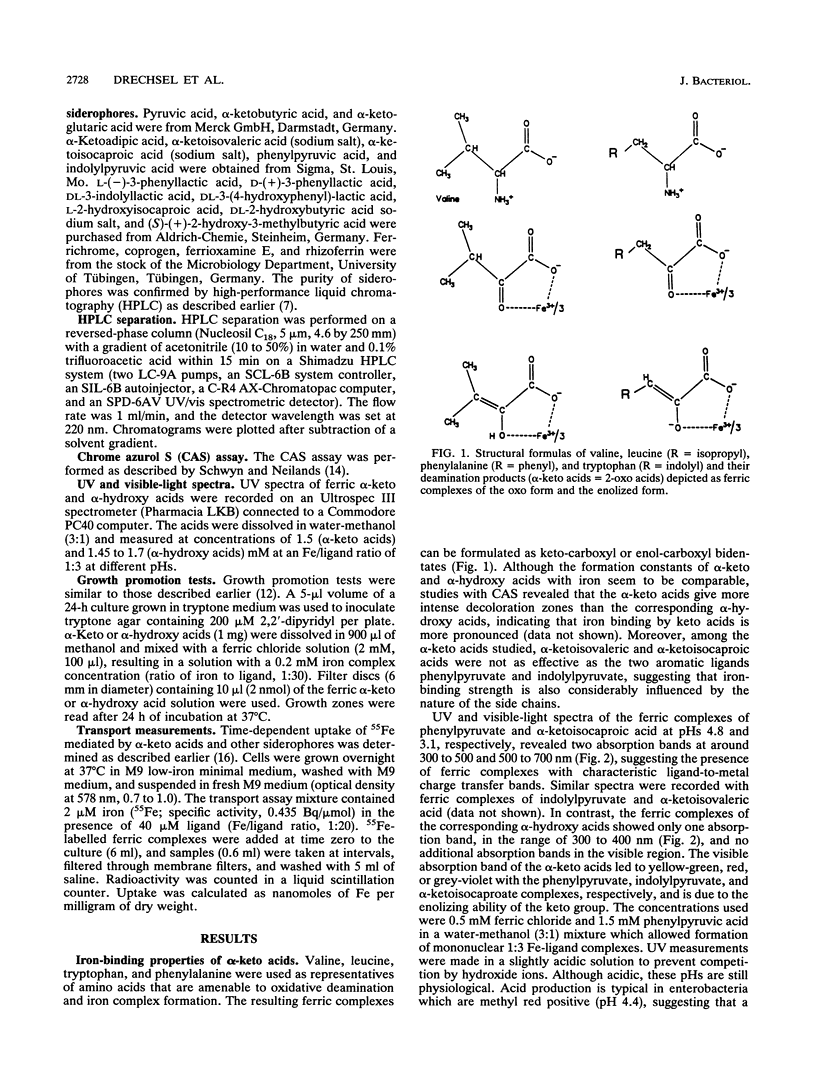
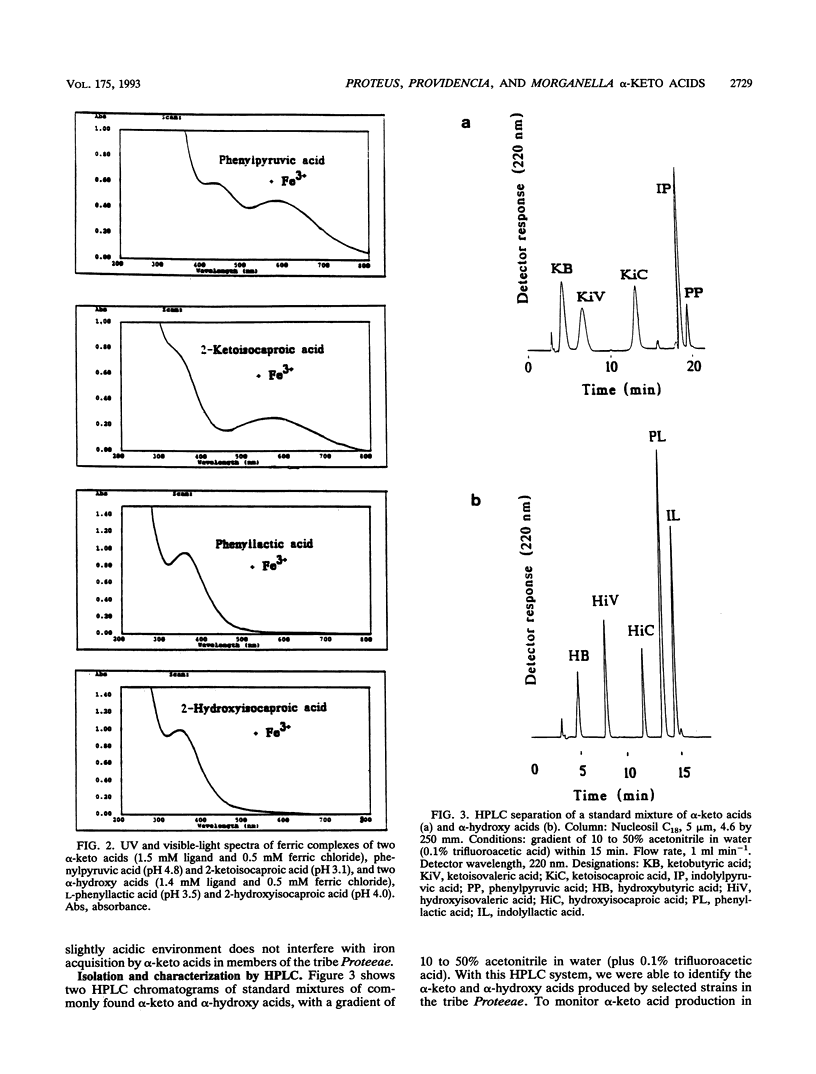
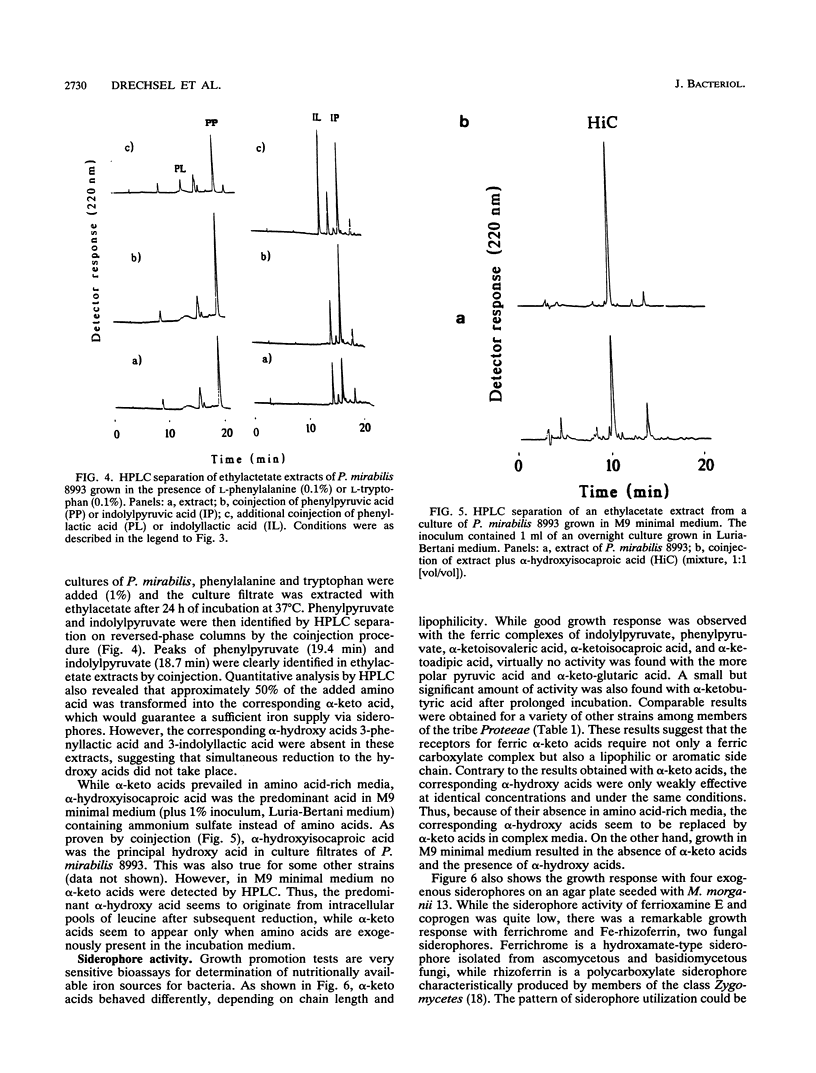
Abstract
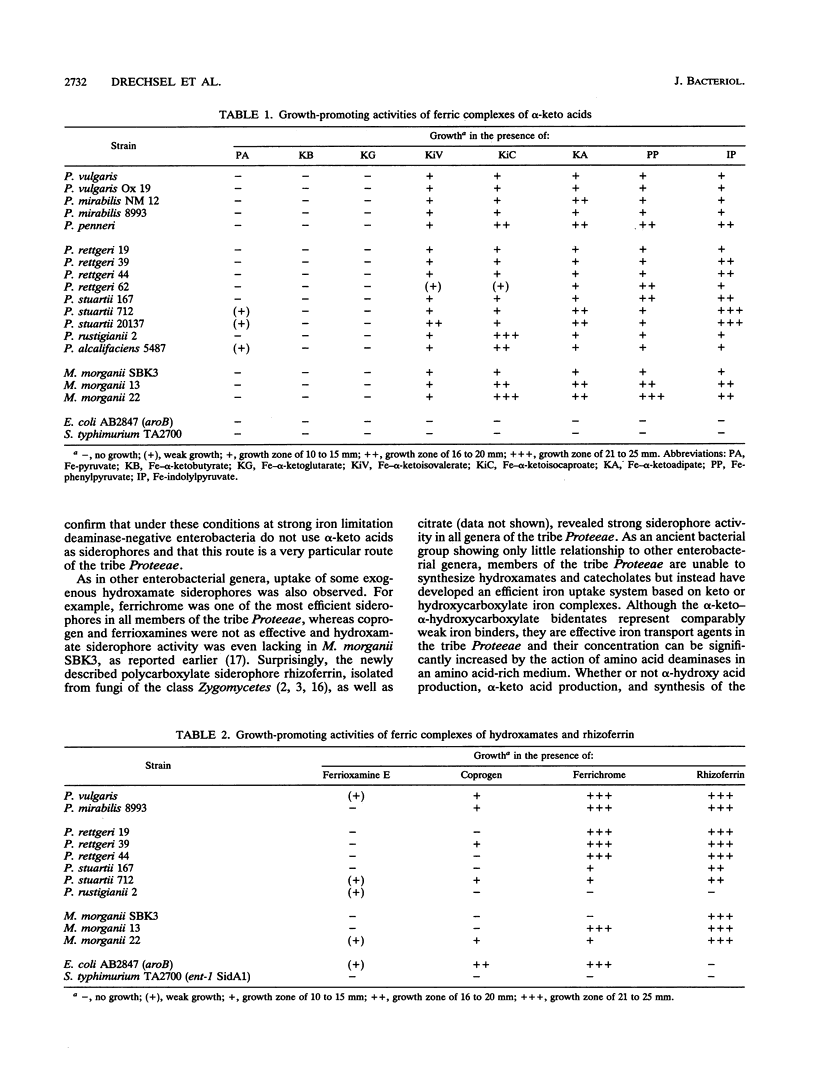
Growth promotion and iron transport studies revealed that certain alpha-keto acids generated by amino acid deaminases, by enterobacteria of the Proteus-Providencia-Morganella group (of the tribe Proteeae), show significant siderophore activity. Their iron-binding properties were confirmed by the chrome azurol S assay and UV spectra. These compounds form ligand-to-metal charge transfer bands in the range of 400 to 500 nm. Additional absorption bands of the enolized ligands at 500 to 700 nm are responsible for color formation. Siderophore activity was most pronounced with alpha-keto acids possessing an aromatic or heteroaromatic side chain, like phenylpyruvic acid and indolylpyruvic acid, resulting from deamination of phenylalanine and tryptophan, respectively. In addition, alpha-keto acids possessing longer nonpolar side chains, like alpha-ketoisocaproic acid or alpha-ketoisovaleric acid and even alpha-ketoadipic acid, also showed siderophore activity which was absent or negligible with smaller alpha-keto acids or those possessing polar functional groups, like pyruvic acid, alpha-ketobutyric acid, or alpha-ketoglutaric acid. The fact that deaminase-negative enterobacteria, like Escherichia coli and Salmonella spp., could not utilize alpha-keto acids supports the view that specific iron-carboxylate transport systems have evolved in members of the tribe Proteeae and are designed to recognize ferric complexes of both alpha-hydroxy acids and alpha-keto acids, of which the latter can easily be generated by L-amino acid deaminases in an amino acid-rich medium. Exogenous siderophores, like ferric hydroxamates (ferrichromes) and ferric polycarboxylates (rhizoferrin and citrate), were also utilized by members of the tribe Proteeae.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berner I., Konetschny-Rapp S., Jung G., Winkelmann G. Characterization of ferrioxamine E as the principal siderophore of Erwinia herbicola (Enterobacter agglomerans). Biol Met. 1988;1(1):51–56. doi: 10.1007/BF01128017. [DOI] [PubMed] [Google Scholar]
- Evanylo L. P., Kadis S., Maudsley J. R. Siderophore production by Proteus mirabilis. Can J Microbiol. 1984 Aug;30(8):1046–1051. doi: 10.1139/m84-163. [DOI] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN S. D. A comparison of the phenylpyruvic acid reaction and the urease test in the differentiation of Proteus from other enteric organisms. J Bacteriol. 1950 Sep;60(3):225–231. doi: 10.1128/jb.60.3.225-231.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg L. A., Wellner D. A sensitive fluorometric assay for amino acid oxidases. Anal Biochem. 1968 Nov;26(2):313–319. doi: 10.1016/0003-2697(68)90343-6. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):453–460. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- Rabsch W., Winkelmann G. The specificity of bacterial siderophore receptors probed by bioassays. Biol Met. 1991;4(4):244–250. doi: 10.1007/BF01141188. [DOI] [PubMed] [Google Scholar]
- SINGER J., VOLCANI B. E. An improved ferric chloride test for differentiating Proteus-Providence group from other Enterobacteriaceae. J Bacteriol. 1955 Mar;69(3):303–306. doi: 10.1128/jb.69.3.303-306.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Thieken A., Winkelmann G. Rhizoferrin: a complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes). FEMS Microbiol Lett. 1992 Jul 1;73(1-2):37–41. doi: 10.1016/0378-1097(92)90579-d. [DOI] [PubMed] [Google Scholar]