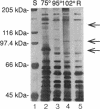
Abstract
The hyperthermophilic archaeon ES4, a heterotrophic sulfur reducer isolated from a deep-sea hydrothermal vent, is capable of protecting itself from thermal stress at temperatures above its optimum for growth. The thermotolerance of ES4 was determined by exposing log-phase cells to various lethal high temperatures. When ES4 was shifted from 95 to 102 degrees C, it displayed recovery from an exponential rate of death, followed by transient thermotolerance. When ES4 was shifted directly from 95 to either 105 or 108 degrees C, only exponential death occurred. However, a shift from 95 to 105 degrees C with an intermediate incubation at 102 degrees C also gave ES4 transient thermotolerance to 105 degrees C. The protein composition of ES4 was examined at temperatures ranging from 75 to 102 degrees C by one-dimensional electrophoresis. Two proteins with molecular masses of approximately 90 and 150 kDa significantly decreased in abundance with increasing growth temperature, while a 98-kDa protein, present at very low levels at normal growth temperatures (76 to 99 degrees C), was more abundant at higher temperatures. The enhanced tolerance to hyperthermal conditions after a mild hyperthermal exposure and the increased abundance of the 98-kDa protein at above-optimal temperatures imply that ES4 is capable of a heat shock-like response previously unseen in hyperthermophilic archaea.
Full text
PDF
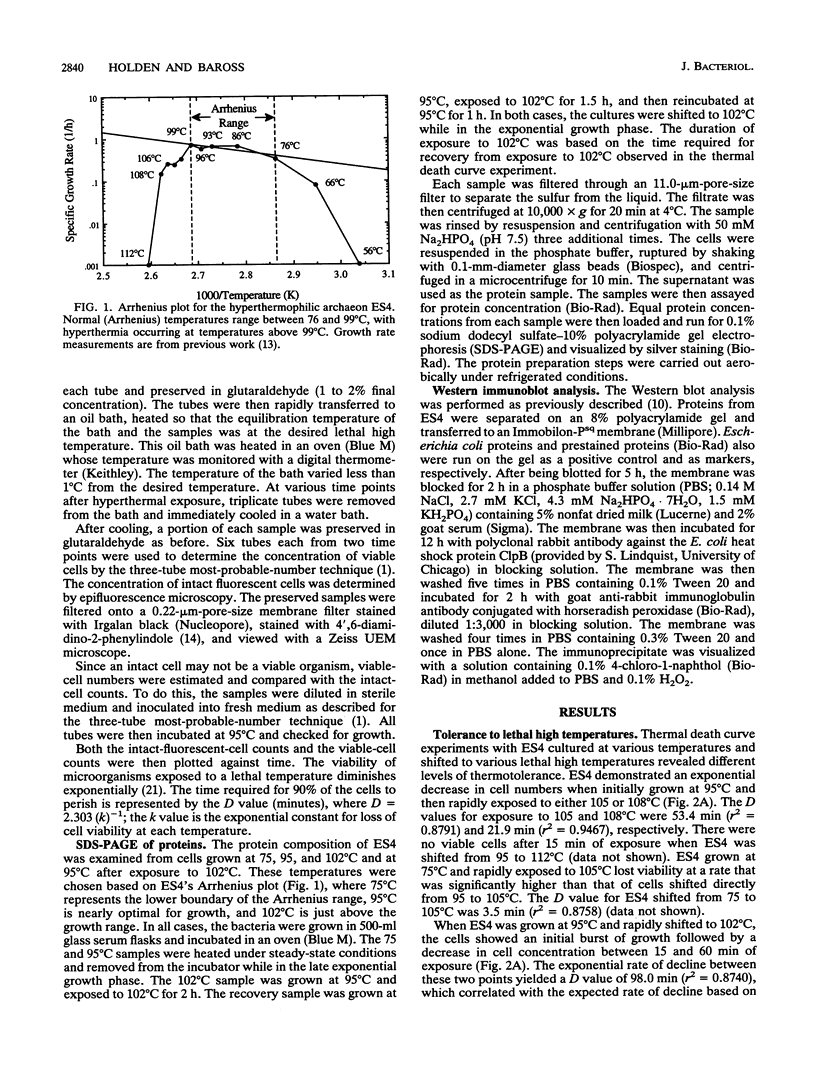
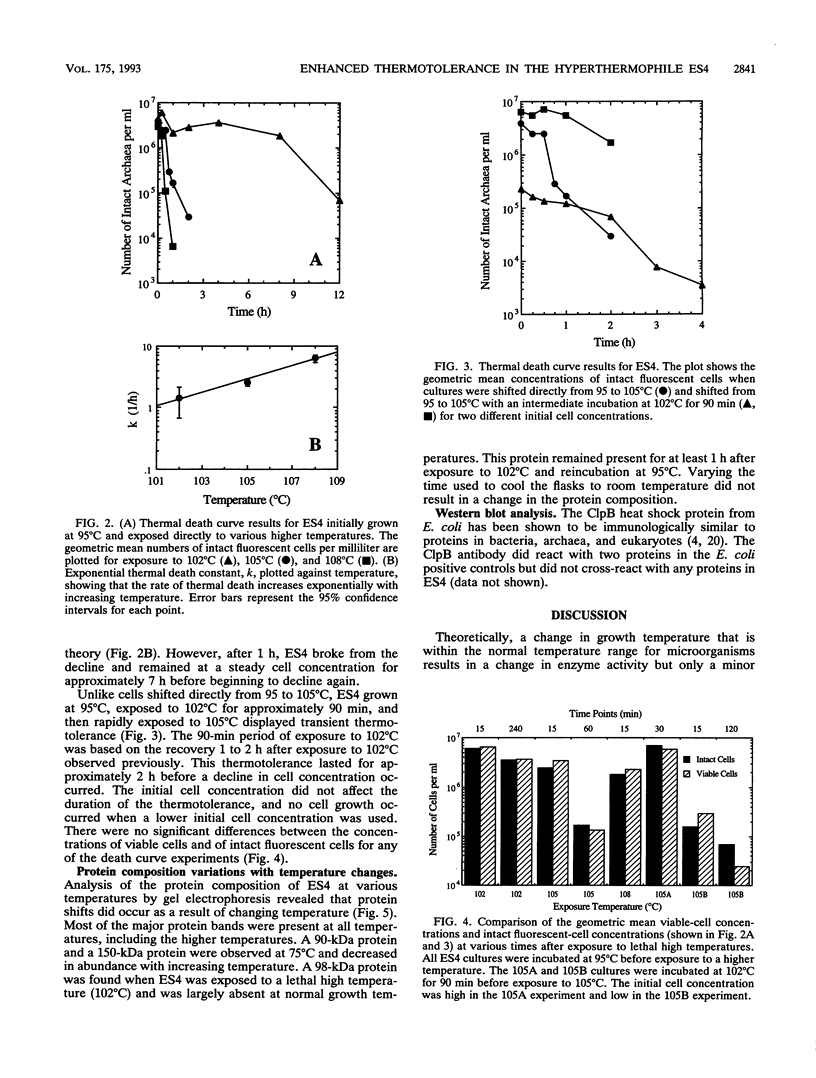


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouthier de la Tour C., Portemer C., Nadal M., Stetter K. O., Forterre P., Duguet M. Reverse gyrase, a hallmark of the hyperthermophilic archaebacteria. J Bacteriol. 1990 Dec;172(12):6803–6808. doi: 10.1128/jb.172.12.6803-6808.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mukund S., Adams M. W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991 Aug 5;266(22):14208–14216. [PubMed] [Google Scholar]
- Parsell D. A., Sanchez Y., Stitzel J. D., Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991 Sep 19;353(6341):270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- Phipps B. M., Hoffmann A., Stetter K. O., Baumeister W. A novel ATPase complex selectively accumulated upon heat shock is a major cellular component of thermophilic archaebacteria. EMBO J. 1991 Jul;10(7):1711–1722. doi: 10.1002/j.1460-2075.1991.tb07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992 Jun;11(6):2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Krzycki J. A., Dobrinski B., Lurz R., Reeve J. N. HMf, a DNA-binding protein isolated from the hyperthermophilic archaeon Methanothermus fervidus, is most closely related to histones. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5788–5791. doi: 10.1073/pnas.87.15.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Meloche M., Richards J. C. Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschii grown at different temperatures. J Bacteriol. 1991 Jun;173(12):3907–3910. doi: 10.1128/jb.173.12.3907-3910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Pedersen S., Ross B. M., Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J Bacteriol. 1991 Jul;173(14):4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. D., Nimmesgern E., Wall J. S., Hartl F. U., Horwich A. L. A molecular chaperone from a thermophilic archaebacterium is related to the eukaryotic protein t-complex polypeptide-1. Nature. 1991 Dec 12;354(6353):490–493. doi: 10.1038/354490a0. [DOI] [PubMed] [Google Scholar]
- Trent J. D., Osipiuk J., Pinkau T. Acquired thermotolerance and heat shock in the extremely thermophilic archaebacterium Sulfolobus sp. strain B12. J Bacteriol. 1990 Mar;172(3):1478–1484. doi: 10.1128/jb.172.3.1478-1484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. Microbial stress proteins. Adv Microb Physiol. 1990;31:183–223. doi: 10.1016/s0065-2911(08)60122-8. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]