Abstract
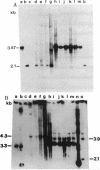
The 47-kb, broad-host-range, streptococcal conjugative transposon Tn5252 is capable of site-specific integration into the pneumococcal chromosome. We present the nucleotide sequence of the terminal regions of the transposon and its target site in the pneumococcal genome. No inverted repeats were found at the termini of the transposon. A 72-bp region of the target was present on either side following the insertion of Tn5252 and appeared to serve as a signal for its integration and excision. The data suggest that the left copy of the 72-bp segment was a part of the conjugative element, the crossover point of integration was nonrandom within this region, and the mechanism of insertion could resemble that of the site-specific temperate phages.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoubi P., Kilic A. O., Vijayakumar M. N. Tn5253, the pneumococcal omega (cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J Bacteriol. 1991 Mar;173(5):1617–1622. doi: 10.1128/jb.173.5.1617-1622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccard F., Smokvina T., Pernodet J. L., Friedmann A., Guérineau M. Structural analysis of loci involved in pSAM2 site-specific integration in Streptomyces. Plasmid. 1989 Jan;21(1):59–70. doi: 10.1016/0147-619x(89)90087-5. [DOI] [PubMed] [Google Scholar]
- Bringel F., Van Alstine G. L., Scott J. R. Transfer of Tn916 between Lactococcus lactis subsp. lactis strains is nontranspositional: evidence for a chromosomal fertility function in strain MG1363. J Bacteriol. 1992 Sep;174(18):5840–5847. doi: 10.1128/jb.174.18.5840-5847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989 Dec 22;59(6):1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet. 1987 Feb;206(2):259–264. doi: 10.1007/BF00333582. [DOI] [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Dang-Van A., Tiraby G., Acar J. F., Shaw W. V., Bouanchaud D. H. Chloramphenicol resistance in Streptococcus pneumoniae: enzymatic acetylation and possible plasmid linkage. Antimicrob Agents Chemother. 1978 Apr;13(4):577–583. doi: 10.1128/aac.13.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. A transposon in Streptococcus faecalis with fertility properties. Nature. 1982 Nov 18;300(5889):281–284. doi: 10.1038/300281a0. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., Bieth G. Conjugative transfer of multiple-antibiotic resistance markers in beta-hemolytic group A, B, F, and G streptococci in the absence of extrachromosomal deoxyribonucleic acid. Plasmid. 1981 Mar;5(2):127–137. doi: 10.1016/0147-619x(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Burdett V. Structural organization of a 67-kilobase streptococcal conjugative element mediating multiple antibiotic resistance. J Bacteriol. 1985 Feb;161(2):620–626. doi: 10.1128/jb.161.2.620-626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Horaud T., Bieth G., Colimon R., Dauguet C. Translocation of antibiotic resistance markers of a plasmid-free Streptococcus pyogenes (group A) strain into different streptococcal hemolysin plasmids. Mol Gen Genet. 1984;194(3):377–387. doi: 10.1007/BF00425548. [DOI] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Molecular analysis of a composite chromosomal conjugative element (Tn3701) of Streptococcus pyogenes. J Bacteriol. 1988 Sep;170(9):3930–3936. doi: 10.1128/jb.170.9.3930-3936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J Bacteriol. 1990 Feb;172(2):727–734. doi: 10.1128/jb.172.2.727-734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Cohen S. N. Structural analysis of plasmid and chromosomal loci involved in site-specific excision and integration of the SLP1 element of Streptomyces coelicolor. J Bacteriol. 1986 Jun;166(3):999–1006. doi: 10.1128/jb.166.3.999-1006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. The integration-excision system of the conjugative transposon Tn 1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990 Sep;4(9):1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Kirchman P. A., Caparon M. G. An intermediate in transposition of the conjugative transposon Tn916. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4809–4813. doi: 10.1073/pnas.85.13.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. R., Korman R. Z., Zahler S. A., Dunny G. M. The conjugative transposon Tn925: enhancement of conjugal transfer by tetracycline in Enterococcus faecalis and mobilization of chromosomal genes in Bacillus subtilis and E. faecalis. Mol Gen Genet. 1991 Mar;225(3):395–400. doi: 10.1007/BF00261679. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M. N., Priebe S. D., Guild W. R. Structure of a conjugative element in Streptococcus pneumoniae. J Bacteriol. 1986 Jun;166(3):978–984. doi: 10.1128/jb.166.3.978-984.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar M. N., Priebe S. D., Pozzi G., Hageman J. M., Guild W. R. Cloning and physical characterization of chromosomal conjugative elements in streptococci. J Bacteriol. 1986 Jun;166(3):972–977. doi: 10.1128/jb.166.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]