Abstract
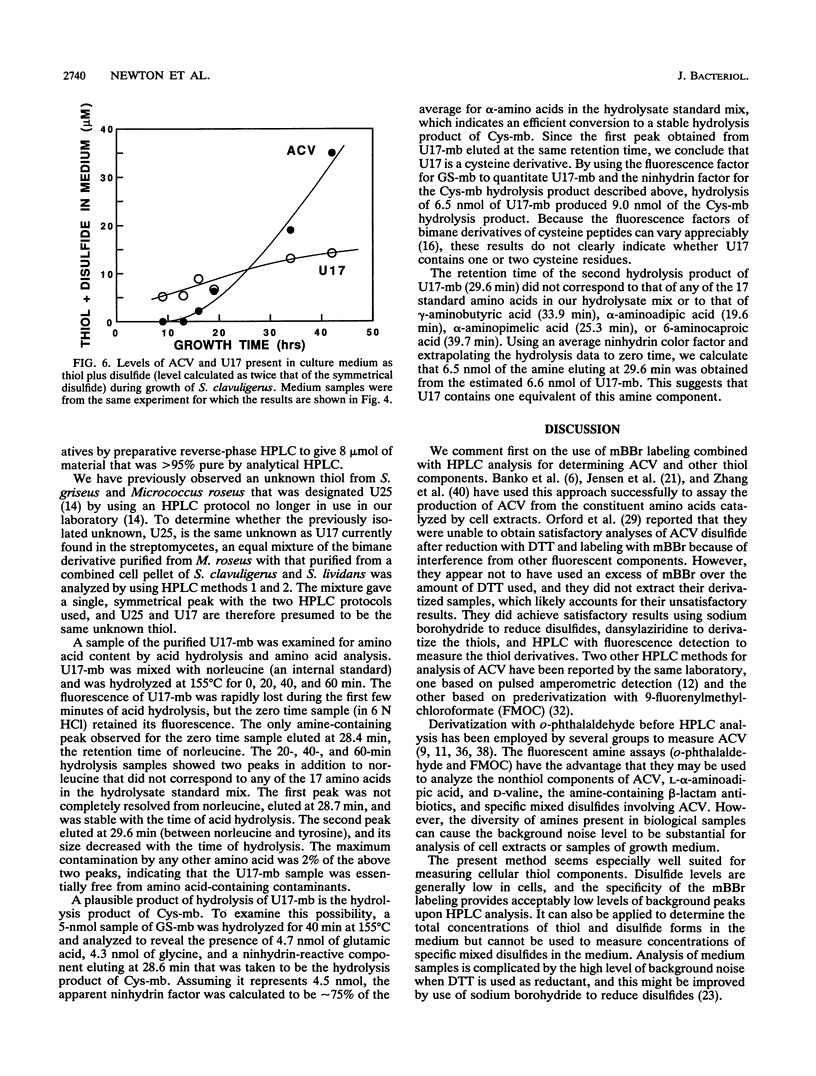
The intracellular low-molecular-weight thiols present in five gram-positive Streptomyces species and one Flavobacterium species were analyzed by high-performance liquid chromatography after fluorescence labeling with monobromobimane. Bacteria were chosen to include penicillin and cephalosporin beta-lactam producers and nonproducers. No significant amount of glutathione was found in any of the streptomycetes. Major intracellular thiols in all strains examined were cysteine, coenzyme A, sulfide, thiosulfate, and an unknown thiol designated U17. Those streptomycetes that make beta-lactam antibiotics also produce significant amounts of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine (ACV), a key intermediate in their biosynthesis. In Streptomyces clavuligerus, a potent producer of beta-lactams, the level of ACV was low during the early phase of growth and increased rapidly toward the end of exponential growth, paralleling that of antibiotic production. These and other observations indicate that ACV does not function as a protective thiol in streptomycetes. U17 may have this role since it was the major thiol in all streptomycetes and appeared to occur at levels about 10-fold higher than those of the other thiols measured, including ACV. Purification and amino acid analysis of U17 indicated that it contains cysteine and an unusual amine that is not one of the common amino acids. This thiol is identical to an unknown thiol found previously in Micrococcus roseus and Streptomyces griseus. A high level of ergothioneine was found in Streptomyces lactamdurans, and several unidentified thiols were detected in this and other streptomycetes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Av-Gay Y., Schreiber R., Cohen G. Characterization of a broad-range disulfide reductase from Streptomyces clavuligerus and its possible role in beta-lactam antibiotic biosynthesis. J Bacteriol. 1993 Feb;175(3):623–629. doi: 10.1128/jb.175.3.623-629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonowitz Y., Cohen G., Martin J. F. Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation, and evolution. Annu Rev Microbiol. 1992;46:461–495. doi: 10.1146/annurev.mi.46.100192.002333. [DOI] [PubMed] [Google Scholar]
- Akanmu D., Cecchini R., Aruoma O. I., Halliwell B. The antioxidant action of ergothioneine. Arch Biochem Biophys. 1991 Jul;288(1):10–16. doi: 10.1016/0003-9861(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Apontoweil P., Berends W. Glutathione biosynthesis in Escherichia coli K 12. Properties of the enzymes and regulation. Biochim Biophys Acta. 1975 Jul 14;399(1):1–9. doi: 10.1016/0304-4165(75)90205-6. [DOI] [PubMed] [Google Scholar]
- Banko G., Wolfe S., Demain A. L. Cell-free synthesis of delta-(L-alpha-aminoadipyl)-L-cysteine, the first intermediate of penicillin and cephalosporin biosynthesis. Biochem Biophys Res Commun. 1986 May 29;137(1):528–535. doi: 10.1016/0006-291x(86)91242-8. [DOI] [PubMed] [Google Scholar]
- Calabro-Jones P. M., Aguilera J. A., Ward J. F., Smoluk G. D., Fahey R. C. Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. Cancer Res. 1988 Jul 1;48(13):3634–3640. [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson M. J., Adlard M. W. Analysis of delta-L-alpha-aminoadipyl-L-cysteinyl-D-valine by ion chromatography and pulsed amperometric detection. J Chromatogr. 1990 Jun 22;509(2):347–356. doi: 10.1016/s0021-9673(01)93092-4. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L., Dorian R., Kosower E. M. Analysis of biological thiols: quantitative determination of thiols at the picomole level based upon derivatization with monobromobimanes and separation by cation-exchange chromatography. Anal Biochem. 1981 Mar 1;111(2):357–365. doi: 10.1016/0003-2697(81)90573-x. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Sundquist A. R. Evolution of glutathione metabolism. Adv Enzymol Relat Areas Mol Biol. 1991;64:1–53. doi: 10.1002/9780470123102.ch1. [DOI] [PubMed] [Google Scholar]
- Genghof D. S. Biosynthesis of ergothioneine and hercynine by fungi and Actinomycetales. J Bacteriol. 1970 Aug;103(2):475–478. doi: 10.1128/jb.103.2.475-478.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E. Ergothioneine as antioxidant. Methods Enzymol. 1990;186:310–318. doi: 10.1016/0076-6879(90)86124-e. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Levels of glutathione in Escherichia coli. Can J Biochem. 1979 Feb;57(2):107–111. doi: 10.1139/o79-013. [DOI] [PubMed] [Google Scholar]
- Mansoor M. A., Svardal A. M., Ueland P. M. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992 Feb 1;200(2):218–229. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Fahey R. C. Purification of thiols from biological samples. Methods Enzymol. 1987;143:96–101. doi: 10.1016/0076-6879(87)43017-6. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Javor B. gamma-Glutamylcysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria. J Bacteriol. 1985 Jan;161(1):438–441. doi: 10.1128/jb.161.1.438-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüesch J., Heim J., Treichler H. J. The biosynthesis of sulfur-containing beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:51–75. doi: 10.1146/annurev.mi.41.100187.000411. [DOI] [PubMed] [Google Scholar]
- Orford C. D., Perry D., Adlard M. W. High-performance liquid chromatographic determination of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine in complex media by precolumn derivatisation with dansylaziridine. J Chromatogr. 1989 Nov 3;481:245–254. doi: 10.1016/s0021-9673(01)96768-8. [DOI] [PubMed] [Google Scholar]
- Palissa H., von Döhren H., Kleinkauf H., Ting H. H., Baldwin J. E. Beta-lactam biosynthesis in a gram-negative eubacterium: purification and characterization of isopenicillin N synthase from Flavobacterium sp. strain SC 12.154. J Bacteriol. 1989 Oct;171(10):5720–5728. doi: 10.1128/jb.171.10.5720-5728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwecke T., Aharonowitz Y., Palissa H., von Döhren H., Kleinkauf H., van Liempt H. Enzymatic characterisation of the multifunctional enzyme delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Streptomyces clavuligerus. Eur J Biochem. 1992 Apr 15;205(2):687–694. doi: 10.1111/j.1432-1033.1992.tb16830.x. [DOI] [PubMed] [Google Scholar]
- Shah A. J., Adlard M. W. Determination of beta-lactams and their biosynthetic intermediates in fermentation media by pre-column derivatisation followed by fluorescence detection. J Chromatogr. 1988 Feb 26;424(2):325–336. doi: 10.1016/s0378-4347(00)81109-3. [DOI] [PubMed] [Google Scholar]
- Smoluk G. D., Fahey R. C., Ward J. F. Equilibrium dialysis studies of the binding of radioprotector compounds to DNA. Radiat Res. 1986 Aug;107(2):194–204. [PubMed] [Google Scholar]
- Sundquist A. R., Fahey R. C. Evolution of antioxidant mechanisms: thiol-dependent peroxidases and thioltransferase among procaryotes. J Mol Evol. 1989 Nov;29(5):429–435. doi: 10.1007/BF02602913. [DOI] [PubMed] [Google Scholar]
- Sundquist A. R., Fahey R. C. The function of gamma-glutamylcysteine and bis-gamma-glutamylcystine reductase in Halobacterium halobium. J Biol Chem. 1989 Jan 15;264(2):719–725. [PubMed] [Google Scholar]
- Usher J. J., Lewis M., Hughes D. W. Determination by high-performance liquid chromatography of some compounds involved in the biosynthesis of penicillin and cephalosporin. Anal Biochem. 1985 Aug 15;149(1):105–110. doi: 10.1016/0003-2697(85)90481-6. [DOI] [PubMed] [Google Scholar]
- White R. L., DeMarco A. C., Shapiro S., Vining L. C., Wolfe S. Measurement of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase activity in Streptomyces clavuligerus by high-performance liquid chromatography after precolumn derivatization with o-phthaldialdehyde. Anal Biochem. 1989 May 1;178(2):399–403. doi: 10.1016/0003-2697(89)90660-x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wolfe S., Demain A. L. Biochemical studies on the activity of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Streptomyces clavuligerus. Biochem J. 1992 May 1;283(Pt 3):691–698. doi: 10.1042/bj2830691. [DOI] [PMC free article] [PubMed] [Google Scholar]



