Abstract
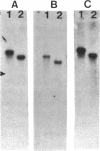
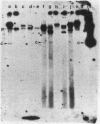
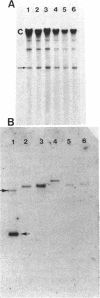
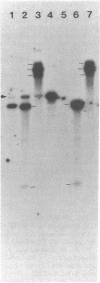
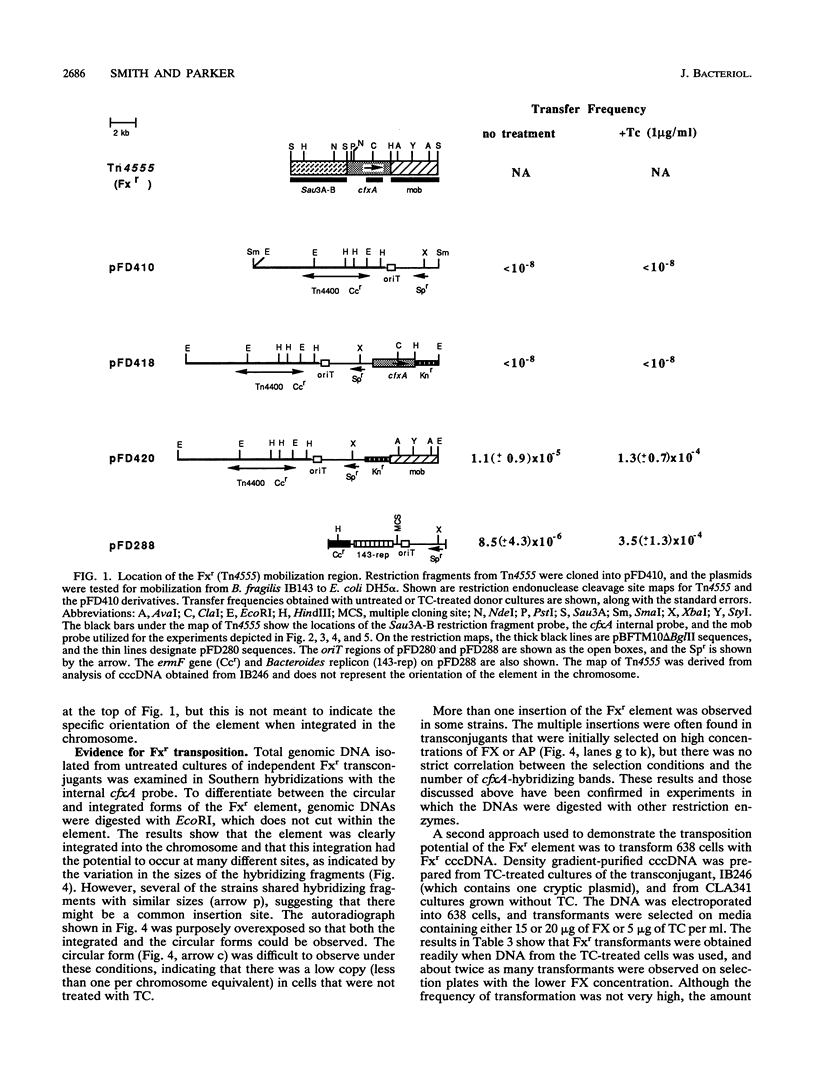
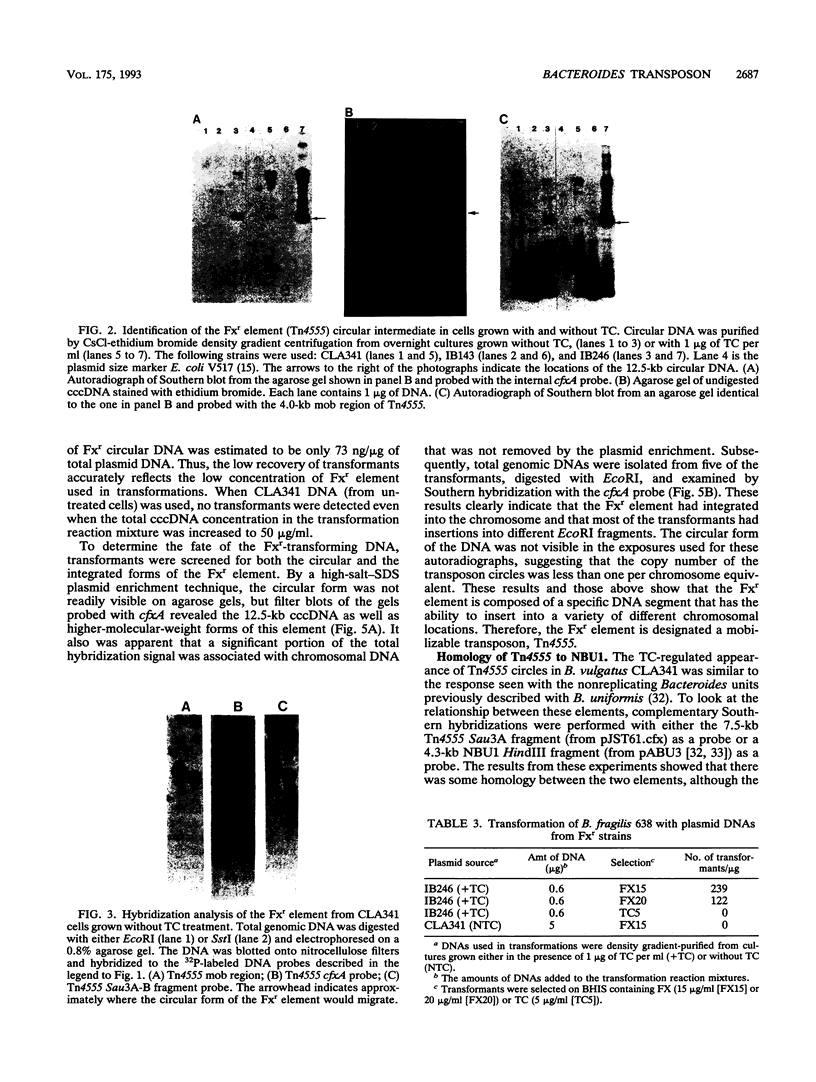
Transmissible cefoxitin (FX) resistance in Bacteroides vulgatus CLA341 was associated with the 12.5-kb, mobilizable transposon, Tn4555, which encoded the beta-lactamase gene cfxA. Transfer occurred by a conjugation-like mechanism, was stimulated by growth of donor cells with tetracycline (TC), and required the presence of a Bacteroides chromosomal Tcr element. Transconjugants resistant to either FX, TC, or both drugs were obtained, but only Fxr Tcr isolates could act as donors of Fxr in subsequent matings. Transfer of Fxr could be restored in Fxr Tcs strains by the introduction of a conjugal Tcr element from Bacteroides fragilis V479-1. A covalently closed circular DNA form of Tn4555 was observed in donor cells by Southern hybridization, and the levels of this circular transposon increased significantly in cells grown with TC. Both the cfxA gene and the Tn4555 mobilization region hybridized to the circular DNA, suggesting that this was a structurally intact transposon unit. Circular transposon DNA purified by CsCl-ethidium bromide density gradient centrifugation was used to transform Tcs B. fragilis 638, and Fxr transformants were obtained. Both the circular form and the integrated Tn4555 were observed in transformants, but the circular form was present at less than one copy per chromosomal equivalent. Examination of genomic DNA from Fxr transformants and transconjugants revealed that Tn4555 could insert at a wide variety of chromosomal sites. Multiple transposon insertions were present in many of the transconjugants, indicating that there was no specific barrier to the introduction of a second transposon copy.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedzyk L. A., Shoemaker N. B., Young K. E., Salyers A. A. Insertion and excision of Bacteroides conjugative chromosomal elements. J Bacteriol. 1992 Jan;174(1):166–172. doi: 10.1128/jb.174.1.166-172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callihan D. R., Young F. E., Clark V. L. Identification of three homology classes of small, cryptic plasmids in intestinal Bacteroides species. Plasmid. 1983 Jan;9(1):17–30. doi: 10.1016/0147-619x(83)90028-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Storey J. R., Malamy M. H. Transfer of beta-lactamase-associated cefoxitin resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986 May;29(5):918–920. doi: 10.1128/aac.29.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. A transposon in Streptococcus faecalis with fertility properties. Nature. 1982 Nov 18;300(5889):281–284. doi: 10.1038/300281a0. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Bouic K., Matthews B. Genetic transfer systems in Bacteroides: cloning and mapping of the transferable tetracycline-resistance locus. Mol Microbiol. 1989 Nov;3(11):1617–1623. doi: 10.1111/j.1365-2958.1989.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hecht D. W., Jagielo T. J., Malamy M. H. Conjugal transfer of antibiotic resistance factors in Bacteroides fragilis: the btgA and btgB genes of plasmid pBFTM10 are required for its transfer from Bacteroides fragilis and for its mobilization by IncP beta plasmid R751 in Escherichia coli. J Bacteriol. 1991 Dec;173(23):7471–7480. doi: 10.1128/jb.173.23.7471-7480.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D. W., Malamy M. H. Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. J Bacteriol. 1989 Jul;171(7):3603–3608. doi: 10.1128/jb.171.7.3603-3608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Inamine J. M. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Sep;35(9):1804–1810. doi: 10.1128/aac.35.9.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Marsh P. K., Malamy M. H., Shimell M. J., Tally F. P. Sequence homology of clindamycin resistance determinants in clinical isolates of Bacteroides spp. Antimicrob Agents Chemother. 1983 May;23(5):726–730. doi: 10.1128/aac.23.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. G., Guiney D. G. Characterization and mapping of regions encoding clindamycin resistance, tetracycline resistance, and a replication function on the Bacteroides R plasmid pCP1. J Bacteriol. 1986 Aug;167(2):517–521. doi: 10.1128/jb.167.2.517-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays T. D., Smith C. J., Welch R. A., Delfini C., Macrina F. L. Novel antibiotic resistance transfer in Bacteroides. Antimicrob Agents Chemother. 1982 Jan;21(1):110–118. doi: 10.1128/aac.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Privitera G., Dublanchet A., Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Rashtchian A., Dubes G. R., Booth S. J. Transferable resistance to cefoxitin in Bacteroides thetaiotaomicron. Antimicrob Agents Chemother. 1982 Oct;22(4):701–703. doi: 10.1128/aac.22.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. L., Odelson D. A., Macrina F. L. Complete nucleotide sequence and transcription of ermF, a macrolide-lincosamide-streptogramin B resistance determinant from Bacteroides fragilis. J Bacteriol. 1986 Nov;168(2):523–533. doi: 10.1128/jb.168.2.523-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Mitsuhashi S. Properties of a new penicillinase type produced by Bacteroides fragilis. Antimicrob Agents Chemother. 1982 Oct;22(4):579–584. doi: 10.1128/aac.22.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Kirchman P. A., Caparon M. G. An intermediate in transposition of the conjugative transposon Tn916. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4809–4813. doi: 10.1073/pnas.85.13.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Sex and the single circle: conjugative transposition. J Bacteriol. 1992 Oct;174(19):6005–6010. doi: 10.1128/jb.174.19.6005-6010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Tetracycline-dependent appearance of plasmidlike forms in Bacteroides uniformis 0061 mediated by conjugal Bacteroides tetracycline resistance elements. J Bacteriol. 1988 Apr;170(4):1651–1657. doi: 10.1128/jb.170.4.1651-1657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. Characterization of Bacteroides ovatus plasmid pBI136 and structure of its clindamycin resistance region. J Bacteriol. 1985 Mar;161(3):1069–1073. doi: 10.1128/jb.161.3.1069-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. Development and use of cloning systems for Bacteroides fragilis: cloning of a plasmid-encoded clindamycin resistance determinant. J Bacteriol. 1985 Oct;164(1):294–301. doi: 10.1128/jb.164.1.294-301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Parker A., Rogers M. B. Plasmid transformation of Bacteroides spp. by electroporation. Plasmid. 1990 Sep;24(2):100–109. doi: 10.1016/0147-619x(90)90012-2. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Spiegel H. Transposition of Tn4551 in Bacteroides fragilis: identification and properties of a new transposon from Bacteroides spp. J Bacteriol. 1987 Aug;169(8):3450–3457. doi: 10.1128/jb.169.8.3450-3457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Welch R. A., Macrina F. L. Two independent conjugal transfer systems operating in Bacteroides fragilis V479-1. J Bacteriol. 1982 Jul;151(1):281–287. doi: 10.1128/jb.151.1.281-287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens A. M., Sanders J. M., Shoemaker N. B., Salyers A. A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992 May;174(9):2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jacobus N. V., Gorbach S. L., Aldridge K. E., Cleary T. J., Finegold S. M., Hill G. B., Iannini P. B., McCloskey R. V. Susceptibility of the Bacteroides fragilis group in the United States in 1981. Antimicrob Agents Chemother. 1983 Apr;23(4):536–540. doi: 10.1128/aac.23.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Shimell M. J., Malamy M. H. Characterization of pBFTM10, a clindamycin-erythromycin resistance transfer factor from Bacteroides fragilis. J Bacteriol. 1982 Aug;151(2):686–691. doi: 10.1128/jb.151.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Macrina F. L. Physical characterization of Bacteroides fragilis R plasmid pBF4. J Bacteriol. 1981 Feb;145(2):867–872. doi: 10.1128/jb.145.2.867-872.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]