Abstract
Study Objective:
To assess usual nightly sleep duration of patients referred for a Multiple Sleep Latency Test (MSLT).
Design:
Retrospective chart review.
Setting:
Military, hospital-based, sleep center.
Patients:
Fifty–four patients with excessive daytime sleepiness referred for an MSLT.
Interventions:
None.
Measurements and Results:
Self-reported average nightly sleep duration (6.13 ± 1.23 hours), sleep log-recorded average nightly sleep duration (6.99 ± 0.85 hours), and actigraphy-measured average nightly sleep duration (5.56 ± 1.50 hours) were compared for the 2-week period immediately preceding an MSLT. One-way analysis of variance revealed a significant difference in the 3 estimates of nightly sleep duration (p < 0.0001), and only actigraphy-measured average nightly sleep duration correlated with mean sleep latency on the MSLT (r = 0.4258, p = 0.0016). Subgroup analysis showed that patients with a mean sleep latency shorter than 8 minutes slept an average of 1.57 hours less per night than did those patients with a mean sleep latency of 8 minutes or longer (4.53 ± 1.37 vs 6.10 ± 1.37 hours per night, p < 0.001) as measured by actigraphy. There was no difference in either self-reported average nightly sleep duration or sleep log-recorded average nightly sleep duration between the 2 subgroups.
Conclusions:
Prolonged actigraphy monitoring may provide useful clinical information about pre-MSLT sleep not always obtainable from patient self-reporting or sleep logs.
Citation:
Bradshaw DA; Yanagi MA; Pak ES et al. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med 2007;3(6):613-619.
Keywords: Actigraphy, sleep log, MSLT, sleep deprivation
The evaluation of patients who complain of excessive daytime sleepiness relies on a meticulously obtained history supplemented by appropriate sleep diagnostic tests. The Multiple Sleep Latency Test (MSLT) is currently recommended in the diagnostic evaluation for narcolepsy, especially when cataplexy is absent, and may be useful in patients clinically suspected to have idiopathic hypersomnolence.1 The MSLT measures the physiologic tendency of an individual to fall asleep in a controlled, sleep-conducive environment. Physiologic sleep tendency is a product of underlying physiologic sleepiness, internal and environmental arousal factors, and one's ability to transition from wakefulness to sleep.2–5 The American Academy of Sleep Medicine (AASM) has provided specific recommendations for conducting the MSLT that are designed to exclude common sleep disorders and minimize environmental and test protocol-related factors known to affect sleep latency.6 For example, the current AASM guideline states that the MSLT “must be performed immediately following polysomnography recorded during the individual's major sleep period.” The purpose of the polysomnogram is to rule out an underlying sleep-fragmenting disorder, such as obstructive sleep apnea, and also to ensure “adequate” sleep duration immediately prior to the MSLT. The AASM guideline suggests that less than 6 hours of sleep could make the diagnosis of narcolepsy problematic, presumably because a single night of restricted sleep duration could reduce the next-day sleep latency into the narcolepsy range.7 Unfortunately, a single night's sleep in the laboratory may not accurately reflect habitual sleep duration or sleep quality at home. Both the AASM guideline and current International Classification of Sleep Disorders Diagnostic and Coding Manual emphasize the importance of ensuring “adequate” sleep in the week prior to the MSLT.6,8
Sleep logs are frequently used to assess sleep-wake patterns prior to the MSLT and may reveal evidence of chronically insufficient sleep. For example, a marked increase in sleep duration on weekends suggests recovery from prior partial sleep deprivation. In order to detect such a weekday-weekend discrepancy, sleep-log entries must be accurately recorded for an extended period of time. Although we routinely request sleep logs in order to assess sleep-wake patterns and sleep quantity in our evaluation of patients with excessive daytime sleepiness, we have been disturbed by the rather common occurrence of watching patients fill out days or weeks of sleep-log entries while sitting in the clinic waiting room. Wrist-based actigraphy offers an alternative or complementary objective measure of sleep-wake patterns that has been validated against polysomnography and is ideally suited for long-term data collection.9–12 Studies comparing sleep logs and actigraphy data have generally found good correlation between the 2 for some sleep indexes, such as sleep duration13–16; however, this presumes careful daily recording of sleep times. Our concern about the diligence of some patients in keeping a reliable daily sleep log prompted the addition of actigraphy to our diagnostic evaluation for excessive daytime sleepiness, and we routinely collect actigraphy data before doing an MSLT. This retrospective review was designed to compare habitual nightly sleep-duration measures for the 2-week period immediately preceding an MSLT. We also sought to compare habitual home sleep duration with sleep on the night of the polysomnogram and to determine whether any of these pre-MSLT sleep measures affected mean sleep latency on the MSLT.
METHODS
Between January 2003 and October 2005, we conducted 147 MSLTs in our sleep laboratory. For the purposes of this review, patients were included only if they had an adequate overnight polysomnogram on the night before the MSLT and complete sleep-log and actigraphy data for 2 weeks immediately preceding the polysomnogram/MSLT. Repeat studies and patients diagnosed with obstructive sleep apnea were excluded. Information extracted from 54 sleep records meeting these criteria included basic demographics, average nightly sleep duration by self-report (defined as the single response to the question “How many hours of sleep do you get on average per night?”), intake Epworth Sleepiness Scale (ESS)17 score, sleep-log entries, actigraphy data, and sleep variables obtained from the polysomnogram and MSLT. The study protocol was reviewed and approved by the Institutional Review Board at Naval Medical Center San Diego.
Demographics
The following demographic and questionnaire information was extracted from the record: age, sex, body mass index, caffeine intake, nap frequency, self-reported usual nightly sleep duration, and ESS score, all recorded on the night of the overnight polysomnogram.
Sleep Logs
Sleep logs were kept concurrently with actigraphy monitoring (see below) for 2 weeks preceding the MSLT. Patients were instructed to fill out the log each morning; however, scheduled reminders or prompts were not provided, and the completed log was turned in at the time of the polysomnogram/MSLT. The sleep log used in our laboratory requires the patient to enter arrows along a horizontal line representing the 24-hour day; a “down arrow” indicates the time he or she goes to bed and an “up arrow” indicates the time he or she gets up. Actual sleep time is estimated with a straight line drawn across a grid divided into 1-hour intervals. Breaks in the line indicate awake time in bed. Data extracted from the sleep log included bedtime, rise time, and sleep duration for each night, which were then converted to means for the 2-week period.
Actigraphy
Patients were outfitted with the Octagonal Sleep Scoring Watch (OSSW), an ambulatory wrist actigraph (Precision Control Design, Ft. Walton Beach, FL). Each actigraph was initialized to record activity in 1-minute epochs and worn for 14 consecutive days prior to the polysomnogram/MSLT. Patients were instructed to wear the actigraph on their nondominant wrist, push a button located on the side of the watch (“event marker”) when they got into bed to sleep and again immediately after arising in the morning, and to remove the device only during periods when it would otherwise be submerged in water (e.g., bathing or swimming).
For the purposes of this study, only the actigraphy data from the overnight sleep period were analyzed, and sleep was scored utilizing the ActionW (version 2.4.20) software program (Ambulatory Monitoring, Inc., Ardsley, NY). The overnight sleep period (defined as the time from attempted sleep-onset until final awakening) was visually identified by tick marks inserted into the tracing by the patient-actuated event marker button. The tick marks were correlated with the sleep log or, when absent, the overnight sleep period was bounded by the first and last epochs scored as sleep by the software program. Sleep epochs were determined based on the Cole-Kripke sleep-scoring algorithm using the zero-crossing mode channel, and sleep efficiency was calculated by dividing time scored as sleep by duration of the overnight sleep period. As mentioned, patients with incomplete actigraphy records (primarily due to repeated removal or malfunction of the watch) were excluded from the study.
Polysomnogram/MSLT
Following 2 weeks of ambulatory actigraphy and sleep-log entries recorded during usual sleep at home, all patients underwent an overnight polysomnogram in the sleep laboratory on the night before the MSLT. Sleep recording and staging were performed utilizing a digital polysomnograph (E Series Polysomnograph, Compumedics Limited, Abbotsford, VIC, Australia) according to standard recommendations.18 Patients also wore the actigraphy watch during the polysomnogram recording and throughout the MSLT. Airflow and respiratory effort were recorded using a nasal/oral thermocouple and piezo-crystal belts placed around the chest and abdomen. Other sensors included a snore microphone, digital pulse oximetry probe, electrocardiogram leads, and leg electromyogram electrodes. Patient hook-ups were generally completed by 2200 with a goal of “lights out” no later than 2230. The following morning, patients were awakened by 0630 and allowed to eat breakfast prior to initiating a 5-nap MSLT protocol. MSLTs were conducted according to commonly accepted standards.19,20 The first nap began approximately 2 hours after awakening, and successive nap trials occurred at 2-hour intervals over the course of the day. Prior to initiating the first nap, patients were asked to dress in comfortable street clothing. Rooms were noise attenuated, dark, and comfortable. Patients were instructed to assume a comfortable sleep position and “try to fall asleep.” Nap sessions were terminated at 20 minutes if no sleep was detected or 15 minutes after the first epoch of any sleep stage. Sleep-onset was defined as the first epoch of any stage of sleep, and mean sleep latency was calculated as the summed time to sleep-onset of each nap divided by 5. A urine sample was obtained from all patients and submitted for illicit drug analysis.
Data Analysis
All data are presented as mean ± SD or number (percentage). Mean nightly sleep duration by 3 measures (self-report, sleep log, and actigraphy) was compared using 1-way analysis of variance. Comparisons between subgroup means (mean sleep latency > 8 minutes vs mean sleep latency ≤ 8 minutes) were performed using either a 2-tailed Student t test or rank sum test depending on distribution of the underlying data. A Fisher Exact test was applied to dichotomous variables. Scatter plots were generated for variables of interest, and correlation coefficients were calculated. A 2-tailed p value < 0.05 was considered statistically significant. Statistical analysis was performed using a commercially available software package (Statistica, Statsoft, Tulsa, Okla).
RESULTS
Overall Group Demographics
The mean age of our 54 patients (47 men, 7 women) was 30.7 ± 10.4 years (range 19–56) (Table 1). The mean ESS score was 16.2 ± 4.7 (range 4–23). Mean caffeine consumption was 2.5 ± 2.4 (range 0–10) caffeinated beverages (cups of coffee or tea or cans of caffeinated soft drinks) per day. Patients also reported taking 4.4 ± 3.3 (range 0–14) naps per week.
Table 1.
Demographics and Sleep Data for the Entire Group and by Subgroups
| Parameter | Subjects |
p Valuea | ||
|---|---|---|---|---|
| All | MSL ≥ 8 | MSL < 8 | ||
| No. | 54 | 35 | 19 | |
| Age, y | 30.74 ± 10.39 | 30.09 ± 10.78 | 31.95 ± 9.79 | 0.3454 |
| Men, % | 87.04 | 82.86 | 94.73 | 0.4001 |
| BMI, kg/m2 | 27.95 ± 4.45 | 27.30 ± 4.87 | 29.14 ± 3.32 | 0.1477 |
| ESS score | 16.15 ± 4.70 | 15.37 ± 4.47 | 17.58 ± 4.89 | 0.0997 |
| Caffeineb | 2.45 ± 2.41 | 2.71 ± 2.67 | 1.97 ± 1.8 | 0.4303 |
| Self-reported naps, no./wk | 4.40 ± 3.26 | 3.99 ± 2.54 | 5.13 ± 4.25 | 0.5200 |
| AHI, no./h | 2.22 ± 3.44 | 2.18 ± 3.45 | 2.31 ± 3.53 | 0.5622 |
| MSL, min | 9.75 ± 4.49 | 12.30 ± 3.53 | 5.05 ± 1.51 | <0.0001* |
| Sleep-onset REM periods, no. | 0.65 ± 1.07 | 0.49 ± 1.07 | 0.95 ± 1.13 | 0.1012 |
| Average nightly sleep duration, h | ||||
| Self-reported | 6.13 ± 1.23 | 6.11 ± 1.36 | 6.18 ± 0.97 | 0.9413 |
| Sleep log-recorded | 6.99 ± 0.85 | 6.94 ± 0.93 | 7.08 ± 0.70 | 0.5914 |
| Actigraphy-measured | 5.56 ± 1.50 | 6.10 ±1.37 | 4.53 ± 1.37 | 0.0001* |
| Differencec | 1.43 ± 1.31 | 0.84 ± 0.83 | 2.55 ± 1.41 | 0.0004* |
| Sleep duration on PSG lab night, h | ||||
| By actigraphy | 7.09 ± 1.40 | 7.17 ± 1.65 | 6.93 ± 1.83 | 0.8779 |
| By PSG | 7.35 ± 0.65 | 7.29 ± 1.36 | 7.47 ± 0.62 | 0.1920 |
| Sleep efficiency, % | ||||
| Measured by actigraphy | ||||
| At home | 83.19 ± 14.13 | 87.36 ± 11.11 | 75.31 ± 16.08 | 0.0071* |
| In lab | 89.62 ± 13.96 | 91.74 ± 9.66 | 85.49 ± 19.56 | 0.5873 |
| Measured by PSG | 91.48 ± 6.29 | 91.69 ± 6.26 | 91.10 ± 6.48 | 0.6969 |
p Value is based on comparison of subgroups with mean sleep latency (MSL) ≥ 8 vs MSL < 8 minutes. Data are presented as mean ± SD unless otherwise indicated. BMI refers to body mass index; AHI, apnea-hypopnea index; REM, rapid eye movement.
Cups of coffee or tea or cans of soft drink per day.
Difference between sleep log and actigraphy-measured average nightly sleep duration.
Statistically significant
Nightly Sleep Duration for the 2-Week Period Preceding the MSLT
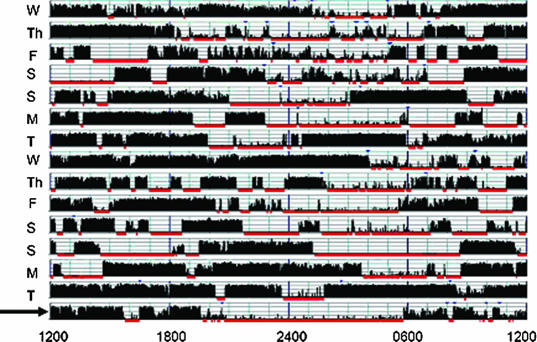
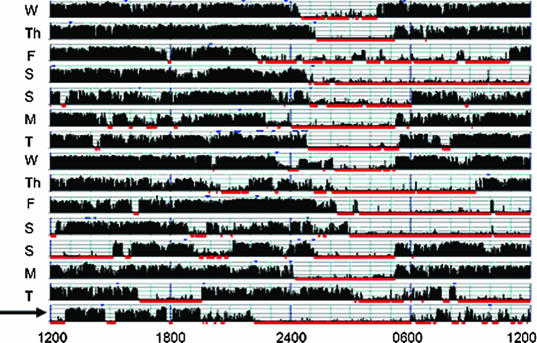
Nightly sleep duration by self-report (defined as the single response to the question “How many hours of sleep do you get on average per night?”), sleep-log entries, and wrist actigraphy for the 2-week period preceding the polysomnogram/MSLT are shown in Table 2. One-way analysis of variance showed a significant difference between the 3 measures (p < 0.0001). Average nightly sleep duration was longest on sleep logs (6.99 ± 0.85 hours), followed by self-reports (6.13 ± 1.23 hours), and then actigraphy (5.56 ± 1.50 hours). Although only 14% of patients recorded an average nightly sleep duration less than 6 hours per night on sleep logs, 33% of patients reported sleeping less than 6 hours per night on average, and 58% of patients had less than 6 hours per night of sleep measured by actigraphy. Significant day-to-day variability with weekend recovery sleep was obvious on many actigraphy tracings (Figure 1).
Table 2.
Average Nightly Sleep Duration by Different Measures for 2 Weeks Preceding a Multiple Sleep Latency Test
| Group | Sleep duration |
p Value | ||
|---|---|---|---|---|
| Self-reporteda | Sleep logb | Actigraphyc | ||
| > 8 | 4 (7) | 5 (10) | 1 (2) | |
| 7–7.9 | 14 (26) | 20 (40) | 8 (15) | |
| 6–6.9 | 18 (33) | 18 (36) | 13 (25) | |
| 5–5.9 | 14 (26) | 7 (14) | 13 (25) | |
| < 5 | 4 (7) | 0 (0) | 17 (33) | |
| Mean, h | 6.13 ± 1.23 | 6.99 ± 0.85 | 5.56 ± 1.50 | < 0.0001 |
Data are presented as number (%) of patients in each group, based on number of hours of sleep, except mean, which is the mean ± SD number of hours of sleep for the total population.
Self-reported average nightly sleep is the patient's response to the question “How many hours of sleep do you get on average per night?”
Data extracted from patient sleep logs.
Actigraphy-measured sleep per Cole-Kripke scoring algorithm.
Figure 1.
Actigraphy Samples. Actigraphy samples were collected for 2 weeks prior to a Multiple Sleep Latency Test (MSLT). In both cases, the night preceding the MSLT (arrow) was spent in the laboratory with concurrent actigraphy and polysomnogram (PSG) monitoring. Sample (a) shows a very irregular sleep-wake pattern and less than 3 hours sleep on the night prior to the PSG. Note the difference in sleep timing and duration on the PSG night compared with the prior 2-week period. The patient recorded sleeping an average of 7.7 hours per night in his sleep log. Sample (b) demonstrates restricted weeknight sleep with obvious recovery on the weekends. The patient's usual bedtime was well after midnight; however, he had no difficulty falling asleep promptly in the lab at 2200, ruling out a primary circadian rhythm disturbance. The mean sleep latency of patients with such pronounced weeknight versus weekend sleep duration might vary significantly depending on the day of week the MSLT is conducted.
a.
b.
Sleep Logs vs Actigraphy
Sleep log-recorded average nightly sleep duration (6.99 ± 0.85 hours) exceeded actigraphy-measured average nightly sleep duration (5.56 ± 1.50 hours) by 1.43 ± 1.31 hours per night for the 2-week period preceding the MSLT (p = 0.0001). The vast majority of patients (50/54) recorded longer sleep duration on their logs than was measured by actigraphy (range from −1.19 to +4.43 hours).
Nightly Sleep Duration for the 2-Week Period Preceding the MSLT vs Sleep Duration in the Laboratory
We next compared actigraphy-measured average nightly sleep duration for the 2-week period preceding the polysomnogram/MSLT (5.56 ± 1.50 hours) to actigraphy- (7.09 ± 1.40 hours) and polysomnography- (7.35 ± 0.65 hours) measured sleep duration in the lab on the night before the MSLT. Sleep duration in the laboratory by either measure (i.e., actigraphy or polysomnography) significantly exceeded actigraphy-measured average nightly sleep duration at home (both p < 0.0001). Actigraphy-measured sleep efficiency in the laboratory (89.62% ±13.96%) also exceeded actigraphy-measured sleep efficiency at home (83.19% ± 14.13%, p = 0.0229).
Actigraphy- vs Polysomnography-Measured Sleep
Significant correlations in total sleep time (r = 0.4599, p = 0.0008) and sleep efficiency (r = 0.5469, p < 0.0001) were found between concurrently recorded actigraphy and polysomnogram in the lab on the night before the MSLT. Exclusion of several outliers identified by visual analysis of the scatter plots (all well outside 2 standard deviations of the group mean) significantly increased correlation for both total sleep time (r = 0.6910, p < 0.0001) and sleep efficiency (r = 0.7108, p < 0.0001).
Nightly Sleep Duration for the 2-Week Period Preceding the MSLT vs Mean Sleep Latency
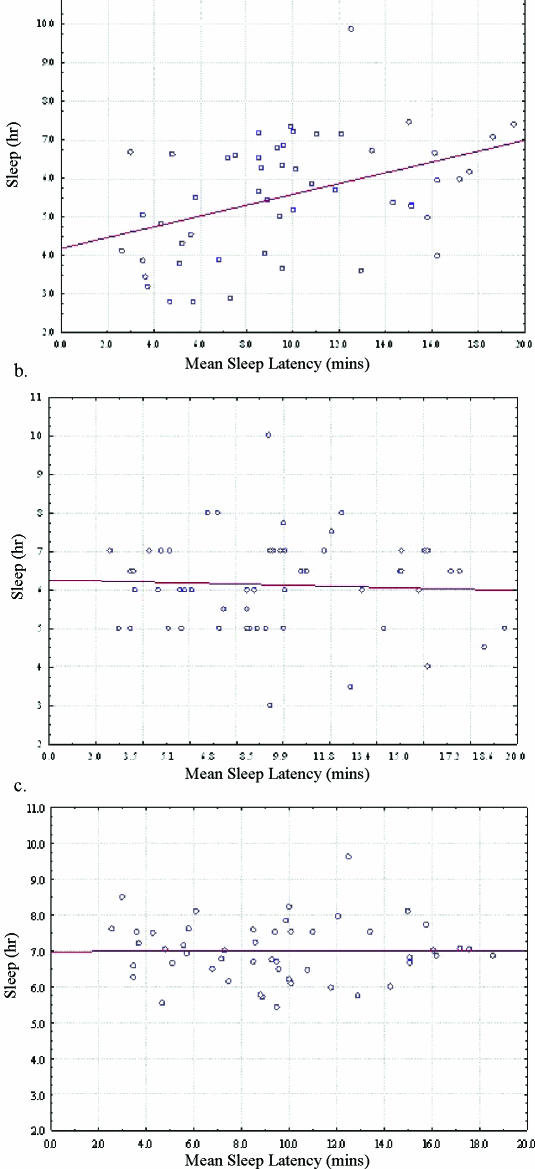
Linear regression analysis showed significant correlation between actigraphy-measured average nightly sleep duration and mean sleep latency on the MSLT (r = 0.4258, p = 0.0016) but not between self-reported average nightly sleep duration and mean sleep latency (r = −0.0515, p = 0.7117) or sleep log-recorded average nightly sleep duration and mean sleep latency (r = 0.0017, p = 0.9908) (Figure 2). We also found a significant inverse correlation when we plotted the difference between actigraphy and log-reported sleep time versus mean sleep latency (r = −0.4969, p = 0.0003). Actigraphy-measured sleep efficiency in the 2-week period preceding the polysomnogram/MSLT also correlated, albeit weakly, with mean sleep latency (r = 0.2760, p = 0.0476).
Figure 2.
Correlation between mean sleep latency (MSL) and average nightly sleep measured by (a) actigraphy, (b) self-report, and (c) sleep log for the 2 weeks prior to the Multiple Sleep Latency Test (MSLT). Average nightly sleep measured by actigraphy correlated with MSL (r = 0.4258, p = 0.0016) whereas self-report (r = −0.0515, p = 0.7117) and sleep log (r = 0.0017, p = 0.9908) did not.
Subgroup Analysis
We divided patients into 2 subgroups: those with a mean sleep latency of shorter than 8 minutes on the MSLT (n = 19) and those with a mean sleep latency of 8 minutes or longer (n = 35) (Table 1). We selected 8 minutes because this is the cutpoint currently recommended when the MSLT is used in the diagnosis of narcolepsy or idiopathic hypersomnia.8 We did not find significant differences in age, sex, body mass index, ESS score, caffeine intake, self-reported nap frequency, self-reported average nightly sleep duration, or sleep log-recorded average nightly sleep duration between the 2 subgroups. Actigraphy-measured average nightly sleep duration was shorter and actigraphy-measured sleep efficiency was lower in the subgroup with a mean sleep latency shorter than 8 minutes than in the subgroup with a mean sleep latency of 8 minutes or longer (sleep duration: 4.53 ± 1.37 vs 6.10 ± 1.37 hours, p = 0.0001; sleep efficiency: 75.31% ± 16.08 vs 87.36% ± 11.11%, p = 0.0071). In addition, patients with a mean sleep latency shorter than 8 minutes showed a much greater discrepancy between sleep log- and actigraphy-measured average nightly sleep duration (2.55 ± 1.41 hours) than those patients with a mean sleep latency of 8 minutes or longer (0.84 ± 0.83 hours, p = 0.0004). Finally, actigraphy-measured sleep duration on the polysomnogram night exceeded average actigraphy-measured nightly sleep duration in the 2 preceding weeks in both subgroups; however, the difference was much greater in those patients with a mean sleep latency shorter then 8 minutes (2.40 ± 1.45) than in those patients with a mean sleep latency of 8 minutes or longer (1.07 ± 1.16 hours p = 0.0014).
DISCUSSION
The MSLT is a “validated objective measure of the ability or tendency to fall asleep.”6 Although the clinical utility of the MSLT is debated,21–23 it is currently recommended as part of the diagnostic evaluation for narcolepsy, especially when cataplexy is absent, and may be useful in the evaluation of patients suspected to have idiopathic hypersomnolence.2 The current AASM guideline standardizes the MSLT protocol and minimizes environmental and test protocol-related factors that are known to affect sleep latency; however, it does not adequately address prior sleep quantity, an obvious determinant of physiologic sleepiness. The requirement to document a minimum of 6 hours of total sleep time on the polysomnogram immediately preceding the MSLT is inadequate because 6 hours may be significantly less than the constitutional sleep requirement of many individuals, thereby introducing an element of acute sleep deprivation, and would not provide adequate recovery sleep time for those patients with accumulated sleep debt.24 Unrecognized acute or chronic partial sleep deprivation increases the likelihood of misdiagnosing sleep-deprived patients with a central hypersomnia, such as narcolepsy or idiopathic hypersomnolence.25
The mean sleep latency of our 54 patients was 9.75 ± 4.49 minutes, which is below the mean sleep latency (11.6 ± 5.2 minutes) of published pooled control normative adult data using a 5-nap protocol.6 Not unexpectedly, there was a clear relationship between prior average nightly sleep duration and mean sleep latency on the MSLT; however, this was true only for actigraphy-measured sleep. Self-reported average nightly sleep duration and sleep log-recorded average nightly sleep duration showed no significant correlation with mean sleep latency. Although individual sleep requirements vary, taking an average nightly sleep requirement of 6 hours as a lower limit of “normal,” we suspect that the majority of patients referred to our laboratory for an MSLT were chronically sleep deprived. In fact, 58% of our patients averaged less than 6 hours of actigraphy-measured sleep per night. In the subgroup of patients with a mean sleep latency of shorter than 8 minutes, 15 of the 19 (79%) averaged less than 6 hours of actigraphy-measured sleep per night (average 4.53 ± 1.37 hours). However, using self-reported average nightly sleep duration, only 6 of 19 (32%) patients would have been identified as sleeping less than 6 hours on average per night, and sleep logs would have identified only 1 of 19 (5%) patients. Strict reliance on self-reported average nightly sleep duration, sleep logs, and polysomnogram/MSLT data would have led to an incorrect assessment of prior nightly sleep duration and potential misdiagnosis of a substantial number of patients.
Although sleep logs are commonly used to document sleep-wake patterns and sleep duration, bed and rise times, as well as time spent awake in bed, must be meticulously recorded over an extended period of time, to include weekends, so that recovery sleep—a hallmark of chronic partial sleep deprivation—can be identified. In our experience, sleep logs provided by clinic patients are often of poor quality and can be, frankly, misleading. Scheduled reminders or prompts would likely improve the quality of sleep log data; however, this is impractical in a clinical setting.
Wrist actigraphy provides reliable and valid detection of sleep in normal and healthy adults and has the added advantage of offering data collection over days, weeks, or even months.26,27 As discussed above, actigraphy-measured average nightly sleep duration correlated with mean sleep latency, whereas self-reported average nightly sleep duration and sleep log-recorded average nightly sleep duration did not. In our study, 50 of 54 patients (93%) recorded longer average nightly sleep duration on their logs than was captured with wrist actigraphy. The average difference between sleep log-recorded and actigraphy-measured nightly sleep duration was 1.43 ± 1.31 hours, although, remarkably, the difference was 2 hours or more in 15 patients, 3 hours or more in 6 patients, and 4 hours or more in 4 patients. The reason for the marked discrepancy between sleep log-recorded and actigraphy-measured sleep duration in our patients is unclear, and, to our knowledge, a comparison between actigraphy and self-reports of usual sleep duration or sleep logs in a cohort of subjectively sleepy patients referred for multiple sleep latency testing has not been previously reported.28 Because some of our patients were referred for evaluation due to job-related performance or disciplinary problems (e.g., oversleeping, sleeping on watch, etc.), we cannot rule out intentional misrepresentation of usual sleep duration in some cases. Whatever the reason, patients with a mean sleep latency of shorter than 8 minutes showed a much greater discrepancy between sleep logs and actigraphy-measured sleep than did patients with a mean sleep latency of 8 minutes or longer. Clearly, relying on self-reported or log-recorded sleep duration may sometimes be misleading.
Surprisingly, patients slept longer and more efficiently in the laboratory on the night before the MSLT than they did on average in the 2 prior weeks. This is contrary to the notion of impaired laboratory sleep due to the unfamiliar environment and monitoring equipment (i.e., the so called “first-night” effect). We speculate that the lack of a first-night effect was at least partially due to increased homeostatic sleep drive from prior partial sleep deprivation; however, it is also possible that some patients simply found the laboratory more comfortable and sleep conducive than their usual home sleep environment. Perhaps home sleep suffered from bedpartner movement or snoring, pets, tobacco and/or alcohol use, television or other environmental disturbances. It is also possible that the increased actigraphy-measured sleep efficiency on the polysomnogram night was the result of restricted movement in the monitored laboratory environment and did not reflect actual differences in sleep efficiency. Additionally, we use “memory foam” mattresses in our laboratory, which are advertised to reduce movement; to our knowledge the performance of wrist actigraphy on different mattresses has not been reported.
This study has several important limitations. First, this is a retrospective review with inherent bias related to patient selection. Second, our military population may be unique in that some of our patients were not self-referred but, rather, “command-directed” because of observed daytime performance deficiencies. Third, a recent epidemiologic study by Lauderdale et al29 of middle-aged adults also found that subjects tended to significantly overreport sleep duration when compared with actigraphy-measured sleep. Of note, in the Lauderdale study were significant sex and race effects on sleep duration, with African American men sleeping only 5.1 hours per night (compared with 5.9 hours for African American women, 6.1 hours for Caucasian men, and 6.7 hours for Caucasian women). Although our military patient population is racially diverse, we did not collect race information for this study. Future studies of sleep duration should certainly include sex, race, and ethnicity data. Fourth, due to the retrospective nature of this study, we lack systematic outcome data, including clinical course and final diagnosis. Fifth, although we did have some self-reported nap data, we were unable to confidently identify and measure nap durations from the actigraph. Hence, our sleep data reflect only the major sleep period recorded each night. Finally, we did not control for the day of week that the MSLT was performed. It seems likely that the day of the week may be an important determinant of mean sleep latency. For example, mean sleep latency on a Monday, following a weekend of recovery sleep, might be quite different from that recorded on a Thursday or Friday.
CONCLUSION
This retrospective study shows that many of our patients referred for an MSLT had actigraphic evidence of chronically insufficient sleep duration that was unrecognized or underappreciated on history, sleep questionnaires, and patient-recorded sleep logs. Our data also show that sleep duration on the polysomnogram night before the MSLT often exceeded average nightly home sleep duration and should not be used as evidence of adequate sleep in the preceding days or weeks. Finally, patients with a mean sleep latency shorter than 8 minutes on the MSLT were much more likely to under record (compared with actigraphy) nightly sleep duration on daily sleep logs. Sleep logs, therefore, may be less reliable in patients with a reduced sleep latency on the MSLT. The impact of pre-MSLT actigraphy monitoring on important clinical outcomes awaits further investigation.
Footnotes
Disclosure Statement
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Wise MS. Objective measures of sleepiness and wakefulness: application to the real world? J Clin Neurophysiol. 2006;23:39–49. doi: 10.1097/01.wnp.0000190416.62482.42. [DOI] [PubMed] [Google Scholar]
- 2.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–144. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet MH, Arand DL. Impact of motivation on multiple sleep latency test and maintenance of wakefulness test measurements. J Clin Sleep Med. 2005;1:386–390. [PubMed] [Google Scholar]
- 4.Bonnet MH, Arand DL. Sleepiness as measured by modified multiple sleep latency testing varies as a function of preceding activity. Sleep. 1998;21:477–483. [PubMed] [Google Scholar]
- 5.Bonnet MH, Arand DL. The impact of music upon sleep tendency as measured by the multiple sleep latency test and maintenance of wakefulness test. Physiol Behav. 2000;71:485–492. doi: 10.1016/s0031-9384(00)00353-x. [DOI] [PubMed] [Google Scholar]
- 6.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal L, Roehrs TA, Rosen A, Roth T. Level of sleepiness and total sleep time following various time in bed conditions. Sleep. 1993;16:226–232. doi: 10.1093/sleep/16.3.226. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and Coding Manual. 2nd ed. Westchester, Ill: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 9.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 10.Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001;72:21–28. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- 11.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 12.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizuma H, Sakamoto T. Wrist activity rhythm and sleep diary of delayed sleep phase syndrome. Psychiatry Clin Neurosci. 1998;52:256–258. doi: 10.1111/j.1440-1819.1998.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Usui A, Ishizuka Y, Obinata I, Okado T, Fukuzawa H, Kanba S. Validity of sleep log compared with actigraphic sleep-wake state II. Psychiatry Clin Neurosci. 1999;53:183–184. doi: 10.1046/j.1440-1819.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 16.Carney CE, Lajos LE, Waters WF. Wrist actigraph versus self-report in normal sleepers: sleep schedule adherence and self-report validity. Behav Sleep Med. 2004;2:134–143. doi: 10.1207/s15402010bsm0203_2. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 19.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 20.Thorpy MJ. The clinical use of the Multiple Sleep Latency Test. The Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1992;15:268–276. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet MH. ACNS clinical controversy: MSLT and MWT have limited clinical utility. J Clin Neurophysiol. 2006;23:50–58. doi: 10.1097/01.wnp.0000190415.83841.17. [DOI] [PubMed] [Google Scholar]
- 22.Arand DL. The MSLT/MWT should be used for the assessment of workplace safety. J Clin Sleep Med. 2006;2:124–127. [PubMed] [Google Scholar]
- 23.Bonnet MH. The MSLT and MWT should not be used for the assessment of workplace safety. J Clin Sleep Med. 2006;2:128–131. [PubMed] [Google Scholar]
- 24.Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–1259. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjua T, Samp T, Cramer-Bornemann M, Hannon H, Mahowald MW. Clinical caveat: prior sleep deprivation can affect the MSLT for days. Sleep Med. 2003;4:69–72. doi: 10.1016/s1389-9457(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 28.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorder: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 29.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]