Abstract
The protooncogene bcl-2 inhibits neuronal apoptosis during normal brain development as well as that induced by cytotoxic drugs or growth factor deprivation. We have previously demonstrated that neurons of mice deficient in Bcl-2 are more susceptible to neurotoxins and that the dopamine (DA) level in the striatum after systemic 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) administration was significantly lower than in wild-type mice. In the present study we have used transgenic mice overexpressing human Bcl-2 under the control of neuron-specific enolase promoter (NSE-hbcl-2) to test the effects of the neurotoxins 6-hydroxydopamine (6-OHDA) and MPTP on neuronal survival in these mice. Primary cultures of neocortical neurons from normal and transgenic mice were exposed to these dopaminergic neurotoxins. Addition of 6-OHDA resulted in cell death of essentially all neurons from normal mice. In contrast, in cultures generated from heterozygous NSE-hbcl-2 transgenic mice, only 69% of the cells died while those generated from homozygous transgenic mice were highly resistant and exhibited only 34% cell death. A similar effect was observed with neurons treated with MPP+. Moreover, while the striatal dopamine level after MPTP injections was reduced by 32% in the wild type, the concentration remained unchanged in the NSE-hbcl-2 heterozygous mice. In contrast levels of glutathione-related enzymes were unchanged. In conclusion, overexpression of Bcl-2 in the neurons provided protection, in a dose-dependent manner, against neurotoxins known to selectively damage dopaminergic neurons. This study provides ideas for inhibition of neuronal cell death in neurodegenerative diseases and for the development of efficient neuroprotective gene therapy.
Parkinson disease is a progressive neurological disorder associated with selective degeneration of the dopaminergic neurons in the substantia nigra (1). Data from postmortem studies of patients’ brains suggest that reactive oxygen species (ROS) may play a critical role in this process (2). ROS may be harmful to macromolecules and other cellular components, causing lipid peroxidation and protein and DNA oxidation. Necropsy studies also provide evidence for specific chemical changes indicative of damaging oxidative events in the substantia nigra. These include higher levels of cholesterol hydroperoxides, malondialdehyde protein adducts of 4-hydroxy-2-nonenal and 8-hydroxy-deoxyguanosine that are indicative of ROS-induced DNA breaks (3).
A number of neurotoxins selectively damage dopaminergic neurons. For example, 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP), selectively destroys cultured dopaminergic neurons (4). In vivo, MPTP, which is converted to the active metabolite MPP+, causes subacute Parkinsonism in humans and other primates (5–6). MPTP was also shown to elicit apoptotic cell death in nigral dopaminergic neurons after systemic administration to mice (7). Another neurotoxin, 6-hydroxydopamine (6-OHDA), is also widely used to damage dopaminergic neurons mainly in vitro but also in animals models (8).
We have previously demonstrated that dopamine (DA), the endogenous neurotransmitter produced by nigrostriatal neurons, and its oxidative metabolites can induce biochemical, histochemical, and morphological changes characteristic of apoptosis in various neuronal and nonneuronal cells (9–12). In addition, recent autopsy reports indicated the presence of morphological and biochemical features of apoptosis in dopaminergic neurons in Parkinsonian patients (13, 14). These findings suggest that in these patients there is disregulation, either inherited or acquired, in the antioxidative protective mechanisms, within or around nigral neurons, leading to pathological activation of apoptosis (9–12, 15).
The protooncogene bcl-2 is one of the key regulators of apoptosis, capable of preventing death induced by cytotoxic drugs and growth factor deprivation (reviewed in ref. 16). Overexpression of the Bcl-2 protein in sensory and sympathetic neurons protects them against apoptosis induced by deprivation of neurotrophic factors or ROS (17, 18). Similarly, we have demonstrated that PC12 cells overexpressing Bcl-2 are highly resistant to DA-induced apoptosis (19–20). In contrast, a Bcl-2- expressing dopaminergic neuronal cell line is resistant to MPP+ but is not protected from death induced by 6-OHDA. Moreover, our studies with mutant mice deficient in the bcl-2 gene showed that cerebellar granule cells are significantly more susceptible to DA and MPTP toxicity, and that the level of DA in the striatum, after systemic MPTP administration was significantly lower in the bcl-2 deficient mice (unpublished observations).
Transgenic mice expressing human bcl-2 gene under the control of the neuron-specific enolase promoter (NSE-bcl-2), which forced expression in neurons, were found to be less susceptible to neuronal death during development and throughout adult life (22–25). Studies of this animal model also indicated that Bcl-2 regulates neuronal cell survival and protects against various cell death stimuli in a dose-dependent manner (24–26).
In the present study we focused on the possible protective effect of Bcl-2 against neurotoxins that are selectively toxic to dopaminergic neurons.
MATERIALS AND METHODS
Animals.
Transgenic mice of the heterozygous and homozygous genotype for the human bcl-2 gene (hbcl-2) were generated as described (22).
Brain Homogenates.
Animals were sacrificed by cervical dislocation. The brains were removed, frozen promptly in liquid nitrogen and stored at −80°C. For preparation of homogenates, the brains were thawed and homogenized in 50 mM potassium phosphate, pH 7.2, containing 1 mM phenylmethylsulfonyl fluoride and 1 μg/ml leupeptin, 1 mM EDTA and 0.1% Triton X-100. The extract was then sonicated (2 × 15 sec) and centrifuged at 150,000 × g for 1 h. The supernatant was used in the enzymatic assays and for glutathione (GSH) determination by using the Calbiochem kit.
Enzymatic Assays.
(i) GSH reductase was assayed spectrophotometrically by following reduced nicotinamide-adenine dinucleotide phosphate oxidation at 340 nm: the reaction mixture contained 100 mM potassium phosphate (pH 7.4), 0.1 mM reduced nicotinamide-adenine dinucleotide phosophate, 1 mM GSH disulfide, and 1 mM EDTA (27). One unit of enzyme activity yields the reduction of 1 μmol GSH disulfide/min.
(ii) GSH peroxidase was determined according to Flohe and Gunzler (28) with t-butyl hydroperoxide as a substrate. The assay is based on determination of reduced nicotinamide-adenine dinucleotide phosphate at 340 nm, in a reaction mixture containing 50 mM potassium phosphate, (pH 7.0), 1 mM GSH, 0.12 mM t-butyl hydroperoxide, 0.15 mM reduced nicotinamide-adenine dinucleotide phosphate, and 0.24 units of GSH reductase. One unit of enzyme activity results in the oxidation of 1 μmol GSH/min.
(iii) GSH transferase was determined according to Habig et al. (29) by using 1-chloro-2,4-dinitrophenol as a substrate. One unit of enzyme activity results in the binding of 1 μmol GSH/min.
Protein concentration was determined by the Bradford method (30) with BSA as a standard.
Determination of DA Levels in the Striatum.
Both striata were dissected out, weighed, and sonicated in 10 vol of ice-cold 0.1 M perchloric acid containing 0.01 M NaHSO3 and 0.001% ascorbic acid containing 1 μg/ml dihydroxybenzylamine (Sigma) as an internal standard. After centrifugation (12,000 × g for 10 min) the supernatants were filtered (0.45 μm nylon filter) and 20 μl were injected onto C18 reverse-phase column (Phenomenex, Belmont, CA). The mobile phase consisted of 0.15 M monochloroacetic acid, 0.1 M NaOH, 0.6 mM EDTA, 0.2 mM sodium acetylsulfate, and 9% methanol pH 2.9. DA was detected by electrochemical oxidation against a glassy carbon electrode (+0.77 V).
Primary Cell Culture.
Primary cell cultures of murine neocortical cells were prepared from fetal mice of gestational day 15–16 (E15–E16), essentially as described by Larm et al. (31). Cells were plated at a concentration of ≈1.5 × 105 cells per cm2 in 24-well plates (Nunc) previously coated with poly-d-lysine (50 μg/ml) in medium containing 10% dialyzed fetal calf serum. Twenty-four hours after plating, the medium was removed and replaced with serum-free growth medium.
Immunocytochemistry.
On day 7 in vitro, cells were fixed in 4% paraformaldehyde, in PBS (pH 7.4) for 15 min at room temperature. Cells were then washed three times with Tris buffered saline (TBS): 50 mM Tris⋅HCl, 0.9% NaCl (pH 7.6), and quenched in 3% H2O2 for 5 min at room temperature. After three washes in TBS the cells were blocked in 10% normal goat serum (0.1% Triton X-100) in TBS for 1 h at 4°C, washed twice with TBS, followed by incubation with mouse anti-human Bcl-2 monoclonal antibody, which does not cross-react with mouse Bcl-2 (32) (1:1 dilution in 2% normal goat serum and 0.1% Triton X-100) overnight at 4°C. Cells were then washed three times with TBS and incubated with sheep anti-mouse IgG conjugated to horse radish peroxidase (1:300) for 3 h at room temperature. Similarly, microtubule-associated protein 2 (1:250) and glial fibrillary acidic protein (1:5) antibodies were incubated overnight at 4°C followed by incubation with mouse anti-rabbit conjugated to horseradish peroxidase. After further washings with TBS the cells were incubated with 0.05% 3,3′-diaminobenzidine, 0.01% H2O2 in TBS until color developed. Finally cells were washed three times with TBS and examined under bright-field microscopy (Olympus IMT-2).
Toxicity Experiments.
Cells were exposed to either 6-OHDA (Research Biochemicals, Natick, MA) or MPP+ (Research Biochemicals) on day 5 in vitro. Drugs were made up in N2 growth medium (31) and concentrations of 0.1–100 μM of both 6-OHDA and MPP+ were tested in triplicate on each of the cultures. Cells were exposed to the drugs for 18–24 h. The trypan blue exclusion assay was used to assess cell viability after drug exposure. Medium was removed and 0.2% trypan blue in TBS added to the cells for 10 min. After the removal of the trypan blue solution the cells were fixed with 4% paraformaldehyde in PBS for 5 min, then replaced by TBS. Cells were then observed under bright field microscopy (Olympus, IMT-2).
Data Analysis.
Three random representative fields of cells were photographed at each concentration of both 6-OHDA and MPP+. Cells stained with trypan blue were considered dead. The cells were counted and percentage of dead cells per photograph calculated (cells stained trypan blue/cells stained trypan blue + nonstained cells) (≈1,500–2,500 cells were counted for each condition). sigma stat (Microsoft) was used to carry out two-way ANOVA and post hoc Newman–Keuls test on the data. The graph pad prism (Microsoft) program was used to calculate mean percent ± SEM of dead cells and the level of statistical significance was set at P < 0.05. The DA levels were analyzed from a group of 5 mice, the two hemispheres separately, while the brain extracts for the enzyme activity were analyzed from a group of 8 mice. Data are represented as mean ± SEM and the P value was calculated by using the t test.
RESULTS
Expression of Human Bcl-2 in Neuronal Cultures of Transgenic Mice.
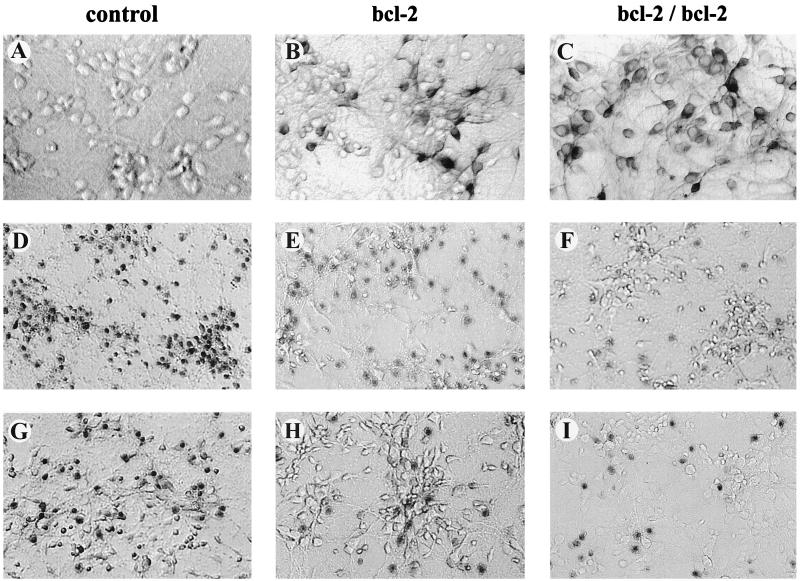
Primary cultures of neocortical neurons were prepared from E15–E16 mouse embryos. Immunocytochemical staining of the cultures for microtubule-associated protein 2, a neuronal marker, and glial fibrillary acidic protein, a glial cell marker, indicated that the cultures used consisted of >90% neurons (data not shown). Cultures derived from transgenic mice expressed the bcl-2 transgene as shown by immunocytochemistry (Fig. 1 A–C).
Figure 1.
Photomicrographs of the murine cortical cultures used to analyze the neurotoxicity of 6-OHDA and MPP+. Immunocytochemical staining for the hBcl-2 protein was found in both the heterozygous (B) and homozygous cultures (C), but was absent in the wild-type cultures (A). After exposure of the cultures to 6-OHDA (10–100 μM) and MPP+ (10–50 μM) for 18–24 h, cell viability was assessed by using the trypan blue exclusion procedure. Cell counts (mean ± SEM) of three random fields (1,500–2,500 cells) revealed the percentage of dead cells. Representative fields of cells treated with 30 μM 6-OHDA (D–F) and 50 μM MPP+ (G–I) are shown for wild-type (D, G), heterozygous (E, H), and homozygous (F, I) cultures overexpressing hBcl-2, respectively. (A–C, 200×; D–I, 150×). In all cases the hbcl-2 transgene conferred significant neuroprotection (P < 0.05; ANOVA) upon the cortical cultures. Further details are given in Figs. 2 and 3.
Effects of MPP+ and 6-OHDA on Cortical Neuronal Cultures from Wild-Type, Heterozygous, and Homozygous NSE-hbcl-2 Transgenic Mice.
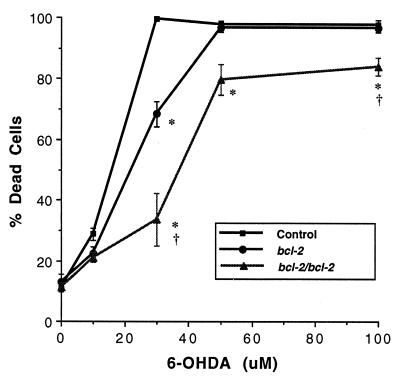
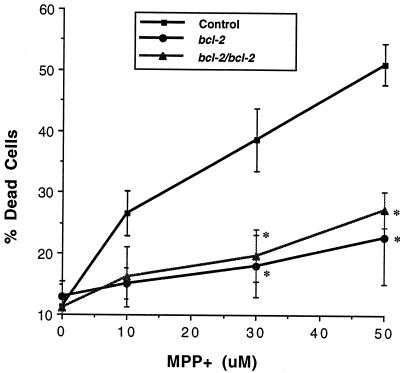
Cortical neurons from E15–E16 mice were prepared, grown in culture and used to investigate the neurotoxicity of MPP+ and 6-OHDA. Initially the concentration-dependence of the neurotoxic effects of MPP+ and 6-OHDA (both 0.1–100 μM) was examined in preliminary experiments in cultures derived from wild-type and from the NSE-hbcl-2 heterozygous mice. Both drugs induced neuronal death in a concentration-dependent manner, which was shown by microscopy (2–4 h after drug administration) to involve rapid neuronal swelling and subsequent cell death. Detailed analyses were then performed by using MPP+ (10–50 μM) and 6-OHDA (10–100 μM) in cortical neuronal cultures derived from wild-type, heterozygous, and homozygous fetal mice expressing the hBcl-2 protein. Three independent experiments were performed for each drug. Data from a single experiment are shown (Figs. 2 and 3). 6-OHDA effected a concentration-dependent neuronal cell death under the conditions tested (P < 0.0001) and there was an overall difference between the genotypes (P < 0.0001; Fig. 2). Representative data are shown in Fig. 1 D–F where neurons were exposed for 18–24 h to 30 μM 6-OHDA and the degree of cell death was 100%, 69%, and 34% in cultures derived from wild-type, heterozygous, and homozygous transgenic mice, respectively, with the neuroprotection conferred by the hbcl-2 transgene being significantly enhanced in the homozygous vs. heterozygous cultures (Fig. 2). Under the conditions tested, MPP+ induced a similar pattern of concentration-dependent cell death (P < 0.0001), but a significant difference was not observed between the homozygous and the heterozygous cultures (Fig. 3). In cultures treated with 50 μM MPP+, 51% of the cells in the wild-type cultures died, while only 23% and 27% of neurons died in cultures derived from heterozygous and homozygous mice, respectively.
Figure 2.
6-OHDA toxicity in neocortical cultures established from wild-type, heterozygous and homozygous E15-E16 transgenic mice. After administration of 6-OHDA (10–100 μM, 18–24 h) cell viability was estimated by using the trypan blue exclusion assay. 6-OHDA exerted a concentration-dependent effect on cell viability (F[4, 44] = 408, P < 0.0001) and significant protection was observed in the hBcl-2 overexpressing cultures relative to the wild-type culture, with the neuroprotection in the homozygous cultures being significantly greater than the heterozygous cultures (P < 0.05). Approximately 1,500–2,500 cells were counted from each condition and percentage of dead cells per condition estimated. Results shown are mean percent of dead cells ± SEM. ∗, Significant relative to wild-type culture; †, significant relative to heterozygous culture as determined by a two-way ANOVA and post hoc Student-Newman-Keuls test (P < 0.05).
Figure 3.
Neurotoxic effects of MPP+ in murine neocortical neurons of wild-type, heterozygous, and homozygous transgenic mice. After administration of MPP+ (10–50 μM, 18–24 h) cell viability was estimated by using the trypan blue exclusion assay. MPP+ effected a concentration-dependent effect (F[3, 35] = 15.3, P < 0.0001) with significant neuroprotection found in both the heterozygous and homozygous cultures at 30 μM and 50 μM of MPP+ when compared with the wild-type cultures (P < 0.05). Approximately 1,500–2,500 cells were counted from each condition and percent of dead cells per condition estimated. Results are shown as mean percent of dead cells ± SEM. ∗, Significant relative to wild-type cultures determined by a two-way ANOVA and post hoc Student-Newman-Keuls test (P < 0.05).
Bcl-2 Protects Striatal DA Level After MPTP Injections.
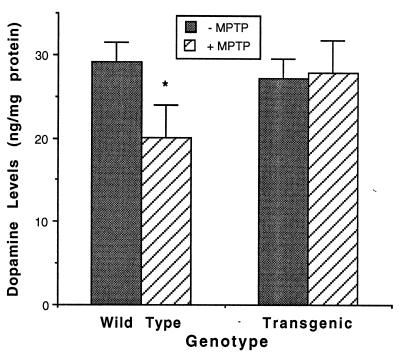
Wild-type and heterozygous adult NSE-hbcl-2 mice were injected (i.p.) with MPTP (10 mg/kg), four times at 24 h intervals. Ten days later, animals were decapitated, brains removed, the striata dissected, and DA concentrations were measured by HPLC. As shown in Fig. 4, DA levels in the striata of untreated heterozygous transgenic mice were similar to those of wild-type. However, after MPTP treatment the DA level in the striata was markedly reduced in the wild-type mice (32%) but remained unchanged in the heterozygous NSE-hbcl-2 mice (P < 0.05).
Figure 4.
DA levels in the striata of wild-type and heterozygous transgenic mice. Striatal homogenates from wild-type (n = 5) and heterozygous (n = 5) adult mice that were injected with MPTP were analyzed by HPLC for the concentration of DA (mean ± SEM). ∗, Significant relative to wild-type and MPTP-treated NSE-hbcl-2 by a two-tailed t test analysis (P < 0.05).
Activities of the GSH-Related Enzymes in Wild-Type and Transgenic Mice.
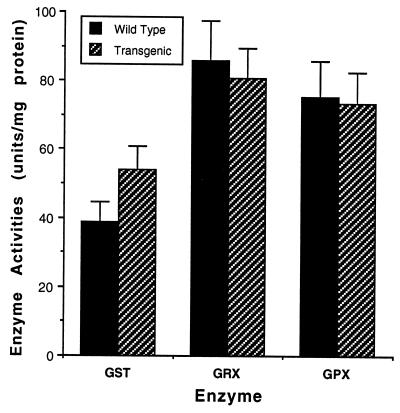
One of the mechanism by which Bcl-2 may act is by decreasing the generation of ROS. To examine this possibility, we measured the activity of the GSH-related enzymes and the level of reduced GSH in homogenates of brain from normal and transgenic mice. The activities of GSH reductase, GSH transferase, and GSH peroxidase in the normal mice were 85, 37, and 75 units/mg protein, respectively, while the level of these enzymes in the transgenic mice was 80, 55, and 74 units/mg protein, respectively. The differences in activities between normal and transgenic mice were not statistically significant (Fig. 5). Similarly, the total GSH level in the brain homogenates of normal and transgenic mice was comparable in the wild-type (16.0 ± 0.75) and in the heterozygous transgenic mice (14.9 ± 1.04).
Figure 5.
Activities of GSH-related enzymes in brain extracts of wild-type and heterozygous transgenic mice. Brain homogenates from wild-type (n = 5) and heterozygous (n = 5) mice were analyzed for the activity of GSH reductase (GRX) GSH transferase (GST), and GSH peroxidase (GPX). The results are shown as mean ± SEM. The differences between the activities of these enzymes in the wild-type and the heterozygous mice were not statistically significant (P > 0.05).
DISCUSSION
In the present study we have used NSE-hbcl-2 transgenic mice as a model system to demonstrate that expression of human Bcl-2 in neurons provided significant protection against death caused by the selective dopaminergic neurotoxins, MPTP and 6-OHDA. The NSE-bcl-2 heterozygous mice were fully resistant to MPTP injections and did not show any reduction of DA levels in their striata. In contrast, injections of MPTP to normal mice resulted in 32% reduction of DA levels. Similar protection was also seen in cultures of cortical neurons generated from brains of the transgenic mice, which showed a higher survival rate after MPP+ and 6-OHDA treatments.
The two neurotoxins differed in their cytotoxicity for cultured cortical neurons. At a concentration of 30 μM, 6-OHDA killed 100% of the neurons in wild-type cultures and Bcl-2 protected the neurons in a concentration-dependent manner. Addition of 6-OHDA to homozygous neurons resulted only in 30% death, whilst addition of the same drug to heterozygous cultures resulted in 65% of dead cells. In contrast, MPP+ is less toxic to the neurons and at a concentration of 50 μM, only half of the neurons in the wild-type cultures died. Moreover, cultures from both heterozygous and homozygous transgenic mice showed similar protection and only ≈25% of the neurons died. These results indicate that the level of protection conferred by Bcl-2 depends on the given insult. The amount of Bcl-2 in the heterozygous mice is sufficient to protect the neurons from death caused by MPP+. However, a higher concentration of Bcl-2 is needed to protect efficiently from death by 6-OHDA and therefore the neurons from homozygous mice are less susceptible to death by this neurotoxin. The present studies clearly show that the protective effect of the Bcl-2 protein is concentration-dependent and consistent with the results of our previous study demonstrating that bcl-2 deficient mice were more sensitive to MPTP and DA treatment than wild-type mice (unpublished results).
Although it was previously shown that Bcl-2 can protect various established neuronal cultures against insults by different neurotoxins (19–21), the advantage of our experimental model is that it allowed us to study the physiological functions of Bcl-2 in primary neuronal cultures and in an in vivo system, where the level of Bcl-2 was increased by genetic manipulation.
It is suggested that Bcl-2 can protect cell death by different mechanisms, one of which is by inhibiting the function of the mammalian homologs of the Caenorhabditis elegans genes ced 3 and ced 4 (34). These genes are essential for all developmental cell death in the worm that is suppressed by ced 9, the homologue of the vertebrate gene bcl-2. The vertebrate homologs of ced 3 encodes caspases and that of ced 4 encodes a protein known as Apaf, that participates in activation of caspase-3 (35). The recent finding that Bcl-2 can interact with CED4 suggests that it may protect cells from death by directly regulating the activation of caspases (34).
Other studies indicate that Bcl-2 is also involved in changes in the cellular oxidation-reduction potential, which was demonstrated to be shifted toward a more reducing level in neural cell lines expressing Bcl-2 (36). In the Bcl-2 expressing cells the reductive shift includes a decrease in the ratio between GSH disulfide/GSH disulfide + GSH, the major determinant of cellular thiol redox, which reflects an alteration in GSH level and redox state. Our previous study suggested that the enhanced DA and MPTP cytotoxicity in bcl-2-deficient mice resulted from decreased antioxidative capacity of the bcl-2-deficient neurons. However, in the present study we were not able to detect any significant alterations in the antioxidant enzymes or in the GSH systems between wild-type and transgenic mice. Because the transgene is expressed only in neurons, which normally comprise only a part of the cell population in the adult brain, it is possible that small changes in the levels of these enzymes in the neurons are masked by the presence of nonneuronal cells.
Another potential mechanism by which Bcl-2 may suppress cell death is by changes in Ca2+ flux and mitochondrial permeability that prevent the release of apoptotic factors (37). Interestingly, decreased activity of the mitochondrial respiratory chain also has been proposed to play a role in the etiology and pathogenesis of Parkinson disease. Several studies demonstrated a decrease in complex I of the mitochondrial electron transfer and loss of the alpha-ketoglutarate dehydrogenase complex in the substantia nigra of Parkinson disease patients (38). Furthermore, human neuroblastoma cells containing mitochondria derived from platelets of Parkinson disease patients (cytoplasmatic hybrid), show a decrease in complex I activity and an increase in sensitivity to MPP+ when compared with cells with control mitochondria (39). In addition, it was recently reported that mitochondrial DNA in Parkinson disease patients is extremely susceptible to hydroxyl radical damage that causes a cellular energy crisis (40).
There are conflicting reports concerning the expression and concentration of Bcl-2 in the substantia nigra of normal and Parkinsonian patients. Mogi et al. (41) used a sensitive immunoassay and found significantly higher Bcl-2 levels in the nigrostriatal dopaminergic regions in Parkinsonian patients. In contrast, Vyas et al. (42) reported that the level of bcl-2 mRNA in the substantia nigra was unaltered in Parkinson disease patients when compared with controls. Our results indicate that Bcl-2 can protect against the effect of neurotoxins that selectively damage the dopaminergic neurons and therefore may be associated with the etiology of Parkinson disease. It is possible that elevated level of Bcl-2 in the substantia nigra of Parkinson disease patients represents selective survival of those cells with the highest levels of Bcl-2.
In conclusion, we have demonstrated that overexpression of Bcl-2 in primary neurons provides neuroprotection, in a dose-dependent manner, against neurotoxins known to selectively cause toxicity to dopaminergic neurons. This study may lay the foundation for new therapeutic approaches to efficiently provide neuroprotection in Parkinson disease and other neurodegenerative disorders.
Acknowledgments
Supported, in part, by The National Parkinson Foundation, Miami, Florida, U.S.A., Teva Pharmaceuticals Ltd., and the Israel Science Foundation. O.B. and P.M.B. were supported by the National Health and Medical Research Council (Australia) and O.B. was also supported by the Australian Cooperative Research Center for Growth Factors and the National Multiple Sclerosis Society of Australia.
ABBREVIATIONS
- MPTP
1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- DA
dopamine
- 6-OHDA 6-hydroxy-DA
NSE, neuron-specific enolase promoter
- ROS
reactive oxygen species
- GSH
glutathione TBS, Tris-buffered saline
References
- 1.Hirsch, E. C., Faucheux, B., Damier, P., Mouatt-Prigent, A. & Agid, Y. (1997) J. Neural Transm. 50 Suppl., 79–88. [DOI] [PubMed]
- 2.Fahn S, Cohen G. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 3.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman E R, Mizuno Y. Proc Natl Acad Sci USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mytilineou C, Cohen G, Heikilla R E. Neurosci Lett. 1985;57:19–24. doi: 10.1016/0304-3940(85)90034-5. [DOI] [PubMed] [Google Scholar]
- 5.Burns R S, LeWitt P A, Ebert M H, Pakkenberg H, Kopin I J. N Engl J Med. 1985;312:1418–1421. doi: 10.1056/NEJM198505303122203. [DOI] [PubMed] [Google Scholar]
- 6.Langston J W, Ballard P, Tetrud J W, Irwin I. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 7.Tatton N A, Kish S J. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 8.Ungerstedt U. Pharmacol Ther. 1976;2:37–40. doi: 10.1016/0306-039x(76)90016-7. [DOI] [PubMed] [Google Scholar]
- 9.Ziv I, Melamed E, Nardi N, Luria D, Achiron A, Offen D, Barzilai A. Neurosci Lett. 1994;170:136–140. doi: 10.1016/0304-3940(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 10.Offen D, Ziv I, Gorodin S, Barzilai A, Malik A, Melamed E. Biochim Biophys Acta. 1995;1268:171–177. doi: 10.1016/0167-4889(95)00075-4. [DOI] [PubMed] [Google Scholar]
- 11.Offen D, Strenin H, Ziv I, Melamed E, Hochman A. Exp Neurol. 1996;141:32–39. doi: 10.1006/exnr.1996.0136. [DOI] [PubMed] [Google Scholar]
- 12.Offen D, Ziv I, Barzilai A, Gorodin S, Glater E, Hochman A, Melamed E. Neurochem Int. 1996;31:207–216. doi: 10.1016/s0197-0186(96)00150-7. [DOI] [PubMed] [Google Scholar]
- 13.Michizuki H, Goto K, Mori H, Mizuno Y. J Neurol Sci. 1996;137:120–123. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins M M, Basgall E J, Zamrini E, Hill W D. Am J Pathol. 1997;150:119–131. [PMC free article] [PubMed] [Google Scholar]
- 15.Ziv, I., Barzilai, A., Offen, D., Nardi, N. & Melamed, E. (1997) J. Neural Transm. 49 Suppl., 69–76. [DOI] [PubMed]
- 16.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 17.Garcia I, Martinou I, Tsujimoto Y, Martinou J. Science. 1992;258:302–324. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- 18.Hockenbery D, Oltvai Z N, Yin X, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 19.Offen D, Ziv I, Panet H, Wasserman L, Stein R, Melamed E, Barzilai A. Cell Mol Neurobiol. 1997;17:289–304. doi: 10.1023/A:1026390201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziv I, Offen D, Barzilai A, Stein R, Melamed E. Apoptosis. 1997;2:4–10. [Google Scholar]
- 21.Oh Y J, Wong S C, Moffat M, O’Malley K L. Neurobiol Dis. 1995;2:157–167. doi: 10.1006/nbdi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 22.Farlie P G, Dringen R, Rees S M, Kannourakis G, Bernard O. Proc Natl Acad Sci USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard R, Farlie P, Bernard O. Dev Neurosci. 1997;19:79–85. doi: 10.1159/000111188. [DOI] [PubMed] [Google Scholar]
- 24.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 25.Dubois-Dauphin M, Frankowski H, Tsujimoto Y, Huarte J, Martinou J C. Proc Natl Acad Sci USA. 1994;91:3309–3313. doi: 10.1073/pnas.91.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfanti L, Strettoi E, Chierzi S, Cenni M C, Liu X H, Martinou J C, Maffei L, Rabacchi S A. J Neurosci. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlberg R, Mannerrik B. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 28.Flohe L, Gunzler W A. Methods Enzymol. 1984;105:115–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 29.Habig W H, Pabst M J, Jacoby W B. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 30.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Larm J A, Cheung N S, Beart P M. Eur J Pharmacol. 1996;314:249–254. doi: 10.1016/s0014-2999(96)00633-4. [DOI] [PubMed] [Google Scholar]
- 32.Pezzella F, Turley H, Kuzu I, Tungekar M F, Dunnill M S, Pierce C B, Harris A, Getter K C, Masson D Y. N Engl J Med. 1993;329:690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- 33.Bottenstein J E, Sato G H. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson M D. Curr Biol. 1997;7:R277–R281. doi: 10.1016/s0960-9822(06)00136-9. [DOI] [PubMed] [Google Scholar]
- 35.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 36.Ellerby L M, Ellerby H M, Park S M, Holleran A L, Murphy A N, Fiskum G, Kane D J, Testa M P, Kayalar C, Bredesen D E. J Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- 37.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno Y, Ikebe S, Hattori N, Nakagawa-Hattori Y, Mochizuki H, Tanaka M, Ozawa T. Biochim Biophys Acta. 1995;1271:265–274. doi: 10.1016/0925-4439(95)00038-6. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow R H, Parks J K, Miller S W, Tuttle J B, Trimmer P A, Sheehan J P, Bennet J P, Jr, Davis R E, Parker W D., Jr Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 40.Ozawa T, Hayakawa M, Katsumata K, Yoneda M, Ikebe S, Mizuno Y. Biochem Biophys Res Commun. 1997;235:58–161. doi: 10.1006/bbrc.1997.6754. [DOI] [PubMed] [Google Scholar]
- 41.Mogi M, Harada M, Kondo T, Mizuno Y, Narabayashi H, Riederer P, Nagatsu T. Neurosci Lett. 1996;215:137–139. [PubMed] [Google Scholar]
- 42.Vyas S, Javoy-Agid F, Herrero M T, Strada O, Boissiere F, Hibner U, Agid Y. J Neurochem. 1997;69:223–231. doi: 10.1046/j.1471-4159.1997.69010223.x. [DOI] [PubMed] [Google Scholar]