Abstract
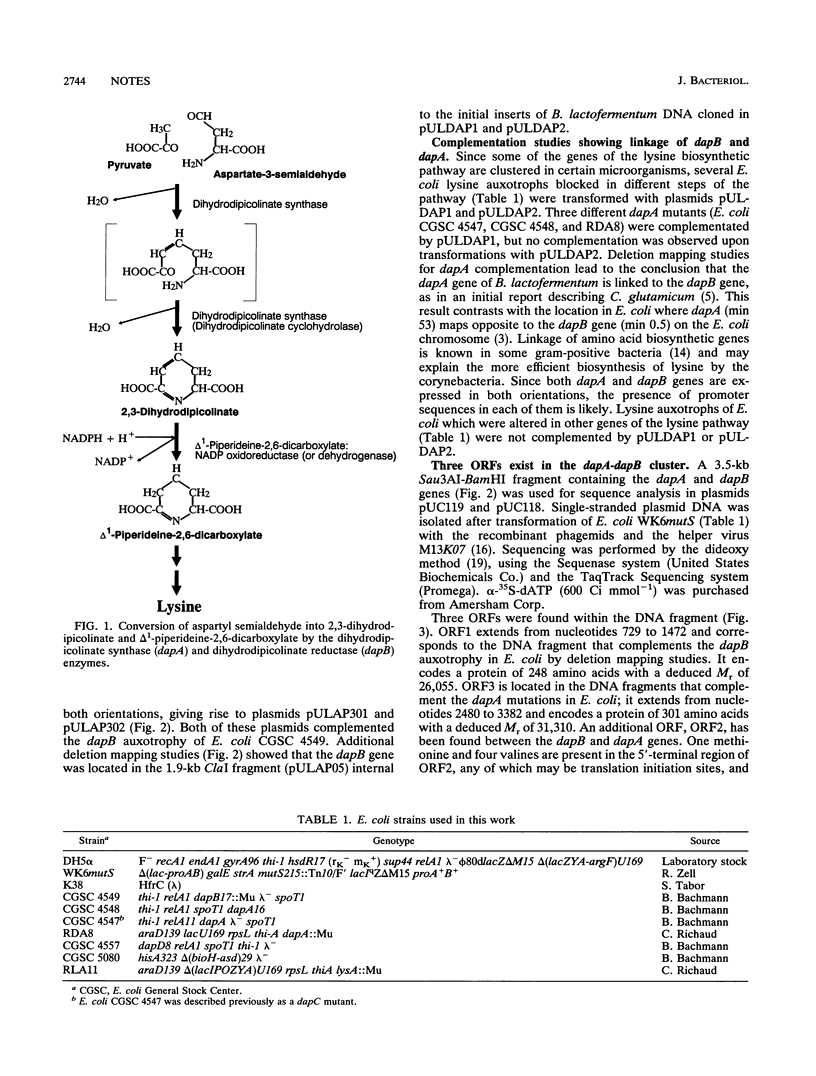
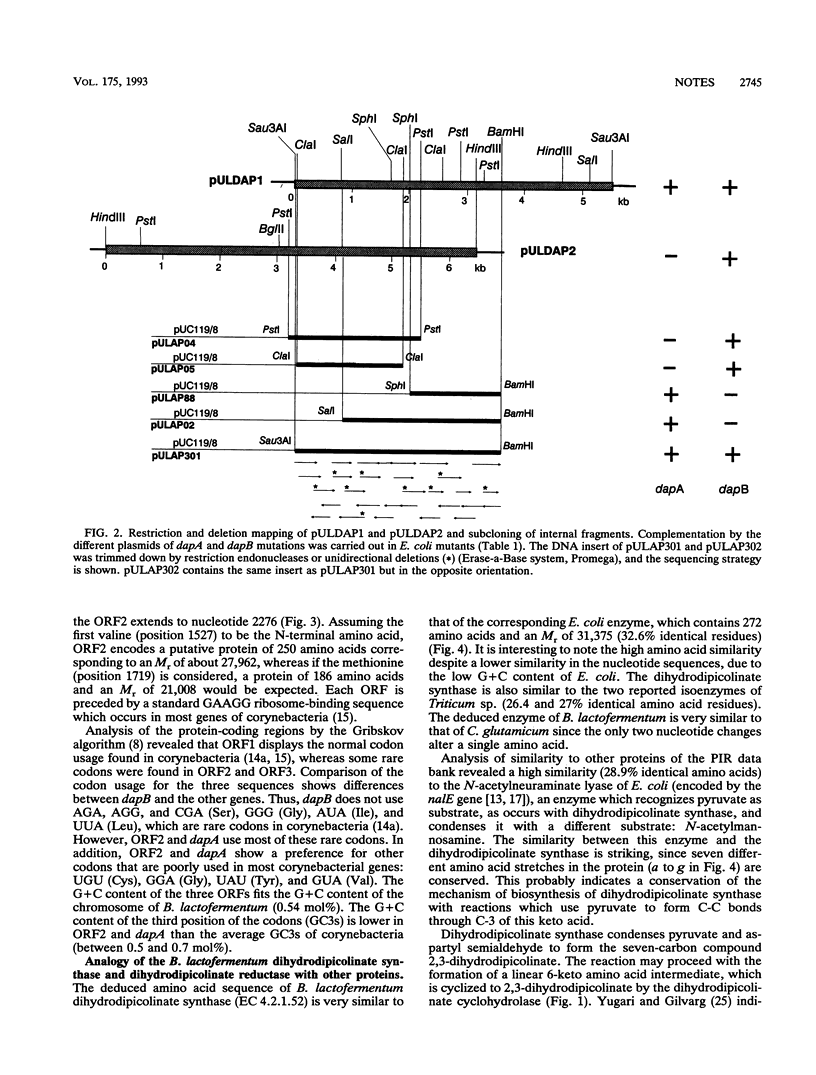
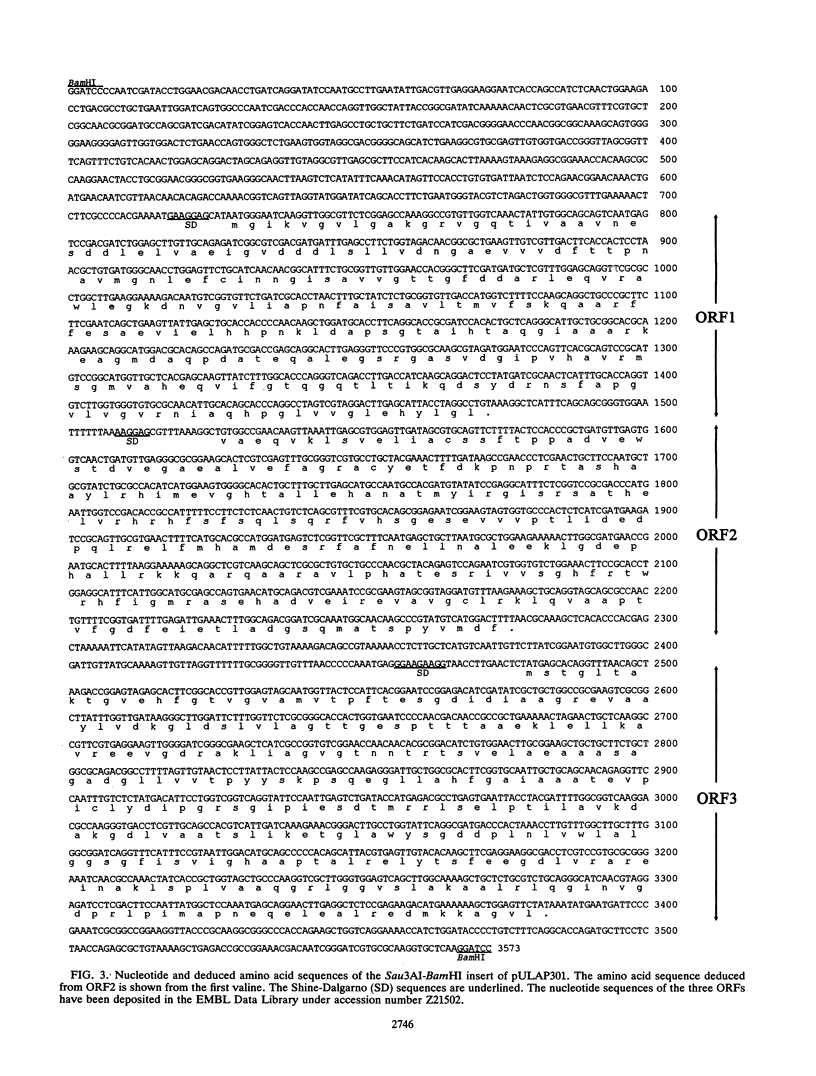
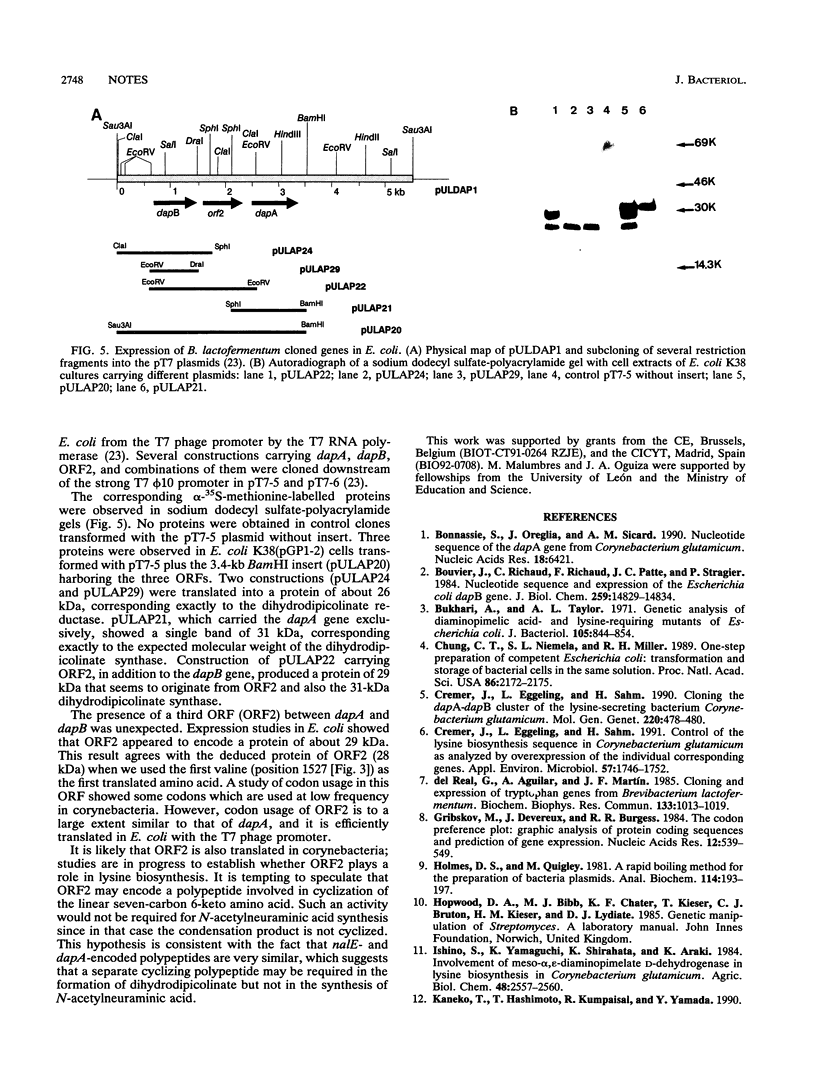
The dapA and dapB genes, encoding, respectively, dihydrodipicolinate synthase and dihydrodipicolinate reductase, the two first enzymes of the lysine branch of the aspartic amino acid family, were cloned from the DNA of the amino acid-producing bacterium Brevibacterium lactofermentum. The two genes were clustered in a 3.5-kb Sau3AI-BamHI fragment but were separated by an open reading frame of 750 nucleotides. The protein encoded by this open reading frame had little similarity to any protein in the data banks, and its function remains unknown. The three genes were translated in Escherichia coli, giving the corresponding polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnassie S., Oreglia J., Sicard A. M. Nucleotide sequence of the dapA gene from Corynebacterium glutamicum. Nucleic Acids Res. 1990 Nov 11;18(21):6421–6421. doi: 10.1093/nar/18.21.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Richaud C., Richaud F., Patte J. C., Stragier P. Nucleotide sequence and expression of the Escherichia coli dapB gene. J Biol Chem. 1984 Dec 10;259(23):14829–14834. [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer Josef, Eggeling Lothar, Sahm Hermann. Control of the Lysine Biosynthesis Sequence in Corynebacterium glutamicum as Analyzed by Overexpression of the Individual Corresponding Genes. Appl Environ Microbiol. 1991 Jun;57(6):1746–1752. doi: 10.1128/aem.57.6.1746-1752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ludovice M., Martin J. F., Carrachas P., Liras P. Characterization of the Streptomyces clavuligerus argC gene encoding N-acetylglutamyl-phosphate reductase: expression in Streptomyces lividans and effect on clavulanic acid production. J Bacteriol. 1992 Jul;174(14):4606–4613. doi: 10.1128/jb.174.14.4606-4613.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Kemper B. Chimeric single-stranded DNA phage-plasmid cloning vectors. Biotechnology. 1988;10:85–102. doi: 10.1016/b978-0-409-90042-2.50010-6. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Watanabe K., Kimura A. Complete nucleotide sequence of the E. coli N-acetylneuraminate lyase. Nucleic Acids Res. 1985 Dec 20;13(24):8843–8852. doi: 10.1093/nar/13.24.8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaud F., Richaud C., Ratet P., Patte J. C. Chromosomal location and nucleotide sequence of the Escherichia coli dapA gene. J Bacteriol. 1986 Apr;166(1):297–300. doi: 10.1128/jb.166.1.297-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrumpf B., Schwarzer A., Kalinowski J., Pühler A., Eggeling L., Sahm H. A functionally split pathway for lysine synthesis in Corynebacterium glutamicium. J Bacteriol. 1991 Jul;173(14):4510–4516. doi: 10.1128/jb.173.14.4510-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]
- del Real G., Aguilar A., Martín J. F. Cloning and expression of tryptophan genes from Brevibacterium lactofermentum in Escherichia coli. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1013–1019. doi: 10.1016/0006-291x(85)91237-9. [DOI] [PubMed] [Google Scholar]