Abstract
Study Objective:
Subjective and objective assessments of sleep may be discrepant due to sleep misperception and measurement effects, the latter of which may change the quality and quantity of a person's usual sleep. This study compared sleep times from polysomnography (PSG) with self-reports of habitual sleep and sleep estimated on the morning after a PSG in adults.
Design:
Total sleep time and sleep onset latency obtained from unattended home PSGs were compared to sleep times obtained from a questionnaire completed before the PSG and a Morning Survey completed the morning after the PSG.
Participants:
A total of 2,113 subjects who were ≥ 40 years of age were included in this analysis.
Measures and Results:
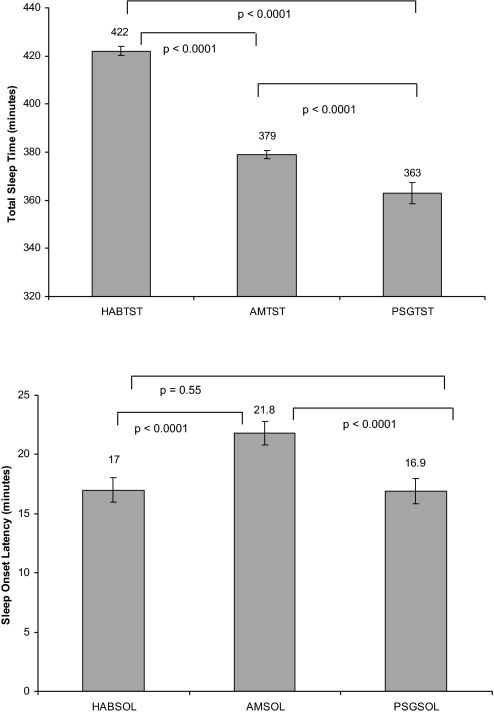
Subjects were 53% female, 75% Caucasian, and 38% obese. The mean habitual sleep time (HABTST), morning estimated sleep time (AMTST), and PSG total sleep times (PSGTST) were 422 min, 379 min, and 363 min, respectively. The mean habitual sleep onset latency, morning estimated sleep onset latency, and PSG sleep onset latency were 17.0 min, 21.8 min, and 16.9 min, respectively. Models adjusting for related demographic factors showed that HABTST and AMTST differ significantly from PSGTST by 61 and 18 minutes, respectively. Obese and higher educated people reported less sleep time than their counterparts. Similarly, small but significant differences were seen for sleep latency.
Conclusions:
In a community population, self-reported total sleep times and sleep latencies are overestimated even on the morning following overnight PSG.
Citation:
Silva GE; Goodwin JL; Sherrill DL; Arnold JL; Bootzin RR; Smith T; Walsleben JA; Baldwin CM; Quan SF. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J Clin Sleep Med 2007;3(6):622-630.
Keywords: Polysomnography, subjective, objective, sleep, time
INTRODUCTION
Subjective and objective assessments of sleep parameters may differ due to sleep misperception and measurement effect. While subjective estimates may be biased by a subject's own sleep perception, objective assessment methods, such as PSGs, may be considered distressing, thus changing the quality and quantity of a person's usual sleep. Exposure to polysomnographic equipment, e.g., head and chest sensors, or sleeping in an unfamiliar setting such as a laboratory may interfere with the subject's habitual sleep time.
Studies comparing subjective and objective estimates of sleep utilizing PSG assessments performed either at home or in the laboratory, have found that sleep time misperception is common.1–4 In these studies, subjects tended to underestimate their amount of total sleep time (TST) and overestimate the amount of sleep onset latency (SOL). In a recent study, a group of subjects with Parkinson disease showed reduced subjective sleep duration and longer SOL compared with healthy subjects.3 Another study showed that subjects with and without sleep apnea tended to overestimate SOL.2 Subjects with sleep apnea, however, made larger SOL overestimations and tended to underestimate their TST compared to those without sleep apnea. Nevertheless, consistency between subjective and objective sleep measures has also been reported.1,5 The aforementioned studies used small samples or subjects from populations with specific somatic or psychiatric disorders, and thus may not reflect the overall estimates for a community population.
In addition to sleep duration and latency being altered by a number of somatic and psychiatric disorders, other social, environmental, or host factors may be influential as well. Advancing age, obesity, gender, and ethnicity have been associated with differences in sleep duration.6–9 Furthermore, lifestyle and behavior changes have influenced the amount of sleep in certain populations. It is estimated that Americans slept one and a half hours less each night in 1975 than they did in 1900, mostly attributed to the invention of electric light and to social and economic pressures.10
The present study was conducted to examine the concordance between self-reported measures of TST and SOL and objective measures of sleep times as determined by ambulatory PSG, using subjects from a large multicenter community-based population. The relation between estimated and PSG sleep measures to possible host and environmental factors, as well as to the subject's perception of sleep time on the night of PSG was also assessed. This study addresses whether subjects' estimation of total sleep time and sleep onset latency differ from an objective measure of sleep and whether sleep times differences are affected by social factors and some medical conditions.
METHODS
The Sleep Heart Health Study (SHHS) is a prospective multicenter cohort study designed to investigate the relationship between sleep disordered breathing and cardiovascular diseases in the United States. Details of the study design have been published elsewhere.11 Briefly, initial baseline recruitment began in 1995, enrolling 6,441 subjects over 40 years of age from several ongoing geographically distinct cardiovascular and respiratory disease cohorts that were initially assembled between 1976 and 1995.12 These included the Offspring Cohort and the Omni Cohort of the Framingham Heart Study in MA; the Hagerstown, MD, and Minneapolis, MN, sites of the Atherosclerosis Risk in Communities Study; the Hagerstown, MD, Pittsburgh, PA, and Sacramento, CA sites of the Cardiovascular Health Study; three hypertension cohorts (Clinic, Worksite, and Menopause) in New York City; the Tucson Epidemiologic Study of Airways Obstructive Diseases and the Health and Environment Study; and the Strong Heart Study (SHS) of American Indians in OK, AZ, ND, and SD. A 5-year SHHS follow-up survey took place between February 2000 and May 2003, enrolling 3,079 of the original participants. As in the baseline study, subjects were recruited to undergo an overnight home polysomnogram, completion of several questionnaires, and collection of a small amount of physical examination data. The follow-up survey including polysomnography occurred continuously throughout the recruitment window without any significant seasonal variation. Unless otherwise noted, data for the present analysis is derived from participants in the SHHS follow-up survey. However, data from participants who had follow-up PSG from the New York City site were excluded because they did not meet quality standards for the follow-up examination.
All participants completed the SHHS Sleep Habits Questionnaire (SHQ).13 The SHQ contained questions regarding sleep habits, smoking status, as well as cardiovascular and respiratory problems. The habitual total sleep time (HABTST) and habitual sleep onset latency (HABSOL) during the weekdays and weekends were derived from specific questions on the SHQ. These questions were: How much sleep do you usually get at night (or in your main sleep period); on weekdays (weekends) or workdays (non-work days)?; and How long does it usually take you to fall asleep at bedtime? Weekend or weekday HABTST was used respectively according to whether the PSG was performed on a weekend or weekday. Height and weight were measured directly to determine body mass index (BMI, kg/m2). BMI was categorized into nonobese (< 30) and obese (≥ 30), according to established clinical guidelines.14
SHHS participants underwent an overnight in-home PSG using the Compumedics Portable PS-2 System (Abbottsville, Victoria, Australia) administered by trained technicians. The methods for obtaining PSG data followed those used during the first SHHS examination cycle. Briefly, after a home visit was scheduled, the SHQs generally were mailed 1–2 weeks prior to the PSG home appointment. Each participant was asked to complete the questionnaire prior to the home visit at which time the SHQ was collected and verified for completeness. The home visits were performed by 2-person mixed-gender teams in visits that lasted 1.5 to 2 hours. There was emphasis on making the night of the PSG assessment as representative as possible of a usual night of sleep. Participants were asked to schedule the visit so that it would occur approximately 2 hours prior to their usual bedtime. Participants' weekday or weekend bedtime routines were encouraged to be consistent with the day of the week the visits were made.
The SHHS recording montage consisted of electroencephalogram (EEG) (C4/A1 and C3/A2); right and left electrooculogram (EOG); a bipolar submental electromyogram (EMG); thoracic and abdominal excursions (inductive plethysmography bands); airflow (detected by a nasal-oral thermocouple [Protec, Woodinville, WA]), oximetry (finger pulse oximetry [Nonin, Minneapolis, MN]), ECG, and heart rate (using a bipolar ECG lead); body position (using a mercury gauge sensor); and ambient light (on/off, by a light sensor secured to the recording garment). Sensors were placed and equipment was calibrated during an evening home visit by a certified technician. Following equipment retrieval, the data, stored in real time on PCMCIA cards, were downloaded to the computers of each respective clinical site, locally reviewed, and forwarded to a central reading center (Case Western Reserve University, Cleveland, OH). Comprehensive descriptions of PSG scoring and quality assurance procedures have been previously published.15,16 In brief, sleep was scored according to guidelines developed by Rechtschaffen and Kales.17 Strict protocols were maintained in order to assure comparability between centers and technicians. Intrascorer and interscorer reliability was high.16 As in previous analyses of SHHS data, an apnea was defined as a complete or almost complete cessation of airflow (at least <25% of baseline), as measured by the amplitude of the thermocouple signal, lasting >10 s. Hypopneas were identified if the amplitude of a measure of flow or volume (detected by the thermocouple or thorax or abdominal inductance band signals) decreased to <70% of the amplitude of baseline breathing for >10 s, but did not meet the criteria for apnea. For this study, only apneas or hypopneas associated with ≥4% oxyhemoglobin desaturation were considered in the calculation of the respiratory disturbance index (RDI4%, apneas+hypopneas per hour of total sleep time). A total of 2,113 subjects had PSGs that were of sufficient quality, in whom the time of lights out and recording time did not begin or end in a sleep state, and in whom reliable determinations of total sleep time (PSGTST) and sleep onset latency (PSGSOL) could be made.
A brief morning questionnaire13 completed by participants the day after the PSG was designed to assess perceived quality of sleep on the night of the study and to record alcohol, tobacco, and caffeine use in the 4-h period before the PSG. The specific sleep questions used were: how much time do you think you actually slept last night? (AMTST), and how long did it take you to fall asleep at bedtime last night? (AMSOL).
Gender, ethnicity, education, obesity, and time zone covariates were derived from data obtained from the SHHS parent cohorts. Ethnic group percentages included 75% Caucasian, 14% Native American, 6% African American, 4% Hispanic, and 1% Asian or Pacific Islander. Because of the small numbers comprising each of the ethnic categories other than Caucasians, this variable was dichotomized into Caucasians and other ethnic groups. Education was divided at the 25th and 75th quartiles and categorized into those with <12 years, those with 12–16 years, and those with >16 years of education. To determine whether late evening network newscasts were associated with differences in total sleep time, time zone was assigned according to the subject's parent study location. Persons enrolled in the Sacramento, CA cohort were included in the Pacific time zone, those in the Tucson and Phoenix, AZ, and SD cohorts were included in the Mountain time zone, those in the OK and MN cohorts were included in the Central time zone, and those enrolled in the Framingham, MA, Hagerstown, MD, and Pittsburgh, PA were included in the Eastern time zone. Time zone was dichotomized into Pacific/Eastern and Mountain/Central time zones. Age was dichotomized at the mean, those ≤67 years of age, and those >67 years of age.
The subject's respiratory disturbance index, self-report of chronic lung or heart diseases, and use of alcohol or caffeine were evaluated to determine if these factors affected differences in reported and measured sleep times and sleep latencies. The RDI4% was categorized into the following groups: <5, 5-<15, 15-<30, and ≥ 30 events per hour of total sleep time. Subjects were classified as having chronic lung disease if they answered yes to having a doctor informing them that they had emphysema, chronic bronchitis, chronic obstructive pulmonary disease, or asthma, and if the asthma was still present. Subjects were classified as having chronic heart disease if they answered yes to having a physician ever telling them they had any of the following: angina, heart attack, stroke, or heart failure; or ever having had any of the following procedures: coronary bypass surgery, coronary angioplasty, insertion of a peacemaker, or any other heart cardiac surgery. Alcohol and caffeine consumption were defined as use of the following within 4 h of the PSG sleep period: wine (4 oz.), hard liquor (1 shot), or beer (12 oz.), and use of one cup or more of caffeinated coffee, tea, or cola. Subjects with amounts equal or greater than these limits were then compared to those with less using binary dummy variables. The SHHS was approved by the respective institutional review boards for human studies, and informed written consent was obtained from all subjects at the time of their enrollment into the study.
Statistics
A logarithmic transformation of the sleep onset latency values was used in the present analyses to normalize the distribution; their geometric means are presented. χ2 tests were used to test for differences in proportions. The Student's t-test was used to compare differences in mean values for HABTST, AMTST, and PSGTST, and the log transformed values for HABSOL, AMSOL, and PSGSOL by gender, age category, ethnicity, BMI, time zone, chronic heart or lung disease, and alcohol or caffeine intake. One-way analysis of variance was used to compare differences in mean values by variables with >2 categories, i.e., education and RDI4%. Pearson's correlation coefficient with Bonferroni's correction was used to test for differences in correlation coefficients between the 3 sleep assessment measures; habitual, morning estimated, and PSG. Two separate multivariate mixed-effects linear regression models were fitted to evaluate mean differences in total sleep time and log-transformed values of sleep onset latency associated with type of sleep assessment. The dependent or outcome variables were TST in one model and SOL in the other consisting of all 3 sleep assessments values from each of the subjects. A categorical variable which specified the type of sleep assessment, either habitual, morning-estimated, or PSG was included as an independent variable in the models using PSG as the reference category. Covariates (gender, race, BMI, education, time-zone, RDI4%, chronic lung or heart disease, and alcohol or caffeine consumption) were then included as fixed effects in the models. Centers and subjects were fitted as random effects, to account for correlation within centers and serial intrasubject correlations. Predictor variables with multiple categories (>2) i.e., education and RDI4%, were entered as indicator variables. Covariates that were not significant were excluded from the final models. In these models we include centers and subjects as random effects. Subjects within centers may be similar, and thus their data may be correlated. Additionally, one has to assume that there will be correlation between the habitual, morning estimated, and PSG values on individual subjects and must be adjusted for in the analysis. Separate models were used to evaluate linear prediction of total sleep time and sleep onset latency with age. Statistical tests were performed using Intercooled Stata, version 9.0 for Windows (Stata Corporation; College Station, TX). A significance level of 0.05 was used for all statistical tests.
RESULTS
A total of 2,113 subjects were included in this study (mean age = 67, SD = 10 years). The demographic characteristics are presented in Table 1. The subjects were 53% female, 75% Caucasian, 38% obese, and 60% had between 12–16 years of education. Women had higher PSGTST compared to men. Caucasians had significantly higher mean HABTST and lower AMTST than all other ethnic groups combined (Table 2). Subjects who were >67 years of age had significantly lower mean AMTST and PSGTST than those who were ≤67 years of age. Obese subjects had lower mean PSGTST than nonobese subjects. Subjects with more years of education reported lower mean values for HABTST and AMTST and higher mean PSGTST than those with less years of education. There were no significant mean differences for HABTST and AMTST by RDI4% categories, chronic heart or lung disease, or alcohol consumption. However, subjects in the highest category of RDI4% had lower mean PSGTST (355 min) compared to those in the lowest category (372 min, p <0.0001). In addition, a higher percentage of obese than nonobese subjects, were found in the highest category of RDI4% (56% vs. 44%) than the lowest category (24% vs. 76%) (p < 0.0001). There were no significant differences for any of the 3 sleep measures for subjects with or without chronic lung disease. Subjects with chronic heart disease had significantly lower PSGTST than those without.
Table 1.
SHHS Basic Demographics
| % | N | |
|---|---|---|
| All | 2,113 | |
| Gender | ||
| Male | 47.0 | 993 |
| Female | 53.0 | 1,120 |
| Age Category | ||
| ≤ 67 years | 52.7 | 1,114 |
| > 67 years | 47.3 | 997 |
| Ethnicity | ||
| Caucasian | 75.2 | 1,588 |
| Others | 24.8 | 525 |
| BMI | ||
| Normal (<30%) | 62.6 | 1,298 |
| Obese (>=30%) | 37.8 | 788 |
| Education | ||
| <12 years | 13.8 | 268 |
| 12–16 years | 60.1 | 1,162 |
| >16 years | 25.1 | 502 |
| Time Zone | ||
| Pacific/Eastern | 50.1 | 1,057 |
| Mountain/Central | 49.9 | 1,055 |
| RDI4% | ||
| <5 | 27.7 | 579 |
| 5 - <15 | 39.4 | 824 |
| 15 - <30 | 20.0 | 417 |
| ≥ 30 | 12.9 | 269 |
| Any Chronic Lung Disease | ||
| No | 87.7 | 1,853 |
| Yes | 12.3 | 260 |
| Any Heart Disease | ||
| No | 79.4 | 1,673 |
| Yes | 20.6 | 434 |
| Any Alcohol Intake | ||
| No | 92.9 | 1,941 |
| Yes | 7.1 | 148 |
| Any Caffeine Intake | ||
| No | 82.7 | 1,728 |
| Yes | 17.3 | 361 |
Table 2.
SHHS Mean Total Sleep Time in Minutes£
| Habitual HABTST |
AM Estimated AMTST |
Polysomnogram PSGTST |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Gender | |||||||||
| Male | 424 | 74 | 980 | 379 | 84 | 970 | 355 | 57 | 993 |
| Female | 421 | 74 | 1,103 | 381 | 86 | 1,092 | 371‡ | 62 | 1,120 |
| Ethnicity | |||||||||
| Caucasian | 424 | 70 | 1,562 | 376 | 83 | 1,544 | 365 | 57 | 1,588 |
| Others | 415† | 87 | 521 | 392† | 90 | 518 | 359 | 68 | 525 |
| Age category | |||||||||
| ≤67 years | 424 | 70 | 1,099 | 384 | 80 | 1,095 | 373 | 58 | 1,114 |
| >67 years | 421 | 79 | 983 | 375† | 90 | 966 | 354‡ | 61 | 997 |
| BMI | |||||||||
| Nonobese (<30%) | 423 | 73 | 1,279 | 378 | 85 | 1,261 | 367 | 59 | 1,298 |
| Obese (>=30%) | 421 | 77 | 779 | 382 | 85 | 775 | 358‡ | 62 | 788 |
| Education | |||||||||
| <12 years | 432 | 95 | 266 | 397 | 102 | 260 | 350 | 67 | 268 |
| 12–16 years | 420 | 73 | 1,147 | 375 | 84 | 1,133 | 363 | 60 | 1,162 |
| >16 years | 428* | 61 | 493 | 385* | 78 | 491 | 369* | 55 | 502 |
| Time Zone | |||||||||
| Pacific/Eastern | 416 | 78 | 1,045 | 370 | 87 | 1,021 | 361 | 60 | 1,057 |
| Mountain/Central | 429‡ | 70 | 1,038 | 389‡ | 82 | 1,041 | 366 | 60 | 1,055 |
| RDI4% | |||||||||
| <5 | 416 | 70 | 571 | 381 | 82 | 566 | 372 | 56 | 579 |
| 5-<15 | 424 | 76 | 812 | 380 | 86 | 801 | 366 | 62 | 824 |
| 15-<30 | 420 | 70 | 410 | 377 | 82 | 406 | 354 | 58 | 417 |
| >=30 | 430 | 79 | 266 | 377 | 90 | 265 | 355§ | 60 | 269 |
| Chronic Lung Disease | |||||||||
| No | 424 | 74 | 1,826 | 380 | 84 | 1,805 | 365 | 58 | 1,853 |
| Yes | 414 | 77 | 257 | 376 | 91 | 257 | 358 | 62 | 260 |
| Chronic Heart Disease | |||||||||
| No | 424 | 71 | 1,649 | 382 | 82 | 1,637 | 368 | 58 | 1,673 |
| Yes | 418 | 85 | 429 | 373 | 96 | 420 | 348‡ | 65 | 434 |
| Any Alcohol | |||||||||
| No | 422 | 75 | 1,916 | 380 | 85 | 1,904 | 364 | 61 | 1,941 |
| Yes | 422 | 66 | 145 | 381 | 83 | 147 | 367 | 52 | 148 |
| Any Caffeine | |||||||||
| No | 422 | 73 | 1,704 | 377 | 86 | 1,696 | 365 | 59 | 1,728 |
| Yes | 423 | 82 | 357 | 393† | 80 | 354 | 360 | 64 | 361 |
t-test was used to compare means between the two groups in gender, age category, ethnicity, BMI, time zone, any chronic lung or heart disease, and any alcohol or caffeine intake in each of the three sleep assessments. Comparisons between the three categories in education and RDI4% were made using one-way analysis of variance (ANOVA) in each of the three sleep assessments.
p-value <0.05 for t-test.
p-value <0.0001 for t-test.
p-values <0.001 for one-way analysis of variance (ANOVA).
p-value <0.0001 for ANOVA.
Although significant, small differences were seen for HABSOL, AMSOL, and PSGSOL. Women reported higher HABSOL and AMSOL than men; however, there were no significant gender differences among PSGSOL (Table 3). Caucasians had significantly lower HABSOL, AMSOL, and PSGSOL (14.7, 19.9, and 15.2 min) than all other ethnic groups combined (19.9 min, 23.8 min, and 18.9 min). Although subjects >67 years of age reported higher HABSOL and AMSOL than those ≤67 years, there were no significant differences among PSGSOL for these groups. Obese subjects had significantly higher SOL for all 3 measures than nonobese subjects. Subjects with >16 years of education consistently had lower mean sleep latencies for all 3 measures than those with fewer years of education. Alcohol consumption was significantly associated with reduced sleep onset latency compared with no alcohol consumption for all 3 measures, HABSOL (15.4 vs. 16.0 min), AMSOL (17.5 vs. 21.1min), and PSGSOL (12.4 vs. 16.2 min).
Table 3.
SHHS Mean Sleep Onset Latency in Minutes£
| Habitual HABSOL |
AM Estimated AMSOL |
Polysomnogram PSGSOL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Gender | |||||||||
| Male | 14.5 | 2.4 | 946 | 19.6 | 2.4 | 940 | 15.6 | 2.3 | 783 |
| Female | 17.3‡ | 2.5 | 1,051 | 22.0† | 2.7 | 1,043 | 16.1 | 2.4 | 902 |
| Ethnicity | |||||||||
| Caucasian | 14.7 | 2.4 | 1,482 | 19.9 | 2.6 | 1,468 | 15.2 | 2.2 | 1,332 |
| Others | 19.9‡ | 2.5 | 515 | 23.8† | 2.5 | 515 | 18.9‡ | 2.5 | 353 |
| Age category | |||||||||
| ≤67 years | 15.1 | 2.4 | 1,085 | 18.3 | 2.5 | 1,080 | 15.56 | 2.42 | 877 |
| >67 years | 17.0† | 2.5 | 911 | 24.3‡ | 2.5 | 902 | 16.39 | 2.19 | 805 |
| BMI | |||||||||
| Nonobese (<30%) | 15.2 | 2.5 | 1,221 | 20.4 | 2.6 | 1,208 | 15.2 | 2.3 | 1,072 |
| Obese (>=30%) | 17.3‡ | 2.5 | 752 | 21.6† | 2.6 | 749 | 17.1† | 2.4 | 593 |
| Education | |||||||||
| <12 years | 18.2 | 2.6 | 242 | 25.1 | 2.6 | 240 | 19.7 | 2.2 | 172 |
| 12–16 years | 16.6 | 2.5 | 1,095 | 20.9 | 2.5 | 1,085 | 16.1 | 2.3 | 980 |
| >16 years | 13.0§ | 2.3 | 483 | 18.5* | 2.5 | 482 | 13.5* | 2.3 | 373 |
| Time Zone | |||||||||
| Pacific/Eastern | 16.5 | 2.5 | 964 | 22.3 | 2.6 | 947 | 15.1 | 2.3 | 939 |
| Mountain/Central | 15.4 | 2.4 | 1,033 | 19.6† | 2.5 | 1,036 | 17† | 2.4 | 745 |
| RDI4% | |||||||||
| <5 | 15.4 | 2.4 | 557 | 19.6 | 2.6 | 548 | 16.3 | 2.3 | 496 |
| 5-<15 | 16.3 | 2.4 | 771 | 21.4 | 2.5 | 767 | 15.8 | 2.3 | 664 |
| 15-<30 | 16.4 | 2.5 | 394 | 21.3 | 2.6 | 391 | 15.8 | 2.3 | 320 |
| >=30 | 15.4 | 2.6 | 252 | 21.1£ | 2.5 | 255 | 15.3 | 2.4 | 183 |
| Chronic Lung Disease | |||||||||
| No | 15.7 | 2.4 | 1,747 | 20.8 | 2.6 | 1,733 | 15.8 | 2.3 | 1,478 |
| Yes | 17.9† | 2.7 | 250 | 21.1 | 2.6 | 250 | 16.2 | 2.2 | 207 |
| Chronic Heart Disease | |||||||||
| No | 15.4 | 2.4 | 1,581 | 20.1 | 2.5 | 1,575 | 15.8 | 2.3 | 1,335 |
| Yes | 18.5‡ | 2.5 | 411 | 24.3† | 2.6 | 403 | 16.3 | 2.2 | 345 |
| Any Alcohol | |||||||||
| No | 16.0 | 2.5 | 1,835 | 21.1 | 2.6 | 1,831 | 16.2 | 2.3 | 1,540 |
| Yes | 15.4† | 2.4 | 142 | 17.5† | 2.5 | 145 | 12.4‡ | 2.2 | 125 |
| Any Caffeine | |||||||||
| No | 15.6 | 2.4 | 1,625 | 20.9 | 2.6 | 1,622 | 15.6 | 2.3 | 1,385 |
| Yes | 17.6† | 2.5 | 352 | 20.5 | 2.5 | 350 | 17.4 | 2.5 | 281 |
t-test was used to compare means between the two groups in gender, age category, ethnicity, BMI, time zone, any chronic lung or heart disease, and any alcohol or caffeine intake in each of the three sleep assessments. Comparisons between the 3 categories in education and RDI4% were made using one-way analysis of variance (ANOVA) in each of the 3 sleep assessments.
Means for HABSOL, AMSOL, and PSGSOL are presented here (values were log transformed for these tests, and converted back by taking the antilogarithm).
p-value <0.05 for t-test.
p-value <0.0001 for t-test.
p-value <0.001 for ANOVA.
p-value <0.0001 for ANOVA.
Correlation between HABTST and PSGTST was relatively weak although significant (r = 0.18, p < 0.0001, 95% CI: 0.14–0.22) as well as between AMTST and PSGTST (r = 0.16, p < 0.0001, 95% CI: 0.12–0.20). Correlation between HABTST and AMTST was stronger (r=0.44, p < 0.0001, 95% CI: 0.40–0.47). Correlations were also weak between the log-transformed values of HABSOL and PSGSOL (r = 0.23, p < 0.0001, 95% CI: 0.17–0.28), and between AMSOL and PSGSOL (r = 0.14, p < 0.0001, 95% CI: 0.08–0.20). Correlation between HABSOL and AMSOL was stronger (r = 0.52, p < 0.0001, 95% CI: 0.48–0.55). Thus, a number of subjects claiming to have high habitual or morning estimated values had low PSG values or vice versa.
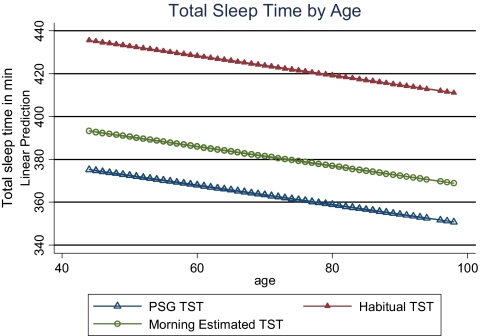
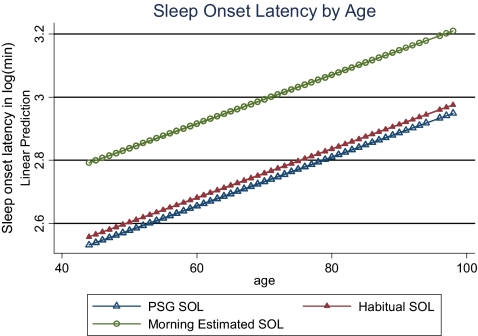
Mixed-effects linear regression models were used to evaluate the overall difference associated with type of attainment for total sleep time and sleep onset latency. Unadjusted models showed that mean PSGTST was significantly lower than mean HABTST (363 min and 422 min, p < 0.0001) and that mean PSGTST was significantly lower than mean AMTST (363 min and 379 min, p < 0.0001). Ranges for HABTST, AMTST, and PSGTST were (90–900 min, 0–720 min, and 110–519 min, respectively) (Table 4 and Figure 1). Adjusted means differed only slightly from unadjusted (Table 4). Unadjusted mean sleep onset latency was significantly different between AMSOL and PSGSOL (21.8 min and 16.9 min, p < 0.0001) but not between HABSOL and PSGSOL (16.7 min and 16.9 min, p = 0.69). Ranges for HABSOL, AMSOL, and PSGSOL were 1–300 min, 1–510 min, and 1–217 min, respectively. Adjusted models showed that on average HABTST and AMTST were higher than PSGTST by 61 and 18 min, respectively (Table 5), after adjusting for other demographic factors. Obese, higher educated people, and those with heart disease had less sleep time than their counterparts. Subjects residing in the Mountain/Central time zone slept 15 minutes more than subjects in the Pacific/Eastern time zone. Similarly, small adjusted differences, although significant, were found for sleep onset latency values (Table 6). Separate mixed models showed a decline in total sleep time of 0.5 min and an increase of 1 min in sleep onset latency for every year increased in age (Figures 2 and 3).
Table 4.
Adjusted and Unadjusted Means and Geometric Means for TST and SOL Values From Mixed-Effects Linear Regression Models.*
| HABTST |
AMTST |
PSGTST |
||||
|---|---|---|---|---|---|---|
| Mean | S.E. | Mean | S.E. | Mean | S.E. | |
| Unadjusted | 422 | 3.9 | 379 | 3.9 | 363 | 3.9 |
| Adjusted | 424 | 3.1 | 381 | 3.2 | 363 | 3.1 |
| HABTST |
AMTST |
PSGTST |
||||
| Mean | S.E. | Mean | S.E. | Mean | S.E. | |
| Unadjusted | 17.0 | 1.04 | 21.8 | 1.04 | 16.9 | 1.04 |
| Adjusted | 15.8 | 1.02 | 20.8 | 1.02 | 16.1 | 1.03 |
Figure 1.
Unadjusted mean total sleep time (TST) and sleep onset latency (SOL) for habitual (HAB), morning estimated (AM), and polysomnogram (PSG) measures from mixed-effects linear regression models.
Table 5.
Mixed-effects Linear Regression Model of Total Sleep Time in Minutes by Type of Assessment (Habitual, AM Estimated, and PSG) and Other Predictive Variables
| Variables | Regression coefficient | p-value | 95% CI* |
|---|---|---|---|
| Habitual | 61.0 | <0.0001 | 57.0 – 65.0 |
| AM Estimated | 17.7 | <0.0001 | 13.7 – 21.8 |
| BMI, obese | −5.2 | 0.041 | −10.2 – −0.22 |
| 12–16 yr of education | −7.5 | 0.049 | −15.0 – −0.02 |
| >16 yr of education | −1.1 | 0.80† | −9.9 – 7.7 |
| Mountain/Central time zone | 14.9 | 0.010 | 3.5 – 26.3 |
| Any Heart Disease | −9.8 | 0.001 | −15.7 – −3.92 |
| Intercept | 363.9 | <0.0001 | 353.3 – 374.5 |
CI = confidence interval. Note: PSG is the reference category for type of assessment, non-obese is the reference category for BMI, <12 years of education is the reference category for education, and Pacific/Eastern is the reference category for time-zone, no heart disease is the reference category for any heart disease.
p-value < 0.01, difference for linear contrast of coefficients between 12–16 and >16 years of education.
Table 6.
Mixed-effects linear regression model of sleep onset latency time in minutes by type of assessment (habitual, AM estimated, and PSG) and by predictive variables
| Variables | Regression coefficient | p-value | 95% CI* |
|---|---|---|---|
| Habitual | 0.98 | 0.552 | 0.93–1.04 |
| AM Estimated | 1.29 | <0.0001 | 1.22–1.37 |
| Female sex | 1.14 | <0.0001 | 1.07–1.22 |
| Ethnicity, other than Caucasian | 1.16 | 0.001 | 1.06–1.27 |
| 12–16 yr of education | 0.93 | 0.179 | 1.18–1.03 |
| >16 yr of education | 0.83 | <0.002 | 0.74–0.94 |
| Any Heart Disease | 1.15 | 0.001 | 1.06–1.24 |
| Intercept | 15.5 | <0.0001 | 13.8–17.4 |
CI = confidence interval. Note: Values for habitual, am estimated, and polysomnogram sleep onset latency time were log transformed for this test, anti-log values are presented here. PSG is the reference category for type of assessment, male is the reference category for sex, Caucasian is the reference category for ethnicity, <12 years of education is the reference category for education, no heart disease is the reference category for any heart disease, and Pacific/Eastern is the reference category for time zone.
Figure 2.
Linear prediction of total sleep time by age.
Figure 3.
Linear prediction of sleep onset latency by age.
To determine whether the extreme values observed in the morning estimated total sleep time had an effect in any of our results, we reevaluated the data. Repeated analyses excluding 6 extreme observations (i.e., those with >3 SDs) yielded no appreciable difference in any result. To determine whether subjects included in the analyses differed from those excluded, comparisons between these 2 groups were made. There were no significant differences in demographic distributions between the 2,113 subjects included in this analysis and the 966 subjects who were excluded when these 2 groups were compared for age, race, BMI, education, time zone, heart or lung disease, or alcohol or caffeine consumption. The only differences seen between the 2 groups were more females than males in the inclusion sample than the exclusion sample (60% and 53%, p < 0.0001) and lower percentage of subjects with RDI4% ≥30 in the inclusion sample than the exclusion sample (9% vs 13%, p = 0.048). In addition, no appreciable differences were seen for any of the results when the whole study sample was used (n = 3,079) in analyses not requiring PSG data as compared to when we included only the selected sample (n = 2,113), thus suggesting a low possibility of bias selection.
DISCUSSION
The current analysis was conducted to assess differences between subjectively and objectively obtained total sleep time and sleep onset latency and to determine the relationship of these measures with other potential sleep determinants. Findings from this large multicenter community study indicate that compared with objectively measured total sleep time, subjects reported higher values for their habitual and morning estimated total sleep times by 61 and 18 minutes respectively. Differences for sleep onset latencies, although significant, were small. Notably, the self-reported and PSG correlations for total sleep time and sleep onset latency were low compared with other studies.1–3 These studies however, had small number of participants and included those in specific populations. The large sample size in the present study is a strength that may help differentiate subjective versus objective correlation of sleep time that may not be found in smaller studies. Although some variability was observed related to gender, ethnicity, education, and time zone of residence, these findings indicate that participants tend to report higher sleep times and sleep latencies compared with their objectively obtained sleep measures. Both males and females reported higher total sleep time estimates compared with their PSG values. Others have reported sex differences between subjectively recorded total sleep time, although results have not been consistent1. Thus, it is unclear whether there are gender differences in subjective reporting of sleep duration.
Ethnic differences in sleep duration have been reported previously.8,18,19 As compared with Caucasians, Blacks had shorter TST in one study,7 while another study showed longer TST for Blacks than for Caucasians.18 In the present study, comparing Caucasians with all other ethnic groups, no significant ethnic differences were found for PSGTST. Interestingly, Caucasians reported longer HABTST but shorter AMTST compared with the combination of other ethnic groups. Although our findings do not clarify whether there are ethnic or racial differences in objectively recorded sleep duration, they suggest the possibility that ethnicity or race may be a factor in self-reported data.
In previous studies, objectively measured TST was lower for those older, obese, and for those with greater amounts of sleep apnea.20–23 Thus, our data provide additional evidence for a decline in nocturnal sleep with age. Obesity is associated with a number of chronic conditions including sleep apnea and insomnia which can potentially reduce TST.2,24,25 A higher RDI4% is synonymous with sleep apnea severity, and consequently it is not surprising that we observed an association with a lower PSGTST. Subjects with more education had higher PSGTST than those with less education, even though they reported lower HABTST and AMTST than those with less education. Therefore, less educated subjects made higher TST estimates. Educational level is a commonly used surrogate for socioeconomic status. Therefore, our data would indicate that self-reported sleep duration is much more likely to be higher in poorer segments of the population.
It has been suggested that subjects with chronic diseases may perceive their sleep differently from healthy subjects including diverging TST and SOL estimates.2,3 In the present study, there were no differences in subjective TST estimations for subjects with and without chronic lung or heart disease, although subjects with chronic heart disease had lower PSGTST than persons without heart disease. However, subjects with chronic lung or heart disease reported higher subjective SOL estimates than those without disease, although no significant differences were found between subjects with and without chronic disease for PSGSOL. Although differences in TST and SOL are relatively small and significance could be attributed to the large sample size, it is worth noting that some clinical trials assessing efficacy of drugs for insomnia consider differences in this range to be clinically significant. In a recently published efficacy trial of ramelteon in patients with chronic primary insomnia (n = 103) the authors considered the results clinically significant and well within the range seen with commonly prescribed sedative hypnotics of an 8–17 min reduction in latency to persistent sleep (LPS) and increase in total sleep time. As compared to placebo, 16 mg of ramelton reduced LPS by a mean of 13.7 min and increased TST by 11.2 min as assessed by PSG.26
Subjects residing in the Mountain/Central time zones tended to estimate longer HABTST and AMTST than those residing in the Pacific/Eastern time zones. In addition, our multivariate analysis indicated that residence in the Mountain/Central time zone was associated with a 15 min longer total sleep time than residence in the Pacific/Eastern time zone. A possible explanation for this finding may be the difference in time zone for broadcasting of late-night news programs. For example, network affiliate newscasts usually occur at 22:00 in the Mountain/Central time zones and 23:00 in the Pacific/Eastern time zones. Empiric observations indicate that many individuals go to sleep after the network newscasts, but nevertheless must awaken at the same time in order to prepare for work. If generally true, this would lead to shorter sleep times for those in the Pacific/Eastern time zones.
The differences between subjective and objective sleep data have been previously analyzed by others.1–3,5,27 In comparison with our data, these studies generally have shown that subjects tend to underestimate total sleep duration and to overestimate SOL compared with objective measurement. However, these studies used small sample sizes or specific populations. Nonetheless, results from this study are consistent with a recent finding that self-reported sleep duration exceeded actigraphic assessment of sleep time by an average of 60 min in a general population sample.28 If verified, they have important implications for future population based studies as well as previously published observations. Many reported associations between sleep duration and obesity, heart disease, mortality, and diabetes have been based on self-report of total sleep time.19,29,30 If subjective reporting of sleep duration varies from PSG measurements, previously observed self-reported associations in population based studies may not be comparable to PSG studies.
We acknowledge that PSGTST and PSGSOL measurements may have been affected by discomfort from PSG sensors and equipment. Consequently, habitual sleep times may not have been maintained, resulting in reduced TST and prolonged SOL; this might explain our observation that HABTST was higher than PSGTST. This “first-night effect” has been addressed previously by other researchers,31,32 who reported sleep variations across several nights of compared PSG assessments. Not all studies, however, show a marked first night effect.33–35 Nevertheless, it is likely that some of the difference we observed between HABTST and PSGTST is a reflection of PSG induced poor sleep. However, our results still suggest that subjects tended to estimate higher values for TST and SOL the morning after the PSG, supporting the presence of a real bias in estimation. Furthermore, using the participant's bed time and wake time as reported on the morning survey, the time in bed was 450 ± 101 min, which is comparable to the habitual sleep time of 420 ± 74 min. This confirms that the total time in bed during the PSG night was not shortened by methodological reasons.
Although we have demonstrated in this population-based sample that self-estimates of sleep times and latencies may be higher than PSG recorded equivalents, the explanation for these disparities is not entirely clear. Our data suggest that self-estimates of sleep parameters may be affected by ethnicity or socioeconomic status. For TST, a greater degree of divergence appeared to be present for those with lower educational level (surrogate for socioeconomic status). Similarly, educational level and other parameters influenced self assessment of SOL. Thus, in accordance with other studies, we would propose that sleep misperception can be enhanced by ethnicity, lower socioeconomic status, or other parameters4,7,36–38.
It is important to emphasize that inherent in the design of SHHS, individual subjects were nested within centers which could potentially result in some interdependence and correlation within our data. To mitigate these potential effects, we fitted a 2-level mixed-effects linear regression model with centers and subjects as random effects to evaluate differences in overall TST and log transformed SOL. These models showed that after adjusting for the nested level correlation structures, residing in the Mountain/Central time zone had significant positive effect on TST, while obesity, more education, and having any heart disease had a negative effect. SOL was affected by being of female sex, non-Caucasian, higher education, and having any heart disease.
In conclusion, results from the present study showed that self-reports of total sleep times, both habitually and on the morning after a PSG tend to be higher than objectively measured sleep times. Estimates of SOL are more accurate, although discrepancies occur as well. The findings from this study suggest that results from studies subjectively assessing sleep times may not be comparable to those using objective determinations.
ACKNOWLEDGEMENTS
Sleep Heart Health Study (SHHS) acknowledges the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), the Framingham Heart Study (FHS), the Cornell/Mt. Sinai Worksite and Hypertension Studies, the Strong Heart Study (SHS), the Tucson Epidemiologic Study of Airways Obstructive Diseases (TES) and the Tucson Health and Environment Study (H&E) for allowing their cohort members to be part of the SHHS and for permitting data acquired by them to be used in the study. SHHS is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. SHHS further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff and their participating institutions is available on the SHHS website, www.jhucct.com/shhs. The authors wish to thank Ms. Hui-Chun Tammy Hsu for her help in analyses verification.
This work was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research). Graciela E. Silva, Ph.D. was supported by NHLBI grant HL 062373-05A2.
The opinions expressed in the paper are those of the author(s) and do not necessarily reflect the views of the IHS.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Quan has received research support from Respironics and is on the speaker's bureau for Takeda. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Carskadon MA, Dement WC, Mitler MM, et al. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 2.McCall WV, Turpin E, Reboussin D, et al. Subjective estimates of sleep differ from polysomnographic measurements in obstructive sleep apnea patients. Sleep. 1995;18:646–50. doi: 10.1093/sleep/18.8.646. [DOI] [PubMed] [Google Scholar]
- 3.Happe S, Klosch G, Lorenzo J, et al. Perception of sleep: subjective versus objective sleep parameters in patients with Parkinson's disease in comparison with healthy elderly controls. Sleep perception in Parkinson's disease and controls. J Neurol. 2005;252:936–43. doi: 10.1007/s00415-005-0785-0. [DOI] [PubMed] [Google Scholar]
- 4.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–9. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 5.Baekeland F, Hoy P. Reported vs recorded sleep characteristics. Arch Gen Psychiatry. 1971;24:548–51. doi: 10.1001/archpsyc.1971.01750120064011. [DOI] [PubMed] [Google Scholar]
- 6.Asplund R. Sleep disorders in the elderly. Drugs Aging. 1999;14:91–103. doi: 10.2165/00002512-199914020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Stepnowsky CJ, Jr, Moore PJ, Dimsdale JE. Effect of ethnicity on sleep: complexities for epidemiologic research. Sleep. 2003;26:329–32. doi: 10.1093/sleep/26.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Klauber MR, Stepnowsky C, et al. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 9.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 10.Webb WB, Agnew HW. Are we chronically sleep-deprived? Bull Psychonomic Soc. 1975;6:47. [Google Scholar]
- 11.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 12.Lind BK, Goodwin JL, Hill JG, et al. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: experience of the Sleep Heart Health Study. Sleep Breath. 2003;7:13–24. doi: 10.1007/s11325-003-0013-z. [DOI] [PubMed] [Google Scholar]
- 13. http://www.jhucct.com/shhs/
- 14.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Int Med. 1998;158:1855–67. doi: 10.1001/archinte.158.17.1855. Anonymous. [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 16.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. Manual of standardized techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service and Brain Research Institute; 1968. [Google Scholar]
- 18.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14:321–6. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- 19.Jean-Louis G, Magai CM, Cohen CI, et al. Ethnic differences in self-reported sleep problems in older adults. Sleep. 2001;24:926–933. doi: 10.1093/sleep/24.8.926. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Int Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 21.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 22.Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Int Med. 2003;253:536–43. doi: 10.1046/j.1365-2796.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 23.Quan SF, Griswold ME, Iber C, et al. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnogaphy--the Sleep Heart Health Study. Sleep. 2002;25:843–9. [PubMed] [Google Scholar]
- 24.Millman RP, Carlisle CC, McGarvey ST, et al. Body fat distribution and sleep apnea severity in women. Chest. 1995;107:362–6. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 25.Tjepkema M. Insomnia. Health Rep. 2005;17:9–25. [PubMed] [Google Scholar]
- 26.Erman M, Seiden D, Zammit G, et al. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Baker FC, Maloney S, Driver HS. A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res. 1999;47:335–341. doi: 10.1016/s0022-3999(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 28.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 29.Jensen E, Dehlin O, Hagberg B, et al. Insomnia in an 80-year-old population: relationship to medical, psychological and social factors. J Sleep Res. 1998;7:183–9. doi: 10.1046/j.1365-2869.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. [see comment] Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 31.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients (≪first night effect⩾) Electroencephalogr Clin Neurophysiol. 1967;22:556–8. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt HS, Kaelbling R. The differential laboratory adaptation of sleep parameters. BiolPsychiatry. 1971;3:33–45. [PubMed] [Google Scholar]
- 33.Chediak AD, Acevedo-Crespo JC, Seiden DJ, et al. Nightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnograms. Sleep. 1996;19:589–92. doi: 10.1093/sleep/19.7.589. [DOI] [PubMed] [Google Scholar]
- 34.Lord S, Sawyer B, O'Connell D, et al. Night-to-night variability of disturbed breathing during sleep in an elderly community sample. Sleep. 1991;14:252–8. [PubMed] [Google Scholar]
- 35.Loredo JS, Clausen JL, Ancoli-Israel S, et al. Night-to-night arousal variability and interscorer reliability of arousal measurements. Sleep. 1999;22:916–20. doi: 10.1093/sleep/22.7.916. [DOI] [PubMed] [Google Scholar]
- 36.Franklin KA, Svanborg E. The accuracy of subjective sleep time in sleep apnoea recordings. Respir Med. 2000;94:569–73. doi: 10.1053/rmed.1999.0777. [DOI] [PubMed] [Google Scholar]
- 37.Gellis LA, Lichstein KL, Scarinci IC, et al. Socioeconomic status and insomnia. J Abnorm Psychol. 2005;114:111–8. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- 38.Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]