing during sleep at least some of the night (Figure 1, stable NREM). Mechanistically, this effect is likely due to changes in the arousal threshold to respiratory stimuli and compensation via UAMs.22,26,27 Together, these findings strongly refute the importance of myopathy in OSA since sustained periods of stable breathing during sleep via UAM activation could not occur if the muscles were truly weak.
DISEASE PROGRESSION AND CHANGES TO UPPER AIRWAY STRUCTURE AND FUNCTION AS AN EPIPHENOMENON OF OSA
Neuropathy versus Myopathy
Impairment at one or multiple levels within a motor unit (i.e., cell body, axon of the peripheral nerve, neuromuscular junction or the muscle fibers innervated) has the potential to lead to decreased muscle contraction output.7 It is important to note however, that neuropathic changes are by definition different from myopathic ones, although both may ultimately contribute to muscle weakness. Nonetheless, changes in UAMs may occur in OSA from repeated pharyngeal trauma/vibration via airway closure/snoring, inflammation, and repeated exposure to hypoxia.28,29 Indeed, numerous studies have revealed the presence of UA neuropathy and histological changes in OSA.30–38 However, others have demonstrated impaired sensory processing of respiratory afferent information in OSA only during sleep, further supporting the state-dependence of the disorder.39,40
Changes in Upper Airway Structure and Muscle Properties Due to Adaptation and Hypoxia: Support for an Epiphenomenon
While structural changes in UAMs may occur in OSA, there are also numerous adaptive changes that take place to preserve UAM function in OSA. Indeed, the histological changes that take place in the UAMs of snorers and OSA patients have been proposed to be an adaptive response without evidence for myopathic changes.12,41,42 Furthermore, one crucial compensatory mechanism mediated by sensory receptors in the UA, the genioglossus negative pressure reflex, appears to be similar if not augmented in OSA patients.43 Although some have suggested that myopathy may be a mechanism of OSA progression, most evidence shows that OSA, once established, is quite stable in the absence of weight gain.44 Thus, the observed UAM changes may be an epiphenomenon of OSA (i.e., a marker of vibration injury but of no major pathophysiological importance). The similarity in UAM fiber area frequency distribution, UA sensory function, and fatigue propensity between OSA and non-OSA snorers strongly suggests that other variables are more important.12,13,30,45,46
Nonetheless, other studies have shown more pronounced neurogenic lesions and impaired UAM force characteristics in OSA patients compared to controls and snorers.34,38 However, the importance of these observed abnormalities could be questioned, since they may reflect a nonspecific consequence of vibration and hypoxemia rather than a cause of OSA. Indeed, nocturnal hypoxemia is believed to result in a generalized neuropathy.47 Furthermore, training at altitude leads to a shift from aerobic to anaerobic muscle energy metabolism with associated histological changes.48 Measurement of serum creatine phosphokinase (CK) concentration (an important enzyme involved in energy metabolism of muscle) is often helpful in diagnosing myopathic syndromes as the sarcoplasm of the affected muscles poorly retains such soluble enzymes leading to elevated serum CK levels.7 Inconsistent with extensive UA myopathy in OSA, the majority of OSA patients have normal CK levels.49 In addition, in the subset of patients that do have modest CK elevations, mainly those with severe OSA, nocturnal hypoxemia appears to play a role in causing systemic skeletal muscle injury.49 Thus, the importance of histological abnormalities in various studies could be questioned unless physiological sequelae are demonstrable.
Individual Variability in Upper Airway Muscle Function
As stated, UAMs are capable of responding to respiratory stimuli during sleep (Figure 1). However, the functional effectiveness of increased UAM activity in restoring airflow during sleep varies substantially between individuals.22 Emerging data strongly suggest varying roles for pathophysiological abnormalities in different patients with OSA. That is, some patients have primarily anatomical abnormalities, while others have major abnormalities in loop gain (ventilatory control instability) while still others may have an UAM function problem or various combinations of these abnormalities.1 An OSA patient highly dependent on UAM activity to maintain pharyngeal patency, may be particularly susceptible to disease progression due to changes in UAM function. In theory, some patients may be more prone to muscle weakness/ineffectiveness due to the various possible perpetuating factors (i.e., vibration, hypoxia, inflammation) than others and thus, these patients may contribute to the modest disease progression that is not attributable to weight gain. However, to date, attempts to identify and characterize such patients have been elusive.
SUMMARY
Clearly, UA myopathy is not a major contributing factor to OSA pathogenesis for most patients. Rather, state-dependent reductions in neural drive to UAMs would appear to be a more critical pathogenic mechanism. While there are subtle changes in UA structure and function, there is little evidence to suggest that myopathy per se is important in OSA. Furthermore, most OSA patients are indeed capable of achieving stable periods of breathing at least part of the night, an effect believed to be importantly mediated via compensation of UA dilator muscles. It is extremely difficult to conceptualize how this may occur if myopathy were fundamentally important in OSA pathogenesis.
Furthermore, disease progression appears to be modest at best and is largely explained by increased weight gain. Nonetheless, it is acknowledged that subtle changes in UAM output due to factors such as repeated UA vibration, trauma, inflammation, and hypoxia may contribute to this effect. However, the current evidence would suggest that, if present, most of these changes would appear to be neurogenic rather than truly myopathic in origin. Adaptive processes to preserve UAM function in OSA in spite of these changes also appear to occur. In addition, these apparent changes may be an epiphenomenon rather than functionally important. Finally, some patients may be more vulnerable to UAM weakness with greater consequential functional effects than others, although this remains scarcely studied. Thus, future studies should carefully explore the functional consequences of UAM abnormalities and define which patients, if any, are susceptible to these potentially detrimental effects.
ACKNOWLEDGMENTS
Dr Eckert is a recipient of the Thoracic Society of Australia and New Zealand/Allen and Hanbury's respiratory research fellowship. Dr. Malhotra is funded by NIH P50 HL060292-09, AG024837-01, RO1-HL73146.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Malhotra has received research support from Respironics and Restore Medical and has consulted for Respironics, Restore Medical, Inspiration Medical, NMT Medical, Cephalon, and Pfizer. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 2.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. doi: 10.1513/pats.200707-114MG. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 4.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 5.Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–6. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland LP. Diseases of the motor unit. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 3rd ed. New York: McGraw-Hill; 1991. [Google Scholar]
- 8.Suresh S, Wales P, Dakin C, Harris MA, Cooper DG. Sleep-related breathing disorder in Duchenne muscular dystrophy: disease spectrum in the paediatric population. J Paediatr Child Health. 2005;41:500–3. doi: 10.1111/j.1440-1754.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 9.Dedrick DL, Brown LK. Obstructive sleep apnea syndrome complicating oculopharyngeal muscular dystrophy. Chest. 2004;125:334–6. doi: 10.1378/chest.125.1.334. [DOI] [PubMed] [Google Scholar]
- 10.Lacomis D, Kupsky WJ, Kuban KK, Specht LA. Childhood onset oculopharyngeal muscular dystrophy. Pediatr Neurol. 1991;7:382–4. doi: 10.1016/0887-8994(91)90071-r. [DOI] [PubMed] [Google Scholar]
- 11.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9:389–93. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 12.Series F, Cote C, Simoneau JA, et al. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest. 1995;95:20–5. doi: 10.1172/JCI117640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Series FJ, Simoneau SA, St Pierre S, Marc I. Characteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorers. Am J Respir Crit Care Med. 1996;153:1870–4. doi: 10.1164/ajrccm.153.6.8665048. [DOI] [PubMed] [Google Scholar]
- 14.Butler JE, Saboisky JP, McKenzie DK, et al. Genioglossus EMG activity in obstructive sleep apnea: single and multiunit analysis. Am J Respir Crit Care Med. 2007;175:A276. [Google Scholar]
- 15.Saboisky JP, Butler JE, McKenzie DK, et al. Genioglossus motor unit activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:A273. [Google Scholar]
- 16.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 17.Lo YL, Jordan AS, Malhotra A, et al. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:798–804. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–6. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 19.Eckert DJ, Malhotra A, Schory K, White DP, Jordan AS. Factors influencing genioglossus muscle control during REM Sleep. Sleep. 2007;30:A202. [Google Scholar]
- 20.Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–7. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–9. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 24.Lo YL, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–7. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 26.Sforza E, Krieger J, Petiau C. Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep. 1999;22:69–75. [PubMed] [Google Scholar]
- 27.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. doi: 10.1152/japplphysiol.00561.2007. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Petrof BJ, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis. Sleep. 1996;19:465–71. doi: 10.1093/sleep/19.6.465. [DOI] [PubMed] [Google Scholar]
- 29.Series F, Marc I. Upper airway mucosa temperature in obstructive sleep apnoea/hypopnoea syndrome, nonapnoeic snorers and nonsnorers. Eur Respir J. 1998;12:193–7. doi: 10.1183/09031936.98.12010193. [DOI] [PubMed] [Google Scholar]
- 30.Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–5. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005;28:585–93. doi: 10.1093/sleep/28.5.585. [DOI] [PubMed] [Google Scholar]
- 32.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 33.Akay M, Leiter JC, Daubenspeck JA. Reduced respiratory-related evoked activity in subjects with obstructive sleep apnea syndrome. J Appl Physiol. 2003;94:429–38. doi: 10.1152/japplphysiol.00018.2001. [DOI] [PubMed] [Google Scholar]
- 34.Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–93. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 35.Friberg D, Gazelius B, Lindblad LE, Nordlander B. Habitual snorers and sleep apnoics have abnormal vascular reactions of the soft palatal mucosa on afferent nerve stimulation. Laryngoscope. 1998;108:431–6. doi: 10.1097/00005537-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Svanborg E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir Physiol Neurobiol. 2005;147:263–72. doi: 10.1016/j.resp.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1960–6. doi: 10.1164/ajrccm.159.6.9809052. [DOI] [PubMed] [Google Scholar]
- 38.Carrera M, Barbe F, Sauleda J, et al. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J. 2004;23:425–9. doi: 10.1183/09031936.04.00099404. [DOI] [PubMed] [Google Scholar]
- 39.Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136:221–34. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 40.Gora J, Trinder J, Pierce R, Colrain IM. Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:1225–34. doi: 10.1164/rccm.2106005. [DOI] [PubMed] [Google Scholar]
- 41.Smirne S, Iannaccone S, Ferini-Strambi L, Comola M, Colombo E, Nemni R. Muscle fibre type and habitual snoring. Lancet. 1991;337:597–9. doi: 10.1016/0140-6736(91)91651-a. [DOI] [PubMed] [Google Scholar]
- 42.Ferini-Strambi LJ, Smirne S, Moz U, Sferrazza B, Iannaccone S. Muscle fibre type and obstructive sleep apnea. Sleep Res Online. 1998;1:24–7. [PubMed] [Google Scholar]
- 43.Berry RB, White DP, Roper J, et al. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol. 2003;94:1875–82. doi: 10.1152/japplphysiol.00324.2002. [DOI] [PubMed] [Google Scholar]
- 44.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 45.Series F, Simoneau JA, St Pierre S. Muscle fiber area distribution of musculus uvulae in obstructive sleep apnea and non-apneic snorers. Int J Obes Relat Metab Disord. 2000;24:410–5. doi: 10.1038/sj.ijo.0801172. [DOI] [PubMed] [Google Scholar]
- 46.Vuono IM, Zanoteli E, de Oliveira AS, et al. Histological analysis of palatopharyngeal muscle from children with snoring and obstructive sleep apnea syndrome. Int J Pediatr Otorhinolaryngol. 2007;71:283–90. doi: 10.1016/j.ijporl.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Mayer P, Dematteis M, Pepin JL, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213–9. doi: 10.1164/ajrccm.159.1.9709051. [DOI] [PubMed] [Google Scholar]
- 48.Howald H, Hoppeler H. Performing at extreme altitude: muscle cellular and subcellular adaptations. Eur J Appl Physiol. 2003;90:360–4. doi: 10.1007/s00421-003-0872-9. [DOI] [PubMed] [Google Scholar]
- 49.Lentini S, Manka R, Scholtyssek S, Stoffel-Wagner B, Luderitz B, Tasci S. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest. 2006;129:88–94. doi: 10.1378/chest.129.1.88. [DOI] [PubMed] [Google Scholar]